Abstract
Background: Timely individualized treatment is essential to improving relapsing-remitting multiple sclerosis (RRMS) patient health outcomes, yet little is known about how patients make treatment decisions. We sought to evaluate RRMS patient preferences for risks and benefits of treatment.
Methods: Fifty patients with RRMS completed conjoint analysis surveys with 16 hypothetical disease-modifying therapy (DMT) medication profiles developed using a fractional factorial design. Medication profiles were assigned preference ratings from 0 (not acceptable) to 10 (most favorable). Medication attributes included a range of benefits, adverse effects, administration routes, and market durations. Analytical models used linear mixed-effects regression.
Results: Participants showed the highest preference for medication profiles that would improve their symptoms (β = 0.81–1.03, P < .001), not a proven DMT outcome. Preventing relapses, the main clinical trial outcome, was not associated with significant preferences (P = .35). Each year of preventing magnetic resonance imaging changes and disease symptom progression showed DMT preferences of 0.17 point (β = 0.17, P = .002) and 0.12 point (β = 0.12, P < .001), respectively. Daily oral administration was preferred over all parenteral routes (P < .001). A 1% increase in death or severe disability decreased relative DMT preference by 1.15 points (P < .001).
Conclusions: Patient preference focused on symptoms and prevention of progression but not on relapse prevention, the proven drug outcome. Patients were willing to accept some level of serious risk for certain types and amounts of benefits, and they strongly preferred daily oral administration over all other options.
With numerous disease-modifying therapy (DMT) choices for relapsing-remitting multiple sclerosis (RRMS) now on the market, health-care decisions require patients and physicians to weigh a more complicated spectrum of treatment risks and benefits.1,2 Although improved outcomes are associated with early adoption and continued adherence to DMTs, patients with RRMS continue to have high discontinuation and low adherence rates.3–6 All approved DMTs decrease relapse rates, and some have been shown to slow disease progression. In addition, most DMTs are injectable, although the newest drugs are oral. Adverse effect risks range from mild (injection-site reactions) to severe (liver failure, serious cardiac events, leukemia, and progressive multifocal leukoencephalopathy).7–10 The differences in medication attributes and patient risk perception make it difficult for patients to select a treatment, resulting in barriers to DMT initiation and adherence.11
Preferences when weighing risks and benefits have been studied by various methods, including standard gamble, willingness to pay, and time trade-off utility scores, all measuring the maximum acceptable risk for a health state in exchange for a chance at either a better health state or death. These widely used techniques, which follow the expected utility framework, are criticized for not accurately representing real decision making because people do not follow linear probability weighting when making decisions.12
Conjoint analysis does not assume linear weighting and has recently been successfully applied to health-related decisions requiring trade-offs of risks and benefits.13,14 We used a ratings-based conjoint analysis to elicit patient preferences for benefit/risk trade-offs necessary when selecting attributes of a DMT. The objective was to develop a conjoint analysis tool to evaluate the preferences of patients with RRMS for a wide range of DMT attributes and to learn how patients with MS trade off risks and benefits. We tested patient preferences for the full range of each attribute inherent in currently available DMTs rather than for a particular single DMT to learn about patient preferences for important drug attributes across all DMTs.
Methods
Sample
Fifty patients with RRMS were selected from all appointments at the University of California, San Francisco, MS clinic between November 1, 2011, and May 31, 2012. Patients were included if they had a diagnosis of RRMS confirmed by medical record review, were aged 18 years or older, were English speaking, and consented to study participation. A ratings-based conjoint analysis survey and a demographic and medical history questionnaire were administered at the participant's home or in the neurology clinic. The study protocol was approved by the University of California, San Francisco, Human Subjects Research Committee.
Measures
The conjoint analysis method for measuring patients' preferences for the risks and benefits of DMTs followed the practice guidelines for conjoint analysis.15 Patients assigned numerical treatment ratings reflecting their preferences for attributes describing important factors of a treatment choice.
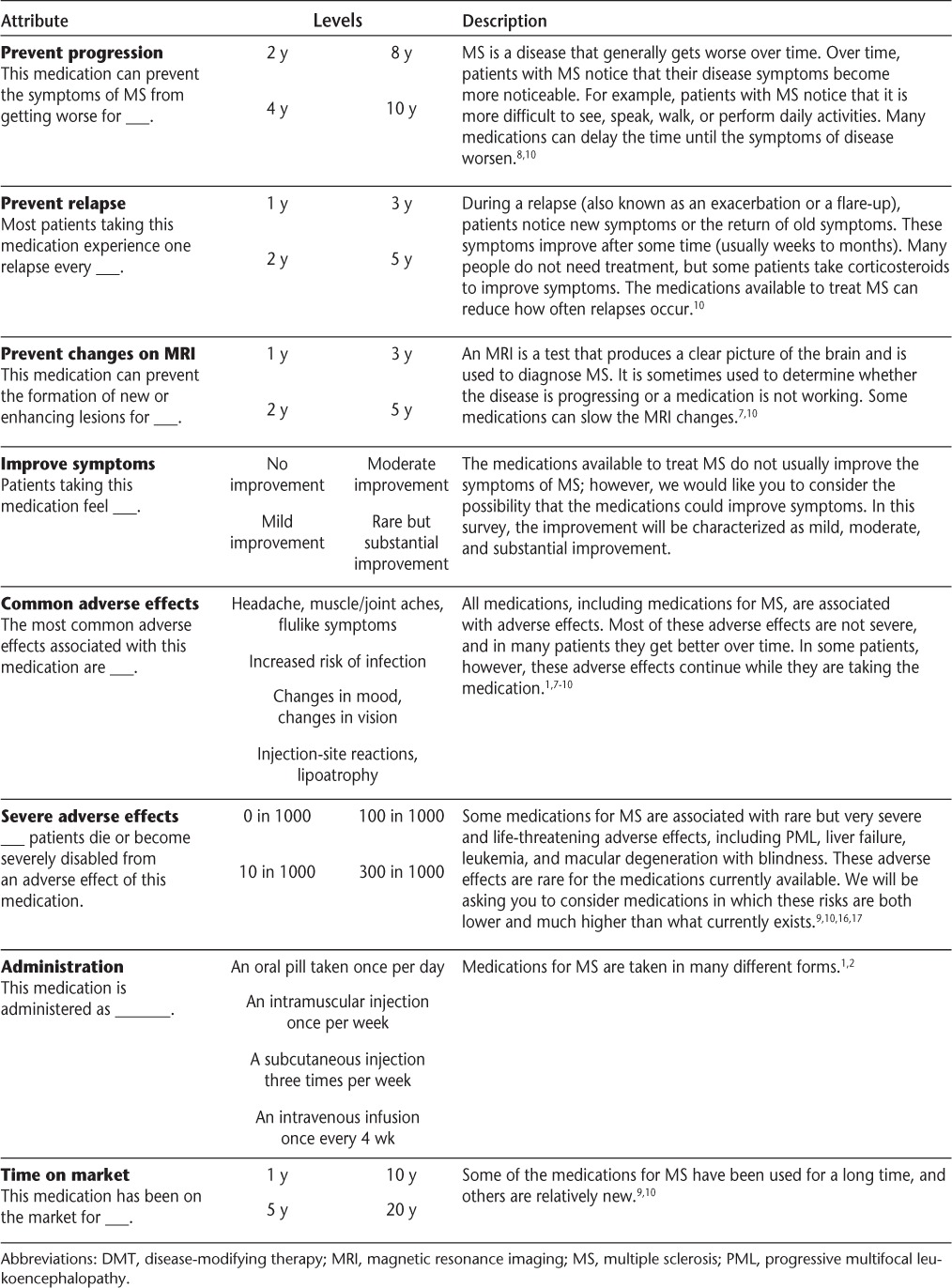
There were eight attributes of DMTs, each with four levels of description. A fractional factorial design was used to construct choice cards containing unique levels of all eight attributes (Table 1). Patients viewed 16 cards and were asked to weigh the level of each attribute combined to evaluate overall preference for each card relative to the other cards by first placing each card on a number line from 0 to 10, with 0 being the worst and 10 being the best possible selection. They then were asked to assign an exact preference number to each card. Patient-assigned rankings for the 16 cards had a hierarchical order that showed which cards are most and least preferred on an arbitrary scale. These rankings produced relative preferences ranging from 1 to 10 as respondents traded off treatment risk and benefit attributes, analogous to real treatment decisions. The ratings-based conjoint analysis using this card-sorting technique was selected because it can produce precise estimates using smaller sample sizes, it is paper based, and individual preferences can be applied.14
Table 1.
Hypothetical DMT attributes and levels for a card ratings-based conjoint analysis
Survey Components
The DMT attributes and levels were developed through a review of current clinical trial literature and with expert clinical and statistical expertise to select the most important actual or perceived attributes of all available DMT risks and benefits (Table 1).
Benefits
The primary efficacy outcome for DMT clinical trials is prevention of relapses, so this attribute was included as a conjoint attribute of benefit. Disease progression (defined as worsening of MS symptoms) and preventing change on magnetic resonance imaging (MRI) (defined as preventing the formation of new or enhancing radiologic lesions) were also included. Both of these outcomes are common secondary clinical outcomes. Prevention of disease progression is a more subjective measure and has been demonstrated only for some DMTs. Progression on MRI represents a less patient-visible marker but an outcome that providers may communicate to patients. Providers may also use this end point to determine the effectiveness of DMTs. Finally, improvement in symptoms was included because some individuals may expect DMTs to make them feel better symptomatically, despite the lack of considerable evidence supporting the ability of DMTs to improve existing symptoms of MS.
Risks
Risk attributes were divided into minor but uncomfortable adverse effects and potentially fatal adverse effects. The four levels of common adverse effects included those affecting appearance (lipoatrophy and injection-site reactions), those affecting the central nervous system (depressed mood and vision changes), generalized discomfort (headache, muscle/joint aches, and flulike symptoms), and episodic yet treatable adverse effects (increased chance of infections) to help us understand whether patients preferred some nonfatal adverse effects over others.7–10 The probability range of life-threatening adverse events was broad to evaluate patients' willingness to tolerate severe risks beyond current limits of DMT values and to determine maximum risk preferences and whether severe risk was linear.16–19 A written description explaining each DMT attribute and the visual risk scale was given to patients for reference (Table 1). A questionnaire assessed patients' current and past DMT use and demographic characteristics.
Analysis
A fractional factorial design (48–6) was used to select a representative subset of four levels in each of the eight attributes for the 16 cards for patients to sort and rate among the full factorial possible combinations. This allowed patients to rate only 16 cards instead of the possible 65,546 cards (48), saving substantial patient effort (SAS software version 9.3, SAS Institute Inc, Cary, NC). Descriptive statistics were used to summarize patient characteristics. Multivariate mixed-effects regression of the conjoint card ratings evaluated the importance of each level of the DMT attributes relative to one another.14 The card DMT preference rating was the dependent variable, the attribute levels were fixed independent variables, and patient identifiers were independent random effects. Multivariate mixed-effects regression using Stata software release 12 (StataCorp LP, College Station, TX) also determined whether patient age, sex, time since diagnosis, and current treatment were associated with willingness to accept various DMT risks and benefits.
Results
Demographic and Disease Characteristics
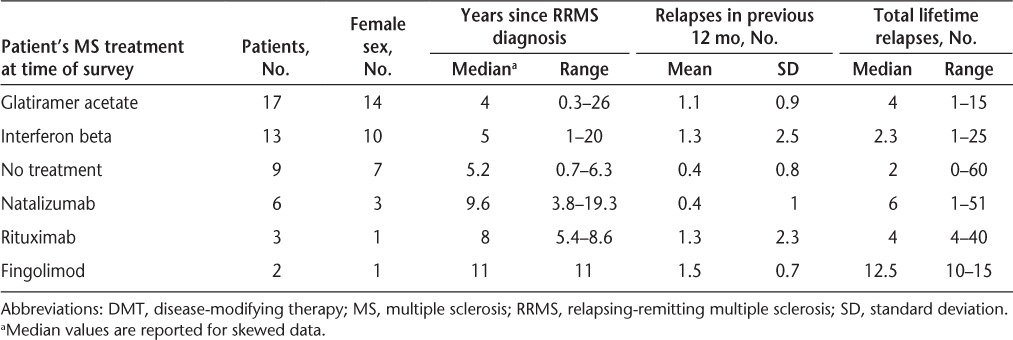
Fifty-two patients with RRMS were approached to participate in the study, with only two refusing to participate. Of the 50 patients with RRMS who completed the study, 74% were women, and the mean age was 42.7 years, comparable with national MS statistics.20 The predominant ethnicity was white (74%), and most patients were employed (64%) and married (62%). The median time since initial RRMS diagnosis was 5.1 years, and the mean number of relapses in the previous 12 months was 1 (Table 2). The most common current DMT treatment was glatiramer acetate (34%), followed by interferon beta (26%), no current treatment (18%), natalizumab (12%), rituximab (6%), and fingolimod (4%). Patients taking glatiramer acetate had the shortest time since RRMS diagnosis (median, 4 years) and fingolimod the longest (median, 11 years). Patients receiving natalizumab and no treatment had the lowest number of relapses in the previous 12 months (mean, 0.4) and fingolimod the highest (mean, 1.5) (Table 3).
Table 2.
Summary of patient and clinical characteristics
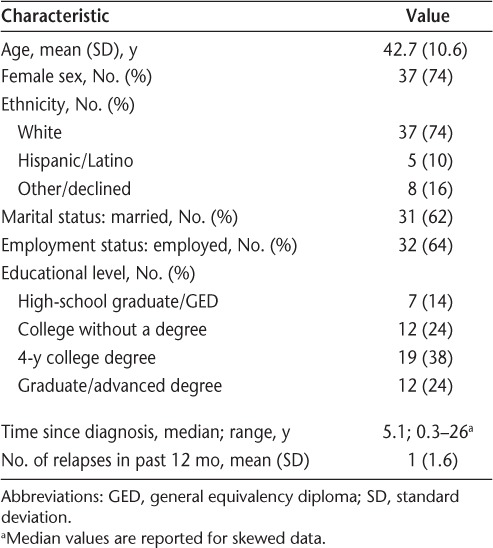
Table 3.
Characterization of current DMT use in the 50 study patients
Preference Ratings for Risks and Benefits
Risks included severe life-threatening adverse effects and more common adverse effects. Patients understandably expressed a negative preference for severe adverse effects at any frequency (P < .001). We found that increasing risk of death or severe disability was nonlinear at the levels presented. When presented with a DMT that had 1%, 10%, and 30% risk of severe disability or death, we found that patients rated these medications 1.15, 3.06, and 3.82 preference points (on a scale from 0 to 10) lower, respectively, than medications that had 0% risk of disability or death (P < .001 for all risk levels). Common adverse effects were compared using increased risk of infection as the reference attribute level. Patients assigned a 0.82 lower rating to DMTs with central nervous system adverse effects relative to DMTs with increased risk of infection (P < .001) (Table 4).
Table 4.
Relative preference for DMT attributes
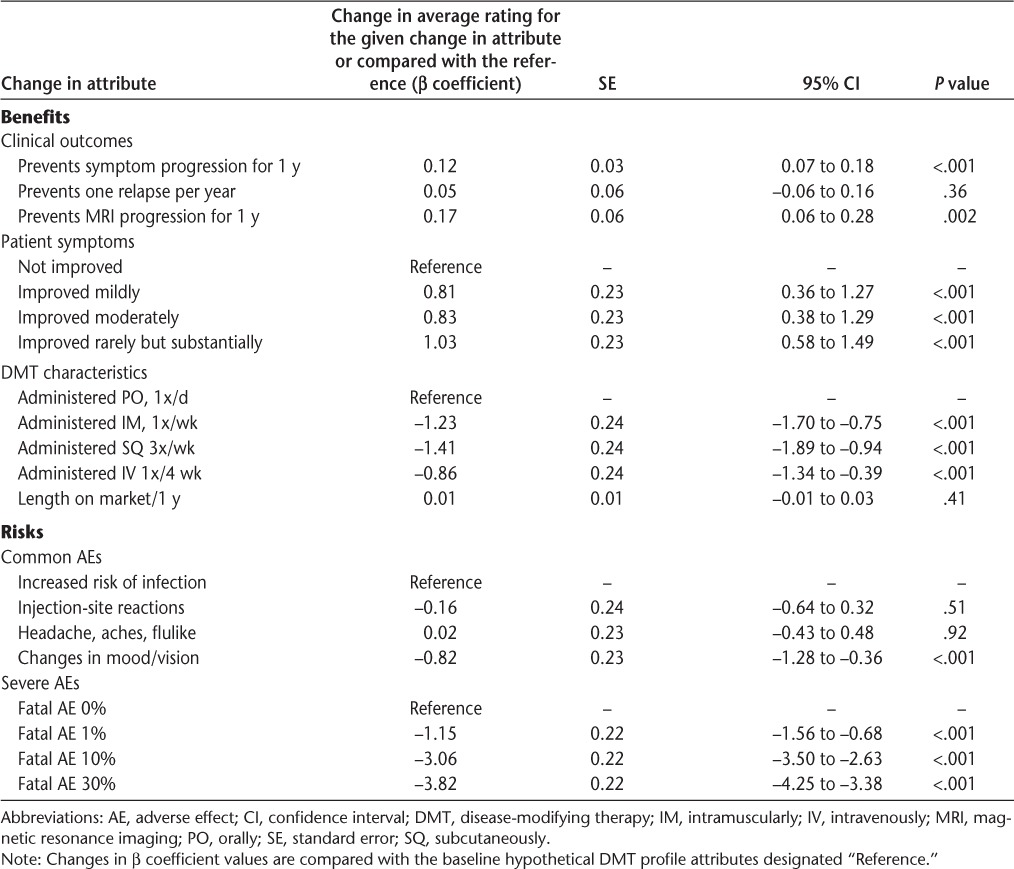
The beneficial attributes were based on treatment outcomes and symptom changes. Patients had no significant preference for decreasing relapse frequency as an attribute for choosing a DMT (P = .36). Patients had the greatest preference for improved MS symptoms compared with annual changes in other benefits. They favored any improvement in their MS symptoms. Patients rated DMTs 0.81, 0.83, and 1.03 (P < .001 for all) higher if they resulted in mild, moderate, and rare but substantial improvement in symptoms, respectively, relative to DMTs that resulted in no improvement in symptoms (Table 4).
When considering benefits that are common secondary end points of DMT clinical trials—preventing progression of symptoms/disability and preventing progression shown by new lesions on MRI—patients had the highest preference for preventing MRI lesions. Preventing changes in MRI lesions for 1 year resulted in a significant increase in the relative DMT rating preference of 0.17 (P = .002) (Table 4). As a result, medications that delayed MRI progression for 10 years would be associated with a 1.7-point increase in preference rating, higher than that of any improvement in symptoms, if considered over 10 years.
Patients preferred preventing disease symptom progression slightly less than progression measured by MRI lesions, with each year of progression prevention providing an increased relative preference rating of 0.12 point (P < .001) (Table 4). These ratings demonstrate that patients prefer more objectively visible disease benefits (MRI results) related to disease progression and benefits affecting daily symptoms.
Patients strongly preferred once-daily oral medication administration compared with all other combinations of injectable routes and frequencies of administration. The preference for an oral agent, even when taken daily, showed a stronger preference rating than, for example, moderate improvement in symptoms. Injecting subcutaneously three times weekly was least preferred, resulting in a preference rating 1.41 points lower than oral administration (P < .001). Intramuscular administration once weekly and intravenous administration once monthly resulted in preference ratings 1.23 and 0.86 points (P < .001 for both) lower, respectively, than oral administration. There was no significant preference for the number of years that a medication had been on the market (P = .41) (Table 4).
Risk/Benefit Trade-offs
We examined serious risks ranging from no risk to a 30% risk, much higher than those associated with current DMTs, which are approximately 0.009% to 8% for liver failure.16,17,19 The present analysis indicated that patients' preference for risk was not linear. There was a substantial decrease in preference when patients compared medications with a 1% risk of severe adverse effects with a medication with a 0% risk of severe adverse effects, but the decreases in ratings became less steep as patients evaluated higher levels of risk.
Based on the present results, we can make some interesting observations about patients' risk/benefit trade-offs. For example, patients were willing to accept a 1% risk of severe adverse effects or death for a medication that prevented symptom progression for 10 years or MRI progression for 7 years. Patients were also willing to accept that 1% risk for a medication that is administered orally or that has the potential for a rare but substantial improvement in symptoms. Patients indicated that they would be willing to accept a much higher risk, a 10% or 30% risk of severe adverse effects or death, for a medication that prevented progression for 25 or 32 years, respectively.
Demographic Characteristics and DMT Preferences
Comparing the descriptive statistics of age, sex, time since RRMS diagnosis, marital status, and current DMT in the mixed-effects model revealed several variations in risk acceptance. Patients were separated into two approximately equal age groups by mean sample age. Those aged 40 years and older significantly preferred preventing progression (β = 0.18, P < .001), whereas patients younger than 40 years did not (β = 0.04, P = .295), with a significant difference between the two age groups (P = .004). A significant difference (P = .036) was seen between patients aged 40 years and older, who showed less aversion to fatal risk (β = −0.098, P < .001), and those aged younger than 40 years (β = −0.13, P < .001) (Figure 1 A–D). These results indicate that older patients are more concerned with preventing disease progression and less concerned with fatal risks than younger patients.
Figure 1 A–D.
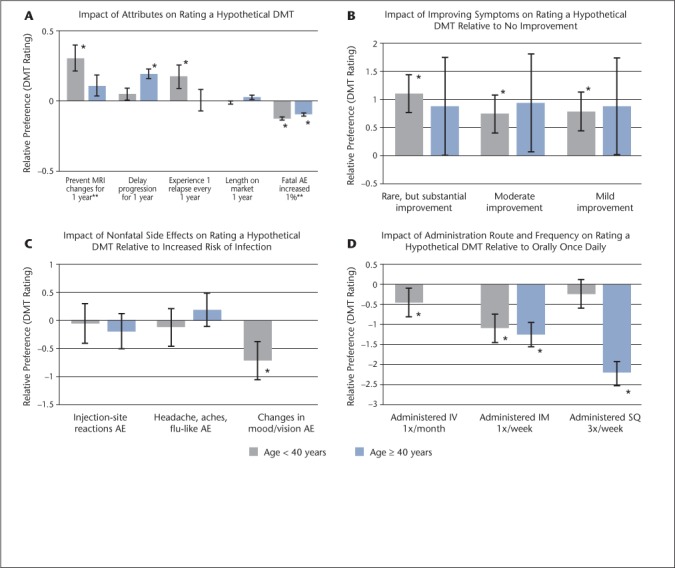
Relative preference for disease-modifying therapy attributes in patients aged younger than 40 years (n = 22) and 40 years and older (n = 28)
*Significant in mixed-effects model. **The age younger than 40 years group was significantly different from those aged 40 years and older. Changes in mood/vision adverse effects (AEs) and administered intravenously (IV) once per month for age 40 years and older were omitted owing to collinearity. Error bars represent standard errors. DMT, disease-modifying therapy; IM, intramuscularly; SQ, subcutaneously.
Analyzing the data by current DMT groups with first-line (interferons and glatiramer acetate), non–first-line (natalizumab, fingolimod, and rituximab), and no treatment revealed that those not receiving treatment had no significant relative preference for preventing disease progression (P = .96), possibly owing to lower disease activity. Patients receiving first-line DMTs displayed more aversion to fatal risk (β = −0.108, P < .001) than those receiving non–first-line DMTs (β = −0.071, P < .001), and this difference was significant (P = .002). This finding could be due to increased disease severity in patients receiving non–first-line DMTs, leading to higher tolerance for severe risk. These data also indicate that patients with current DMT or no drug choice may be reflecting their preferences for risk/benefit trade-offs.
Discussion
Treating practitioners should note that these data suggest that patients had a high preference for DMTs that could improve how they feel (symptoms), despite the lack of this benefit being demonstrated in clinical trials. In contrast, patients did not show a significant preference for decreasing relapse rates. This emphasizes the divergence between patient preferences and measurable clinical outcomes because relapse prevention is the main efficacy outcome for many DMT clinical trials. Relapse rate was the least significant factor for patients' choices in two previous studies.18,21 Of the benefit attributes that have been demonstrated as secondary efficacy end points in DMT clinical trials, patients significantly preferred objectively tested benefits, such as preventing MRI lesion progression. Finally, patients preferred, although slightly less, preventing symptom-measured disease progression, which is another key end point in pivotal trials of MS drugs.
The inconsistency between the primary measures of pharmaceutical efficacy and patient preferences raises the concern that patients' desire and expectations for symptom improvement are not being met by current DMTs and other treatments. This inconsistency might partially explain the low drug adherence in some patients with RRMS.5,6 The National Multiple Sclerosis Society states that DMTs can be viewed as an investment in the future rather than as a treatment that makes a patient feel better immediately.22 The present results show partial consistency with patients' acceptance of this message by their strong preference for preventing disease progression rather than preventing relapses.
This study showed that patients perceived acceptance of a 1% increase in severe risk from no severe risk to be comparable with the benefits of preventing symptom progression for 10 years or MRI progression for 7 years. Findings from other studies suggest that both type and amount of risk are important in risk perception.18 A wide range of severe adverse drug risks (0%–30%) was tested in this study, and the analysis indicated that patients' preference for risk was not linear. Patients were willing to accept a 1%, 10%, or 30% risk of severe adverse effects or death for a medication that prevented MRI progression for 7, 18, or 23 years, respectively. Further investigation is warranted between no risk and 1% risk, where preference exhibits the steepest decline.
We found that a ratings-based conjoint analysis was a successful method for quantifying the relative preferences of patients with RRMS for levels of DMT risk and benefit attributes. Patients strongly preferred daily oral medication administration over all parenteral routes regardless of their frequency. This finding further reinforces the existence of barriers to injectable drug use in the RRMS population illustrated in the literature.23 Injecting subcutaneously three times weekly was least preferred, followed by intramuscularly once weekly and intravenously once monthly. A stronger preference for the added discomfort of intramuscular administration than for subcutaneous injections shows that there may be a preference for less frequent administration (once rather than three times per week). Intravenous infusion once monthly was preferred to all other parenteral administration attributes, despite its inconvenience of infusion center visits, suggesting a preference for decreased frequency, a dislike for self-injecting, or both. Research supports the present findings of a strong preference for oral medications over injectables as well as decreased patient preference for frequent injections.24
The present study had a variety of potential limitations. First, the sample was only 50 patients, preventing extensive subgroup analyses. Despite the small sample size, we successfully screened a variety of DMT attributes. The confidence intervals of the attributes in the regression analysis were narrow and enabled significance on attribute levels. The small sample size, however, allowed patients to be tested in person rather than using the more common Web-based sampling used for conjoint analysis studies with large sample sizes. Another limitation was that the study was conducted in one university-based practice, and its results may not be generalizable to a community practice. However, the mean age, sex proportions, and relapse rates were comparable with those in the published literature.18,20,25 In addition, the numbers of patients receiving first-line and non–first-line DMTs were similar to national market shares, although we saw a slightly higher proportion of patients taking glatiramer acetate and fewer taking interferons.26 At the levels presented, patient preference was not linear for risk of death or severe disability. It seems that at the lowest ranges of risk, actual patient preferences diverged most from the linear model. In a follow-up study we will test this linearity more specifically in this lower risk range. The categorical attribute testing symptom change did not specify the length of time the benefit would be experienced. Therefore, it is difficult to compare the level of this preference with the linear benefits that are gained for specified periods. Last, the methods of this study account for the inherent variability between participants' preferences. As such, these results represent the average perspective of all patients surveyed and not the perspective of any specific individual.
A growing body of literature supports shared decision making as a preferential way for patients and providers to individualize treatment choice and improve efficacy through risk/benefit discussions.27 Studies show that patients with MS are often willing to accept different amounts of risks than providers, indicating disparities between these two groups and further supporting the need for a collaborative approach.28 To optimize shared decision-making models, patients and clinicians may draw on patient preference information to more efficiently and effectively make treatment decisions, leading to improved health outcomes. Regulatory bodies may benefit from the present results because creating new policies and approving new medications require evaluation of acceptable risks and benefits to the intended population. This research will enhance our understanding of patient preference, a key factor in improving medication risk management approaches, and will foster more productive communication between patients and clinicians.
PracticePoints.
Patients with relapsing-remitting MS strongly preferred preventing long-term disability progression and magnetic resonance imaging changes over preventing relapses, a major outcome in clinical trials.
Patients strongly preferred disease-modifying therapies that can substantially improve how they feel, which is not a proven outcome for MS medications.
Daily oral medication administration was preferred over all injected routes and frequencies, especially when self-injected and administered more frequently than monthly.
Acknowledgments
We thank Melissa Azarraga and Claudia Gonzalez for their support.
Footnotes
Financial Disclosures: Dr. Wilson received funding for this study from Novartis Pharmaceuticals Corp and has family members employed by other drug companies that have MS drug products. The other authors have no conflicts of interest to disclose.
Funding/Support: This study was funded by Novartis Pharmaceuticals Corp, East Hanover, NJ.
References
- 1.Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler. 2012;18:932–946. doi: 10.1177/1352458511433302. [DOI] [PubMed] [Google Scholar]
- 2.Morris K, Yiannikas C. Treatment update in multiple sclerosis. Curr Allergy Asthma Rep. 2012;12:246–254. doi: 10.1007/s11882-012-0256-5. [DOI] [PubMed] [Google Scholar]
- 3.Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–2212. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- 4.Putzki N, Fischer J, Gottwald K et al. Quality of life in 1000 patients with early relapsing-remitting multiple sclerosis. Eur J Neurol. 2009;16:713–720. doi: 10.1111/j.1468-1331.2009.02572.x. [DOI] [PubMed] [Google Scholar]
- 5.Devonshire V, Lapierre Y, Macdonell R et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18:69–77. doi: 10.1111/j.1468-1331.2010.03110.x. [DOI] [PubMed] [Google Scholar]
- 6.Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Patient and community preferences for treatments and health states in multiple sclerosis. Mult Scler. 2003;9:311–319. doi: 10.1191/1352458503ms903oa. [DOI] [PubMed] [Google Scholar]
- 7.Kappos L, Ernst-Wilhelm R, Paul O et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 8.Ford C, Goodman A, Johnson K et al. Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. Mult Scler. 2010;16:342–350. doi: 10.1177/1352458509358088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold R, Rieckmann P, Chang P, Abdalla J. The long-term safety and tolerability of high-dose interferon beta-1a in relapsing-remitting multiple sclerosis: 4-year data from the PRISMS study. Eur J Neurol. 2005;12:649–656. doi: 10.1111/j.1468-1331.2005.01083.x. [DOI] [PubMed] [Google Scholar]
- 10.Ebers GC, Traboulsee A, Li D et al. Analysis of clinical outcomes according to original treatment groups 16 years after the pivotal IFNB-1b trial. J Neurol Neurosurg Psychiatry. 2010;81:907–912. doi: 10.1136/jnnp.2009.204123. [DOI] [PubMed] [Google Scholar]
- 11.Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. The relationship between risk attitude and treatment choice in patients with relapsing-remitting multiple sclerosis. Med Decis Making. 2002;22:506–513. doi: 10.1177/0272989X02238299. [DOI] [PubMed] [Google Scholar]
- 12.Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–292. [Google Scholar]
- 13.Marshall D, Bridges JFP, Hauber B et al. Conjoint analysis applications in health: how are studies being designed and reported? an update on current practice in the published literature between 2005 and 2008. Patient. 2010;3:249–256. doi: 10.2165/11539650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Orme BK. Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research. 2nd ed. Madison, WI: Research Publishers LLC; 2010. [Google Scholar]
- 15.Bridges JFP, Hauber AB, Marshall D et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Bloomgren G, Richman S, Hotermans C et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;20:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 17.Ramkumar B, Chadha MK, Barcos M, Sait SNJ, Heyman MR, Baer MR. Acute promyelocytic leukemia after mitoxantrone therapy for multiple sclerosis. Cancer Genet Cytogenet. 2008;182:126–129. doi: 10.1016/j.cancergencyto.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Johnson FR, Van Houtven G, Ozdemir S et al. Multiple sclerosis patients' benefit-risk preferences: serious adverse event risks versus treatment efficacy. J Neurol. 2009;256:554–562. doi: 10.1007/s00415-009-0084-2. [DOI] [PubMed] [Google Scholar]
- 19.Samson K. Health officials launch investigation in deaths of patients taking fingolimod. Neurol Today. 2012;12:27–28. [Google Scholar]
- 20.O'Brien JA, Ward AJ, Patrick AR, Caro J. Cost of managing an episode of relapse in multiple sclerosis in the United States. BMC Health Serv Res. 2003;12:1–12. doi: 10.1186/1472-6963-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartung HP, Aktas O. Evolution of multiple sclerosis treatment: next generation therapies meet next generation efficacy criteria. Lancet Neurol. 2011;10:293–295. doi: 10.1016/S1474-4422(11)70043-6. [DOI] [PubMed] [Google Scholar]
- 22.National Multiple Sclerosis Society. The MS disease-modifying medications: general information. http://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Brochure-The-MS-Disease-Modifying-Medications.pdf. Published January 2015. Accessed January 2015.
- 23.Cohen B, Rieckmann P. Emerging oral therapies for multiple sclerosis. Int J Clin Pract. 2007;61:1922–1930. doi: 10.1111/j.1742-1241.2007.01561..x. [DOI] [PubMed] [Google Scholar]
- 24.Zambanini A, Newson RB, Maisey M, Feher MD. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract. 1999;46:239–246. doi: 10.1016/s0168-8227(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 25.Ramagopalan SV, Sadovnick AD. Epidemiology of multiple sclerosis. Neurol Clin. 2011;29:207–217. doi: 10.1016/j.ncl.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Johnson GS, Lomb D. Multiple sclerosis: too crowded, or still room to play? DefinedHealth website. http://knowledgebase.definedhealth.net/wp-content/uploads/2011/07/DH-WEBINAR-MS-JGDL-2011.pdf. Published July 7, 2011. Accessed February 2, 2014.
- 27.Heesen C, Kasper J, Segal J, Köpke S, Mühlhauser I. Decisional role preferences, risk knowledge and information interests in patients with multiple sclerosis. Mult Scler. 2004;10:643–650. doi: 10.1191/1352458504ms1112oa. [DOI] [PubMed] [Google Scholar]
- 28.Heesen C, Kleiter I, Nguyen F et al. Risk perception in natalizumab-treated multiple sclerosis patients and their neurologists. Mult Scler. 2010;16:1507–1512. doi: 10.1177/1352458510379819. [DOI] [PubMed] [Google Scholar]


