Abstract
In this paper, ultra-weak photon emission (UPE) was used to distinguish cold patients from healthy subjects. The UPE intensity of fingertips of two hands from healthy subjects and cold patients was measured using a two-hand UPE detecting system and a group of cut-off filters. We found a significant difference in the maximum spectral peak and photon emission ratio between the filter of 550nm and 495nm, which can be used in distinguish cold patients from healthy people. Methods and results in this work could be useful for developing a new optical diagnostic tool for early disease diagnosis in the future.
OCIS codes: (170.6280) Spectroscopy, fluorescence and luminescence; (170.0170) Medical optics and biotechnology; (170.4580) Optical diagnostics for medicine; (300.6550) Spectroscopy, visible; (330.5370) Physiological optics; (330.6180) Spectral discrimination
1. Introduction
All the biological systems, including humans, send ultra-weak photon emission (UPE) to their surroundings [1], and spontaneous ultra-weak photon emission is generated from the relaxation of electronically excited species formed during oxidative metabolic processes [2–5].Ultra-weak photon emission is a common phenomenon and carries information about its generating processes, and closely related to photosynthesis, lipid per-oxidation, catabolism, free radical reactions, radiation effects, detoxification and carcinogenic effects, aging and death process [6]. There has been a lot of evidence that the features of UPE including intensity, spectral distribution, photon count distribution, photon spatial distribution, and photon statistical properties contain and carry information about the biological systems that result in its production [7]. Therefore, measurement and analysis of these parameters can reveal the detail changes within biological systems and assess the essential characteristics of internal physical and chemical reactions.
Research on human ultra-weak photon emission (UPE) started in the 1970’s.In the early research, it was generally descriptive and the photon emission was thought to reflect the physiology of the human body [8–11]. Previous reports have focused on the photon intensity from the dorsum and palm of hands and multi-anatomic body sites of healthy and some diseased subjects [12–15]. It has been demonstrated that the photon intensity of healthy persons was different over body sites, and photon intensity varied in different physiological and pathological states [9, 16]. Data suggested that biological rhythms, age, and gender could affect the photon emission intensity, and in disease condition, the UPE intensity and its left-right symmetry differed from healthy conditions, and different consciousness states would lead to changes in photon emission [12, 17, 18]. Due to very weak intensity of the human body and the limitation of equipment specifications, the analysis of the spectral distribution of the human body has received relatively less attention. In 1989, Edwards et al. [19] used color filters positioned between a hand and a photomultiplier and divided the visible spectrum into the following four bands: 410-495nm, 495-540 nm, 540-570nm, and 570-650nm. However, they were not able to describe the detailed shape of the spectral curve. Spectral analysis was described in more detail by Van Wijk in 2005 [20], who analyzed the wavelength spectrum of spontaneous photon emission in the 200-650nm range of a male healthy subject at the superior frontal part of the right upper leg, forehead, and palm of the left and right hands. Their experimental results demonstrated that specific spectral differences were present among the four body sites especially for the two hands. Data revealed that the right hand emitted a higher intensity in the short (<360 nm) wavelength range, and the left hand in the 470nm range. Yang et al. [21] investigated spectral distribution of some points of healthy subjects and 30 patients with essential hypertension. Data indicated that spectral peak of healthy subjects was in the 450-490nm range, and that of patients with essential hypertension was in the 530-570nm range at the attack state, and returned closer to normal in the 490-530nm rate during the remission state. Zhu [22] tested the fingernails powder of normal people and cancer patients with Fourier transform infrared (FTIR) spectroscopy and found a significant difference in spectra parameters, such as frequency, intensity, and band shape between cancer patients and normal people. Chen [23] distinguished serum samples of patients with acute lymphoblastic leukemia from those of healthy volunteers.
However, spectral distribution of human UPE in pathological compared to healthy states has been studied less. In addition, subjects and body locations were not sufficient, and left and right symmetry was also not considered. Therefore, previous reports needed further research. In this paper, we report the spontaneous ultra-weak photon emission spectral of fingertips of the left and right hands from healthy subjects and patients with a cold by utilizing a high-sensitivity two-hand ultra-weak photon detecting system and a series of cut-off filters. We hope to find the difference in the spectral distribution between healthy subjects and patients to provide quantitative data that can be useful in clinical diagnosis in the future.
2. Material and methods
2.1 Experimental setup
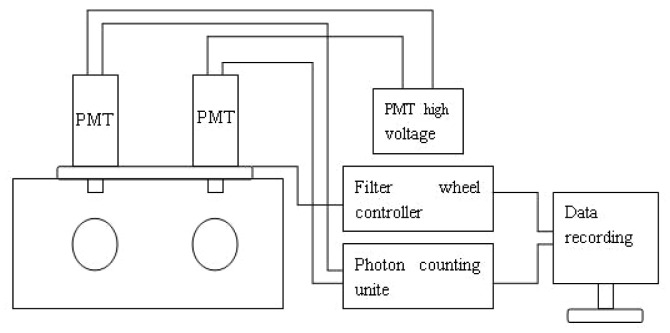
We used a two-hand ultra-weak photon emission detecting system, which was a table-top model specifically designed for both hands. The components that were used in the system included the following: dark box consisting of two chambers; two photomultipliers tubes, with two detector heads located on top of two dark chambers; high voltage supply; a photon counting unit; and a computer with photon count data software. Figure 1 illustrates the components of the two hand detecting system. At the front of the box, two openings are present to position a hand inside a chamber, so both hands can be measured simultaneously. At the top of the dark box, there are two detector heads and each detector head consists of a shutter and a photomultiplier tube. The photomultiplier tube (Electron Tube 9235QA) was sensitive in the spectral range of 290-630 nm and has a 51 mm (2 in.) diameter window. To secure the distance, angle, and surface areas of fingertips, which are measured to be the same for all the measurements, we created a mounting system with a black thick paper cover that has a hole of diameter 10 mm on the PMT.
Fig. 1.
Schematic representation of the two-hand photon detecting system.
Because they are not internally cooled, the device must be placed in a room with a standardized temperature, in our experiment, the room temperature was maintained at 20 ± 1.0°C.The shutter of the device is a large round plate regulating synchronously the opening/closing of the light transmission pathways of both hands. The top plate of the dark box contains a wheel with openings, and the turning of the filter wheel is mechanical, and regulated by a computer to ensure that the wheel moves in one specific direction. The openings of this wheel are specifically made to contain color filters, and the cut-off filters were used in the experiments. The high voltage power supplies (Sens Tech PM20) are utilized for the PMT and regulate the electronic background. Two photon counting unites (C9744) are used for photon counting. The detection time of every photon was recorded and stored digitally by a computer with a counter card and photon counting software.
2.2 Color filters
The spectral analysis utilizes a set of cut-off filters, which were GG395, GG455, GG495, OG550, and RG610(Schott Glaswerke AG, Mains, Germany), with cut-off wavelengths at 395 nm, 455 nm, 495 nm, 550 nm, and 610 nm, and one opening had no filter. The auto-luminescence of every filter was measured before detection of the hands. Auto-luminescence between the different filters fluctuated between 7.54 and 18.04 cps, including electronic noise. Quantum efficiencies of the photomultiplier for different spectral bands were 27%(290-395nm), 23%(395-455nm), 15%(455-495nm), 6%(495-550nm), 3%(550-610nm),and 2%(610-630nm).
2.3 Subjects
Twenty healthy volunteers (13 females and 7 males, age 23-40 years) and ten cold patients (6 females and 4 males, age 24–35 years) participated voluntarily in the spontaneous UPE measurement. The twenty healthy subjects were interviewed to exclude physical or emotional disorders. The ten cold patients were all diagnosed with wind cold according to TCM diagnostic criteria of common cold [24]. TCM diagnostic criteria of wind cold was the following: (1) chills, and no sweat, or fever; (2) nasal congestion, and runny nose; (3) headache, or limbs rheumatic pain; and (4) pulse floating or tight. The subjects were diagnosed with wind cold if only the experienced symptoms agreed with both of (1), (2) and either of (3), (4). All these subjects adequately understood the recording procedure and gave verbal informed consent. According to the Medical Research Involving Human Subjects Act (2006), this study did not require a medical ethics review.
2.4 Measuring procedure
The dark counts of without and with filters in a sequence from 395nm to 610nm in both left and right photomultipliers was recorded simultaneously for 2min at intervals of 1s before the measurement of fingertips. The spontaneous UPE of ten fingertips was then recorded. The detection was conducted between 3 P.M. and 5 P.M. to reduce the influence of diurnal rhythms. The subject was asked to wash their hands with clean tap water, and dry them for 5 min, then they wore light tight gloves on both hands for half an hour before the UPE measurement to eliminate the influence of delayed luminescence caused by light exposure. Subsequently, the subject placed the thumb, index finger, middle finger, ring finger, and little finger of both hands sequentially below the two photomultiplier tubes to record signals. The two particular fingertips of both hands were measured synchronously constantly utilizing one PMT for one hand and the other for the other hand. In the procedure, normally small fluctuations in photon intensity will have no influence on the differences between the left and right hands. The detection time for each filter was 2 min at intervals of 1s, and each finger recording time with the full set of filter took approximately 12min, and it took approximately 1h for a complete measurement. Immediately after one measurement, a second measurement identical with the first one was performed.
2.5 Data analysis
Statistical analysis of photon count data was performed with SPSS 16.0 software (SPSS, USA), and data calculation and graphing were performed with Origin 9.0 (OriginLab Corporation, Northampton, USA).
3. Results
3.1 Spectral distribution of fingertips from healthy subjects
Ten fingertips of each subject were measured for spectral analysis, including the thumb, index finger, middle finger, ring finger, little finger of the left and right hands. Photon emission of each fingertip with different cut-off filters and filters alone from 20 healthy subjects are averaged in mean ± variance. Results are presented in the Table 1 . The data demonstrated that the UPE intensity of fingers decreased gradually from the thumb to the little, and showed a balance in photon emission from the left and right hands for healthy subjects. The high variance of each finger suggested that photon emission fell within a wide range for different age subjects. For the same finger, the variance of photon emission with different cut-off filters decreased from 395nm to 610nm. From the table, dark counts of the individual filters (column 2) are different and thus in the UPE intensity calculation of a particular wavelength range, each filter value should be corrected for its own dark counts.
Table 1. Spontaneous photon emission of fingertips of left and right hand from 20 healthy subjects with different cut-off filtersa.
| Wavelength | Filter alone | Thumb | Index finger | Middle finger | Ring finger | Little finger |
|---|---|---|---|---|---|---|
| None-L | 7.98 ± 0.85 | 33.42 ± 11.63 | 28.53 ± 10.52 | 26.10 ± 10.52 | 22.66 ± 11.67 | 21.31 ± 10.34 |
| -R | 7.54 ± 1.15 | 35.32 ± 13.83 | 29.01 ± 10.28 | 25.87 ± 8.11 | 23.54 ± 11.96 | 22.56 ± 11.80 |
| 395nm-L | 18.04 ± 0.89 | 41.30 ± 12.59 | 37.24 ± 11.73 | 34.32 ± 11.40 | 31.92 ± 10.85 | 30.95 ± 10.15 |
| -R | 15.81 ± 0.86 | 41.59 ± 13.25 | 35.39 ± 9.30 | 32.06 ± 8.92 | 31.78 ± 12.64 | 29.46 ± 9.32 |
| 455nm-L | 16.94 ± 1.09 | 36.09 ± 11.01 | 33.85 ± 10.62 | 30.87 ± 9.15 | 28.38 ± 9.75 | 27.92 ± 7.58 |
| -R | 16.80 ± 1.14 | 38.42 ± 12.15 | 34.14 ± 10.13 | 31.80 ± 8.81 | 28.47 ± 8.29 | 28.51 ± 9.04 |
| 495nm-L | 11.19 ± 0.80 | 23.85 ± 7.31 | 21.03 ± 5.80 | 20.51 ± 6.19 | 18.51 ± 4.86 | 19.05 ± 5.58 |
| -R | 11.35 ± 0.85 | 25.83 ± 7.52 | 22.61 ± 6.04 | 21.73 ± 5.13 | 19.48 ± 5.18 | 20.91 ± 7.01 |
| 550nm-L | 8.80 ± 0.59 | 13.19 ± 2.54 | 12.36 ± 2.39 | 12.36 ± 2.52 | 11.41 ± 2.24 | 11.67 ± 2.07 |
| -R | 9.25 ± 0.92 | 14.02 ± 2.78 | 13.11 ± 2.21 | 12.67 ± 1.95 | 12.38 ± 2.18 | 12.53 ± 2.81 |
| 610nm-L | 11.55 ± 0.84 | 12.68 ± 0.68 | 12.48 ± 1.08 | 12.54 ± 1.05 | 12.43 ± 1.05 | 12.47 ± 1.02 |
| -R | 7.81 ± 1.13 | 8.58 ± 1.12 | 8.39 ± 1.12 | 8.58 ± 0.96 | 8.50 ± 1.16 | 8.33 ± 1.19 |
L represents left hand, R represents right hand.
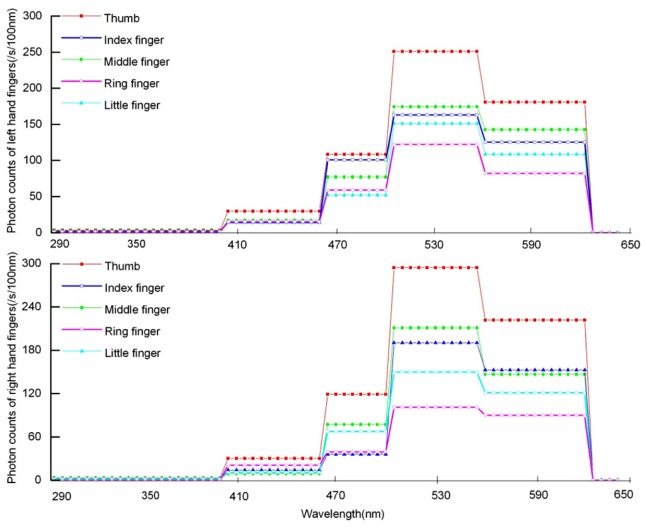
Table 1 gives photon intensity of each fingertip with different cut-off filters and filters alone. In the subsequent calculation, the photon intensity of each fingertip is first calculated by subtracting the auto-luminescence of corresponding filters, i.e., [(fingertips + filter)-filter]; then, the difference in each fingertip between two filters with successive wavelength cut-off filters is mathematically combined with the transmittance of the filters and quantum efficiency of the photomultiplier. The final estimation of the spectral distribution of the fingertips of the left and right hands from healthy subjects is expressed in cps per100 nm. The calculation method is the same as that reported by Van Wijk in 2006 [20], the result of the spectral distribution of fingertips of left and right hand from 20 healthy subjects is shown in Fig. 2 . Figure 2 illustrates that photon emission of all the fingertips demonstrated higher intensities in the 495-550nm range than others for both hands. In other words, ten fingertips of both hands demonstrated maximal peak in the range of 495-550 nm. The right hand demonstrates higher intensities than the left hand in the whole wavelength range. For the same wavelength, the photon emission of five fingertips differed between the left hand and right hands. For example in the 495-550nm wavelength range, photon emission from the highest to the lowest are thumb, middle finger, index finger, little finger, and ring finger for the left hand, and thumb, middle finger, little finger, index finger, and ring finger.
Fig. 2.
Spectral distribution of spontaneous photon emission of fingertips of left and right hand from healthy subjects.
3.2 Spectral distribution of fingertips from patients with a cold
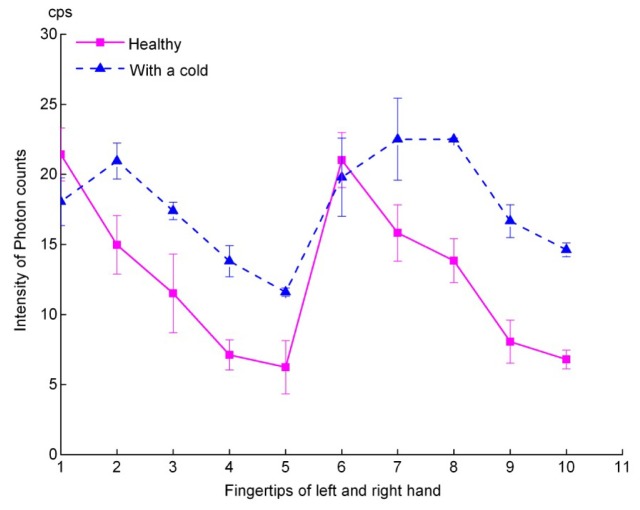
Figure 3 illustrates typical UPE intensity of ten fingertips without cut-off filters (dark counts subtracted) from subjects before and after a common cold. In the figure, a dotted line indicates UPE intensity of fingertips from subjects with a cold, and a thick line from the corresponding healthy state. Numbers 1 to 5 in the axis represent thumb, index finger, middle finger, ring finger and little finger of the left hand, respectively, and 6-10 represent those of the right hand. Data demonstrated that the photon emission of fingertips from a cold condition is significantly higher than the normal (Paired t test, p = 0.003) and no longer show a gradual decrease from thumb to little finger.
Fig. 3.
Spontaneous ultra-weak photon emission intensity of fingertips from patients before and after a cold.
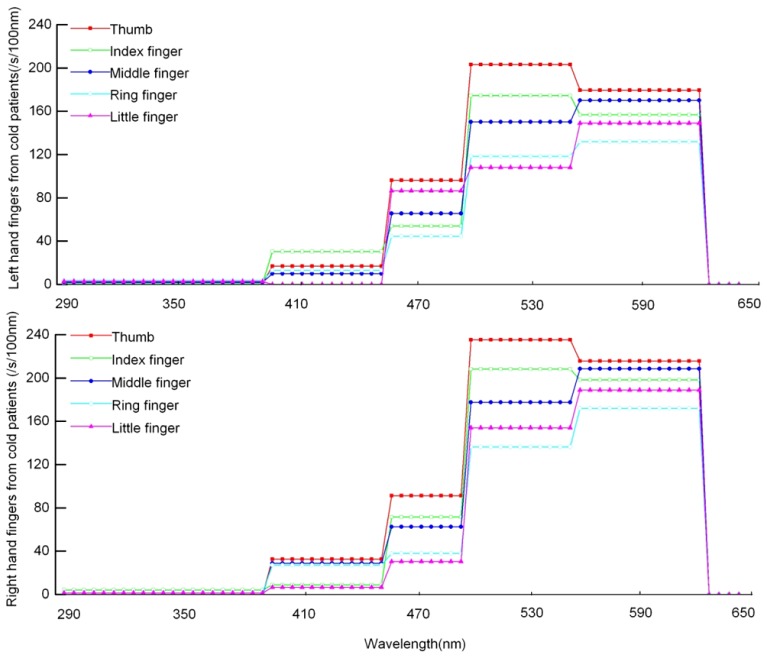
Figure 4 shows the distribution of fingertips of the left and right hands from ten patients with a cold. In comparison with that from healthy subjects, obvious differences exist between healthy subjects and patients with a cold. For both hands, the spectra distribution of the thumb and index finger basically remain unchanged, but the maximum spectral peak of the middle finger, ring finger, and little finger move from the495-550nm range to the 550-610nm range.
Fig. 4.
Spectral distribution of spontaneous photon emission of fingertips from patients with a cold.
Table 2 shows the ratio of fingertips photon emission between the ranges of 550-610nm and 495-550nmof both hands from healthy subjects and cold patients. The table shows a clear difference between the middle, ring, and little fingers of both hands between healthy subjects and cold patients, with the value for patients being almost twice as high as that for healthy subjects(left hand: Paired t test, p = 0.007;right hand: Paired t test, p = 0.005.). These differences that are absolutely outside the experimental errors could probably be used to distinguish cold patients from healthy subjects. The different spectral component 550nm/495nm ratio of middle, ring, and little finger in the healthy people and cold patients has been indicated as a parameter suitable for diagnostic purposes.
Table 2. Ratio of fingertips photon emission between the ranges of 550-610 nm and 495-550 nm for healthy subjects and cold patients.
| Left hand
|
Right hand
|
||||
|---|---|---|---|---|---|
| Body sites | Healthy | Cold | Healthy | Cold | |
| Thumb | 0.72 ± 0.18 | 0.88 ± 0.19 | 0.75 ± 0.16 | 0.92 ± 0.17 | |
| Index finger | 0.77 ± 0.10 | 0.90 ± 0.20 | 0.81 ± 0.12 | 0.95 ± 0.23 | |
| Middle finger | 0.82 ± 0.28 | 1.13 ± 0.15 | 0.69 ± 0.25 | 1.17 ± 0.19 | |
| Ring finger | 0.67 ± 0.11 | 1.11 ± 0.13 | 0.89 ± 0.16 | 1.26 ± 0.21 | |
| Little finger | 0.72 ± 0.19 | 1.38 ± 0.26 | 0.80 ± 0.20 | 1.23 ± 0.38 | |
4. Discussion and conclusion
Ultra-weak photon emission is an inherent property of biological systems, and it is associated with the oxidative metabolic processes of biological macromolecules. Physical and chemical principle studies of bioluminescence have suggested that the photon emission phenomenon happens only when molecules transition from an excited state to the ground state. The photons released by the electronically excited products of oxidative reactions can be displayed by the ultra-weak photon emission of human body surface. Previous studies reported that the dismutation of superoxide anion radical (O2•-) is accompanied by the formation of hydrogen peroxide (H2O2) and further hydroxyl radical (HO•) via Fenton reaction [25, 26]. The highly reactivehydroxyl radical (HO•) has the capability to oxidize all types of biomolecules such as lipids, proteins and nucleic acids [27, 28]. The oxidation of these biomolecules and decomposition of high-energy intermediates could finally generate electronically excited species such as triplet-excited carbonyl (3R = O*), singlet (1P*), and triplet (3P*) excited pigments, and singlet oxygen (1O2). The photon emission of (3R = O*) is at near UVA and blue-green regions of the spectrum (350-550 nm) [29]. The photon emission of singlet (1P*) and triplet (3P*) excited pigments are in the green-red (550–750 nm) and red-near (750-1000nm) [28].
Spectral peak may serve as an indicator of the body's metabolism, and measurements with broad-band spectral analysis with and without specific diseases might be very useful. In our study, the spectral distribution of healthy subjects and patients with a cold were measured. Results demonstrated that there were obvious differences in spectral distribution between healthy subjects and the patients with a cold in the visible spectrum.The455-550nm range and 550-610nm range correspond to the photon emission of the triplet excited carbonyl (3R = O*) and singlet excited pigment (1P*) transition to the ground state during radiation. The spectral maximum peak of fingertips moved towards red (550-610nm) range suggests that a change in the complex biochemical reactions and energy transition occurred in the cold patients, which resulted in the type of photon emission change. This shift phenomenon may be associated with corresponding energy metabolism disorders related to diseases, and the differences may be caused by the structural change of the emitter [28, 30].
In conclusion, we measured spontaneous ultra-weak photon emission of fingertips from both healthy people and cold patients using a two-hand ultra-weak photon detecting system. We found a significant difference in the spectral distribution of middle, ring, and little fingertips for both the left and right hand between healthy and cold patients. A comparison of the position of maximum spectral peak and the ratio of fingertips photon emission between the ranges of 550-610nm and 495-550nm can be used to distinguish cold patients from healthy people. Next, the spectral distribution of many more subjects and different types of common cold need to be studied. Further accumulation of data could lead to construction of a database that will be useful for developing a new diagnostic tool. In addition, results also suggest that ultra-weak photon emission detection technology is expected to become a new method of early and rapid diagnosis of other diseases.
Acknowledgments
This work was supported by the grant from the National Natural Science Foundation of China ( 81273997) and Ministry of Science and Technology of China ( 2014DFA30380). The special equipment was constructed under supervision of Dr. E.P.A. Van Wijk and Y.Yan (Meluna Research). The authors also thank them for their technical assistance.
References and links
- 1.Wijk R. V., Wijk E. P., “An introduction to human biophoton emission,” Forsch. Komplementarmed. Klass. Naturheilkd. 12(2), 77–83 (2005). 10.1159/000083763 [DOI] [PubMed] [Google Scholar]
- 2.Van Wijk R., Van Wijk E. P. A., Wiegant F. A. C., Ives J., “Free radicals and low-level photon emission in human pathogenesis: State of the art,” Indian J. Exp. Biol. 46(5), 273–309 (2008). [PubMed] [Google Scholar]
- 3.Rastogi A., Pospísil P., “Spontaneous ultraweak photon emission imaging of oxidative metabolic processes in human skin: effect of molecular oxygen and antioxidant defense system,” J. Biomed. Opt. 16(9), 096005 (2011). 10.1117/1.3616135 [DOI] [PubMed] [Google Scholar]
- 4.Sławinski J., “Biophotons from stressed and dying organisms: toxicological aspects,” Indian J. Exp. Biol. 41(5), 483–493 (2003). [PubMed] [Google Scholar]
- 5.Prasad A., Pospíšil P., “Towards the two-dimensional imaging of spontaneous ultra-weak photon emission from microbial, plant and animal cells,” Sci. Rep. 3, 1211 (2013). 10.1038/srep01211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang J. J., “Physical Properties of biophotons and their biological functions,” Indian J. Exp. Biol. 46(5), 371–377 (2008). [PubMed] [Google Scholar]
- 7.Bajpai R. P., “Quantum coherence of biophotons and living systems,” Indian J. Exp. Biol. 41(5), 514–527 (2003). [PubMed] [Google Scholar]
- 8.Usa M., Inaba H., “Spontaneous photon emission from human body,” Med. Imaging. Technol. 13, 47–54 (1995). [Google Scholar]
- 9.Jung H. H., Woo W. M., Yang J. M., Choi C., Lee J., Yoon G., Yang J. S., Lee S., Soh K. S., “Left-right asymmetry of biophoton emission from hemiparesis patients,” Indian J. Exp. Biol. 41(5), 452–456 (2003). [PubMed] [Google Scholar]
- 10.Van Wijk E. P., Ackerman J., Van Wijk R., “Effect of Meditation on Ultraweak Photon Emission from Hands and Forehead,” Forsch. Komplementarmed. Klass. Naturheilkd. 12(2), 107–112 (2005). 10.1159/000084028 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M., Kokubo H., Parkhomtchouk D. V., Chen W., Tanaka M., Zhang T., Kokado T., Yamamoto M., Fukuda N., “Biophoton and temperature changes of human hand during Qigong,” J. Int. Soc. Life Inf. Sci. 18, 418–422 (2000). [Google Scholar]
- 12.Van Wijk R., Kobayashi M., Van Wijk E. P., “Anatomic characterization of human ultra-weak photon emission with a moveable photomultiplier and CCD imaging,” J. Photochem. Photobiol. B 83(1), 69–76 (2006). 10.1016/j.jphotobiol.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 13.Choi C., Woo W. M., Lee M. B., Yang J. S., Soh K. S., Yoon G., Kim M., Chang J. J., “Biophoton Emission from the Hands,” J. Korean Phys. Soc. 41(2), 275–278 (2002). [Google Scholar]
- 14.Kim T. J., Nam K. W., Shin H. S., Lee S. M., Yang J. S., Soh K. S., “Biophoton emission from fingernails and fingerprints of living human subjects,” Acupunct. Electrother. Res. 27(2), 85–94 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Yang J. M., Choi C., Hyun-hee W. M., Woo S. H., Yi K. S., Soh J. S., Yang, Choi C., “Left-Right and Yin-Yang Balance of Biophoton Emission from Hands,” Acupunct. Electrother. Res. 29(3-4), 197–211 (2004). [PubMed] [Google Scholar]
- 16.Lee C., Yang J. M., Yi S. H., Cho H. J., Kang M. J., Yang J. S., Soh K. S., “Biophoton emission from patients with a cold,” J. Int. Soc. Life Inf. Sci. 22(2), 362–365 (2004). [Google Scholar]
- 17.Cifra M., Van Wijk E., Koch H., Bosman S., Wijk R. V., “Spontaneous ultra-weak photon emission from human hands is time dependent,” Radioengineering. 16(2), 15–19 (2007). [Google Scholar]
- 18.Kobayashi M., Kikuchi D., Okamura H., “Imaging of Ultraweak Spontaneous Photon Emission from Human Body Displaying Diurnal Rhythm,” PLoS ONE 4(7), e6256 (2009). 10.1371/journal.pone.0006256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edward R., Ibisin M. C., Jessel-Kenyon J., Taylor R. B., “Light emission from the human body,” Comp. Med. Res. 3, 16–19 (1989). [Google Scholar]
- 20.Wijk E. P., Wijk R. V., “Multi-site recording and spectral analysis of spontaneous photon emission from human body,” Forsch. Komplementarmed. Klass. Naturheilkd. 12(2), 96–106 (2005). 10.1159/000083935 [DOI] [PubMed] [Google Scholar]
- 21.Yang W. Y., Ni D. X., Zhang H. M., Sun K. X., Su L., “Spectral analysis of ultraweak photon emission of acuponits of patients with essential hypertension,” Chin. J. Basic Med. Tradi. Chin. Med. 4(8), 49–51 (1998). [Google Scholar]
- 22.Zhu N., “FTIR spectra of finger nails of normal people and cancer patients,” Res. Explor. Lab. 31(10), 246–247 (2012). [Google Scholar]
- 23.Chen P., Zhang L., Zhang F., Liu J. T., Bai H., Tang G. Q., Lin L., “Spectral discrimination between normal and leukemic human sera using delayed luminescence,” Biomed. Opt. Express 3(8), 1787–1792 (2012). 10.1364/BOE.3.001787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Professional Committee of Chinese Medical Association , “Traditional Chinese medicine diagnostic criteria for common cold (Version 2013),” J. Tradit. Chin. Med. 5(4), 350–351 (2014). [Google Scholar]
- 25.Halliwell B., Clement M. V., Long L. H., “Hydrogen peroxide in the human body,” FEBS Lett. 486(1), 10–13 (2000). 10.1016/S0014-5793(00)02197-9 [DOI] [PubMed] [Google Scholar]
- 26.Komatsu S., Kamal A. H., Makino T., Hossain Z., “Ultraweak photon emission and proteomics analyses in soybean under abiotic stress,” Biochim. Biophys. Acta 1844(7), 1208–1218 (2014). 10.1016/j.bbapap.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 27.Lambert A. J., Brand M. D., “Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane,” Biochem. J. 382(2), 511–517 (2004). 10.1042/BJ20040485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pospíšil P., Prasad A., Rác M., “Role of reactive oxygen species in ultra-weak photon emission in biological systems,” J. Photochem. Photobiol. B 139, 11–23 (2014). 10.1016/j.jphotobiol.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 29.Fedorova G. F., Trofimov A. V., Vasil’ev R. F., Veprintsev T. V., “Peroxy-radical-mediated chemiluminescence: mechanistic diversity and fundamentals for antioxidant assay,” ChemInform 38(9), 163–215 (2007). 10.1002/chin.200709275 [DOI] [Google Scholar]
- 30.Van Wijk R., Schamhart D. H., “Regulatory aspects of low intensity photon emission,” Experientia 44(7), 586–593 (1988). 10.1007/BF01953306 [DOI] [PubMed] [Google Scholar]