Abstract
We examined neural indices of pre-attentive phonological and attentional auditory discrimination in children with developmental language disorder (DLD, n=23) and typically developing (n=16) peers from a geographically isolated Russian-speaking population with an elevated prevalence of DLD. Pre-attentive phonological MMN components were robust and did not differ in two groups. Children with DLD showed attenuated P3 and atypically distributed P2 components in the attentional auditory discrimination task; P2 and P3 amplitudes were linked to working memory capacity, development of complex syntax, and vocabulary. The results corroborate findings of reduced processing capacity in DLD and support a multifactorial view of the disorder.
Keywords: developmental language disorder, specific language impairment, attention, working memory, auditory discrimination, phonological processing, event-related potentials, MMN, P3, PCA
1. Introduction
Although most children acquire their native language rapidly and effortlessly, a sizeable number of them exhibit protracted or atypical language development despite having normal hearing, average nonverbal cognitive ability, and no obvious sensory, psychiatric, neurologic or genomic pathology. Clinical taxonomies such as DSM-IV-TR (American Psychiatric Association, 2013) place this heterogeneously manifesting condition in a category of neurodevelopmental communication disorders, broadly referred to as developmental language disorders1 (DLD). Language deficits in DLD span multiple domains, including articulatory phonology, lexical development and morphosyntax.
Like other complex disorders, etiologically, DLD is likely multifactorial and polygenic, i.e., caused by multiple genetic liabilities that interact with each other and environmental risk factors, and no definite cause of the disorder has been identified to date. The search for the etiology of DLD has been boosted in the past decades by application of neurobiological methods, specifically, non-invasive pediatric neuroimaging techniques such as fMRI, EEG, and event-related potentials (ERPs). Neurophysiological indices of brain activity such as ERPs reduce the complexity of end-point behavioral phenotypes and provide an intermediate neurocognitive link between biological and behavioral-syndromic levels of analysis, thus representing so-called endophenotypes (Cannon & Keller, 2006). The increase of interest in the neurophysiological endophenotypes of complex disorders in general, and DLD in particular, was accompanied by both theoretical and methodological changes in the approach to these disorders that put emphasis on multivariate, dimensional and continuous investigations of facets of cognitive functioning that might be implicated in their etiology (Grigorenko et al., 1997; Smith et al., 2010). This approach parallels recent theoretical developments in the field of psychiatric genetics that suggest viewing complex developmental disorders as extreme cases on the continua of both phenotypic and genotypic variation (Plomin, Haworth, & Davis, 2009). Crucially, for what can be called an individual differences approach (as opposed to the case-control approach), EEG and ERPs permit a componential analysis of neurocognitive processing with the potential for quantifying the individual differences at different stages of processing on a fine-grained time-scale, presumably linked to various facets of language functioning in DLD.
Most research on the neurocognitive underpinnings of DLD has been largely motivated by domain-general accounts of DLD that argued that language difficulties in DLD stem from deficits in perceptual and cognitive processing not limited to the language domain, such as deficits in general speed of processing (Miller, Kail, & Leonard, 2001) and rapid auditory processing (e.g., Tallal & Piercy, 1976). Thus, to date, most published ERP studies of DLD focused on auditory and phonological processing in children with DLD, specifically, on the pre-attentive index of general auditory discrimination, the mismatch negativity (MMN; Naatanen & Alho, 1995), which reflects the processing of auditory information at stages following initial sound change detection supported by sensory memory. Early studies found significantly reduced MMN amplitudes in children with DLD for tone stimuli that differed in frequency (Holopainen, Korpilahti, Juottonen, Lang, & Sillanpää, 1998; Korpilahti & Lang, 1994). Although later studies suggested that auditory processing deficits in DLD might be circumscribed to stimuli presented at rapid interstimulus intervals (ISIs; Benasich et al., 2006), overall atypical responses to tone stimuli were also found at longer ISIs when the overall shape of the ERP waveform rather than the MMN amplitude was considered (Bishop, Hardiman, Uwer, & Von Suchodoletz, 2007; Bishop & McArthur, 2005). In addition, children with or at risk for DLD present with atypical neural pre-attentive discrimination responses to phonological2 stimuli, e.g., CV syllables that differ in consonant’s place of articulation (Kraus et al., 1996; Uwer, Albrecht, & Von Suchodoletz, 2002). Some studies suggested that deficits in the MMN response in DLD may be circumscribed to phonological rather than general auditory processing (Uwer et al., 2002). However, other studies found atypical brain responses to both phonological and tone stimuli, including the MMN (Davids et al., 2011), and the N1-P2 components (McArthur, Atkinson, & Ellis, 2009) in DLD, suggesting a generalized auditory processing deficit potentially stemming from a maturational lag in the development of the auditory cortex in children with DLD (Bishop & McArthur, 2005). However, as Bishop (2007) noted in her review of MMN studies in children with spoken and written language disorders, the overall landscape of findings from such studies is highly variable. This variability is likely caused by methodological differences across studies, the recruitment of heterogeneous groups of children with DLD, and poor individual reliability of the MMN response.
Fewer studies have examined attentional auditory processing in children with DLD by focusing on the P3 component3, typically elicited in oddball tasks that require attentive monitoring of task-related acoustic events. The P3 component is most frequently linked to working memory functioning, potentially indicating the efficiency of the process of updating mental representations in the working memory buffer (Donchin & Coles, 1988), or event categorization broadly supported by the activation of neural populations that underlie attention and working memory functioning (Kok, 2001). Early studies of auditory P3 in DLD by Courchesne and colleagues (1989) and Adams and colleagues (1987) found similar tone P3 latencies and increased P3 amplitudes in children with DLD. In contrast, Ors and colleagues (2002) found prolonged P3 latencies to tone and phonological stimuli and reduced P3 amplitudes to speech stimuli in children with DLD. In a recent study by Evans, Selinger, and Pollak (2011), adolescents with DLD showed decreased P3 latencies in an auditory n-back task, suggesting that the auditory processing deficits found in this group might be attributed to working memory and attentional capacity limitations, consistent with the claims that DLD might involve deficits in general processing capacity (Miller et al., 2001).
Overall, this concise review of ERP studies of general auditory and phonological discrimination and processing in children with DLD indicates substantial heterogeneity in the patterns of results from different studies. This is not surprising given the heterogeneous nature of the disorder. One important avenue for clarifying the role of general auditory and phonological processing in DLD is by focusing on the relationship between ERP indices of processing and symptom severity across different facets of cognitive and language development in children with DLD (Bishop, 2007). Importantly, only a few studies have investigated relationships between the observed neural processing deficits and specific facets of language functioning of children with DLD. For example, Benasich et al. (2006) found that the latency of the N250 (the component preceding the auditory mismatch response in infants) at 6 months predicted expressive and receptive language abilities of at-risk children at 24 months. Weber-Fox and colleagues (2010) found that the auditory P3 amplitude was weakly to moderately related to the amplitude of a different neural response to verb agreement violations in adolescents with DLD. Taken together, these studies warrant further investigation of the functional role of pre-attentive and attentional auditory and phonological processing and discrimination components in the atypical language development in DLD.
The current study aims to address some of the inconsistencies and gaps in previous research on the neural indices of low-level pre-attentive and attentional general auditory and phonological processing in DLD mentioned above. Specifically, we a) investigated whether children with DLD showed atypical ERPs to tone and phonological stimuli, and b) examined the continuous relationships between these ERPs and a set of behavioral measures of cognitive and language development. While not directly pitting auditory, in general, and phonological, in particular, discrimination accounts of DLD against each other, we focused on a set of well-studied ERPs that tapped into pre-attentive and attentional general auditory and phonological processing: MMN and P3. Given that memory deficits are well documented in children DLD (Graf Estes, Evans, & Else-Quest, 2007), we hypothesized that children with DLD are more likely to show discrimination and processing deficits when they are examined in paradigms that draw on attentional and working memory resources. The two experiments reported in the manuscript are part of a large epidemiological study of DLD in a Russian-speaking geographically isolated population. Importantly, since the population in question is environmentally and, presumably, genetically homogenous (Rakhlin et al., 2013c), it allowed us to overcome some of the methodological difficulties related to studying heterogeneous referred samples of children with DLD.
2. Experiment 1: pre-attentive phonological discrimination
2.1. Methods and materials
2.1.1. Participants
The participants for the current study come from a small geographically isolated Russian-speaking population (AZ; total n=861) that resides in a small cluster of villages in northwestern Russia. In comparison to a nearby-residing comparison population, similar to AZ on a set of socioeconomic indicators, the AZ population is characterized by an atypically high prevalence of DLD (Rakhlin et al., 2013c). More than 31% of children aged 3 to 18 and adults over the age of 18 in this community exhibit atypical language development despite the absence of apparent neurobiological, cognitive, or sensory pathology, compared to a rate of 9% in the comparison population (who speak the same dialect of Russian; see Rakhlin et al., 2013c, for details).
Although initial estimates of the prevalence of DLD in the AZ population were based on a set of expressive language measures, the affected children in the AZ population also present with marked deficits in receptive language (Kornilov et al., 2012; Rakhlin et al., 2013b) and phonological working memory capacity (Kavitskaya, Babyonyshev, Walls, & Grigorenko, 2011), consistent with the manifestations of DLD documented in other languages. The characterization of the population’s behavioral and neural (endo)phenotype is part of an ongoing family-based epidemiological investigation of genetic bases of DLD in AZ.
For the purpose of the present study, we recruited 39 children aged 7.17 to 15.83 years (M = 10.54, SD = 2.34; 23 boys). Of these, 23 were classified as having DLD (M = 10.12, SD = 2.40; 16 boys) and 16 as typically developing (TD; M = 11.14, SD = 2.18; 7 boys) on indices of expressive and receptive language obtained using two standardized language development measures (the classification procedure is described below). The two groups did not differ with respect to gender, χ2(1) = 2.60, p = .107, or age, t(37) = 1.35, p = .186. The 39 children who participated in the study represented 54% of all children aged 7 to 16 in the population. The recruitment was targeted with a goal of obtaining EEG data from children who were previously classified as either having DLD or TD. Thirty three additional children and their parents were approached but either declined to participate (n=12) or were temporarily unavailable at the time of the ERP (n=18) or behavioral (n=3) data collection. Informed consents were obtained from the children’s parents and oral assents from the children at the time of the data collection.
2.1.2. Behavioral measures
2.1.2.1. Language measures
Children’s language development was assessed using two diagnostic tools: a standardized elicited narrative task developed for establishing the language development status in rural Russian populations (Rakhlin et al., 2013c) and the Assessment of the Development of Russian (Kornilov et al., 2012).
2.1.2.1.1. Narrative task
Expressive language skills were assessed using two wordless storybooks: for children under 13, these were “Frog, Where Are You?” and “One Frog too Many” (Meyer, 1969); for those over 13, these were “Free Fall” (Wiesner, 2008) and “Tuesday” (Wiesner, 1997). Both the audio and the transcripts of the elicited speech samples were analyzed by two native-Russian linguists and rated on a number of characteristics in phonological, syntactic, and semantic/pragmatic domains. These measures (descriptive statistics are presented in Table 1) included: 1) Phonetic/Prosodic Characteristics (i.e., phonological simplifications and omissions, misarticulations and prosodic abnormality), 2) Wellformedness (frequency of lexical and grammatical errors and false starts adjusted for the length of the narrative), 3) Number of Complex Structures (e.g., embedded and conjoined clauses, passives, participial constructions), 4) Mean Length of Utterance (in words; MLUw), 5) Number of Semantic/pragmatic Errors, and 6) Lexical Richness (i.e., number of distinct lexemes adjusted for length). Robust length- and age-adjusted z scores were computed for each measure separately using data from a larger sample of children from a comparison rural population mentioned above (see Rakhlin et al., 2013c, for more details).
Table 1.
Descriptive Statistics for Study Measures
| TD (n=16) | DLD (n=23) | Cohen’s d | t(37) | p | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Demographics | |||||||
| Age | 11.14 | 2.19 | 10.12 | 2.40 | .44 | 1.35 | .186 |
| WHQ-R | 43.44 | 16.73 | 48.92 | 26.15 | −.24 | −.74 | .465 |
| Cognitive Measures | |||||||
| Digit Span | 14.19 | 3.78 | 11.65 | 2.96 | .77 | 2.35 | .024 |
| Word Span | 12.06 | 2.14 | 10.43 | 1.67 | .87 | 2.66 | .011 |
| Nonverbal IQ | 104.06 | 13.18 | 97.35 | 14.60 | .48 | 1.47 | .150 |
| Narratives Scales | |||||||
| Phonetic/Prosodic Characteristics | 11.00 | 1.03 | 9.78 | 1.24 | 1.06 | 3.22 | .003 |
| Wellformedness | .047 | .011 | .068 | .030 | −.90 | −2.59 | .014 |
| Number of Complex Structures | .085 | .022 | .067 | .023 | .80 | 2.44 | .020 |
| MLUw | 5.19 | 1.49 | 3.69 | .97 | 1.26 | 3.81 | .001 |
| Number of Semantic/Pragmatic Errors | .015 | .023 | .030 | .025 | −.62 | −1.90 | .066 |
| Lexical Richness | 53.56 | 7.10 | 45.35 | 5.91 | 1.29 | 3.93 | .001 |
| ORRIA | 92.94 | 8.66 | 85.91 | 9.46 | .78 | 2.36 | .024 |
| Experiment 2 - Behavioral Performance | |||||||
| Hit rate | .988 | .018 | .916 | .139 | .67 | 2.04 | .049 |
| Miss rate | .012 | .018 | .084 | .139 | −.67 | −2.04 | .049 |
| False alarm rate | .007 | .006 | .055 | .146 | −.44 | −1.32 | .194 |
| Correct rejection rate | .993 | .006 | .945 | .146 | .44 | 1.32 | .194 |
| d′ | 5.04 | .63 | 4.15 | 1.59 | .70 | 2.13 | .040 |
| Reaction Time (RT) | 514 | 59 | 581 | 144 | −.59 | −1.78 | .083 |
| ln(RT) | 6.24 | .12 | 6.33 | .26 | −.47 | −1.43 | .161 |
Note. For Wellformedness and Semantic/Pragmatic Errors measures higher scores indicate lower performance. p-values below .05 are shown in bold. The d′ sensitivity measure was calculated using the traditional adjustments for the perfect hit and zero false alarm rates, i.e., the minimum p was set to p = 1/N where N is the number of trials used in the calculation, and the maximum p was set to p = (N-1)/N.
2.1.2.1.2. Assessment of the Development of the Russian Language
In order to get a more complete picture of children’s language abilities, they were also assessed with the Assessment of the Development of Russian Language (ORRIA; Kornilov et al., 2012), a standardized Russian language development test comparable to the Clinical Evaluation of Language Fundamentals (Semel, Wiig, & Secord, 1995), and the Test of Language Development (Newcomer & Hammill, 1982). ORRIA is aimed at assessing language development in the areas of morphology, syntax, semantics, and lexicon, both in comprehension and production, as well as phonological awareness. We used the following 5 subtests: passive vocabulary; active vocabulary; linguistic operators (assessment of semantic knowledge of temporal and logical operators in comprehension of complex sentences); sentence structure (assessment of grammatical knowledge in comprehension); and word structure (assessment of morphosyntactic competence in production). Standardized age-adjusted scores for overall language development (M = 100, SD = 15) were calculated using an external sample (n=484) representative of the general population of Russian children.
2.1.2.1.3. Cut-offs for establishing group status
Language status (DLD or TD) was determined by using the cut-off criterion of a Z score at or below −1.25 on at least two of the six narratives scales listed above or an overall ORRIA score below 82 (roughly corresponding to the epiSLI criterion, see Tomblin et al., 1997).
As can be seen from Table 1, children with DLD significantly underperformed on almost all of the language measures with the effect sizes for the significant differences ranging from d = .62 to 1.29. The largest effect sizes were observed for the narrative scales of Phonetic Development (d = 1.06), MLUw (d = 1.26), and Lexical Richness (d = 1.29).
2.1.2.2. Cognitive measures
To ensure that all children had typical nonverbal cognitive functioning, they were administered Scale 2 of the Culture-Fair Intelligence Test (CFIT; Cattell & Cattell, 1973), a normed measure of nonverbal intelligence. A standardized nonverbal IQ score was obtained for each child (all scored above the cut-off score for intellectual disability, IQ > 70), and the the group difference in IQ scores was not significant, t(37) = 1.47, p = .150.
We also used a 32-item Digit Span (backward and forward) measure and a 21-item Word Span measure to quantify individual and group differences in verbal short-term and working memory capacity. The groups differed in their mean scores on both the Digit Span, t(37) = 2.35, p = .024, d = .77, and on the Word Span, t(37) = 2.66, p = .011, d = .87.
The participants were also assessed using the Waterloo Handedness Questionnaire – Revised (WHQ-R; Elias, Bryden, & Bulman-Fleming, 1998). All but one (in the DLD group) were predominantly right-handed, and the group difference was not significant, t(37) = −.74, p = .465. The descriptive statistics for the cognitive measures and the WHQ-R scores are presented in Table 1.
2.1.3. Hearing screening and other exclusionary criteria
To ensure normal hearing acuity, all participants were administered a bilateral hearing screening with a Beltone 119 (Beltone New England) portable audiometer at 500, 1000, 2000, and 4000 Hz. All children passed the screening at 25 dB. According to their medical records, none of the children were diagnosed with hearing loss, intellectual disability, autism or any other neurodevelopmental or genomic disorders.
2.1.4. Experimental stimuli and procedure
We used a passive pre-attentive phonological discrimination paradigm (e.g., Naatanen, 2001) to elicit the MMN component(s). The stimuli consisted of one standard canonical synthetic CV syllable /bɑ:/ (/ba/) and two synthetic deviant CV syllables, classified as easy (/gɑ: / or /ga/) or difficult (/dɑ:/ or /da/) based on their distance from the standard on a place of articulation continuum.
All of the stimuli (/ba/, /da/, and /ga/) were 251 ms in duration with the onset at ~40 ms (including the burst or prevoicing and transition) followed by ~210 ms of steady state vowel. The stimuli were presented aurally at 70 dB (SPL) via Etymotic insert headphones (Etymotic Research, Inc). A total of 1280 standard /ba/ syllables and 320 deviant syllables (160 /da/ and 160 /ga/, at the standard to deviant ratio of 8:1:1) were presented at a SOA of 600 ms in a pseudorandom order, such that there were at least 3 standards between deviants. The children sat in front of a small display and watched a silent cartoon during the data acquisition. There were two short breaks after the first 550 and 1100 trials.
Although published studies of MMN in children with DLD generally used only one deviant to elicit the MMN (e.g., a deviant /da/ with /ba/ as a standard), we included a second deviant /ga/ for two reasons. First, group differences in MMN might be overlooked when the difference between the standard and deviant is large or not present if it does not elicit a reliable MMN component (Bishop, 2007), justifying the use of multiple deviants. Second, although we expected to see a particular reduction of the MMN to the more difficult to detect /da/ deviant (mirroring the behavioral discrimination deficits observed for children with DLD; Tallal & Stark, 1981), we were intrigued by findings of atypical neural responses to /ga/ but not /da/ or /ba/ in the right hemisphere in children at risk for dyslexia (Guttorm et al., 2005; Guttorm, Leppanen, Richardson, & Lyytinen, 2001), a condition frequently comorbid with DLD in the spoken language modality.
2.1.5. EEG recordings
The EEG was recorded using the ActiveTwo system (BioSemi, Inc.) with 64 sintered Ag/AgCl electrodes mounted on an elastic cap, approximating the standard 10–20 system, using a standard electrolyte gel (SignaGel, Parker Laboratories, Inc.). An additional seven electrodes were used to record the EEG signal at two mastoids, at the nose tip (data from the nose electrode was not used in this study), the vertical electrooculogram (VEOG; electrodes placed above and below the left eye), and the horizontal electrooculogram (HEOG; electrodes positioned lateral to the outer canthi of both eyes). Impedances were kept below 25 kΩ for all electrodes but one (Iz, which had mechanical faults that could not be corrected on-site; the data from this electrode was excluded from the analysis).
2.1.6. EEG/ERP processing and analysis
The EEG signal was sampled at 1024 Hz, stored on a hard drive and referenced offline to the linked mastoids. The pre-processing of the data and the averaging were carried out using EMSE Suite 5.5 (Source Signal Imaging, Inc.).
For each participant, a few channels were identified as containing a high amount of technical artifacts (such as AC power line noise and/or loss of contact) on the basis of visual inspection. These channels were reconstructed using a spline interpolation procedure (no more than five channels per subject). Then, a digital IIR bandpass filter of .50 to 30 Hz and a data-driven spatial ocular artifact correction algorithm (Pflieger, 2001) were applied to the signal. Trials with EEG activity greater than ±110 μV were excluded from further analysis and averaging.
The EEG was epoched from −150 to 600 ms relative to stimulus onset using a 150 ms prestimulus baseline correction. For the standard /ba/ condition, trials that immediately followed the deviant trials were excluded. Overall, 82% of the remaining trials were included in the analysis. There was no significant difference in the proportion of trials included in the analysis between the TD and the DLD group for the easy /ga/ deviants, χ2(1)= 3.71, p = .054, and the difficult /da/ deviants, χ2(1) = 2.52, p = .112. Although there was a significant group difference in the proportion of standard /ba/ trials included, χ2(1)= 16.1, p < .001, the magnitude of this difference was negligible: 83% for the TD group, and 81% for the DLD group (see also Table S1 in the Online Supplement for detailed trials statistics for both experiments).
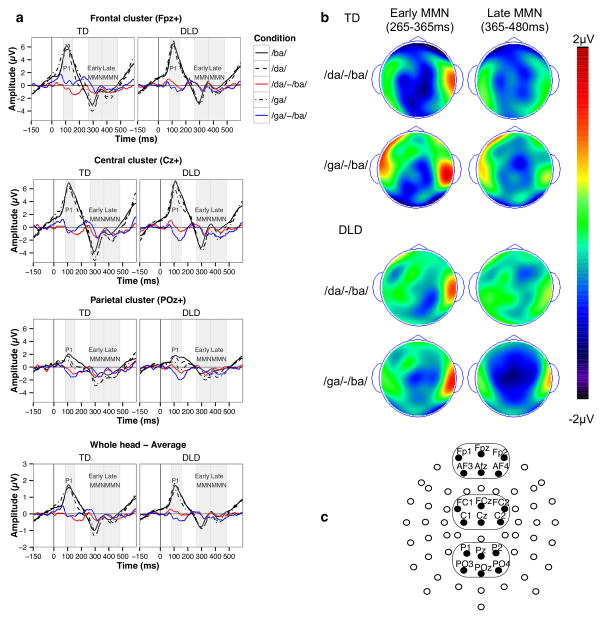
The waveforms were first averaged separately for each condition (/ba/, /da/, and /ga/); then two difference waveforms were obtained by subtracting the standard condition waveform from the two deviant waveforms (i.e., /da/-/ba/ and /ga/-/ba/). The identification of ERPs was guided by a combination of visual inspection of grand-averaged raw and difference waveforms and prior literature regarding the timing and spatial topography of the potentials. Given the fronto-central distribution of the MMN component in school-aged children and adults (Naatanen, Paavilainen, Rinne, & Alho, 2007), in our analysis we focused on the amplitude and latency measures from three clusters of electrodes along the midline corresponding to frontal (FPz+; electrodes Fp1, Fpz, Fp2, AF3, Afz, and AF4), central (Cz+; FC1, FCz, FC2, C1, Cz, and C2), and parietal (POz+; P1, Pz, P2, PO3, POz, and PO4) clusters. Although parietal electrodes are not normally the focus of MMN analyses, we included the parietal cluster to make our analyses parallel to those employed for Experiment 2.
Three time windows of interest were selected for further analysis based on visual inspection of waveforms and topographic plots. Given that there might be differences in the early obligatory components (preceding MMN) between standard and deviant stimuli driven by physical stimulus differences and probability-related refractoriness differences (Naatanen et al., 2007), we first quantified the obligatory auditory P1 potential in the frontal and central electrode clusters as the most positive peak during the 82–152 ms post stimulus (35 ms to the each side of the peak at around 117 ms). We chose 265–365 ms post stimulus as the time window for the early MMN component in the central cluster, followed by a late MMN component in the adjacent 365–480 ms time window. The two adjacent time windows were chosen both to maximize the size of both MMN components and to correspond to the MMN latencies reported in previous research (Barry, Hardiman, & Bishop, 2009; Bishop, 2007; Korpilahti, Lang, & Aaltonen, 1995).
ERP data were analyzed using a set of mixed linear models (Baayen, 2008) as implemented in the lme4 R package (Bates & Maechler, 2010). The amplitude was measured as peak amplitude for P1 and average amplitude in the time window for the two MMN components. Peak latency was used for the P1 potential, and fractional area latency (i.e., timing of the point that divides the area under the curve into two equal fractions) was used for the MMNs. The analyses included fixed effects of condition (with /ba/ as a reference), electrode cluster (with Cz+ as a reference), language group (with TD children as a reference), age, gender (with girls as a reference), and nonverbal intelligence. The models also included random effects of participants, electrodes, and sibpairs (to account for close relatedness) on the intercept4. Quantitative predictors (age and intelligence) were mean-centered. The p-values were adjusted for multiple comparisons using Bonferroni corrections at the study-wide level.
2.2. Results
2.2.1. Amplitude and latency analysis
The analysis of the early obligatory P1 potential did not reveal any significant differences in either latency or amplitude between the two groups (see Online Supplement for the summary of fixed effects). The P1 amplitude was significantly related to age (Est = − .658, SE = .155, pBonferroni = .048) and was smaller in the parietal compared to the central electrode cluster (Est = − 4.960, SE = .575, pBonferroni = .000003). No other effects reached statistical significance. The analysis of P1 latency also did not reveal any significant group-related effects.
The analyses of the early MMN amplitude (sample waveforms and topographies are presented in Figure 1; a summary of the fixed effects is available in the Online Supplement) found significantly more negative amplitudes in response to /da/ deviant compared to the standard /ba/ (Est = − 1.046, SE = .250, pBonferroni = .008). Overall, the amplitude in the early MMN time window was more negative in amplitude the central compared to the parietal electrode cluster (Est = 1.137, SE = .258, pBonferroni = .003). No other effects were significant.
Figure 1. Pre-attentive phonological discrimination ERPs (Experiment 1).
a – sample averaged waveforms for three electrode clusters and the whole head; b – topographic maps for the two MMN components; c – electrode montage showing three electrode clusters used in the analyses.
We observed a similar pattern of results for the late MMN component. The MMN amplitudes were significantly more negative in both /da/ (Est = − .754, SE = .180, pBonferroni = .007) and /ga/ (Est = − .864, SE = .180, pBonferroni = .0004) conditions compared to the standard /ba/ condition, indicating the presence of a significant MMN effect. No other effects were significant for either amplitude or latency analyses. Together, the results of Experiment 1 indicated that children with DLD and TD children did not differ in amplitude or timing of the pre-attentive phonological early and late MMN components.
2.2.2. PCA and source localization analysis
The visual analysis of the topographic plots of the early and late MMN suggested the presence of group differences in these components’ topographies and, potentially, the localization of neural sources for these components in children with DLD and TD children. Given this possibility, we conducted two additional analyses.
First, given that we used a high-density EEG setup, we investigated whether a data-driven approach might reveal additional effects not apparent in the conventional waveform analysis. Thus, we subjected the data to the two-step temporo-spatial principal components analysis (PCA) as outlined by Dien (2012) and implemented in the MATLAB PCA Toolkit (Dien, 2010). At the first step, the individual participants’ average waveforms that represented three data cells (one per condition) were subjected to a temporal PCA with Promax rotation. The scree test suggested that six temporal factors (explaining 90% of variance) should be retained. The factor scores from the first step were then subjected to a spatial ICA with Infomax rotation, revealing three spatial factors that explained 75% of variance. Thus, the temporo-spatial PCA analysis revealed a total of 6 (temporal) x 3 (spatial) = 18 temporo-spatial factors. Only eleven of these eighteen factors explained more than 1% of variance and were further analyzed using a set of 3 (condition) x 2 (group) mixed linear models. These analyses, combined with the visual examination of factor topographies and latencies, revealed that the PCA identified the components that were the focus of our main analysis as well as additional components (generally small in magnitude and potentially subject-specific); however, no group effects or group interactions reached statistical significance (all p’s > .05).
Second, we performed a tomographic analysis to investigate potential group differences in neural sources underlying the two MMN components using the sLORETA approach (Pascual-Marqui, 2002) as implemented in the sLORETA-KEY software (http://www.uzh.ch/keyinst/loreta.htm). sLORETA estimates the standardized 3D distribution of cortical current density based on a realistic head model using the MNI152 template. At the first step, we obtained four standardized sLORETA images for each child: the sLORETA solutions were obtained separately for the /da/-/ba/ and /ga/-/ba/ difference waveforms and for the early and late MMN time windows, and represented estimates of standardized electric activity at each voxel in the MNI space. Four voxel-by-voxel analyses of log-transformed data using the t-statistic for independent groups were performed. The thresholds for statistical significance were estimated using the Statistical non-Parametric Mapping (SnPM) approach. The sLORETA analysis did not reveal any voxels that would reach statistical significance for the group difference (all p’s > .05; the resulting at-maxima images are presented in Figure S2 in the Online Supplement). These analyses indicate that early and late MMN components were similar in topography and source localization patterns in two groups of children.
2.4. Discussion
In Experiment 1, children with DLD displayed neural responses to phonological mismatches similar to those of their TD peers. Intact amplitudes and latencies for both early and late MMN components indicated both comparable time-course and efficiency of pre-attentive phonological processing and discrimination in the two groups. Our findings contradict some earlier reports on atypical MMN responses to phonological stimuli in DLD – i.e., reports of attenuated MMN in children with DLD in response to CV syllables that differed in place of articulation (Kraus et al., 1996; Uwer et al., 2002).
Although no overall pooled effect size was reported in the latest meta-analysis of MMN studies spoken and written DLD (Bishop, 2007), it is worth pointing out that the effect sizes for various stimuli and ISIs varied greatly across the meta-analyzed studies. One of the emergent patterns from this meta-analysis was a seemingly larger MMN difference between the clinical and control (TD) groups when the clinical group consisted of children with spoken rather than written language difficulties. This seems to not be the case for our sample – although presenting with significant spoken language development problems, the DLD group displayed electrophysiological responses to phonological stimuli similar to those of the TD group. Interestingly, we found intact MMN responses (with similar topographies) to both easy /ga/ and difficult /da/ deviant CV syllables. This pattern is intriguing given Guttorm et al.’s (2005) findings of specific abnormalities in neural responses to /ga/ deviants in infants at risk for dyslexia, which were longitudinally associated with poor receptive language skills.
Overall, the results of Experiment 1 suggest that children with DLD are capable of forming stable and precise transient neural representations of brief phonological stimuli and that deficits in sensory memory and the related processes of pre-attentive phonological discrimination and their automaticity might not lie at the core of atypical language development in children with DLD. Thus, Experiment 2 investigated whether children with DLD exhibit atypical neural responses in an auditory oddball task that required attention-modulated discrimination of auditory stimuli (simple tones) that differed in frequency, guided by recent proposals that attention and working memory loaded tasks might be especially sensitive to auditory processing deficits in DLD.
3. Experiment 2: attentional auditory discrimination
3.1. Methods and materials
3.1.1. Participants
The same sample of 39 children participated in Experiment 2.
3.1.2. Experimental stimuli and procedure
We used a standard auditory oddball paradigm (e.g., Polich, 2007) to elicit the auditory P3 component. A series of pure sine tones that differed in frequency were presented at 70 dB (SPL) via Etymotic insert headphones (Etymotic Research, Inc.). A total of 255 frequent standard tones (2000 Hz) and 45 rare target tones (1000 Hz) with a duration of 138 ms each were presented at the SOA of 2000 ms in a pseudorandom order, such that there was at least 1 standard between targets. The 2000 Hz and 1000 Hz tones were chosen because the auditory ERPs in oddball tasks that utilized these stimuli were shown to be atypical in clinical populations with memory and auditory processing deficits (Golob, Johnson, & Starr, 2002; Shutara et al., 1996).
The children participated in Experiment 2 on the same day as Experiment 1. They sat in front of a PC laptop and were instructed to actively listen to the sounds and press the mouse button (with their and) when they heard the target sound. Children were familiarized with the stimuli and went through a training phase that provided them with feedback prior to the test phase.
3.1.3. EEG recordings
The same acquisition setup was used in Experiment 2. The pre-processing of the data for Experiment 2 followed the procedure outlined for Experiment 1.
3.1.4. EEG/ERP processing and analysis
The EEG for correct trials was epoched from −150 to 800 ms relative to stimulus onset using a 150 ms prestimulus baseline correction. Trials with EEG activity greater than ±110 μV were excluded from further analysis and averaging. For standard tone trials, trials that immediately followed the target tone were also excluded from the analysis. Overall, 60% of the trials were included in the analysis. There were no significant differences in the proportions of trials included in the analysis between the TD and the DLD groups: χ2(1)= 3.43, p = .072, for standard tones, and χ2(1) = 2.05, p = .153, for target tones.
The waveforms were averaged separately for the standard and target conditions. Similarly to Experiment 1, we used a combination of the visual inspection of grand-averaged waveforms and prior knowledge regarding the timing and the spatial topography of the potentials to select the time window for each of the ERPs. The same three midline electrode clusters (frontal, central, parietal) were used in the analysis.
The N1 and P2 potentials were maximal in the electrode cluster in the central scalp region (Cz+). Within this cluster, peaks were defined as the most negative peak occurring during the 60–150 ms post stimulus (N1; 45 ms to each side of the peak occurring approximately at 105 ms) and the most positive peak occurring 130–230 ms post stimulus (P2; 50 ms to each side of the peak at 180 ms). The N2 potential was quantified as the most negative peak 210–310 ms post stimulus (50 ms to each side of the peak at 260 ms), and was maximal in the frontal region (FPz+). Finally, the P3 was defined in the parietal region (POz+) as the most positive peak occurring 300–450 ms post stimulus (75 ms to each side of the wide peak at 375 ms). Overall, the selected time windows and electrode clusters corresponded to previously reported latencies, peak maxima, and topographies of the N1 and P2 (e.g., Crowley & Colrain, 2004), N2 (e.g., Patel & Azzam, 2005), and P3 (e.g., Conroy & Polich, 2007).
Data were analyzed using a set of mixed linear models. Peak amplitudes and latencies were estimated separately for each of the ERPs. The analyses included fixed effects of condition (with the standard tone as a reference), electrode cluster (with Cz+ as a reference), language group (with TD children as a reference), age, gender (with girls as a reference), and nonverbal intelligence. Parallel to Experiment 1, the models also included random effects of participants, electrodes, and sibpairs (to account for close relatedness) on the intercept. The p-values were adjusted for multiple comparisons using Bonferroni corrections.
Additionally, we ran a set of exploratory partial correlational analyses that related the measures of neural activity that discriminated between the two language groups to our behavioral measures of cognitive and language development.
3.2. Results
3.2.1. Behavioral performance
While the behavioral performance (see Table 1 for descriptive statistics) of both groups of children was close to ceiling, children with DLD were less accurate overall (for d′, t(37) = 2.13, p = .040, d = .70), with a lower hit rate for target tones, t(37) = 2.04, p = .049, d = .67, compared to the TD children. However, the groups did not differ significantly with respect to their (natural log) reaction time, t(37) = 1.43, p = .161.
3.2.2. Amplitude and latency analysis
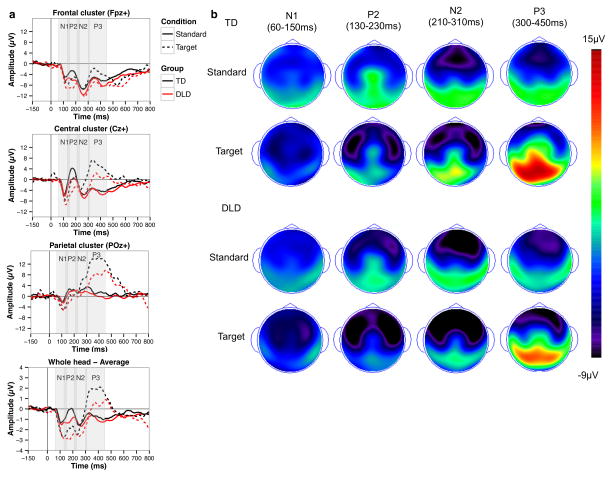
The analysis of the N1 component (Figure 2 shows the ERP waveforms and topographies for the auditory oddball task) indicated that children with DLD did not differ from TD children in either amplitude or latency of the N1 response, suggesting similar early neural auditory processing responses in both groups. The N1 was overall more negative in amplitude for the target compared to the standard tones (Est = −3.399, SE = .629, pBonferroni = .000019) and was more positive in the parietal compared to the central electrode cluster (Est = 3.238, SE = .681, pBonferroni = .001). No other effects were statistically significant.
Figure 2. Attentional auditory discrimination ERPs (Experiment 2).
a – sample averaged waveforms for three electrode clusters and the whole head; b – topographic maps for the the N1, P2, N2, and P3.
Children with DLD showed atypical scalp topography of the P2 component. The interaction between group status and electrode cluster was statistically significant (Est = 3.462, SE = .815, pBonferroni = .006) and suggested that while for TD children the P2 amplitude was similar in the central and parietal electrode clusters (Est = −1.387, SE = .778, pBonferroni = 1.000), for children with DLD it was more positive in the parietal compared to the central electrode cluster relative to TD children. We also found that P2 peaked later for the target compared to the standard tones (Est = 15.444, SE = 2.851, pBonferroni = .000018). No other effects were statistically significant.
For the N2, the only two statistically significant effects were the effect of cluster for N2 amplitude (Est = 7.344, SE = .959, pBonferroni < .000001) and the effect of condition for N2 latency (Est = −12.228, SE = 2.999, pBonferroni = .012), suggesting that the N2 was less negative in amplitude in the parietal compared to the central electrode cluster and peaked earier for the target compared to the standard tones. No other effects were statistically significant.
Finally, we found several significant effects for the P3 amplitude. The P3 amplitude was more positive in the parietal compared to the central electrode cluster (Est = 5.979, SE = .904, pBonferroni < .000001) and, as expected, more positive in response to target compared to standard tones (Est = 11.589, SE = .795, pBonferroni < .000001). Crucially, we found that children with DLD showed an attenuated P3 effect (Est = −4.087, SE = 1.035, pBonferroni = .020), i.e., a smaller increase in the P3 amplitude for the target condition compared to TD children. We did not find any significant effects for the P3 latency.
3.2.3. PCA and source localization analysis
The two-step temporo-spatial PCA was also applied to the data from Experiment 2 as an exploratory complement to the main analyses reported above. At the first step, the individual participants’ average waveforms that represented two data cells (standard and target) were subjected to a temporal PCA with Promax rotation. Seventeen temporal factors (explaining 97% of variance) were retained based on the scree test. The factor scores were then subjected to a spatial ICA with Infomax rotation, revealing four spatial factors that explained 82% of variance. Thus, the temporo-spatial PCA analysis revealed a total of 17×4 = 68 temporo-spatial factors for Experiment 2. Only twenty-two of these factors were further analyzed using a set of 2 (condition) x 2 (group) mixed linear models. Similarly to Experiment 1, the PCA correctly identified the components that were the focus of our main analyses and also revealed some additional components.
We found significant group differences in factor scores for two out of twenty-two temporo-spatial factors. The first factor (TF1SF4) that differentiated the two groups of children represented the combination of the first temporal factor (TF1; peaking at around 428ms) and the fourth spatial factor (SF4; with a maximum in the occipital region, i.e., O2 electrode) and was characterized by a parieto-occipitally distributed negativity in the P3 time window (see Figure S4 in the Online Supplement). The main effect of condition was not significant for this factor, Est = −1.038, SE = 1.141, p = .369, suggesting similar amplitudes for target and standard stimuli. However, children with DLD showed marginally stronger negativity, as manifested in lower factor scores overall, Est = −2.697, SE = 1.376, p = .054. A set of follow-up one-sample t-tests (against the zero value) suggested that while this negative occipital component was present for the DLD children (p = .003 and .032 for the standard and target conditions, respectively), it was absent for the TD children (p = .165 and .845, respectively).
The second factor that differentiated between children with DLD and TD children was a combination of the fifth temporal factor and the first spatial factor (TF5SF1). This factor was characterized by a pronounced fronto-central distribution and peaked at 170 ms post-stimulus, roughly corresponding to P2. We found a significant effect of condition, Est = −7.101, SE = 1.318, p = .000004: standard tones elicited a generally positive response, and the target tones elicited a negative response. We found that children with DLD exhibited overall more negative factor scores (i.e., amplitudes) for this factor, Est = −4.324, SE = 1.669, p = .01199, compared to the TD children.
Thus, the PCA analysis confirmed that children with DLD display atypical P2 – in addition to the topographic difference identified in the main analyses, we also found a general reduction of the P2 amplitude using the PCA. It also suggested that decreased P3 amplitude in this group of children might stem from an overlap between the P3 component and an additional negative component with a parieto-occipital distribution (absent in TD children). Although the functional significance of this negative occipital component is unknown, its topographic distribution is largely consistent with a neural source in the temporoparietal junction (with the occipital negativity representing the negative part of the dipole), a group of brain regions that has been prominently featured in studies of the neural sources of P3 (Linden, 2005), and implicated in auditory working memory (Leff et al., 2009).
To follow-up on the results from the PCA analysis, we performed a tomographic analysis of the potential group differences in neural sources of P3 using sLORETA following the same procedure as for Experiment 1. Standardized sLORETA solutions were obtained for the ERP waveform in the target condition for each child. The images were averaged for 403–453 time window (TF1SF4 peak ±25 ms) and normalized for each subject. The results of sLORETA analysis indicated that no voxel-by-voxel group comparisons reached statistical significance using the SnPM thresholds. However, a number of voxels displayed nominal p-values below .01 using standard t-test thresholds. The vast majority of these voxels were located in the temporal lobe, specifically, left superior temporal gyrus and supramarginal gyrus (see Figure S5 in the Online Supplement), with DLD children showing higher levels of activation than TD children, tentatively supporting the hypothesis that the reduction in P3 amplitude in this group could be attributable to additional activation of brain regions involved in auditory processing, attention, and memory.
3.2.4. Individual differences analysis
To investigate the potential sources of the group differences observed for the P2 and P3 components and to further examine the role of P2 and P3 in cognitive and language functioning in DLD, we ran a correlational analysis between the language and cognitive measures used in the study and the group-differentiating amplitude measures for P2 and P3 (controlling for age, gender, and nonverbal intelligence). Table 2 shows the interocorrelations between ERP and behavioral measures.
Table 2.
Intercorrelations between the group-differentiating attentional auditory discrimination ERPs and the behavioral cognitive and language development measures
| P2 amplitude | P3 amplitude | |||||
|---|---|---|---|---|---|---|
| S | T | DP-C | S | T | DT-S | |
| Cognitive Measures | ||||||
| Digit Span | .234 | .033 | −.015 | −.017 | .287 | .349* |
| Word Span | .215 | .004 | .041 | .036 | .374* | .351* |
| Narratives Scales | ||||||
| Phonetic/Prosodic Characteristics | .179 | .233 | −.074 | −.036 | .195 | .114 |
| Wellformedness | −.092 | .148 | .013 | .265 | −.131 | −.206 |
| Number of Complex Structures | .065 | −.017 | .097 | −.005 | .287 | .344* |
| MLUw | .243 | .342* | −.201 | .119 | .171 | .121 |
| Number of Semantic/Pragmatic Errors | −.108 | .079 | .123 | .075 | .065 | .064 |
| Lexical Richness | .377* | .120 | −.326† | −.040 | .210 | .240 |
| ORRIA | .118 | −.079 | −.142 | −.056 | −.206 | −.163 |
| Behavioral Performance (Experiment 2) | ||||||
| d′ | .146 | −.246 | −.123 | .025 | .360* | .439** |
| Ln(RT) | −.232 | −.068 | .013 | −.118 | −.372* | −.382* |
Note.
p < .05,
p < .01,
p = .053.
Partial correlation coefficients (controlled for age, gender, and nonverbal IQ) are shown. S – standard, T – target, D – difference. For P2, S and T amplitude measures were calculated for the central cluster, D was calculated as a difference between the amplitude in the parietal and the central electrode clusters; for P3, the amplitudes were calculated for the parietal cluster.
Among the cognitive measures, P3 target amplitude and difference waveform correlated with Word Span (r = .374 and .351, p < .05, respectively), and the difference waveform was also positively related to Digit Span (r = .349, p < .05). Consistent with prior literature, the P3 difference amplitude also positively correlated with behavioral accuracy in the tone detection task (r = .439, p < .01) and negatively with reaction time (r = − .372, p < .05).
We found significant positive correlations between the neural measures that discriminated between groups and the measures of expressive lexical and syntactic development: P2 standard amplitude positively correlated with Lexical Richness (r = .377, p < .05), P2 target amplitude positively correlated with MLUw (r = .342, p < .05), and the P3 difference waveform amplitude positively correlated with the Number of Complex Structures in the elicited speech samples adjusted for the length of the narrative (r = .344, p < .05). The increase in P2 amplitude in the parietal relative to the central electrode cluster (i.e., indexing the atypical topography of the P2 in children with DLD) was also marginally significantly associated with Lexical Richness (r = −.326, p = .053).
Neither P2 nor P3 amplitudes were related to the measures of Phonetic/Prosodic Characteristics, Wellformedness, or Number of Semantic/Pragmatic Errors, thus circumscribing the relationship between the neural indices of auditory discrimination and processing and cognitive/language development in the population to the areas of vocabulary, syntactic complexity, and verbal working memory.
3.3. Discussion
Experiment 2 investigated neural and behavioral indices of auditory discrimination of children with DLD. We found that children with DLD were less accurate in detecting the target tones, consistent with previous reports on elevated frequency discrimination thresholds in children with DLD (e.g., McArthur & Bishop, 2004). Importantly, this deficit was observed at a relatively slow stimulus presentation rate, indicating that the deficit is not limited to rapid presentation rates and instead represents a broader deficit in auditory working memory and processing.
The key findings from Experiment 2 revealed that children with DLD had significantly attenuated neural responses to tones in an auditory oddball task that draws on auditory, attentional, and working memory resources. Specifically, we found that P3 amplitudes were significantly attenuated in the DLD group; children with DLD also showed evidence for atypical topographic distribution and attenuated amplitudes of the P2 component. These findings are consistent with previously reported prolonged P3 latencies and reduced P3 amplitudes to non-phonological stimuli (Evans et al., 2011; Ors et al., 2002). Coupled with significant group differences in verbal working memory and significant correlations between the latter and the P3 amplitude, these results suggest that auditory discrimination problems in DLD might be due to deficits in the allocation of attentional resources in auditory working memory. Consistent with this possibility, the P2 component has been linked to detection of attended stimuli and mobilization of attentional resources (e.g., Crowley & Colrain, 2004), while P3 has been conceptualized as indexing working memory processes related to updating of representations in working memory . More recently, Kok (2001) argued that P3 reflects a broader event categorization and processing capacity that reflects joint functioning of attentional and working memory resources. Taken from this perspective, our results provide partial support for theories of DLD that argue that the locus of spoken language impairments in DLD lies in domain-general processing limitations. Our results point specifically to attentional and working memory capacity and processes that support auditory discrimination and processing.
4. General Discussion
In Experiments 1 and 2, we found that while children with DLD and TD children did not differ in the electrophysiological indices of pre-attentive phonological discrimination (indexed by the two MMN components), children with DLD displayed significantly attenuated neural responses (i.e., P3) in the attentional auditory discrimination oddball task. Moreover, group-differentiating ERP measures were related to measures of verbal working memory, expressive vocabulary, and syntactic complexity.
Our study reveals new details about the relative strengths and weaknesses of phonological vs. general auditory processing in DLD, which lead to several suggestive implications. First, although numerous attempts have been made to investigate whether processing deficits in DLD are limited to phonological stimuli, results from these studies have been largely inconsistent with respect to evidence for either specific phonological or general auditory processing deficits (for a review, see McArthur et al., 2009). The rapid auditory processing account of DLD postulates that deficits in auditory processing are crucial for future language development specifically because they preclude children from forming stable and precise phonological representations and detecting rapid changes in the acoustic environment, such as formant transitions (e.g., Tallal & Piercy, 1974). Under this view, any deficits in auditory processing should lead to deficits in the processing of phonological information. Alternatively, language deficits in children with DLD might stem not from general auditory processing deficits but from deficits in the development of phonological representations and phonological processing. In contrast, the current study showed that while auditory processing deficits can indeed be identified as a salient endophenotypic feature of DLD, they are not necessarily accompanied by deficits in phonological processing, suggesting that auditory and phonological processing deficits represent relatively independent areas of weakness in DLD.
Second, we found that neural indices linked to the recruitment of attentional resources during auditory working memory processing discriminated between children with DLD and TD children. It is important to note that to date only a few ERP studies reported individual differences analyses linking the observed neural processing deficits with impairments in specific facets of language development. We found that atypical auditory P3 amplitudes observed in the DLD group were linked to behavioral measures of verbal working memory and measures of complex syntax, and that atypical P2 topography and P2 amplitude were linked to syntactic and lexical development. These results are noteworthy because they indirectly support working memory accounts of DLD. Specifically, the phonological working memory account postulates that language deficits in children with DLD stem from their limited phonological loop capacity (e.g., Baddeley, Gathercole, & Papagno, 1998). In our study, the MMN responses to phonological stimuli, presumably indexing a combination of basic phonological processing, activation of long-term memory traces, and change detection supported by sensory memory (Pulvermuller & Shtyrov, 2006), were generally not related to behavioral working memory performance (data not shown) and did not differentiate children with and without DLD. It is thus possible that the MMN and auditory P2 and P3 tap into different sources of variability in processing capacity. Under this assumption, P2 and P3 might index the functioning of attention-supported auditory working memory rather than sensory auditory storage and automatic change detection per se. This interpretation is consistent with the conceptualization of P2 and P3 as reflecting the mobilization and joint functioning of attentional and working memory resources (Kok, 2001; Lijffijt et al., 2009). It is also consistent with recent findings of decreased P3 amplitudes in children with DLD in the n-back paradigm (Evans et al., 2011) and the association between P3 and language measures reported by Shaheen, Shohdy, Abd Al Raouf, Mohamed El Abd, and Abd Elhamid (2011). However, this interpretation is speculative given the complexity of the processes involved in both P2 and P3, and further research examining the conditions under which children with DLD show atypical auditory P2 and P3 is needed to isolate the locus of these deficits.
The specific pattern of associations of neural measures obtained in Experiment 2 and language measures roughly corresponds to previous findings of positive relationships between working memory, vocabulary, and complex syntax in children with and without DLD (A. M. Adams, 1996; Gray, 2006; Montgomery & Evans, 2009). Note, however, that in the present study neither behavioral nor electrophysiological indices of working memory were related to a measure of basic morphosyntactic performance (i.e., Wellformedness) indexed by number of grammatical errors in elicited speech samples. Thus, although this measure indexes what can be considered a central deficit in DLD, and the DLD children significantly underperformed on this measure compared to the TD children, it was essentially independent from the deficits in attentional auditory processing. This suggests that the former might constitute a separate facet of language development and deficit relatively unaffected by vulnerabilities in the systems that underlie working memory functioning. In this case, the latter represent just one area of possible deficits in the disorder but not the (single) core deficit, as is frequently assumed in research that focuses on the contraposition of potential causal mechanisms, including domain-general and domain-specific accounts of DLD. Our results provide some evidence for a dissociation of neurocognitive mechanisms behind impairments in the domains of early-maturing core morphosyntactic skills and later-maturing complex syntax (Dittmar, Abbot-Smith, Lieven, & Tomasello, 2008; Hahne, Eckstein, & Friederici, 2004).
Of note is that the behavioral heterogeneity observed in DLD in conjunction with some recent findings from ERP studies has led some researchers (McArthur & Bishop, 2004) to acknowledge that such heterogeneity is likely also present at the level of neurophysiology and etiology of the disorder. We would like to emphasize that the DLD phenotype in the AZ population and in the sample recruited for this study is comparable of what is viewed as a specific DLD phenotype in the field. However, it is possible that the absence of significant effects with respect to the MMN responses to phonological stimuli in Experiment 1 could stem from the differences in a) the relative profiles of strengths and weaknesses across different facets of cognitive and language functioning, b) the overall severity of the disorder and the nature of the samples (i.e., clinically referred vs. population-based), and, tentatively, c) the genetic etiologies of the disorder. The participants of our study come from a highly genetically and environmentally homogenous population and thus present a unique window into the possible mechanisms that underlie DLD, warranting further behavioral, genetic, and psychophysiological characterization of this population.
At the moment, based on the existing body of literature on the localization of the source generators for the auditory P2 and P3 components (Crowley & Colrain, 2004; Linden, 2005) and the overlap of neural networks that support auditory attention, working memory, and language, we can speculate that our pattern of results is consistent with the general view of DLD as involving atypical functioning of temporal and parietal cortical regions, most notably left STG and supramarginal gyrus. Although speculative, this view is tentatively supported by the combination of results from the PCA and the source localization analysis, which revealed that children with DLD show an atypical parieto-occipital negativity component in the P3 time window in addition to the parietally distributed P3 present in both TD and DLD groups. This negative component could potentially account for the reduction of the P3 amplitude in the DLD group. Moreover, both its topographic distribution and the results from the sLORETA analysis suggested the presence of group differences in neural sources active during the oddball task performance in the P3 time window, mapped to brain regions in temporo-parietal area. Thus, the nominally significant estimated overactivation of left STG and supramarginal gyrus in the DLD group might reflect more effortful processing of auditory stimuli in the presence of attentional and working memory demands and is also consistent with several structural and functional neuroimaging reports that have highlighted the role of perisylvian regions in language deficits in DLD (Badcock, Bishop, Hardiman, Barry, & Watkins, 2012; Guibert et al., 2011).
This research has several limitations, which should be addressed in future research. First, although the sample size in the current study is typical for clinical neurophysiological studies, further characterization of processing deficits in DLD would require larger samples to detect weaker effects, e.g., those related to pre-attentive processing that does not tax the working memory systems. Correspondingly, while we controlled the rate of Type I error in our group analyses using a conservative approach, the results of our exploratory correlational analyses should be treated with caution. Increasing sample size for such studies is particularly important given the relatively low reliability of the MMN component. Second, evidence for the primacy of the engagement of working memory and attention over stimulus types (i.e., general auditory vs. phonological) should be obtained using paradigms that directly pit the two factors against each other and control for the differences in spectral complexity between the phonological and non-phonological stimuli, especially given existing ERP evidence for the role of spectral complexity in auditory processing deficits in DLD (McArthur & Bishop, 2005). Third, although the findings from the current study (and most studies of cognitive processing in DLD in general) were interpreted within the endophenotype model, it is worth pointing out that there exist viable alternatives to this view. As Bishop, Hardiman, and Barry (2012) pointed out, the nature of relationships found in such studies is correlational, and cognitive deficits found in DLD might be a consequence rather than an endophenotype of the disorder. Currently, the empirical evidence in favor of this neuroplasticity view is still emerging, and future studies are likely to improve our understanding of the role of domain-general cognitive deficits in this causal chain with the use of appropriate methodology, e.g., by employing longitudinal designs that attempt to clarify the temporal relationships between emerging language ability and deficits in auditory processing and working memory. Finally, the current study heavily relied on expressive language development phenotypes as salient markers of DLD in the target population. Expanding the range of behavioral language and cognitive phenotypes, for example, by including other measures in the domains of comprehension (e.g. comprehension of complex syntax) and memory (e.g., sentence and nonword repetition) could further illuminate the endophenotypic role of working memory in the etiology of DLD.
In sum, the results from our study suggest that language difficulties in children with DLD can be at least partially attributed to decreased working memory and processing capacity. We hypothesize that the distinct profile of group differences and absence thereof across the two experiments is related to the nature of processing demands (i.e., pre-attentive vs. attentional and working memory-supported) rather than the nature of the stimuli (i.e., phonological vs. general auditory). In addition, the results of our study point to the differential relationships between working memory deficits and specific facets of language development (i.e., complex syntactic and lexical but not phonological or basic morphosyntactic development). This pattern of results is largely consistent with a multifactorial view of DLD, with deficits in working memory and attention-supported processing constituting one of the domain-general areas of weakness and, potentially, an endophenotype for the disorder.
Supplementary Material
Acknowledgments
This research was supported by NIH grant R01 DC007665 (E. Grigorenko, PI), NSF grant CAREER 0748684 (JSM, PI), NSF IGERT Training Grant 114399 (J. Magnuson, PI), and NIH grant PO1 HD1994 (to Haskins Laboratories, J. Rueckl, PI). Grantees undertaking such projects are encouraged to express freely their professional judgment. This article, therefore, does not necessarily reflect the position or policies of the National Institutes of Health or the National Science Foundation, and no official endorsement should be inferred. We thank the children who participated in the study and their families for their cooperation and the local medical officials of the AZ community for their help with data collection
Footnotes
Although the term most commonly used in the literature to refer to a developmental (rather than acquired) disorder of language development in the absence of obvious explanatory factors is specific language impairment (SLI), we will use the DLD label when referring to this condition to preserve the continuity between the published empirical reports on the unique population that we sampled from (Kornilov, Rakhlin, & Grigorenko, 2012; Rakhlin, Cardoso-Martins, Kornilov, & Grigorenko, 2013a; Rakhlin, Kornilov, & Grigorenko, 2013b; Rakhlin et al., 2013c; Rakhlin et al., 2011) with an understanding that it is similar to the categories of expressive and mixed expressive-receptive language disorders in the DSM-IV-TR. We would like to emphasize that all of the children classified, as DLD in this study would satisfy the conventionally used inclusion and exclusion criteria for SLI.
We will refer to as phonological to all speech stimuli used in these studies, although in some of them the experimental manipulations were not phonological in nature (but, for example, acoustic).
In oddball paradigms, this is a neural response to rare relevant target stimuli with a prominent parietocentral topography, also known as P3b (Kok, 2001).
For both experiments, the cell means (M and SE) and the summaries of fixed effects for each ERP are available in the Online Supplement. For Experiment 1, due to a technical issue, we did not acquire EEG data for one child. Given that this data missingness arose from a purely technical issue, the data were considered missing completely at random (MCAR, i.e., the probability of data missinginess did not depend on either observed or unobserved measurements). The exclusion of this child from Experiment 2 dataset did not change the pattern of the results. Here, we report the results of the analyses of Experiment 1 data based on n=38 (see descriptive statistics for the sample in the Online Supplement) and the analyses of Experiment 2 data based on n=39 (Table 1).
References
- Adams AM. Phonological Working Memory and Spoken Language Development in Young Children. The Quarterly Journal of Experimental Psychology Section A. 1996;49(1):216–233. doi: 10.1080/713755610. [DOI] [Google Scholar]
- Adams J, Courchesne E, Elmasian R, Lincoln AJ. Increased amplitude of the auditory P2 and P3b components in adolescents with developmental dysphasia. In: Johnson R, Rohrbaugh JW, Parasuraman R, editors. Current trends in event-related potential research. New York: Elsevier; 1987. pp. 577–583. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: Author; 2013. [Google Scholar]
- Baayen RH. Analyzing linguistic data: A practical introduction to statistics. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Badcock NA, Bishop DVM, Hardiman MJ, Barry JG, Watkins KE. Co-localisation of abnormal brain structure and function in specific language impairment. Brain and Language. 2012;120(3):310–320. doi: 10.1016/j.bandl.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105(1):158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Barry JG, Hardiman MJ, Bishop DVM. Mismatch response to polysyllabic nonwords: A neurophysiological signature of language learning capacity. PLoS ONE. 2009;4(7) doi: 10.1371/journal.pone.0006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DM, Maechler M. lme4: Linear Mixed-Effects Models Using S4 Classes. R package. (Version 1.0–6) 2010 Retrieved from http://R-Forge.R-project.org/projects/lme4/
- Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: Predicting from event-related potentials to behavior. Neuropsychologia. 2006;44(3):396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM. Using Mismatch Negativity to Study Central Auditory Processing in Developmental Language and Literacy Impairments: Where Are We, and Where Should We Be Going? Psychological Bulletin. 2007;133(4):651–672. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Hardiman M, Uwer R, Von Suchodoletz W. Atypical long-latency auditory event-related potentials in a subset of children with specific language impairment. Developmental Science. 2007;10(5):576–587. doi: 10.1111/j.1467-7687.2007.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM, Hardiman MJ, Barry JG. Auditory deficit as a consequence rather than endophenotype of specific language impairment: Electrophysiological evidence. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0035851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM, McArthur GM. Individual differences in auditory processing in specific language impairment: A follow-up study using event-related potentials and behavioural thresholds. Cortex. 2005;41(3):327–341. doi: 10.1016/s0010-9452(08)70270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- Cattell RB, Cattell AKS. A culture-fair intelligence test. Champaign, IL: Institute for Personality and Ability Testing; 1973. [Google Scholar]
- Conroy MA, Polich J. Normative variation of P3a and P3b from a large sample - Gender, topography, and response time. Journal of Psychophysiology. 2007;21(1):22–32. doi: 10.1027/0269-8803.21.1.22. [DOI] [Google Scholar]
- Courchesne E, Lincoln AJ, Yeung-Courchesne R, Elmasian R, Grillon C. Pathophysiologic findings in nonretarded autism and receptive developmental language disorder. Journal of Autism and Developmental Disorders. 1989;19(1):1–17. doi: 10.1007/BF02212714. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clinical Neurophysiology. 2004;115(4):732–744. doi: 10.1016/J.Clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Davids N, Segers E, van den Brink D, Mitterer H, van Balkom H, Hagoort P, Verhoeven L. The nature of auditory discrimination problems in children with specific language impairment: An MMN study. Neuropsychologia. 2011;49(1):19–28. doi: 10.1016/j.neuropsychologia.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010;187(1):138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J. Applying Principal Components Analysis to Event-Related Potentials: A Tutorial. Developmental Neuropsychology. 2012;7(6):497–517. doi: 10.1080/87565641.2012.697503. [DOI] [PubMed] [Google Scholar]
- Dittmar M, Abbot-Smith K, Lieven E, Tomasello M. German Children’s Comprehension of Word Order and Case Marking in Causative Sentences. Child Development. 2008;79(4):1152–1167. doi: 10.1111/j.1467-8624.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral Brain Sciences. 1988;11:343–356. [Google Scholar]
- Elias LJ, Bryden MP, Bulman-Fleming MB. Footedness is a Better Predictor than is Handedness of Emotional Lateralization. Neuropsychologia. 1998;36(1):37–43. doi: 10.1016/s0028-3932(97)00107-3. [DOI] [PubMed] [Google Scholar]
- Evans JL, Selinger C, Pollak SD. P300 as a measure of processing capacity in auditory and visual domains in specific language impairment. Brain Research. 2011;1389:93–102. doi: 10.1016/j.brainres.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob EJ, Johnson JK, Starr A. Auditory event-related potentials during target detection are abnormal in mild cognitive impairment. Clinical Neurophysiology. 2002;113(1):151–161. doi: 10.1016/s1388-2457(01)00713-1. [DOI] [PubMed] [Google Scholar]
- Graf Estes K, Evans JL, Else-Quest NM. Differences in the nonword repetition performance of children with and without specific language impairment: A meta-analysis. Journal of Speech, Language and Hearing Research. 2007;50(1):177. doi: 10.1044/1092-4388(2007/015). [DOI] [PubMed] [Google Scholar]
- Gray S. The Relationship Between Phonological Memory, Receptive Vocabulary, and Fast Mapping in Young Children With Specific Language Impairment. Journal of Speech, Language and Hearing Research. 2006;49(5):955–969. doi: 10.1044/1092-4388(2006/069). [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, Wood FB, Meyer MS, Hart L, Speed WC, Shuster A, Pauls DL. Susceptibility loci for distinct components of developmental dyslexia on chromosomes 6 and 15. American Journal of Human Genetics. 1997;60:27–39. [PMC free article] [PubMed] [Google Scholar]
- Guibert C, Maumet C, Jannin P, Ferre JC, Treguier C, Barillot C, Biraben A. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia) Brain. 2011;134(10):3044–3058. doi: 10.1093/brain/awr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PH, Poikkeus A, Eklund KM, Lyytinen P, Lyytinen H. Brain Event-Related Potentials (ERPs) Measured at Birth Predict Later Language Development in Children with and Without Familial Risk for Dyslexia. Cortex. 2005;41(3):291–303. doi: 10.1016/S0010-9452(08)70267-3. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppanen PH, Richardson U, Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. Journal of Learning Disabilities. 2001;34(6):534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- Hahne A, Eckstein K, Friederici AD. Brain Signatures of Syntactic and Semantic Processes during Children’s Language Development. Journal of Cognitive Neuroscience. 2004;16(7):1302–1318. doi: 10.1162/0898929041920504. [DOI] [PubMed] [Google Scholar]
- Holopainen IE, Korpilahti P, Juottonen K, Lang H, Sillanpää M. Abnormal frequency mismatch negativity in mentally retarded children and in children with developmental dysphasia. Journal of Child Neurology. 1998;13(4):178–183. doi: 10.1177/088307389801300406. [DOI] [PubMed] [Google Scholar]
- Kavitskaya D, Babyonyshev M, Walls T, Grigorenko EL. Investigating the effects of syllable complexity in Russian-speaking children with SLI. Journal of Child Language. 2011;38(5):979–998. doi: 10.1017/S0305000910000413. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38(3):557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kornilov SA, Rakhlin N, Grigorenko EL. Morphology and developmental language disorders: New tools for Russian. In: Zinchenko YP, Petrenko VF, editors. Psychology in Russia: State of the Art. Moscow: Russian Psychological Society; 2012. pp. 371–387. [Google Scholar]
- Korpilahti P, Lang AH. Auditory ERP components and mismatch negativity in dysphasic children. Electroencephalography and Clinical Neurophysiology. 1994;91(4):256–264. doi: 10.1016/0013-4694(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Korpilahti P, Lang H, Aaltonen O. Is there a late-latency mismatch negativity (MMN) component? Electroencephalography and Clinical Neurophysiology. 1995;95(4):96. doi: 10.1016/0013-4694(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273(5277):971–973. doi: 10.1126/Science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, Price CJ. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132(12):3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg L, Swann AC. P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology. 2009;46(5):1059–1068. doi: 10.1111/j.1469-8986.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DEJ. The P300: Where in the Brain Is It Produced and What Does It Tell Us? Neuroscientist. 2005;11(6):563–575. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Atkinson C, Ellis D. Atypical brain responses to sounds in children with specific language and reading impairments. Developmental Science. 2009;12(5):768–783. doi: 10.1111/j.1467-7687.2008.00804.x. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Bishop DVM. Which people with specific language impairment have auditory processing deficits? Cognitive Neuropsychology. 2004;21(1):79–94. doi: 10.1080/02643290342000087. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Bishop DVM. Speech and non-speech processing in people with specific language impairment: A behavioural and electrophysiological study. Brain and Language. 2005;94(3):260–273. doi: 10.1016/j.bandl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Miller CA, Kail R, Leonard LB. Speed of Processing in Children With Specific Language Impairment. Journal of Speech, Language, and Hearing Research. 2001;44:416–433. doi: 10.1044/1092-4388(2001/034). [DOI] [PubMed] [Google Scholar]
- Montgomery JW, Evans JL. Complex Sentence Comprehension and Working Memory in Children With Specific Language Impairment. Journal of Speech Language and Hearing Research. 2009;52(2):269–288. doi: 10.1044/1092-4388(2008/07-0116). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R. The perception of speech sounds by the human brain as reflected by the mismatch negativity (MMN) and its magnetic equivalent (MMNm) Psychophysiology. 2001;38(1):1–21. doi: 10.1017/S0048577201000208. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Alho K. Mismatch negativity--a unique measure of sensory processing in audition. International Journal of Neuroscience. 1995;80(1–4):317–337. doi: 10.3109/00207459508986107. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology. 2007;118(12):2544–2590. doi: 10.1016/J.Clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Newcomer P, Hammill D. Test of Language Development - Primary. Austin, TX: Pro-Ed; 1982. [Google Scholar]
- Ors M, Lindgren M, Blennow G, Nettelbladt U, Sahlén B, Rosén I. Auditory event-related brain potentials in children with specific language impairment. European Journal of Paediatric Neurology. 2002;6(1):47–62. doi: 10.1053/ejpn.2001.0541. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods and Findings in Clinical Pharmacology. 2002;24:5–12. [PubMed] [Google Scholar]
- Patel SH, Azzam PN. Characterization of N200 and P300: selected studies of the Event-Related Potential. International Journal of Medical Sciences. 2005;2(4):147–154. doi: 10.7150/ijms.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflieger ME. Theory of a Spatial Filter for Removing Ocular Artifacts With Preservation of EEG. Paper presented at the EMSE Workshop; Princeton. 2001. [Google Scholar]
- Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat Rev Genet. 2009;10(12):872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An Integrative Theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermuller F, Shtyrov Y. Language outside the focus of attention: The mismatch negativity as a tool for studying higher cognitive processes. Progress in Neurobiology. 2006;79:49–71. doi: 10.1016/j.pneurobio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Rakhlin N, Cardoso-Martins C, Kornilov SA, Grigorenko EL. Spelling Well Despite Developmental Language Disorder: What Makes it Possible? Annals of Dyslexia. 2013a;63(3–4):253–273. doi: 10.1007/s1181-013-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhlin N, Kornilov SA, Grigorenko EL. Gender and Gender Agreement Processing in Children with Developmental Language Disorder. Journal of Child Language. 2013b doi: 10.1017/S030500091200058X. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhlin N, Kornilov SA, Palejev D, Koposov RA, Chang JT, Grigorenko EL. The language phenotype of a small geographically isolated Russian-speaking population: Implications for genetic and clinical studies of developmental language disorder. Applied Psycholinguistics. 2013c;34(5):971–1003. doi: 10.1017/S0142716412000094. [DOI] [Google Scholar]
- Rakhlin N, Kornilov SA, Reich J, Babyonyshev M, Koposov RA, Grigorenko EL. The relationship between syntactic development and Theory of Mind: Evidence from a small-population study of a developmental language disorder. Journal of Neurolinguistics. 2011;24(4):476–496. doi: 10.1016/j.jneuroling.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical evaluation of language fundamentals. San Antonio, CA: Psychological Corporation; 1995. [Google Scholar]
- Shaheen EA, Shohdy SS, Abd Al Raouf M, Mohamed El Abd S, Abd Elhamid A. Relation between language, audio-vocal psycholinguistic abilities and P300 in children having specific language impairment. International Journal of Pediatric Otorhinolaryngology. 2011;75(9):1117–1122. doi: 10.1016/j.ijporl.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Shutara Y, Koga Y, Fujita K, Takeuchi H, Mochida M, Takemasa K. An Event-Related Potential Study on the Impairment of Automatic Processing of Auditory Input in Schizophrenia. Brain Topography. 1996;8(3):285–289. doi: 10.1007/BF01184786. [DOI] [PubMed] [Google Scholar]
- Smith SD, Grigorenko EL, Willcutt E, Pennington BF, Olson RK, DeFries JC. Etiologies and molecular mechanisms of communication disorders. Journal of Developmental & Behavioral Pediatrics. 2010;31(7):555–563. doi: 10.1097/DBP.0b013e3181ee3d9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: rate of auditory processing and selective impairment of consonant perception. Neuropsychologia. 1974;12(1):83–93. doi: 10.1016/0028-3932(74)90030-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE. Speech acoustic_cue discrimination abilities of normally developing and language_impaired children. Journal of the Acoustical Society of America. 1981;69(2):568–574. doi: 10.1121/1.385431. [DOI] [PubMed] [Google Scholar]
- Tomblin BJ, Records NL, Buckwalter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language & Hearing Research. 1997;40(6):1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwer R, Albrecht R, Von Suchodoletz W. Automatic processing of tones and speech stimuli in children with specific language impairment. Developmental Medicine and Child Neurology. 2002;44(8):527–532. doi: 10.1017/s001216220100250x. [DOI] [PubMed] [Google Scholar]
- van der Lely HKJ, Jones M, Marshall CR. Who did Buzz see someone? Grammaticality judgements of wh-questions in typically developing children and children with Grammatical-SLI. Lingua. 2011;121(3):408–422. doi: 10.1016/j.lingua.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox C, Leonard LB, Wray AH, Tomblin BJ. Electrophysiological correlates of rapid auditory and linguistic processing in adolescents with specific language impairment. Brain and Language. 2010;115(3):162–181. doi: 10.1016/j.bandl.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.