Abstract
Treatment with an epidermal growth factor receptor inhibitor (egfri) in patients having non-small-cell lung cancer can cause frequent and diverse skin toxicities, an acneiform rash being one of the commonest. Although the exact pathophysiology of this rash and its development mechanisms remain unknown, investigators have noted that egfri-induced skin toxicity might be partly associated with sebaceous gland function. Sebum is composed mainly of the lipids squalene (sq), wax ester (we), triglyceride, free fatty acid, and cholesterol, which are secreted mostly from the sebaceous glands and by keratinocytes. We therefore investigated the lipid composition of sebum before and after administration of egfri and whether sebum composition was associated with the development of acneiform rash. To investigate any associated changes in sebum gland activity, we focused especially on alterations in the amounts of sq and we, which are secreted solely from the sebaceous glands.
In contrast to our expectations, we observed no substantial changes in the lipid composition of sebum before and after administration of egfri. Composition varies with the individual; however, the proportion of sq and we derived from the sebaceous glands was significantly lower in regions that did not develop acneiform rash than in regions that did. Our results suggest that development of an acneiform rash after administration of egfri could be related to sebaceous gland activity. Measurement of the lipid composition of sebum before therapy with egfri might predict which patients will be prone to acneiform rash.
Keywords: Skin toxicity, egfr inhibitor, sebum, sebaceous glands, lipids, skin
1. INTRODUCTION
Epidermal growth factor receptor inhibitor (egfri) is increasingly being used in the treatment of non-small-cell lung cancer1. Administration of egfri often results in skin toxicity, and some reports indicate that such toxicity could be associated with a therapeutic response. However, adverse cutaneous drug reactions from egfri can lead to discontinuation of therapy, with attendant loss of benefit2–4.
Acneiform rash, a typical skin toxicity, is a relatively early-onset side effect, usually occurring after 1 week of treatment and reaching maximum intensity after 2–3 weeks. The rash is characterized by erythematous inter- and intrafollicular papulopustules, which are sometimes itchy and painful, and which can lead to physical and psychosocial discomfort. Little is known about the mechanism by which this skin toxicity develops. Investigators have noted that egfri-induced skin toxicity might be associated with the sebaceous glands, where epidermal growth factor receptor (egfr) is expressed5. Meanwhile, no publications have looked into the development of skin toxicity with respect to skin physiology, such as sebum lipid profile. Sebum is composed mainly of the lipids squalene (sq), wax ester (we), triglyceride, free fatty acid, and cholesterol, which are secreted by the sebaceous glands and through the intercellular lipid bilayer6. Secretion of sq and we comes mostly from the sebaceous glands, which suggests that the proportions of those lipids to sebum lipids overall might serve as an indicator of sebaceous gland activity. In the present report, we investigated the association between the lipid composition of sebum and acneiform rash before and after administration of egfri.
2. METHODS
After obtaining approval from the Kyushu University Hospital ethics committee and written consent from the subjects, we studied 6 patients (2 men, 4 women; mean age: 70.8 years) diagnosed with non-small-cell lung cancer who were candidates for gefitinib or erlotinib treatment. No patient had a prior history of acne. Two patients were taking a daily antihypertensive drug; no patient was taking any other relevant medication.
Sebum-absorbing sheets were used to collect sebum samples from the face and chest of patients before, and 2 and 6 weeks after, administration of egfri. Samples were collected during the day in an air-conditioned examination room at Kyushu University Hospital under normal ambient conditions. Room temperature was 24°C–28°C, and humidity was 25%–58%. High-performance liquid chromatography was used to determine the amount of each sebum lipid (sq, we, triglyceride, cholesterol, free fatty acid) in the samples. At the time of sampling, the presence or absence of acneiform lesions on the face and chest was noted.
3. RESULTS
3.1. Sebum Lipids Before and After Administration of EGFRI
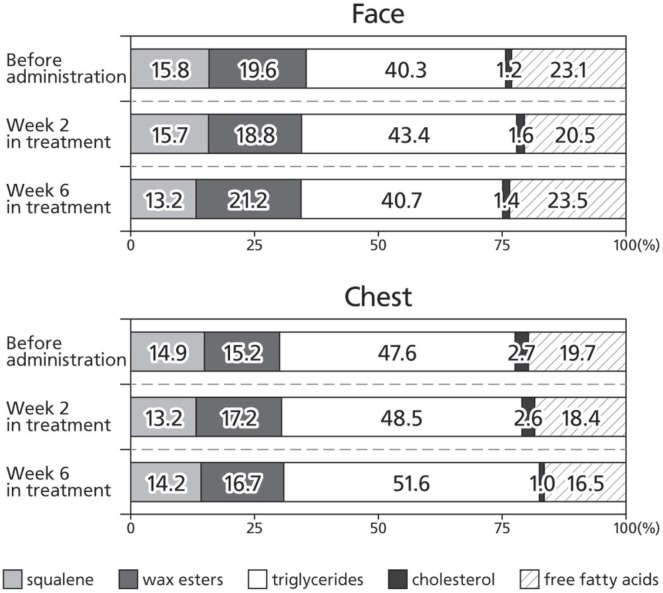
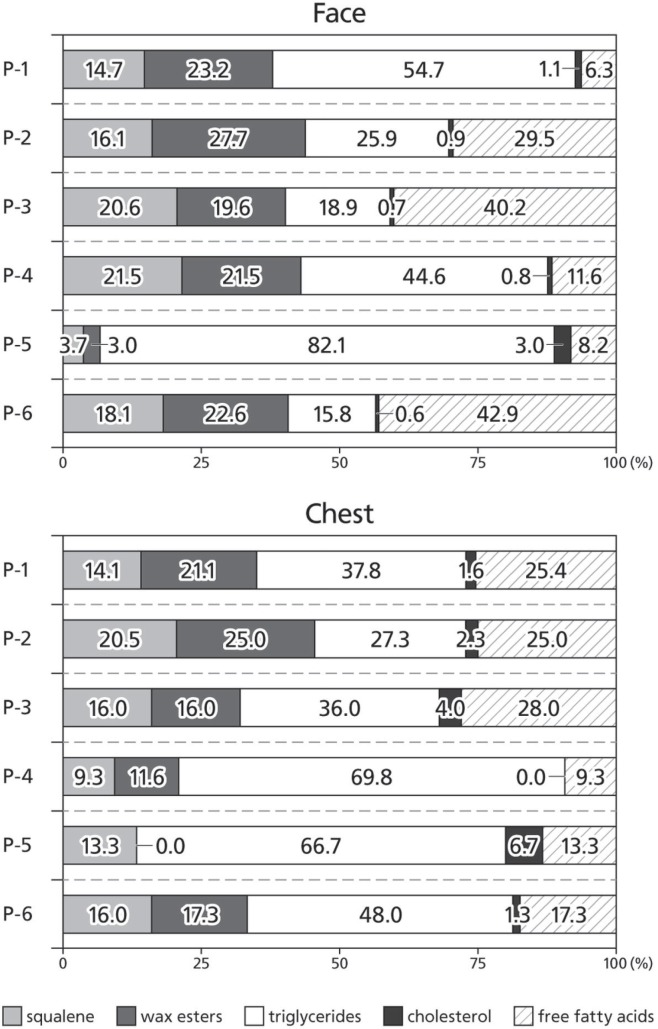
The lipid composition of sebum showed no substantial change after administration of egfri (Figure 1); specifically, we observed no change in the proportion of sq and we to total sebum lipids after egfri administration. Because the intensity of skin toxicity varies with the individual patient, we also performed inter-patient comparisons of the lipid composition of sebum before egfri administration. We observed remarkable inter-patient variability in the proportion of sq and we, the lipids of sebaceous gland origin (Figure 2).
FIGURE 1.
Alterations in the lipid composition of sebum in all patients during therapy with epidermal growth factor receptor inhibitor (egfri). Before administration of egfri, and at 2 weeks and 6 weeks after administration, sebum-absorbing sheets were used to collect sebum from the face and chest of treated patients. High-performance liquid chromatography was then used to determine the proportion of each lipid in the sebum.
FIGURE 2.
Lipid composition of sebum in each study patient before administration of epidermal growth factor receptor inhibitor.
3.2. Proportion of SQ and WE and Development of Acneiform Rash
Because the inter-patient comparison revealed such variability in lipid proportions, we also investigated possible associations between the ratio of sq plus we to total sebum lipids and the development of acneiform rash after initiation of treatment with egfri by anatomic region in each patient at each time point. A low ratio was significantly associated with regions in which acneiform rash did not appear during the study (Wilcoxon signed-rank p < 0.01, Table i, Figure 3).
TABLE I.
Association of the ratio of squalene (sq) plus wax ester (we) to total sebum lipids (tsl) in patients treated with epidermal growth factor receptor inhibitor, by presence of rash, body region, and time point
| Patient | Rash characteristics | sq+we:tsl ratio | p Value | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Group | id | Site | Time point (weeks)a | Value | Mean | |
| With rash | P-01 | Face | 2 | 41.3 | ||
| 6 | 40.8 | |||||
| Chest | 2 | 37.9 | ||||
| P-02 | Face | 2 | 41.4 | |||
| P-03 | Face | 6 | 44.9 | |||
| P-04 | Face | 2 | 39.4 | 38.1±10.3 | ||
| 6 | 33.3 | |||||
| P-05 | Face | 6 | 7.1 | |||
| P-06 | Face | 2 | 42.0 | |||
| 6 | 45.6 | <0.01 by Wilcoxon signed rank test | ||||
| Chest | 2 | 39.0 | ||||
| 6 | 44.7 | |||||
| Without rash | P-01 | Chest | 6 | 38.3 | ||
| P-02 | Chest | 2 | 14.2 | |||
| P-03 | Chest | 6 | 29.6 | |||
| P-04 | Chest | 2 | 32.3 | 24.2±11.1 | ||
| 6 | 30.8 | |||||
| P-05 | Face | 2 | 8.7 | |||
| Chest | 2 | 28.6 | ||||
| 6 | 11.1 | |||||
Sebum lipid measurements are missing at 2 weeks for patient P-03 with rash (because of the patient’s physical condition) and at 6 weeks for patient P-02 with rash (because of failure to visit the hospital).
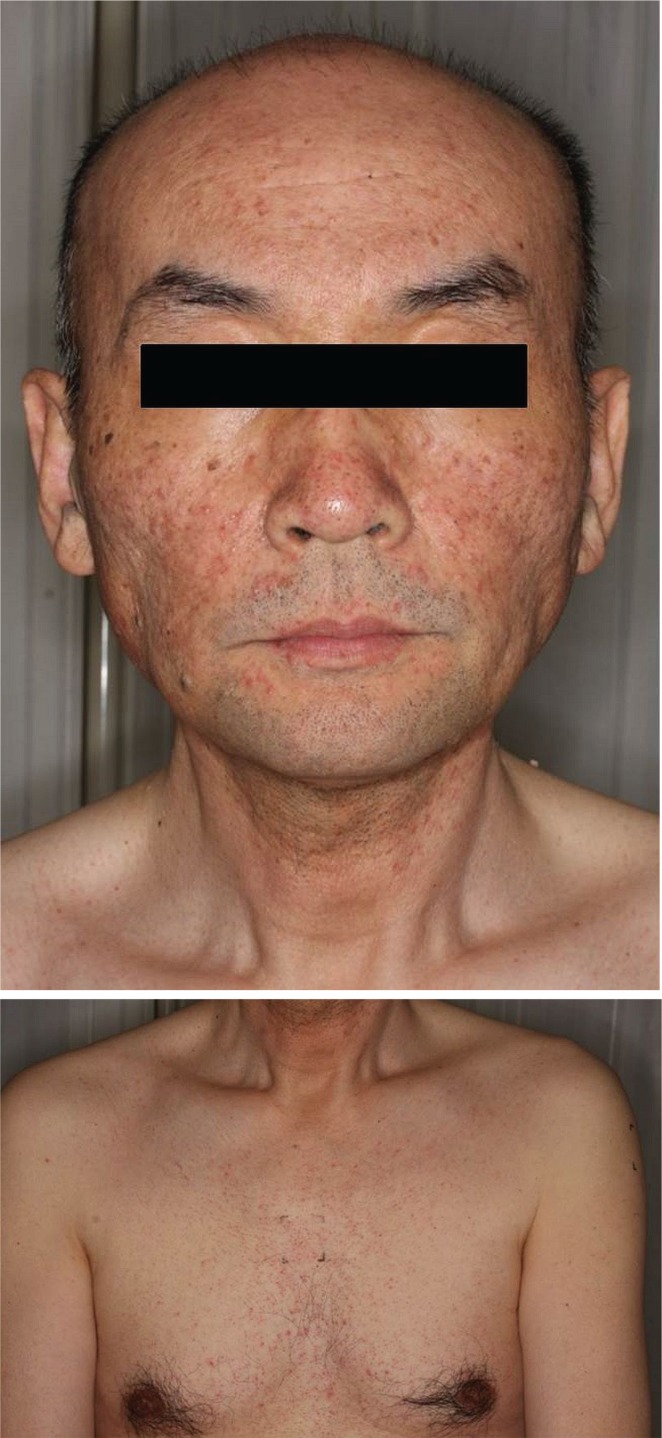
FIGURE 3.
Typical acneiform rash of face and chest in this study.
4. DISCUSSION AND CONCLUSIONS
Epidermal growth factor inhibitor seems to predispose to cutaneous toxicity by affecting the sebaceous glands and causing hair follicle inflammation and dyskeratosis7. Sebaceous gland dysfunction appears to contribute most to the eruptions, because the acneiform rash develops in the early phase of egfri treatment5,7. Recently, a study in a mouse model demonstrated increased secretion of sebum from the sebaceous glands because of constant egfr activation8,9. Further nonclinical studies confirmed that activation of egfr induces sebaceous gland enlargement and sebocyte proliferation by upregulating the transcription c-Myc10–12, indicating a pivotal role for egfr signalling in maintaining the size, cell number, and sebum activity of sebaceous glands. The foregoing work indicates a close correlation between sebum activity and egfr signalling.
The present research shows that regions with low sq and we of sebaceous gland origin could have a lower frequency of acneiform rash, which in turn supports the possibility that high sebaceous gland activity could predispose patients to acneiform rash. Thus, our results suggest that pre-treatment analyses of the lipid composition of sebum could potentially provide a means to predict which patients will be highly predisposed to acneiform rash. In such patients, medications effective in reducing sebum activity—including retinoic acid (tretinoin) and adapalene—might be beneficial, in addition to anti-inflammatory drugs such as steroids and antibiotics, for preventing and treating acneiform rash. The possible association between the development of skin toxicity and the patient’s therapeutic response could warrant further analyses with additional patients, but our investigation points to the predictability of acneiform rash development based on a measurement of sebum lipid composition (and, potentially, the predictability of clinical benefit from egfri).
The conclusions in this report are based on research involving a limited number of patients and skin regions, and thus further investigations with additional skin regions in a larger patient population are warranted.
5. ACKNOWLEDGMENTS
This study was supported by unrestricted educational grant from the nonprofit organization jasmin.
6. CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
7. REFERENCES
- 1.Cufer T, Ovcaricek T, O’Brien ME. Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. Eur J Cancer. 2013;49:1216–25. doi: 10.1016/j.ejca.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Orditura M, De Vita F, Galizia G, et al. Correlation between efficacy and skin rash occurrence following treatment with the epidermal growth factor receptor inhibitor cetuximab: a single institution retrospective analysis. Oncol Rep. 2009;21:1023–8. doi: 10.3892/or_00000319. [DOI] [PubMed] [Google Scholar]
- 3.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 4.Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase iii studies. Clin Cancer Res. 2007;13:3913–21. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 5.Takata T, Tarutani M, Zouboulis CC, Sano S. Sebaceous glands as the primary target of egfr-inhibitors in the development of papulopustular eruption. J Dermatol Sci. 2012;66:165–8. doi: 10.1016/j.jdermsci.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Picardo M, Ottaviani M, Camera E, Mastrofrancesco A. Sebaceous gland lipids. Dermatoendocrinol. 2009;1:68–71. doi: 10.4161/derm.1.2.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guttman–Yassky E, Mita A, De Jonge M, et al. Characterisation of the cutaneous pathology in non-small cell lung cancer (nsclc) patients treated with the egfr tyrosine kinase inhibitor erlotinib. Eur J Cancer. 2010;46:2010–19. doi: 10.1016/j.ejca.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Dahlhoff M, de Angelis MH, Wolf E, Schneider MR. Ligand-independent epidermal growth factor receptor hyperactivation increases sebaceous gland size and sebum secretion in mice. Exp Dermatol. 2013;22:667–9. doi: 10.1111/exd.12219. [DOI] [PubMed] [Google Scholar]
- 9.Zouboulis CC. Epidermal growth factor receptor and the sebaceous gland. Exp Dermatol. 2013;22:695–6. doi: 10.1111/exd.12220. [DOI] [PubMed] [Google Scholar]
- 10.Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–8. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- 11.Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–68. doi: 10.1016/S0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 12.Bull JJ, Pelengaris S, Hendrix S, Chronnell CM, Khan M, Philpott MP. Ectopic expression of c-Myc in the skin affects the hair growth cycle and causes an enlargement of the sebaceous gland. Br J Dermatol. 2005;152:1125–33. doi: 10.1111/j.1365-2133.2005.06458.x. [DOI] [PubMed] [Google Scholar]