Summary
Autism spectrum disorder (autism) is a highly prevalent and heterogeneous family of neurodevelopmental disorders of genetic origins with potentially devastating implications for child, family, health and educational systems. Despite advances in paper-and-pencil screening and in standardization of diagnostic procedures, diagnosis of autism in the US still hovers around the ages of four or five years, later still in disadvantaged communities, and several years after the age of two to three years when the condition can be reliably diagnosed by expert clinicians. As early detection and treatment are two of the most important factors optimizing outcome, and given that diagnosis is typically a necessary condition for families to have access to early treatment, reducing age of diagnosis has become one of the greatest priorities of the field. Recent advances in developmental social neuroscience promise the advent of cost-effective and community-viable, performance-based procedures, and suggest a complementary method for promoting universal screening and much greater access to the diagnosis process. Small but critical studies have already reported on experiments that differentiate groups of children at risk for autism from controls, and at least one study so far could predict diagnostic classification and level of disability on the basis of a brief experiment. Although the road to translating such procedures into effective devices for screening diagnosis is still a long one, and premature claims should be avoided, this effort could be critical in addressing this worldwide public health challenge.
Keywords: Autism, Autism Spectrum Disorder, Social Visual Engagement, Eye Fixation, Infancy, Prodromal, Eye-Tracking
Autism as a public health challenge: the need to reduce age of diagnosis
Autism is a biologically-based but highly complex neurodevelopmental disorder [1]. It is one of the most heritable of psychiatric conditions [2] but no single molecular marker defines its diagnosis. Instead, research estimates suggest that greater than three to five hundred distinct genes—the majority of which are still unknown—may each play a role in etiology [3–5]. No single gene has yet been associated with more than a fraction of patient cases (<1% [6]), and the extent to which any pattern or patterns of gene variants or expression can reliably indicate risk of the condition remains unclear. There are numerous hoped-for future insights into the developmental neurobiology of autism [7], but the condition is still diagnosed behaviorally by the presence of early-emerging, persistent deficits in social interaction and communication skills, and by the presence of restricted and repetitive behavior [8]. The most robust markers for early diagnosis of autism include reduced interaction with and attention to others [9,10]; reduced attention to others’ eyes; failure to respond to the calling of one’s own name; and inability to join in imitative games and reciprocal vocalizations [11–13].
Autism affects approximately 1 in every 68 individuals [14]. More children struggle with autism than with all childhood cancers, juvenile diabetes, cystic fibrosis, and muscular dystrophy combined [15]. Autism is also a lifelong disability, impairing a person’s social and communicative function throughout the entire lifespan [16]. Individual manifestations of autism vary in severity, but all are associated with significant impairments in social and communicative functioning and all require some form of specialized support [17,18]. Beyond the clinical and daily living challenges faced by individuals with autism and their families, the yearly economic cost to society is estimated to be in excess of $136 billion dollars in the US alone [19–21], and the cost of lifetime care for one individual and the economic burden to his or her family is estimated to be $ 2,400,000 [22].
The early identification and early treatment of children with autism are consensually regarded as two of the most important factors for improving lifetime outcomes for individuals impacted by the disorder [23–26]. The earlier a diagnosis can be established, the better the long-term outcome [23]. Because symptoms of ASD are present already by 18 and 24 months in the majority of cases [27], the American Academy of Pediatrics recommends universal screening for autism at 18 and 24 months [28]. Unfortunately, only 8% of primary care providers routinely evaluate the toddlers in their practices for autism in the US [29,30]. Screening for other neurodevelopmental delays are also far from universal. As a result, only 20% of children who require special education services later in life are identified prior to the age of 3 years [31]. Time restrictions [32] and the lack of any well-performing, cost-effective screening or diagnostic device for autism and related developmental delays [33] have been highlighted as key factors in primary care providers’ failure to screen. Care providers are more likely to take a ‘wait-and-see’ approach that has the effect of delaying diagnosis until a point at which symptoms can no longer be missed or denied. In a (US) Center for Disease Control and Prevention study of surveillance records [34], even children who received an initial evaluation for ASD at the mean age of 4 years were not finally diagnosed until a mean age of later than 5 years. That delay in diagnosis is directly contradicted by the presentation of behavioral symptoms: 30% of parents of children with ASD suspected developmental problems before their child’s first birthday; 50% suspected problems by 18 months; and 80% of parents suspected problems by 2 years [35–37]. Nevertheless, the median age of diagnosis in the US remains 5.5 years of age [38]. This late age of diagnosis is even later for those who lack resources and have limited access to expert clinicians: in the US, diagnoses for lower income, minority, and rural families lag on average by another year and a half [39–41]. In all children, delay in diagnosis leads directly to delayed intervention and treatment. Thus the point at which a child can be accurately diagnosed with autism moves from within a window of tremendous neuroplasticity [42]—the period from birth until age three—to a point several years hence, when many years of development have already played a large role in shaping the course of a child’s condition [43]. This marks the loss of a potentially critical opportunity for improving treatment efficacy and associated outcome [26].
Advances in developmental social neuroscience of autism: social engagement in typically developing infants and toddlers and derailment thereof in children with autism
Within the first hours of life, typically-developing babies attend preferentially to people. They distinguish and prefer their own mother’s voice to that of an unknown woman, but prefer the sound of even an unknown woman’s voice to that of silence [44]. Human newborns preferentially fixate on faces gazing at them rather than faces looking away [45], and by 3 months they are drawn to the eye region when viewing speaking faces [46]. Infants are also capable of imitating the facial gestures of a person [47] while not mimicking similar movements made by a mechanical device [48]. This evidence suggests that typically-developing babies have a predisposition to engage with the social aspects of the world around them: the social dimension is what is most behaviorally salient and what consequently commands the greatest portion of the typically-developing child’s attention.
For infants with autism, the available evidence suggests that this is not the case. The most robust markers for early diagnosis of children with autism center on disruptions to typical engagement with the social world: reduced interaction with and looking at others [12]; failure to respond to the calling of one’s own name; diminished eye contact; and inability to join in imitative games and reciprocal vocalizations [11,49]. While, until recently, most insights into the first two years of the lives of children with autism were gained via retrospective parental reports and analyses of home movies made by parents prior to their children’s diagnosis [11,12], in the past 5 years years we have witnessed a surge of prospective studies of children at high-risk for autism, at times from birth. Capitalizing on the high recurrence rate within sibships – 1 or 2 out of 5 among the younger siblings of children with autism are also diagnosed with autism or show transient or subthreshold symptoms of autism [50], there have been tens of prospective studies focused on “baby siblings” [51] which have shed light on the unfolding of autism in the first 2 years of life. While most of the studies so far have focused on the emergence of early symptoms [52–54], several experimental studies have focused on abnormalities in normative processes of socialization. Using behavioral probes, eye-tracking, electrophysiological, functional and diffusion tensor magnetic resonance imaging, investigators have been documenting derailment of fundamental social engagement processes from the first year of life [55–60]. The vast majority of these studies, however, have only been able to report group differences – when comparisons are made between high-risk siblings (with or without ascertained autism) and controls. In other words, these studies were unable to predict an individual baby’s outcome, or his or her level of disability, on the basis of their experimental measures. While prodromal group results generate the promise of performance-based measures predictive of the disability – typically ascertained clinically by the age of 24 to 36 months, they do not have immediate relevance to the goal of developing a screening or diagnostic test that might help identify children at risk before the emergence of symptoms, or in place of a diagnostic process conducted by expert clinicians. There are signs, however, that this situation is now changing. These efforts are focused on abnormalities of eye gaze: the way babies preferentially orient to, and are sensitive to the social and communicative value of, the eyes of others, to which we now turn.
Eye gaze in infants later diagnosed with autism
In autism, deficits in eye gaze are a defining feature of the condition [8] and a key item in standardized diagnostic tests [61]. These deficits have been extensively demonstrated in eye-tracking studies [55,62,63]; in electrophysiological reports [57,64], including intracranial recordings [65]; and also in functional MRI studies [66–68]. The conserved nature [69,70], early onset [45,46], and critical role of eye fixation in socialization [69,71] prompted our group to examine preferential looking to the eyes of approaching adults in infants and toddlers with autism. In an early study [63], we presented 2-year-old children with videos showing an actress looking directly into the camera, playing the role of caregiver, and engaging the viewer in typical infant-directed interaction games such as “pat-a-cake” and “peek-a-boo” while the children’s visual fixation patterns were measured by eye tracking. There were three groups: toddlers with autism (ASD), typically developing (TD) controls, and non-autistic but developmentally delayed (DD) controls. Children with ASD exhibited significantly less eye fixation relative to the two other groups: median eye fixation was in fact less than half that of TD and DD children. Two additional observations added importance to this finding. First, eye fixation in the toddlers with ASD was significantly correlated with their level of social disability (as measured via standardized clinical instruments), thus imbuing this behavioral assay with clinical validity. Second, toddlers with ASD also displayed significantly increased mouth-fixation relative to controls. In light of our studies of preferential orientation to biological motion [72] (the motion of living beings) [73,74] – in which visual behavior of toddlers with autism appeared to be guided primarily by audiovisual synchrony rather than the social nature of the stimuli – we raised the hypothesis that their mouth-fixation resulted from their engaging with the video stimuli as a composite of physical characteristics, without social meaning, given that the mouth is the locus of greatest audiovisual synchrony in speaking faces (i.e., speech sounds and lip movements covary) [72].
Our results supported the contention made in the very first description of autism by Leo Kanner [75], who characterized “autistic disturbances of affective contact” as “congenital”, from birth. Our group and others have long advocated the notion that disruptions of typical, extremely early-emerging mechanisms of adaptive social action give rise to the social disability known as autism [43,76,77]. However, this hypothesis is still only supported by indirect evidence since knowledge of the first two years of life of children with autism has, until recently been quite limited (though growing), primarily because children with autism are diagnosed between two and three years of life at the earliest [49]. The word “congenital” is intended to refer to the behavioral instantiation of genetic liabilities in observable and measurable ways.
Although several studies show atypical neural processing of social stimuli in infants at risk for autism, or in infants who were subsequently diagnosed with autism [56,57], direct observation and quantification of the early developmental progression of autism have not, until recently, been available [78], including possible disruptions in early-emerging social adaptive behaviors. This gap in clinical and research knowledge has been a critical one. The first two years of a baby’s life encompass the most substantial and rapid period of neural and behavioral growth in postnatal human development [79]. For a condition as strongly heritable as autism [2], and for one in which multifactorial genetic etiologies are likely to begin their impact on development from birth if not before [6], a thorough mapping – of social behavior, brain changes, and gene processes – in the first two years of life is a critical step for understanding the pathogenesis of the condition and constraining gene-brain-behavior hypotheses [1].
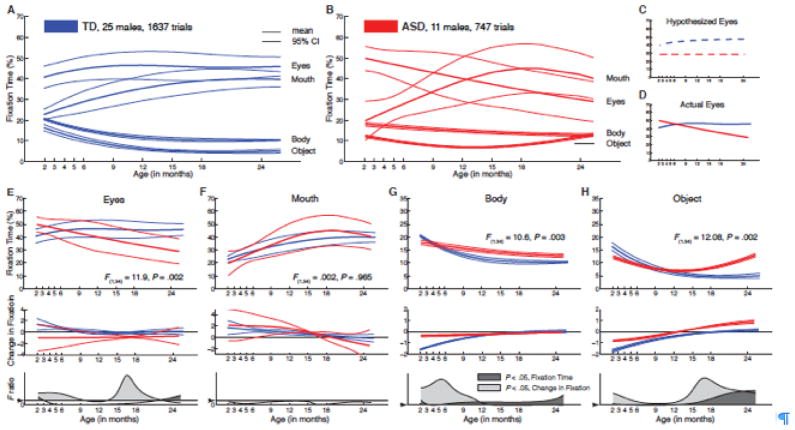
This state of affairs was the impetus for our most recent study of eye fixation in autism [80]. Infants who were later diagnosed with autism and typically developing infants were shown pre-recorded video scenes of actresses playing the role of caregivers while engaging their children in infancy games. Like in our previous study [63], the children’s visual scanning was measured by eye tracking. Data were collected monthly, from two to six months of age, and then every three months until the age of 18 months, with a final data point at 24 months (10 time points overall). Ascertainment of diagnostic status and its stability happened at 24 and 36 months, respectively. Eye-fixation data for the typical children delineated “growth charts” of social visual engagement (Figure 1A) against which we compared the data for the infants later diagnosed with autism (Figure 1B). Typically developing children, from two to six months, looked more at the eyes than at any other region of the screen (mouth, body, objects); eye fixation increased steadily during this period and remained rather stable until the age of 24 months.
Figure 1.
Growth charts of social visual engagement for typically-developing children (TD) relative to children diagnosed with autism spectrum disorder (ASD). (A) Fixation to eyes, mouth, body, and object from 2 until 24 months in TD males (in blue) and (B) in males with ASD (in red). Contrary to a hypothesis of congenital reduction in preferential attention to the eyes in ASD, infants with ASD exhibit mean decline in eye fixation from 2 until 24 months of age. Hypothesized (C) and actual (D) mean eye fixation curves are plotted in blue for TD children and in red for children with ASD. Longitudinal change in fixation to (E) eyes; (F) mouth; (G) body; and (H) object regions. Dark lines of each color represent mean growth curves, while light lines indicate pointwise 95% confidence intervals. Top panel in each section plots percent fixation over time; middle panel plots change in fixation over time (the first derivative, in units of % change per month); and the bottom panel plots F value functions for pointwise comparisons of fixation and change in fixation between groups. Pointwise comparisons with F values greater than Fcrit (for 1,34 dof = 4.13, P = 0.05, marked by arrowhead on F ratio axis) are shaded in medium gray (for comparison of fixation data) and light gray (for comparison of change-in-fixation data).
Given our hypothesis that children with autism have a congenital deficit impairing their ability to preferentially orient to others’ eyes, our expectation was that their levels of eye fixation would be reduced relative to those of typically developing infants from the earliest time of data collection (Figure 1C). Our results falsified this hypothesis (Figure 1DE): eye fixation began at a level similar to typically developing controls but then declined steadily from the two-month starting point, arriving at a level that was approximately half that of controls by the 24-month endpoint. This decline in eye fixation was already underway within the first 6 months.
Two additional observations added significance to this finding. First, the decline in eye fixation within the first six months alone was strongly and significantly associated with diagnostic outcome at the age of 36 months. Thus developmental differences in level of preferential attention to the eyes of other people was a strong marker of later diagnosis one and a half years before the children could be diagnosed conventionally and two and half years before they would be diagnosed stably [80]. Second, in the children with autism, the degree of decline in eye looking was a strong predictor of level of social disability at outcome (as measured with standardized clinical instruments): children whose levels of eye looking declined most rapidly were also most socially disabled in later life [80].
This was the first time that a performance-based, experimental procedure was shown to have clinical utility relative to individual young children, predicting both the child’s diagnosis (i.e., autism vs. non-autism), and severity of the condition (i.e., where, in the spectrum of autistic disability, the individual child’s presentation falls). Clearly, this study requires replication and corroboration that can only come from much larger studies. But it points to the possibility that, in the not too distant future, performance-based measures might be available that can be deployed as measurements of autism. For this technology to be made relevant to clinical practice, however, there is substantial work to be done. It does, nevertheless, carry the potential to contribute to eventual solutions for the immense public health challenge represented by the current late age of diagnosis and treatment of autism.
Developmental social neuroscience meets public health challenge
The translation from experimental procedures conducted in the lab to performance-based, objectified and quantitative measurements of autism deployed in the community will require many steps but this long road has been accomplished in other areas of medicine and science. While our eye-tracking procedure with infants and toddlers is the first to show relevance to individual children [80], substantial research using many other procedures based on eye-tracking, EEG and MRI technology is currently underway, and these procedures too may reach a level of significance to individual children in the near future. In all cases, however, studies are likely to be relatively small and intended to generate hypotheses and explore developmental mechanisms associated with autism pathogenesis. Larger, multi-site studies will be required to substantiate a procedure’s diagnostic efficacy (i.e., demonstrating that the procedure is beneficial in a clinical trial). And yet, to make these kinds of technologies relevant to clinical practice, the true test will come from research on their effectiveness (i.e., demonstrating that benefits of the procedure can be shown in clinical practice). This translational effort will require gathering information from administrators, clinicians and parents about the barriers and facilitators of moving an evidenced-based procedure into real-world practice. Implementation strategies are required to identify methods that increase the probability of success of the proposed solution. This dilemma of implementation is present in many fields of medicine. In the field of autism, however, there is a pressing need for synergy between clinical investigators who build the evidence base and front-line clinicians who are called upon to embrace such solutions. Factors of importance include the realities of clinical practice, culture and level of resources, as well as pressures and incentives extant in the targeted clinical environment. These are core topics of an emerging and critical area of research called implementation science [81], which is at the intersection of science advances and large-scale changes of clinical practice. The very best solutions achieved in academic laboratories will have little relevance to children and families affected by autism if they are not translated into community-viable solutions for public health challenges [82,83].
There are two overlapping ways in which developmental social neuroscience procedures developed in the lab may advance the goal of reducing the age of autism diagnosis in the community: as universal screeners and as proxies for diagnostic ascertainment. Screening efforts have advanced markedly in the past 10 years [84]. Paper-and-pencil screening instruments are available and their dissemination in primary care offices is growing [84], but, as noted, they are still far from being universally adopted despite the directives of the American Academy of Pediatrics [17,18,84]. Autism is sufficiently highly prevalent and potentially devastating to children and families, as well as systems of care, the educational system, and lifespan support systems [19–22] to justify the aspiration for universal screening. And universal screening is the ultimate solution to reduce the current community disparities in access to clinical services still plaguing large sectors of the population in the US [28–30]. Although these paper-and-pencil screening methods are cost-effective, they do not follow the more typical medical model for procedure coverage provided by the health insurance system nor are they accepted as “medical tests”. It would be hard to conceive of a paper-and-pencil screening procedure for medical conditions such as childhood cancer or diabetes. It is in this sense that validated experimental procedures can complement current efforts to disseminate screening for autism as routine care in primary care practices. But this can only happen if these experimental procedures and technologies are shown to be cost-effective and viable within the context of the busy and typically overly taxed primary care office.
The possibility that experimental procedures can act as proxies for clinical diagnosis would, at face value, go against everything that we know about gold standards for clinical practice, which involve the deployment of standardized instrumentation by expert clinicians [2008]. ‘Gold-standard’ (or ‘reference standard’) diagnostic instruments are standardized, validated assessments that measure the presence of autistic social disability through both clinician-based behavioral observation (e.g., using the Autism Diagnostic Observation Schedule (ADOS)[85]), and through parent interview (using the Autism Diagnostic Interview – Revised (ADI-R) [86]). Best practice guidelines for comprehensive diagnostic evaluation also call for standardized assessments of the child’s cognitive functioning and language skills [87]. Unfortunately, the actual community usage of these gold-standard instruments is quite limited. In the US, primary care providers provide the majority of autism diagnoses. Usage of gold-standard diagnostic instruments is almost entirely absent from their practices: for example, the ADI-R and ADOS are used in fewer than 0.1% and 2.1% (respectively) of diagnostic evaluations for autism [88]. Lesser quality (i.e., inferior to the gold-standard) instruments, such as parent questionnaires and checklists, are used in only 30% of such evaluations [88]; and the remainder of diagnostic evaluations for autism—more than 67% of all such evaluations—use no standardized or validated instruments. The usage of gold-standard instruments is confined to specialty clinics with limited patient capacity. The factors accounting for the low adoption of otherwise effective instruments in community settings could be predicted by implementation scientists: for example, gold-standard instruments require lengthy periods for patient testing (e.g., approximately 1.5 to 2 hours for the ADI-R, and 1 hour for the ADOS); they also require extensive training and expertise for reliable administration and scoring, as they depend upon an expert clinician’s subjective assessment of both the informant report (as in the ADI-R) and the patient behavioral observation ( as in the ADOS). These factors place major limitations on the effectiveness of these instruments in two ways: they prevent the wide dissemination and adoption of such gold standard procedures, and they gravely constrain access to the diagnostic process and the attainment of diagnosis for purposes of eligibility for services because the number of expert clinicians in the community is very limited. This is the diagnostic landscape in which cost-effective experimental procedures and technologies could facilitate faster and more widely available diagnostic process.
Whereas one advances the notion that procedures originating from developmental social neuroscience can be translated as “universal screeners” or as “diagnostic proxies”, it will be critical to ensure that premature claims of success are avoided – as noted, the road from small efficacy studies in the lab to “roll-out ready” practices for the clinic, is a long and arduous one. Adequately powered and rigorously conducted clinical trials deployed in community practices set the standard for yielding evidence for the effectiveness for any medical procedure [89,90]. And yet, the promise of translational research that can move science into the community justifies this effort [91].
Conclusion
Autism is a highly prevalent, lifelong and potentially devastating neurodevelopmental disorder of genetic origins whose behavioral symptoms are instantiated in the first two years of life. Despite the evidence indicating that early diagnosis and early treatment can significantly improve outcome, age of diagnosis in the community is still late relative to the window of opportunity afforded by neuroplasticity in the first two years of life. Reducing age of diagnosis in order to provide children with autism with access to early treatment has become one of the key priorities for research in this field. New advances in developmental social neuroscience have already yielded experimental procedures shown to identify markers for the condition even before the emergence of overt symptoms and much before expert clinicians can reliably diagnose the condition. The potential is great for these new technologies to be translated into objectified, quantitative, efficacious and cost-effective practices, capable of reaching wide dissemination. The key aspect of this process, however, will be to ensure that premature claims are not made based on small, lab-based efficacy studies until much larger, multi-site clinical trials are conducted, accompanied by implementation science efforts aimed at making this new science into community-viable solutions. Nevertheless, this bold new prospect was unthinkable even 5 years ago. The success of this effort calls for a concerted and seamless effort bringing together social neuroscientists, clinical practitioners, and implementation scientists [91].
Acknowledgments
This work was supported by grants from the Simons Foundation and the National Institute of Mental Health (R01 MH083727; P50-MH100029). Additional support was provided by the Marcus Foundation, the J.B. Whitehead Foundation, the Cox Foundation, and the Georgia Research Alliance. We wish to thank the families and children for their time and participation in our various studies reviewed here. We also wish to thank our colleagues and students for their contributions to the experimental and clinical work reported here.
References
- 1.DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26(26):6897–906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Constantino JN, Todorov A, Hilton C, Law P, Zhang Y, Molloy R, et al. Autism recurrence in half siblings: strong support for genetic mechanisms of transmission in ASD. Mol Psychiatry. 2013;18(2):137–8. doi: 10.1038/mp.2012.9. [DOI] [PubMed] [Google Scholar]
- 3.O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7397):246–50. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey J, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485(7397):237–41. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485(7397):242–5. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9(5):341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.State MW, Šestan N. Neuroscience. The emerging biology of autism spectrum disorders. Science. 2012;337(6100):1301–3. doi: 10.1126/science.1224989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 9.Merin N, Young GS, Ozonoff S, Rogers SJ. Visual Fixation Patterns during Reciprocal Social Interaction Distinguish a Subgroup of 6-Month-Old Infants At-Risk for Autism from Comparison Infants. J Autism Dev Disord. 2007;37(1):108–21. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- 10.Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, et al. Visual orienting in the early broader autism phenotype: disengagement and facilitation. J Child Psychol Psychiatry. 2009;50(5):637–42. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels AM, Mandell DS. Explaining differences in age at autism spectrum disorder diagnosis: a critical review. Autism. 2013;18(5):583–597. doi: 10.1177/1362361313480277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol. 2002;14(2):239–51. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- 13.Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch Gen Psychiatry. 2007;64(7):853–64. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. Morb Mortal Wkly Rep. 2014;63(SS02):1–21. [PubMed] [Google Scholar]
- 15.Exhorn KS. The Autism Sourcebook. Everything You Need to Know About Diagnosis, Treatment, Coping, and Healing. New York: Regan Books; 2005. p. 77. [Google Scholar]
- 16.Volkmar F, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics C on C with D. The pediatrician’s role in the diagnosis and management of autistic spectrum disorder in children. Pediatrics. 2001;107:1221–1226. doi: 10.1542/peds.107.5.1221. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics C on C with D. Technical report: The pediatrician’s role in the diagnosis and management of autistic spectrum disorder in children. 2001;E85:107. doi: 10.1542/peds.107.5.e85. [DOI] [PubMed] [Google Scholar]
- 19.Peacock G, Amendah D, Ouyang L, Grosse SG. Autism spectrum disorders and health care expenditures: the effects of co-occurring conditions. J Dev Behav Pediatr. 2012;33(1):2–8. doi: 10.1097/DBP.0b013e31823969de. [DOI] [PubMed] [Google Scholar]
- 20.Cidav Z, Marcus SC, Mandell DS. Implications of childhood autism for parental employment and earnings. Pediatrics. 2012;129:617–623. doi: 10.1542/peds.2011-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Mandell DS, Lawer L, Cidav Z, Leslie DL. Healthcare service use and costs for autism spectrum disorder: a comparison between Medicaid and private insurance. J Autism Dev Disord. 2013;43:1057–1064. doi: 10.1007/s10803-012-1649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States of America. JAMA Pediatr. 2014 doi: 10.1001/jamapediatrics.2014.210. [DOI] [PubMed] [Google Scholar]
- 23.Warren Z, McPheeters ML, Sathe N, Foss-Feig JH, Glasser A, Veenstra-Vanderweele J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127(5):e1303–11. doi: 10.1542/peds.2011-0426. [DOI] [PubMed] [Google Scholar]
- 24.Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwaigenbaum L, Bryson S, Garon N. Early identification of autism spectrum disorders. Behav Brain Res. 2013;251:133–46. doi: 10.1016/j.bbr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Dawson G, Jones EJH, Merkle K, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51(11):1150–9. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osterling J, Dawson G. Early recognition of children with autism: A study of first birthday home video tapes. J Autism Dev Disord. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- 28.Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120(5):1183–215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 29.Dosreis S, Weiner CL, Johnson L, Newschaffer CJ. Autism spectrum disorder screening and management practices among general pediatric providers. J Dev Behav Pediatr. 2006;27(2 Suppl):S88–94. doi: 10.1097/00004703-200604002-00006. [DOI] [PubMed] [Google Scholar]
- 30.Heidgerken AD, Geffken G, Modi A, Frakey L. A survey of autism knowledge in a health care setting. J Autism Dev Disord. 2005;35(3):323–30. doi: 10.1007/s10803-005-3298-x. [DOI] [PubMed] [Google Scholar]
- 31.US Department of Education. 30th Annual Report to Congress on the Implementation of the Individuals with Disabilities Education Act. 2011 [Google Scholar]
- 32.Honigfeld L, Chandhok L, Spiegelman K. Engaging pediatricians in developmental screening: the effectiveness of academic detailing. J Autism Dev Disord. 2012;42(6):1175–82. doi: 10.1007/s10803-011-1344-4. [DOI] [PubMed] [Google Scholar]
- 33.Al-Qabandi M, Gorter JW, Rosenbaum P. Early autism detection: are we ready for routine screening? Pediatrics. 2011;128(1):e211–7. doi: 10.1542/peds.2010-1881. [DOI] [PubMed] [Google Scholar]
- 34.Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. J Dev Behav Pediatr. 2006;27(2 Suppl):S79–87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- 35.Chawarska K, Paul R, Klin A, Hannigen S, Dichtel LE, Volkmar F. Parental recognition of developmental problems in toddlers with autism spectrum disorders. J Autism Dev Disord. 2007;37(1):62–72. doi: 10.1007/s10803-006-0330-8. [DOI] [PubMed] [Google Scholar]
- 36.Young RL, Brewer N, Pattison C. Parental identification of early behavioural abnormalities in children with autistic disorder. Autism. 2003;7(2):125–43. doi: 10.1177/1362361303007002002. [DOI] [PubMed] [Google Scholar]
- 37.Wetherby AM, Brosnan-Maddox S, Peace V, Newton L. Validation of the Infant-Toddler Checklist as a broadband screener for autism spectrum disorders from 9 to 24 months of age. Autism. 2008;12(5):487–511. doi: 10.1177/1362361308094501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shattuck PT, Durkin M, Maenner M, et al. Timing of identification among children with an autism spectrum disoder: findings from a population-based surveillance study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):474–483. doi: 10.1097/CHI.0b013e31819b3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandell DS, Listerud J, Levy SE, Pinto-Martin JA. Race differences in the age at diagnosis among medicaid-eligible children with autism. J Am Acad Child Adolesc Psychiatry. 2002;41(12):1447–53. doi: 10.1097/00004583-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;116(6):1480–6. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandell DS, Wiggins LD, Carpenter LA, Daniels J, DiGuiseppi C, Durken MS, et al. Racial/ethnic disparities in the identification of children with autism spectrum disorders. Am J Public Health. 2009;99(3):493–8. doi: 10.2105/AJPH.2007.131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson MH. Cortical plasticity in normal and abnormal cognitive development: evidence and working hypotheses. Dev Psychopathol. 1999;11(3):419–37. doi: 10.1017/s0954579499002138. [DOI] [PubMed] [Google Scholar]
- 43.Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):345–60. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’ voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- 45.Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. PNAS. 2002;99(14):9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haith MM, Bergman T, Moore MJ. Eye contact and face scanning in early infancy. Science. 1977;198(4319):853–855. doi: 10.1126/science.918670. [DOI] [PubMed] [Google Scholar]
- 47.Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- 48.Meltzoff AN, Moore MK. Early imitation within a functional framework: The importance of person identity, movement, and development. Infant Behav Dev. 1992;15(4):479–505. doi: 10.1016/0163-6383(92)80015-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chawarska K, Volkmar FR, Klin A, editors. Autism spectrum disorders in infants and toddlers: Diagnosis, Assessment and Treatment. New York: Guilford Press; 2008. [Google Scholar]
- 50.Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–95. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, et al. Studying the emergence of autism spectrum disorders in high-risk infants: methodological and practical issues. J Autism Dev Disord. 2007;37(3):466–80. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]
- 52.Macari SL, Campbell D, Gengoux GW, Saulnier CA, Klin A, Chawarska K. Predicting developmental status from 12 to 24 months in infants at risk for autism spectrum disorder: a preliminary report. J Autism Dev Disord. 2012;42(12):2636–47. doi: 10.1007/s10803-012-1521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozonoff S, Losif AM, Baugio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49(3):256–66. [PMC free article] [PubMed] [Google Scholar]
- 54.Zwaigenbaum L, Bryson S, Garon N. Early identification of autism spectrum disorders. Behav Brain Res. 2013;251:133–46. doi: 10.1016/j.bbr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol Psychiatry. 2013;74(3):195–203. doi: 10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luyster RJ, Wagner JB, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Neural correlates of familiar and unfamiliar face processing in infants at risk for autism spectrum disorders. Brain Topogr. 2011;24(3–4):220–228. doi: 10.1007/s10548-011-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, et al. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Curr Biol. 2012;22:338–342. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elison JT, Paterson S, Wolff JJ, Reznick S, Sasson NJ, Gu H, et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry. 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klin A, Shultz S, Jones W. Social visual engagement in infants and toddlers with autism: Early developmental transitions and a model of pathogenesis. Neurosci Biobehav Rev. 2014 doi: 10.1016/j.neubiorev.2014.10.006. S0149-7634(14):00255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Courchesne E, Redcay E, Morgan JT, Kennedy DP. Autism at the beginning: microstructural and growth abnormalities underlying the cognitive and behavioral phenotype of autism. Dev Psychopathol. 2005;17(3):577–97. doi: 10.1017/S0954579405050285. [DOI] [PubMed] [Google Scholar]
- 61.Lord C, Rutter M, DiLavore P, Risi S. ADOS Toddler Module: ADOS-T: Manual. Los Angeles, CA: Western Psychological Services; 2008. [Google Scholar]
- 62.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59(9):809–16. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 63.Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-olds with autism. Arch Gen Psychiatry. 2008;65(8):946–54. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 64.Elsabbagh M, Volein A, Csibra G, Homboe K, Garwood H, Tucker L, et al. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biol Psychiatry. 2009;65(1):31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 65.Rutishauser U, Tudusciuc O, Wang S, Mamelak AN, Ross IB, Adolphs R. Single-neuron correlates of atypical face processing in autism. Neuron. 2013;80(4):887–899. doi: 10.1016/j.neuron.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dalton KM, Nacewicz BM, Johnston T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kliemann D, Dziobek I, Hatri A, Baudewig J, Heekeren HR. The role of the amygdala in atypical gaze on emotional faces in autism spectrum disorders. J Neurosci. 2012;32(28):9469–9476. doi: 10.1523/JNEUROSCI.5294-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davies MS, Dapretto M, Sigman M, Sepeta L, Bookheimer SY. Neural bases of gaze and emotion processing in children with autism spectrum disorders. Brain Behav. 2011;1(1):1–11. doi: 10.1002/brb3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 70.Bard KA. Primate parenting. In: Bornstein M, editor. Handbook of parenting, vol 2. Biology and ecology of parenting. 2. Mahwah, NJ: Erlbaum; 2002. pp. 99–140. [Google Scholar]
- 71.Kampe KKW, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413(6856):589–590. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- 72.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism fail to orient towards human biological motion but attend instead to non-social, physical contingencies. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fox R, McDaniel C. The perception of biological motion by human infants. Science. 1982;218:486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- 74.Johnson MH. Biological motion: a perceptual life detector? Curr Biol. 2006;16 (10):R376–7. doi: 10.1016/j.cub.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 76.Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- 77.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Res. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson M. Functional brain development in humans. Nat Rev Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- 80.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–31. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Curran JA, Grimshaw JM, Hayden JA, Campbell B. Knowledge translation research: the science of moving research into policy and practice. J Contin Educ Health Prof. 2011;31(3):174–80. doi: 10.1002/chp.20124. [DOI] [PubMed] [Google Scholar]
- 82.Freed GA. Society for Pediatric Research – 2007 Presidential Address: Expanding the research continuum – from bench to implementation. Pediatr Res. 2007;62:370–373. doi: 10.1203/PDR.0b013e318140b02a. [DOI] [PubMed] [Google Scholar]
- 83.Locke J, Olsen A, Wideman R, Downey MM, Kretzmann M, Kasari C, et al. A tangled web: the challenges of implementing an evidence-based social engagement intervention for children with autism in urban public school settings. Behav Ther. 2015;46(1):54–67. doi: 10.1016/j.beth.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daniels AM, Halladay AK, Shih A, Elder LM, Dawson G. Approaches to enhancing the early detection of autism spectrum disorders: a systematic review of the literature. J Am Acad Child Psy. 2014;53(2):141–152. doi: 10.1016/j.jaac.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 85.Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- 86.Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview - Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 87.Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH, Dawson G, et al. Practice parameter: Screening and diagnosis of autism. Report of the quality standards subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- 88.Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. J Dev Behav Pediatr. 2006;27(2 Suppl):S79–87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- 89.Mansi BA, Clark J, David FS, Gesell TM, Glasser S, Gonzalez J, et al. Ten recommendations for closing the credibility gap in reporting industry-sponsored clinical research: a joint journal and pharmaceutical industry perspective. Mayo Clin Proc. 2012;87(5):424–429. doi: 10.1016/j.mayocp.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.TDR Diagnostics Evaluation Expert Panel. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2010;8(12 Suppl):S17–29. [PubMed] [Google Scholar]
- 91.Insel TR, Gogtay N. National Institute of Mental Health Clinical Trials: new opportunities, new expectations. JAMA Psychiatry. 2014;71(7):745–6. doi: 10.1001/jamapsychiatry.2014.426. [DOI] [PubMed] [Google Scholar]