Abstract
Background: Pain is common in multiple sclerosis (MS). Duloxetine has a potential therapeutic role in treating MS-related pain.
Methods: Thirty-eight MS patients were randomized 1:1 to receive duloxetine (n = 18) or matched placebo (n = 20). The dosing regimen was 30 mg daily for 1 week, then 60 mg daily for 5 weeks. The primary outcome measure was change in worst pain for week 6 relative to baseline recorded on a daily pain diary.
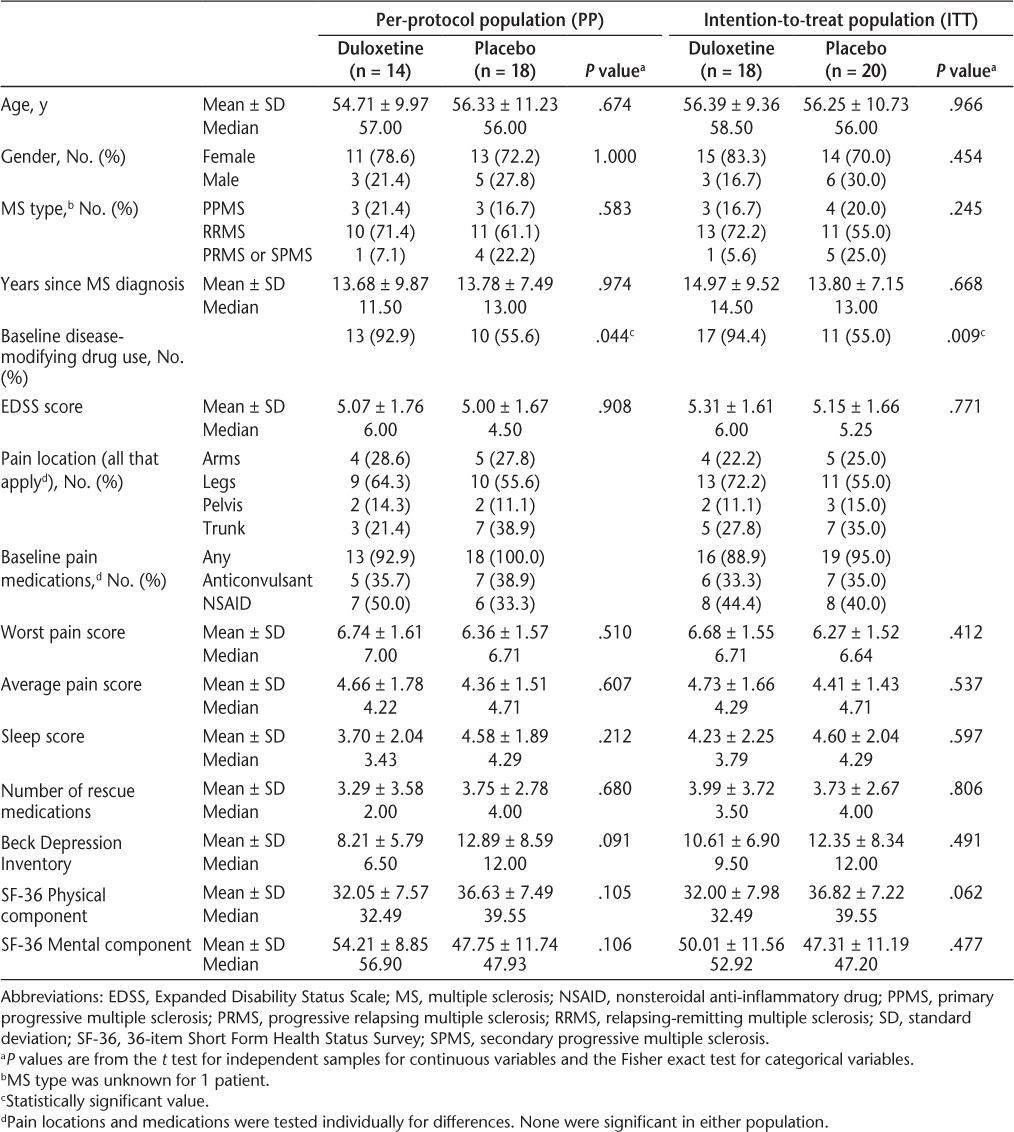
Results: Of 38 randomized patients, 14 (78%) patients randomized to duloxetine and 18 (90%) randomized to placebo completed treatment per protocol. These participants had an average age of 55.5 years, 25% were male, and 66% had relapsing-remitting MS (RRMS). Baseline characteristics were similar. Discontinuations were due primarily to drug intolerance. Among those who completed treatment, worst pain at 6 weeks was reduced by 29% (±20%) for duloxetine versus 12% (±18%) for placebo (P = .016). Average daily pain at 6 weeks was reduced by 39% (±29%) in the duloxetine group compared to 10% (±18.8%) in the placebo group (P = .002). There were no significant changes (week 6 vs. baseline) or between-group differences for subject global impression, Beck Depression Inventory, 36-item Short Form Health Status Survey (SF-36), or sleep quality score.
Conclusions: Fewer patients could tolerate duloxetine compared to placebo. Among patients who completed 6 weeks of treatment, there were significant reductions in average and worst daily pain scores with duloxetine compared to placebo. This study suggests that duloxetine has a direct pain-relieving effect in MS.
There are at least 400,000 people in the United States living with multiple sclerosis (MS), a chronic neurologic disease characterized by demyelination and axonal degeneration. Pain is an important symptom of MS, reported in 44% to 80% of patients.1 Many different types of pain have been associated with MS.2 Central pain, that is, pain initiated or caused by a primary lesion or dysfunction of the central nervous system (CNS), has a reported prevalence of 33% in MS.3,4 Serotonin and norepinephrine have been implicated in the modulation of endogenous analgesic mechanisms via the descending inhibitory pain pathways in the brain and spinal cord and may be a therapeutic target for analgesia. Duloxetine (Cymbalta; Lilly, Indianapolis, IN) is a balanced dual reuptake inhibitor of serotonin and norepinephrine (SNRI). Duloxetine is approved by the US Food and Drug Administration (FDA) for use in the treatment of painful diabetic neuropathy, fibromyalgia, anxiety, depression, and chronic musculoskeletal pain. Because the analgesic mechanism of action may be within the CNS, there is reason to believe that duloxetine may also be effective for MS-related central pain. There is currently no US FDA-approved therapy for patients experiencing pain related to MS (nabiximols [Sativex; GW Pharmaceuticals, Cambridge, UK], a cannabinoid, is approved in Canada and the United Kingdom for this indication).
Duloxetine may be well suited for use in MS because of its effectiveness in other neuropathic conditions, 12-hour half-life that allows for daily dosing, mild adverse effect profile, and potential for supplementary benefits in mood and bladder function (it has been approved in Europe for the treatment of incontinence). A number of studies have found no evidence of any harmful effects of serotonin reuptake inhibitors on MS disease activity, and there is no evidence to suggest such an adverse effect with SNRI use.5–8 Therefore, a clinical trial of duloxetine for the treatment of central pain in MS is warranted.
Our primary outcome measure was change in worst pain between baseline and week 6 for the two treatment groups. Change in average pain was a secondary outcome measure. Our hypotheses were that the duloxetine group would experience mean reductions in the weekly 24-hour worst pain and average pain ratings exceeding 30% and significantly greater than reductions achieved in the placebo group.
Methods
Patients were recruited from our clinic and local community using newspapers, MS newsletters, and other media. All patients provided written informed consent as approved by an institutional review board (Western IRB). Outside medical records were reviewed, and treating physicians were requested to verify the diagnosis and subject qualifications, as needed.
All patients had to have a diagnosis of MS based on McDonald or Poser criteria at least 3 months prior to screening, be aged older than 18 years, and have no MS exacerbation or change in disease-modifying therapy for 90 days prior to screening. Patients were required to have daily pain that was attributed to MS by the treating physician. Pain had to be present for a minimum of 2 months prior to screening, with a score of 4 or greater on the 24-hour worst pain score rated on an 11-point (0–10) Likert scale on the majority of recorded days. At least five valid daily scores were required. Patients with pain that could not clearly be differentiated from causes other than MS, such as diabetic neuropathy, peripheral vascular disease, arthritis or other musculoskeletal condition, chronic headache, visceral pain, and transient pains, were excluded. Other exclusion criteria were current or historical diagnosis of mania, bipolar disorder, or psychosis; and concomitant use of monoamine oxidase (MAO) inhibitors, selective serotonin reuptake inhibitors (SSRIs), SNRIs, tryptophan, St. John's wort, medical marijuana, and any analgesic medication taken on an as-needed basis, except study-related rescue therapy. Regularly scheduled opiates or anticonvulsants for neurogenic pain were allowed. Excluded coexisting health conditions were uncontrolled narrow-angle glaucoma, depression with suicidality, alcohol abuse, history of chronic hepatic insufficiency or alanine aminotransferase (ALT) or aspartate aminotransferase (AST) more than twice the upper limit of normal, renal insufficiency (creatinine clearance <30 mL/min or serum creatinine >1.9), uncontrolled hypertension (systolic blood pressure [SBP] >180, diastolic blood pressure [DBP] >105), breast-feeding or pregnancy in females, or any other serious and/or unstable medical condition.
This study was a parallel-group, double-blind randomized controlled study. After 2 weeks of baseline data acquisition, qualifying patients were randomized at the clinical research pharmacy of Evergreen Hospital Medical Center. The treating physician and examining research coordinators were blinded to treatment arm until the end of the trial, when the code was broken. Patients were randomly assigned 1:1 to duloxetine 30 mg/day for 1 week followed by 60 mg/day for 5 weeks and 30 mg/day for 1 week or identical placebo for 7 weeks under double-blind conditions. The first 6 weeks were considered the acute therapy phase, and week 7 was for drug tapering to limit discontinuation-emergent adverse events. The drug was administered daily as a morning dose with food. Unused study drug and ibuprofen/acetaminophen were returned and counted at the final visit.
Analgesic “rescue medication” was provided in order to prevent intolerable pain and to minimize extraneous analgesic use during this placebo-controlled study. Patients were allowed to take a maximum of 2400 mg/day (12 tablets of 200 mg each) of ibuprofen provided by the research pharmacy. Patients unable to tolerate ibuprofen owing to medical history or study-emergent ibuprofen-related adverse effects could substitute acetaminophen, up to 2000 mg/day (6 tablets of 325 mg each). With this exception, no “as-needed” analgesic medication or medicinal marijuana was allowed during the 7 days prior to baseline visit until after the acute phase (end of week 6). To avoid duplication of rescue medication therapy, patients received a list of common nonsteroidal anti-inflammatory or acetaminophen-containing medications that were not allowed during the study.
At screening, patients were instructed in how to use a pain diary to record their worst pain, average pain, sleep quality rating, and use of rescue medication daily for the duration of the study. A body map was used to identify the location of pain attributed to MS, and patients were instructed to focus on that pain location in their daily pain ratings, using 0–10 visual analogue scales (VAS) for average and worst pain. Sleep rating was based on a 0–10 VAS with 0 indicating that pain did not disrupt sleep at all, slept normally; and 10 indicating that pain completely disrupts sleep, unable to sleep at all. Research visits were done at screening, randomization, and final visit (6 weeks post randomization) with three additional phone visits, including one that was 2 weeks after the final visit. The intention-to-treat (ITT) population was defined as all randomized patients; the per-protocol population was defined as patients with adherence to study medication who completed the study through week 6.
The primary outcome was percent change in worst pain score from baseline to week 6 in the per-protocol population. Secondary outcomes included percent change in average pain score, change in sleep score, and change in number of rescue medications. There is no adjustment for multiple comparisons, so all statistical tests beyond the primary outcome should be considered hypothesis-generating.
Change from baseline was compared using the t test. Baseline characteristics were compared using the t test for independent samples or the Fisher exact test. For worst and average pain reduction thresholds at week 6, subjects who experienced decreases from baseline of greater than 30% were classified as responders. The percentage of responders was compared across groups using the Fisher exact test.
The sample size calculation was based on an earlier study of MS pain using 1:1 randomization and assuming the theoretical difference of 1.75 between treatment groups on a 0–10 scale with a standard deviation of 2.1 and a significance level of 5% (two-sided); a sample size of 54 patients ensured a power of 85%.9 Slow recruitment and funding limitations led to termination of enrollment after 38 patients had been randomized.
Results
There were 38 patients who enrolled and were randomized to duloxetine (n = 18) or placebo (n = 20). There were 6 patients who terminated early and for whom there are no final visit/week 6 data available for analysis. This left 32 patients in the per-protocol population, who completed the study through week 6 with good adherence to study drug and diary requirements (Figure 1).
Figure 1.
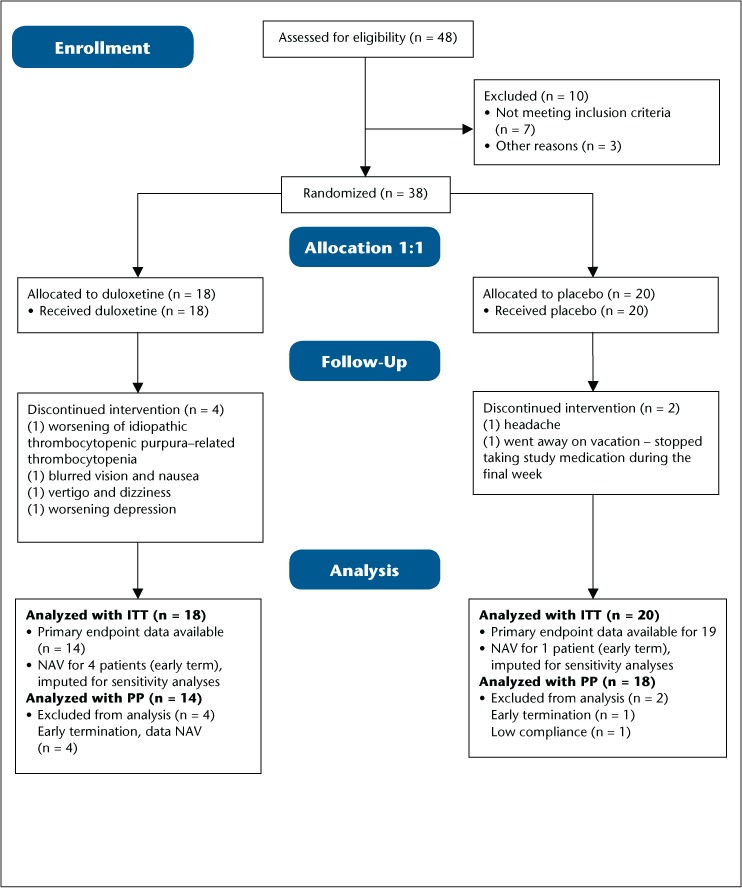
Flow diagram of study patient enrollment through completion
ITT, intention to treat; NAV, not available; PP, per protocol.
Baseline characteristics are shown in Table 1. Age, MS type, disease duration, and baseline Expanded Disability Status Scale (EDSS) scores were similar across groups. More duloxetine patients than placebo patients were on disease-modifying therapy (13 [93%] vs. 10 [56%]; P = .044). Most patients were taking a pain medication regularly, and the legs were the most commonly reported location of pain. Mean worst pain scores were between 6 and 7, and average pain scores were between 4 and 5. There were no significant differences in any of the outcome measures at baseline.
Table 1.
Demographic and baseline clinical characteristics
Patients randomized to duloxetine had a significantly greater reduction in worst pain compared to placebo patients (Figure 2). The mean reduction was −29.1% (SD 20.4%) for duloxetine and −11.5% (SD 18.2%) for placebo (P = .016). The difference in the proportion of patients with a reduction greater than 30% did not reach statistical significance (33% vs. 10%, P = .117).
Figure 2.
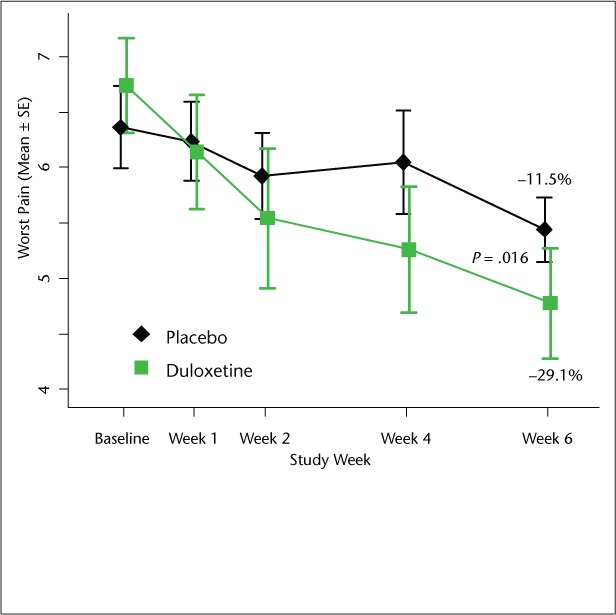
Comparison of worst pain score between duloxetine and placebo groups
The graph depicts raw scores. The text displays mean percent change from baseline in worst pain score. P value compares percent change in worst pain score based on the t test for independent samples.
Average pain was reduced by −38.5% (SD 29.1%) for duloxetine and −10.4% (SD 18.9%) for placebo (P = .002, Figure 3). The percentage with a reduction greater than 30% in average pain was significantly different, with 44% of duloxetine patients and 5% of placebo patients reaching this threshold (P = .007).
Figure 3.
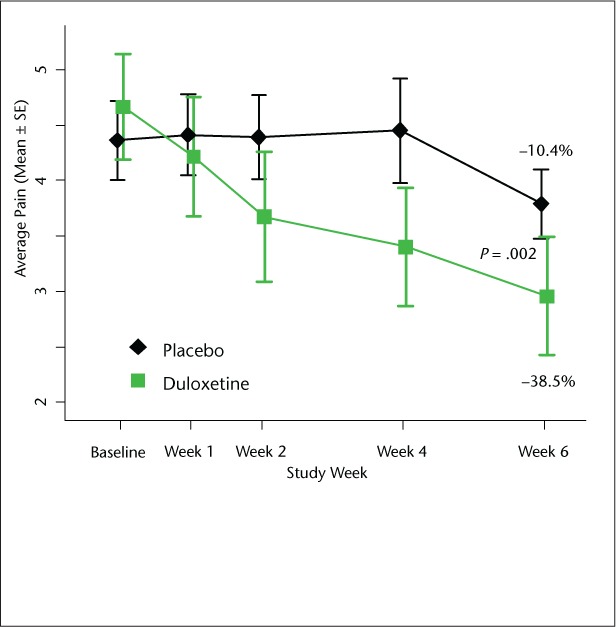
Comparison of average pain score between duloxetine and placebo groups
The graph depicts raw scores. The text displays mean percent change from baseline in average pain score. P value compares percent change in average pain score based on the t test for independent samples.
To determine the impact of missing data, we did ITT analyses, setting the missing data first to the mean in the placebo group and second to the worst observed value. For worst pain and imputing the placebo mean for missing data, the result remained significant, but imputing the worst observed value (gain of +40.1) did result in a nonsignificant P value. For average pain, the difference remained significant for both imputation strategies.
There were no significant differences in changes for other outcome measures (Supplementary Table 1). Sleep score declined slightly, and there were slight improvements in Beck Depression Inventory and 36-item Short Form Health Status Survey (SF-36). The average number of rescue medications was reduced by −1.9 from baseline for duloxetine and −0.7 for placebo (not significant). Subject global impression approached a significant difference at week 6 (mean 4.8 for duloxetine and 3.9 for placebo, P = .074).
Four patients terminated early in the duloxetine group, owing to worsening of pre-existing idiopathic thrombocytopenic purpura–related thrombocytopenia (1 patient), blurred vision and nausea (1 patient), vertigo and dizziness (1 patient), and worsening depression (1 patient). Among the placebo patients, one terminated early owing to headache and one stopped taking study medication during the final week of the study but continued study participation. The most commonly reported adverse events with possible or probable relationship to study drug among the duloxetine group were nausea (2 patients), dizziness (2 patients), headache (2 patients), increased fatigue (2 patients), constipation (2 patients), and urinary incontinence or hesitancy (3 patients). Three falls occurred in both the duloxetine and placebo groups, and none were suspected to be due to the study medication. The next most common adverse event in the placebo group was headache (2 patients). Thrombocytopenia occurred in one subject in the duloxetine group (with history of idiopathic thrombocytopenic purpura) and one subject in the placebo group. Liver function test abnormalities occurred in one placebo subject and no duloxetine patients. There were no serious adverse events.
Discussion
Antiepileptic drugs and tricyclic antidepressants are the most frequently used treatments in neuropathic pain conditions, such as spinal cord injury and MS. It is unknown whether differences in disease mechanism causing nerve pain alter the responsiveness to analgesia.10 There have been few randomized controlled trials (RCTs) of pharmacotherapeutics for MS pain, and most of those studies have focused on treating trigeminal neuralgia in MS.11–15 Three RCTs examined the use of cannabinoid derivatives (oral or oropharyngeal spray preparations) for MS-related pain including dysesthetic limb pain and painful spasms. The studies found some evidence of effectiveness in pain relief, but the utility of these medications is limited by safety concerns, adverse effects, and their status with the Drug Enforcement Agency.9,16,17 A crossover RCT of lamotrigine for central MS pain found no evidence of benefit.18
We found that the SNRI duloxetine significantly reduced average and worst pain ratings versus placebo at 6 weeks. Treatment effect was evident at 2 weeks for average pain and at 4 weeks for worst pain. Duloxetine treatment was associated with a higher percentage of patients with a clinically significant pain reduction, which was defined a priori as a drop of 30% or more. Furthermore, the mean reduction for duloxetine was −2.0 points or 29% for worst pain and −1.7 points or 38.5% for average pain at week 6. The results at week 6 were still significant (P = .0043 for average and P = .0204 for worst pain) after the adjustments for baseline use of disease-modifying therapy mentioned previously. A meta-analysis of more than 2700 patients with various painful conditions suggested that a 30% or 2-point reduction in an 11-point pain scale score was clinically meaningful.19 This study did not specifically address neuropathic pain, and others have suggested that a lower change, such as 1.5, is clinically meaningful in neuropathic pain.20
Duloxetine was associated with better subject global impression (although the difference did not reach statistical significance), and there were no significant differences for sleep quality, Beck Depression Inventory, or SF-36. The on-treatment period of 6 weeks may not have been long enough to produce an antidepressant effect. The findings of pain relief in MS without changes in mood or sleep suggest a direct pain-relieving effect in the CNS.
Duloxetine is approved for other pain conditions and has been tested before in MS in one industry-sponsored multicenter placebo-controlled study, unrelated to the present study.21 Vollmer et al. reported a reduction in average pain intensity of 44% at 6 weeks, similar to the 38.5% that we found. Both studies found that more duloxetine participants had a 30% or greater reduction in average pain, identifying a clinically meaningful pain reduction. Both studies encountered tolerance issues and higher dropout rates with duloxetine.
Tolerance issues may limit the clinical utility of duloxetine for MS pain. Side effects, including nausea, dizziness, fatigue, constipation, and urinary retention, were observed, sometimes leading to treatment discontinuation. We excluded many drugs presenting possible interactions with duloxetine, including all SSRIs, SNRIs, MAO inhibitors, and St. John's wort. We followed a duloxetine titration schedule beginning at 30 mg for 1 week followed by 60 mg for 5 weeks and 30 mg taper for 1 week at the end of the study. It is possible that a higher dose of duloxetine, such as 120 mg daily, might provide greater analgesia, at the expense of greater adverse effects, and that a lower dose, such as 30 mg daily, would be better tolerated, but less effective. Current practice is to start pharmacotherapy for MS pain with anticonvulsants or tricyclic antidepressants, which may be better tolerated than duloxetine. Our study supports a treatment algorithm including duloxetine 60 mg daily as a second-line agent for MS-related pain.
Limitations of this study include the small sample size, which was less than target enrollment. Efforts were made to exclude non-neurogenic causes of pain; however, our approach did not guarantee that the etiology was neuropathic or related to MS in all cases. Change in average pain was robust to several imputation strategies for missing data, but change in worst pain may have been affected by the high withdrawal rate. The study was of short duration, only 6 weeks of titration and full dose. In two phase 3 studies of duloxetine for neuropathic pain, there was a leveling-off of analgesic effect by 6 to 8 weeks.22,23 Other studies have used treatment periods of 5 to 12 weeks. This study excluded patients with a low level of pain. For such MS patients the benefit-to-risk ratio may be less favorable for treatment with duloxetine owing to tolerability issues. In conclusion, we found evidence that duloxetine effectively relieves pain in MS and should be considered as a therapeutic option. However, it should be anticipated that not all patients will tolerate the medication.
PracticePoints.
Duloxetine is a serotonin-norepinephrine reuptake inhibitor that may have analgesic properties.
This randomized controlled trial found that duloxetine significantly reduced average and worst pain in people with MS. The analgesic effect was evident by 4 weeks.
More patients on duloxetine than placebo withdrew owing to adverse effects.
Acknowledgments
The authors thank Carey Gonzales for coordinating the study. The study was the subject of an oral presentation at the Fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis; May 29–June 1, 2013; Orlando, FL.
Footnotes
Financial Disclosures: Dr. Brown has served as a consultant for Acorda, Biogen, Genzyme, Pfizer, and Teva Neuroscience; has received honoraria from Acorda, Biogen, Pfizer, and Teva; and has been funded by research grants from Biogen, Lilly Inc, Nuvo Research, and Teva Neuroscience. Ms. Slee has no conflicts of interest to disclose.
Funding/Support: This study was funded by an independent medical grant from Lilly Inc.
References
- 1.Ehde DM, Osborne TL, Jensen MP. Chronic pain in persons with multiple sclerosis. Phys Med Rehabil Clin N Am. 2005;16:503–512. doi: 10.1016/j.pmr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Solaro C, Brichetto G, Amato MP et al. The prevalence of pain in multiple sclerosis. Neurology. 2004;63:919–921. doi: 10.1212/01.wnl.0000137047.85868.d6. [DOI] [PubMed] [Google Scholar]
- 3.Mersky H, Bogduk N. Classification of Chronic Pain. Seattle, WA: IASP Press; 1994. [Google Scholar]
- 4.Boivie J. Central pain. In: Wall PD, Melzack R, editors. Textbook of Pain. New York, NY: Churchill Livingstone; 1999. pp. 879–914. [Google Scholar]
- 5.Flax JW, Gray J, Herbert J. Effect of fluoxetine on patients with multiple sclerosis. Am J Psychiatry. 1991;148:1603. doi: 10.1176/ajp.148.11.1603a. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti F, Campori E, Colombo C, Smeraldi E. Fluvoxamine treatment of major depression associated with multiple sclerosis. J Neuropsychiatry Clin Neurosci. 2004;16:364–366. doi: 10.1176/jnp.16.3.364. [DOI] [PubMed] [Google Scholar]
- 7.Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69:942–949. [PubMed] [Google Scholar]
- 8.Ehde DM, Kraft GH, Chwastiak L et al. Efficacy of paroxetine in treating major depressive disorder in persons with multiple sclerosis. Gen Hosp Psychiatry. 2008;30:40–48. doi: 10.1016/j.genhosppsych.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–819. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- 10.Rowbotham MC. Mechanisms of neuropathic pain and their implications for the design of clinical trials. Neurology. 2005;65(suppl 4):S66–73. doi: 10.1212/wnl.65.12_suppl_4.s66. [DOI] [PubMed] [Google Scholar]
- 11.Khan OA. Gabapentin relieves trigeminal neuralgia in multiple sclerosis patients. Neurology. 1998;51:611–614. doi: 10.1212/wnl.51.2.611. [DOI] [PubMed] [Google Scholar]
- 12.Leandri M, Lunardi G, Inglese M et al. Lamotrigine in trigeminal neuralgia secondary to multiple sclerosis. J Neurol. 2000;247:556–558. doi: 10.1007/s004150070157. [DOI] [PubMed] [Google Scholar]
- 13.Solaro C, Messmer-Uccelli M, Uccelli A et al. Low-dose gabapentin combined with either lamotrigine or carbamazepine can be useful therapies for trigeminal neuralgia in multiple sclerosis. Eur Neurol. 2000;44:45–48. doi: 10.1159/000008192. [DOI] [PubMed] [Google Scholar]
- 14.Zvartau-Hind M, Din MU, Gilani A et al. Topiramate relieves refractory trigeminal neuralgia in MS patients. Neurology. 2000;55:1587–1588. doi: 10.1212/wnl.55.10.1587. [DOI] [PubMed] [Google Scholar]
- 15.DMKG Study Group. Misoprostol in the treatment of trigeminal neuralgia associated with multiple sclerosis. J Neurol. 2003;250:542–545. doi: 10.1007/s00415-003-1032-1. [DOI] [PubMed] [Google Scholar]
- 16.Svendsen K, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? randomized double blind placebo controlled crossover trial. BMJ. 2004;329:253–258. doi: 10.1136/bmj.38149.566979.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langford RM, Mares J, Novotna A et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260:984–997. doi: 10.1007/s00415-012-6739-4. [DOI] [PubMed] [Google Scholar]
- 18.Breuer B, Pappagallo M, Knotkova H et al. A randomized, double-blind, placebo-controlled, two-period, crossover, pilot trial of lamotrigine in patients with central pain due to multiple sclerosis. Clin Therapeutics. 2007;29:2022–2030. doi: 10.1016/j.clinthera.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Farrar JT, Young JP, LaMoreaux L et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 20.Rowbotham M, Harden N, Stacey B et al. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 21.Vollmer T, Robinson M, Risser R et al. A randomized, double-blind, placebo-controlled trial of duloxetine for the treatment of pain in patients with multiple sclerosis. Pain Pract. 2014;14:732–744. doi: 10.1111/papr.12127. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DJ, Lu Y, Detke MJ et al. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116:109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Wernicke JF, Pritchett YL, D'Souza DN et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67:1411–1420. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]


