Abstract
Amoebic keratitis, a sight-threatening corneal infection, mainly occurs in contact lens wearers who wash their eyes with tap water. The present research was conducted to identify the occurrence of potentially pathogenic free-living amoebae (FLA) in tap water sources on Kish Island, a tourist region in Iran. Amoebae were detected using a culture-enriched method and by polymerase chain reaction (PCR)/sequencing of the diagnostic fragment 3 region of the 18S rRNA gene of Acanthamoeba. In the case of other free-living amoebae species, PCR/sequencing analysis of the 18S rDNA was conducted. Results of this study showed the presence of Acanthamoeba belonging to T3, T4, T5, and T11 genotypes in tap water sources. Additionally, Vermamoebae vermiformis was detected in three water samples. This is the first report of the Acanthamoeba genotypes T3, T4, T5, and T11 and V. vermiformis species in tap water sources in a tourist region in Iran.
Keywords: free-living amoebae, Kish Island, sequencing analysis, Acanthamoeba, Vermamoeba vermiformis
Introduction
Free-living amoebae (FLA) include genera with pathogenic potential, such as Acanthamoeba, Balamuthia, Naegleria, and Sappinia. These ubiquitous FLA are widespread in the environment, including different types of water sources.1–3 Contamination of water sources, such as pools, sea, and recreational waters, particularly those associated with human activity, is an important risk factor for amoebic infections.2,4,5 One of the important issues in water contamination is the presence of pathogenic amoebae in tap or drinking water supply sources. Tap water goes through filtration and chlorination processes before human use; thus, this may reflect in the lower microbial contamination of such waters. However, this depends on many factors, such as effective filtration and disinfection systems of the mentioned water sources.5–9 Amoebae belonging to Acanthamoeba, Vermamoebae (previously known as Hartmannella spp.), and Vahlkampfiids may be the cause of amoebic keratitis (AK) in high-risk populations, such as contact lens wearers and individuals who have undergone eye surgery.10 Prognosis of AK is very poor, and most patients show episodes of recurrence even after effective therapy with a combination of drugs, such as propamidine isethionate and polyhexamethylene biguanide (0.02% PHMB). Moreover, most of the reported cases of AK in cosmetic soft contact lens wearers in Iran as well as worldwide are caused by Acanthamoeba and Vermamoeba genera.8,11–13 Interestingly, these patients report a history of contact with tap water before wearing their lenses. Improper maintenance of lenses, including cleaning of contact lenses with tap or distilled water, is still one of the main causes of AK.8
Unfortunately, FLA-related infections continue to increase in Iran.14–16 The first reported cases in Iran were approximately 20 years ago; since then, several studies indicating an increased incidence of the disease in Iran have been published. However, there are still many unreported cases because of the lack of knowledge regarding the diagnosis of AK.14,16 Other diseases related to FLA have been reported as cases of mixed infections caused by Acanthamoeba and Hartmannella or Acanthamoeba and Vahlkampfia.12,13 There are no reports of granulomatous amoebic encephalitis (GAE) in the country yet; however, there has been a single report of primary amoebic meningoencephalitis (PAM) in Qhum city (central Iran) isolated from the cerebrospinal fluid (CSF) of a 6-month-old-boy with a history of washing his nasal passages with contaminated tap water.17
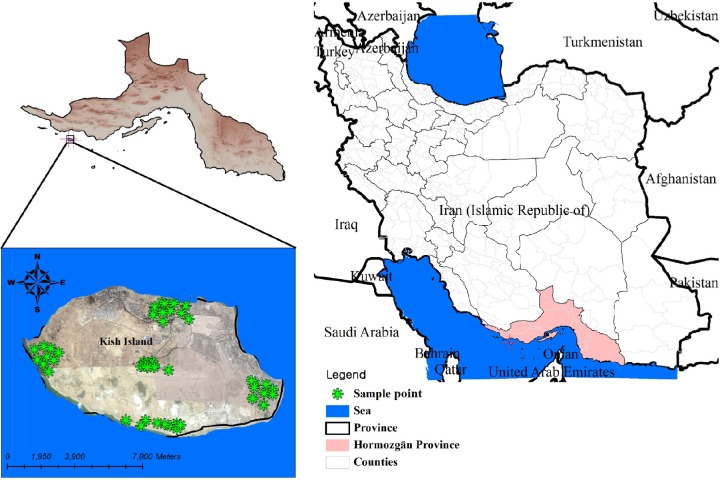
Kish Island is located in Hormozgan Province in southern Iran (Fig. 1) and attracts many people annually due to its climate and tourist interest. Because of the warm climate and the manner in which tap water is stored in the region, it is suspected that the presence of amoebae may be high in water storage tanks. In this connection, it should be mentioned that there are also reported cases of AK infections caused by washing contact lenses in tap water sources in this region.
Figure 1.
Kish Island location in Hormozghan province, Southern Iran (sampling points from five different districts of the region are shown by stars).
Therefore, tap water sources were surveyed to address the occurrence of FLA in the water sources of Kish Island, Iran, using both morphologically and molecular-based methods. To the best of our knowledge, this is the first study reporting the presence of potentially pathogenic FLA in this region.
Methods
Sampling geographical area
This study was conducted in Kish Island, southern Iran. Kish Island is part of the Hormozgan province and is located in the Persian Gulf 19 km (12 mi) from mainland Iran and has an area of approximately 91 km2 (Fig. 1). The island is positioned along the 1,359-km (844-mi)-long Iranian north coastline in the Persian Gulf at the first quarter from the Hormuz entrance to the Persian Gulf (Fig. 1). The island is 15.45 km long from west to east coast. The average temperature in summer is 40°C. Tap water supplies in Kish Island are mainly maintained using reservoir tanks located in houses or resort yards. All tap water is chlorinated and filtered by the city officials in a drinking water plant, according to World Health Organization-defined criteria (0.2–0.7 ppm).9
Processing samples and morphological identification
Fifty-five water samples were collected randomly from the tap water of five districts of Kish Island (north, south, east, west, and central). All tested tap water had been stored in water tanks in the yard of houses, resorts, or malls, and was in direct contact with sunlight all day. Notably, water storage tanks are drained and cleaned approximately every 6 months. Processing of the samples was performed within eight hours of collection. Each sample was filtered using nitrocellulose membranes (0.22 m pore size and 45 mm diameter) and cultured using an enrichment cultivation method. Briefly, culturing was performed using 1.5% non-nutrient bactoagar (Difco, USA) along with heat-inactivated Escherichia coli as food sources of amoebae.3,14 Because the medium was non-nutrient, unwanted organisms could not grow.
Plates were then monitored daily by inverted microscopy.18 Positive plates were then cloned to eliminate bacterial and fungal contamination. Axenic culture of the isolated strains was performed according to previous work.4,16
DNA extraction, PCR analysis, and sequencing
DNA extraction was performed on positive plates using the Instagene matrix (Chelex; Biorad)19 for trophozoites or phenol–chloroform method for cysts. Based on the morphological identification of the amoebae in cultures, primer sets were used for further molecular analysis. Morphological identification of Acanthamoeba genus was based on the presence of flat trophozoites with acantopodia and double-walled cysts with various shapes of endocysts, such as stars and triangles. Vermamoebae were detected morphologically by their elongated, thin trophozoites, their length exceeding their girth by approximately sixfold, and cysts were detected by their smooth, spherical, or slightly oval appearance with a single wall.
Polymerase chain reactions (PCRs) were performed using genus-specific primers targeting the diagnostic fragment 3 (DF3) of 18s rRNA gene for Acanthamoeba20,21 and NA primers targeting various genera of amoebae such as Vermamoebae.22 The sequences of primer sets used in the present study were as follows: JDP1 5′-GGCCCAGATCGTTTACCGTGAA-3′ and JDP2 5′-TCTCACAAGCTGCTAGGGAGTCA-3′ and NA1 5′-GCT CCA ATA GCG TAT ATT AA-3′ and NA2 5′-AGA AAG AGC TAT CAATCT GT-3′. PCR was performed in 30 μL Ampliqone (Taq DNA Polymerase Master Mix RED, Denmark) as a readymade mixture. Twenty-five microliters of Taq Master mix was used with 5 ng template DNA, 0.1 μM of each primer, and distilled water. The thermal cycling conditions were an initial denaturing step of 94°C for 1 minute and 35 repetitions at 94°C for 35 seconds; annealing steps were carried out at 56°C and 50°C for 45 seconds for Acanthamoeba and Vermamoeba, respectively, and 72°C for 1 minute. The extension time was prolonged for five minutes at 72°C. PCR products were electrophoresed on 2% agarose gel stained with a solution of ethidium bromide and visualized under ultraviolet light.
Genus and genotype/species identification
Identification of Acanthamoeba genotypes and other FLA genera was based on alignment analysis of the obtained sequences with the genes available in the basic alignment search tool of the U.S. National Center for Biotechnology Information. All sequences were then submitted to the Genebank database, and are under the following accession numbers: KP337292–KP337308.
Results and Discussion
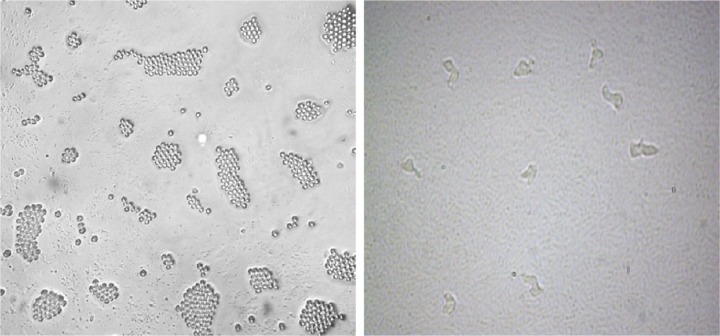
Overall, out of 55 tap water samples collected from five different part of Kish Island, 21 (38.2%) isolates were recovered for FLA after 3 days to 1 month of incubation. The most contaminated water sources were in the northern district of Kish Island, which the endemic population inhabits. However, water contamination in the central part of the island was less in comparison with other districts (Table 1). Fourteen samples (66.7%) contained Acanthamoeba spp. with double wall cysts and flat trophozoites with acanthopodia. Axenic cultivation was successful in all isolated strains (Fig. 2). The cysts were different regarding the size and morphology of the endocysts and included star-, triangle-, and round-shaped cysts (Figs. 3 and 4). Three plates (14.2%) contained elongated amoebae and small round cysts measuring between 10 and 15 μm. These amoebae were identified as members of the Hartmannellidae family (V. vermiformis species) by morphological (Fig. 5) and molecular tools. Moreover, two samples positive for Acanthamoeba, T3 and T4 (NL19 and NL27), also contained V. vermiformis. Furthermore, other FLA, such as Miniamoebae and Thecamoebae genera, were detected in four samples (19%) but were excluded from our research.
Table 1.
Percent contamination of tap water sources in different district of Kish Island, Iran.
| DISTRICT OF THE ISLAND |
TOTAL | POSITIVITY n (%) |
|---|---|---|
| North | 11 | 8 (72.7) |
| South | 11 | 4 (36.3) |
| West | 11 | 3 (27.2) |
| East | 11 | 4 (36.3) |
| Center | 11 | 2 (18.1) |
| Total | 55 | 21 (38.2) |
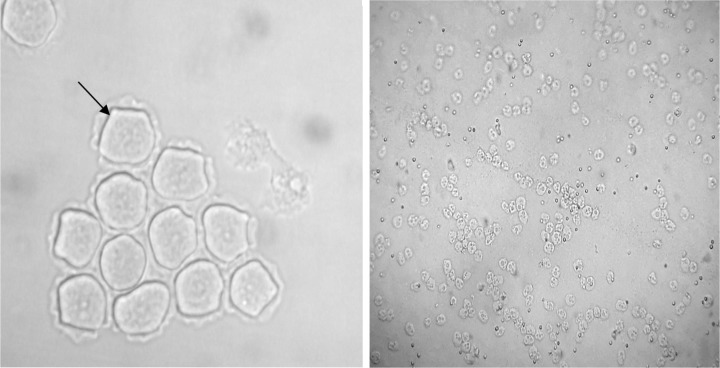
Figure 2.
Light photomicrograph of Acanthamoeba cysts (left: ×400) and trophozoites (right: ×100).
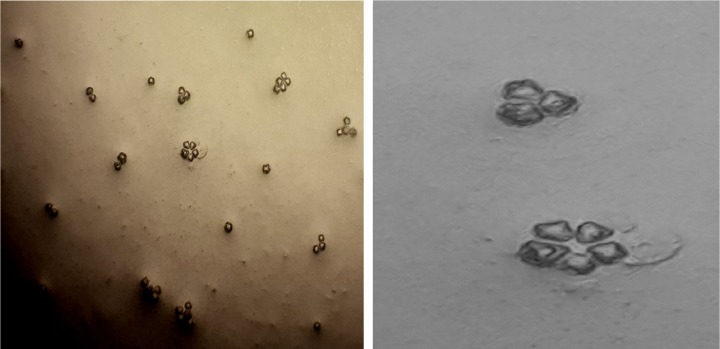
Figure 3.
Acanthamoeba belonging to T4 genotype with star-shaped endocysts (left: ×100; right: ×400) isolated from tap water in the north of Kish Island, Iran.
Figure 4.
Acanthamoeba belonging to T5 genotype with round-shape endocysts (×400) isolated from tap water in the north of Kish Island, Iran.
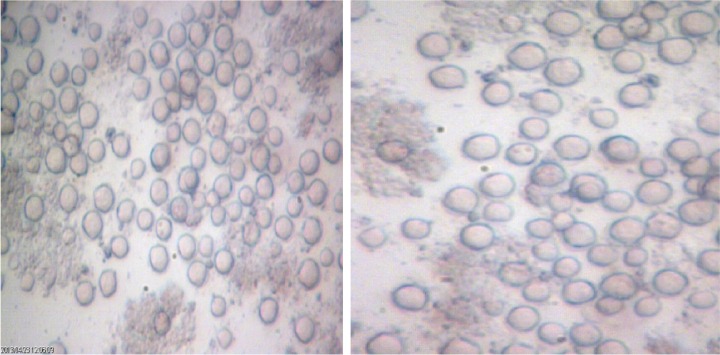
Figure 5.
Vermamoeba vermiformis trophozoites (right) and cysts (left) isolated from tap water in the north of Kish Island, Iran.
The 14 Acanthamoeba isolates amplified a 500-bp product using the DF3 region primers, and the sequencing results revealed the presence of various genotypes, including T3 (NL15, NL19) (corresponding to A. griffini) (14.2%), T4 (57.1%), T5 (NL14, NL18, NL33) (corresponding to A. lenticulata) (21.42%), and T11 (NL17) (7.1%) (Table 2). T4 was the most prevalent detected genotype. It should be mentioned that no Balamuthia, Vahlkampfiids, or Sappinia isolates were detected in this survey.
Table 2.
Location and distribution of free-living amoebae in tap water sources of Kish Island.
| CODE | LOCALITY | ORIGIN | GENOTYPE | QUERY COVERAGE/MAX. IDENTITY | ACCESSION NO. |
|---|---|---|---|---|---|
| NL7 | North | House | T4 | 94/97 | KP337297 |
| NL12 | Center | Resort | T4 | 96/99 | KP337304 |
| NL14 | North | House | T5 | 100/100 | KP337299 |
| NL15 | North | House | T3 | 100/100 | KP337292 |
| NL17 | East | House | T11 | 100/100 | KP337301 |
| NL18 | Center | Resort | T5 | 100/100 | KP337302 |
| NL19 | North | House | T3/V. vermiformis | 100/100 | KP337303/KP337306 |
| NL26 | Center | Resort | T4 | 96/99 | KP337298 |
| NL27 | North | Hospital | T4/V. vermiformis | 97/96 99/99 |
KP337293/KP337307 |
| NL32 | East | House | V. vermiformis | 96/98 | KP337308 |
| NL33 | North | House | T5 | 100/100 | KP337300 |
| NL34 | North | House | T4 | 96/99 | KP337305 |
| NL36 | South | Mall | T4 | 92/99 | KP337294 |
| NL37 | West | Mall | T4 | 98/96 | KP337295 |
| NL48 | North | House | T4 | 95/96 | KP337296 |
This work was performed to detect the presence of FLA in the tap water supplies in Kish Island, southern Iran. The obtained results revealed that 38.2% of the tested water sources were contaminated with potentially pathogenic amoebae. This may highlight that, despite the filtration, chlorination, and treatment processes, amoebae are able to colonize the water distribution systems in the area probably by biofilm formation, as previously suggested in other regions by Cabral et al.23 The most contaminated water was in the northern region, which also presents one of the oldest water distribution systems on the Island. There are several reports regarding contamination of recreational water sources in Iran and around the world, including hot springs, recreational river waters, ponds, and swimming pools.21,22,24 The range of contamination in different kinds of water sources has been recorded from 23% to even 89%.4,24,25 Worldwide, a few studies have also shown the presence of potentially pathogenic Acanthamoeba spp. in tap water sources.23,26,27 A study conducted by Hoffmann and Michel in Germany revealed that water sources are widely contaminated with FLA even after filtration processes.28 In addition, Ozcelic et al showed that FLA were observed in 30% of tap and recreational water sources in Turkey.27 However, chlorine may destroy other FLA, such as Vahlkampfiids, Sappinia, and Balamuthia. Water purification in Kish Island consists of both filtration and chlorination procedures. However, according to this study, these processes are not eliminating these amoebae, most likely because of the ability of these organisms to form resistant cysts. Notably, chlorine has been reported not to eliminate Acanthamoeba in the doses used for water treatment.7,10,11 In addition, Thomas et al in 2008 showed that there is a high biodiversity of FLA and associated bacteria in water treatment plants. Their study reflected that potentially pathogenic amoebae were recovered after a treatment chain of sand filtration, ozonation, chlorination, and sand filtration.
Notably, pipe materials and pores of filters are also important in the retention and proliferation of such amoebae.29 The presence of Acanthamoeba and Vermamoeba reflects that these FLA can survive the purification process and pass through the filter pores. Indeed, the pore size of the filtration membranes is not capable of preventing amoebae from passing through the pores due to the smaller size of the amoebae.
Accordingly, various Acanthamoeba genotypes have been reported in the present study. Moreover, all isolated genotypes have been recorded as agents of AK in Iran and worldwide. In previous research conducted by Niyyati et al, genotypes belonging to T3, T4, and T11 were the causative agents of AK cases.16
Overall, the occurrence of 38.2% of potentially pathogenic FLA, including Acanthamoeba T3, T4, T5, and T11 genotypes and Vermamoebae, in tap water sources in this tourist region in Iran reflects the urgent need to improve water treatment procedures to prevent FLA-related infections.
Acknowledgments
The authors wish to thank Virayesh Center for the English edit of the manuscript.
Footnotes
ACADEMIC EDITOR: Raul Rivas, Editor in Chief
FUNDING: JLM was supported by the Ramón y Cajal Sub program of the Spanish Ministry of Economy and Competitivity (RYC201108863). This work was supported by a grant from Department of Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran (Grant number: 1390-1-91-7783). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments and wrote the first draft of the manuscript: MN. Performed the experiments: ZL. Made critical revisions and approved the final version: JLM. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 3.Khan NA. Acanthamoeba, Biology and Pathogenesis. Caister Academic Press; London: 2009. [Google Scholar]
- 4.Lorenzo-Morales J, Miranda CA, Jimenez C, Tejedor ML, Valladares B, Ortega-Rivas A. Evaluation of Acanthamoeba isolates from environmental sources in Tenerife, Canary Islands, Spain. Ann Agric Environ Med. 2005;12:233–236. [PubMed] [Google Scholar]
- 5.Yousuf FA, Siddiqui R, Subhani F, Khan NA.Status of free-living amoebae (Acanthamoeba spp., Naegleria fowleri, Balamuthia mandrillaris) in drinking water supplies in Karachi, Pakistan. J Water Health 2013112371–375. [DOI] [PubMed] [Google Scholar]
- 6.Winck MA, Caumo K, Rott MB. Prevalence of acanthamoeba from tap water in rio grande do Sul, Brazil. Curr Microbiol. 2011;63(5):464–469. doi: 10.1007/s00284-011-0003-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Masters S, Hong Y. Effect of disinfectant, water age, and pipe material on occurrence and persistence of Legionella, mycobacteria, Pseudomonas aeruginosa, and two amoebas. Environ Sci Technol. 2012;46(21):11566–11574. doi: 10.1021/es303212a. [DOI] [PubMed] [Google Scholar]
- 8.Legarreta JE, Nau AC, Dhaliwal DK. Acanthamoeba keratitis associated with tap water use during contact lens cleaning: manufacturer guidelines need to change. Eye Contact Lens. 2013;39(2):158–161. doi: 10.1097/ICL.0b013e31827a79ee. [DOI] [PubMed] [Google Scholar]
- 9.Coşkun KA, Ozçelik S, Tutar L, Elaldı N, Tutar Y. Isolation and identification of free-living amoebae from tap water in Sivas, Turkey. Biomed Res Int. 2013;2013:675145. doi: 10.1155/2013/675145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30:564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 11.Hassan A, Farouk H, Hassanein F, Abdul-Ghani R, Abdelhady AH. Acanthamoeba contamination of hemodialysis and dental units in Alexandria, Egypt: a neglected potential source of infection. J Infect Public Health. 2012;5(4):304–310. doi: 10.1016/j.jiph.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Niyyati M, Lorenzo-Morales J, Rezaie S, et al. First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp Parasitol. 2010;126:89–90. doi: 10.1016/j.exppara.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Abedkhojasteh H, Niyyati M, Rahimi F, Heidari M, Farnia S, Rezaeian M. First report of Hartmannella keratitis in a cosmetic soft contact lens wearer in Iran. Iran J Parasitol. 2013;8(3):481–485. [PMC free article] [PubMed] [Google Scholar]
- 14.Rezaeian M, Farnia SH, Niyyati M, Rahimi F. Amoebic keratitis in Iran 1997–2007. Iran J Parasitol. 2007;2:1–6. [Google Scholar]
- 15.Rezaeian M, Niyyati M. Pathogenic Free Living Amoebae. Tehran: Tehran University of medical Sciences; 2011. [Google Scholar]
- 16.Niyyati M, Lorenzo-Morales J, Rezaie S, et al. Genotyping of Acanthamoeba isolates from clinical and environmental specimens in Iran. Exp Parasitol. 2009;121:242–245. doi: 10.1016/j.exppara.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Movahedi Z, Shokrollahi MR, Aghaali M, Heydari H. Primary amoebic meningoencephalitis in an Iranian infant. Case Rep Med. 2012;2012:782854. doi: 10.1155/2012/782854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page FC. A New Key to Freshwater and Soil Gymnamoebae. Ambleside, UK: Freshwater Biological Association; 1988. [Google Scholar]
- 19.Lasjerdi Z, Niyyati M, Haghighi A, et al. Potentially pathogenic free-living amoebae isolated from hospital wards with immunodeficient patients in Tehran, Iran. Parasitol Res. 2011;109(3):575–580. doi: 10.1007/s00436-011-2288-5. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder JM, Booton GC, Hay J, et al. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoeba from humans with keratitis and from sewage sludge. J Clin Microbiol. 2001;39:1903–1911. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nazar M, Haghighi A, Niyyati M, et al. Genotyping of Acanthamoeba isolated from water in recreational areas of Tehran, Iran. J Water Health. 2011;9(3):603–608. doi: 10.2166/wh.2011.152. [DOI] [PubMed] [Google Scholar]
- 22.Niyyati M, Lasjerdi Z, Nazar M, Haghighi A, Nazemalhosseini Mojarad E. Screening of recreational areas of rivers for potentially pathogenic free-living amoebae in the suburbs of Tehran, Iran. J Water Health. 2012;10(1):140–146. doi: 10.2166/wh.2011.068. [DOI] [PubMed] [Google Scholar]
- 23.Marciano-Cabral F, Jamerson M, Kaneshiro ES. Free-living amoebae, Legionella and Mycobacterium in tap water supplied by a municipal drinking water utility in the USA. J Water Health. 2010;8:71–82. doi: 10.2166/wh.2009.129. [DOI] [PubMed] [Google Scholar]
- 24.Kilvington S, Gray T, Dart J, Morlet N, Beeching JR, Frazer Matheson M. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci. 2004;45:165–169. doi: 10.1167/iovs.03-0559. [DOI] [PubMed] [Google Scholar]
- 25.Shoff ME, Rogerson A, Kessler K, Schatz S, Seal DV. Prevalence of Acanthamoeba and other naked amoebae in south Florida domestic water. J Water Health. 2008;6:99–104. doi: 10.2166/wh.2007.014. [DOI] [PubMed] [Google Scholar]
- 26.Thomas V, Loret JF, Jousset M, Greub G. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ Microbiol. 2008;72:2428–2438. doi: 10.1111/j.1462-2920.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 27.Ozçelik S, Coşkun KA, Yünlü O, Alim A, Malatyalı E. The prevalence, isolati and morphotyping of potentially pathogenic free-living amoebae from tap water and environmental water sources in Sivas. Turkiye Parazitol Derg. 2012;36(4):198–203. doi: 10.5152/tpd.2012.48. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann R, Michel R. Distribution of free living amoebae during propagation and supply of drinking water. Int J Hyg Environ Health. 2001;203:215–219. doi: 10.1078/S1438-4639(04)70031-0. [DOI] [PubMed] [Google Scholar]
- 29.Loret JF, Jousset M, Robert S, et al. Amoebae-resisting bacteria in drinking water: risk assessment and management. Water Sci Technol. 2008;58(3):571–577. doi: 10.2166/wst.2008.423. [DOI] [PubMed] [Google Scholar]