Abstract
Humoral immunity depends on the germinal centre (GC) reaction during which somatically mutated high-affinity memory B cells and plasma cells are generated. Recent studies have uncovered crucial cues that are required for the formation and the maintenance of GCs and for the selection of high-affinity antibody mutants. In addition, it is now clear that these events are promoted by the dynamic movements of cells within and between GCs. These findings have resolved the complexities of the GC reaction in greater detail than ever before. This Review focuses on these recent advances and discusses their implications for the establishment of humoral immunity.
Introduction
Germinal centres (GCs) are transient structures that form within peripheral lymphoid organs in response to T cell-dependent antigen1. Within GCs, B cells expressing high-affinity antibodies develop and differentiate into antibody-secreting plasma cells and memory B cells that mediate and sustain protection against invading pathogens. The importance of the GC reaction is best shown by the immunodeficiency syndromes that are observed in patients who are unable to form GCs.
Initiation of the GC reaction occurs via a coordinated cascade involving several different cell types that drive antigen-engaged B cells into the GC reaction. Within the GCs, GC B cells proliferate at a rate that is unparalleled in mammalian tissues and their immunoglobulin variable region (IgV) genes are diversified by somatic hypermutation (SHM)2,3. This process results in the generation of mutant clones that have a broad range of affinities for the immunizing antigen. It has been known for a long time that GC-derived memory B cells and plasma cells express a highly selected antibody repertoire, the affinity of which increases over time. This phenomenon is known as affinity maturation and indicates the presence of effective selection processes within the GC that ensure that inferior antibody mutants or those with autoreactive specificities are outcompeted by higher affinity competitors. Antigen-specific memory B cells and plasma cells appear within 1 week after antigen encounter4, which indicates that the GC reaction is remarkably efficient. This efficiency is facilitated by the specialized GC microenvironment that supports the close interaction and the rapid movements of various cell types in a confined space5. These features facilitate several iterative rounds of mutation and selection and, following differentiation into post-GC cells, produce a stepwise increase in the antigen affinity of secreted antibodies.
Elucidating the cellular dynamics of the GC reaction, the mechanics of high-affinity B cell selection and the molecular control of these processes is a major focus in the fields of adaptive immunity, immunodeficiency and B cell diseases. In this Review, we focus on new developments in the rapidly evolving field of GC dynamics and discuss their implications for the establishment of humoral immunity.
Initiation of the GC reaction
The lymph node structure is broadly characterized by follicles that are mostly comprised of IgM+IgD+ naive B cells and are separated by an interfollicular region. T cell-rich areas (also known as T cell zones) border these follicles. GCs form within the centre of these follicles, which contain a network of follicular dendritic cells (FDCs). The first step in this process is the activation of naive B cells by exogenous antigen within the follicle6. The B cells migrate to the border of the T cell zone and B cell zone or the interfollicular region, where they proliferate and form long-lived interactions with antigen-specific T cells7,8 to become fully activated. However, not all of these antigen-activated B cells eventually enter the GC reaction. Following their interaction with the T cells, a subset of the selected B cells moves to specialized areas in the lymph nodes, known as the medullary chords, where they differentiate into short-lived plasmablasts that secrete antibodies that have low affinity for the invading pathogen9. Of note, it seems that among the pool of responding B cells, those with high-affinity antibody specificities predominantly differentiate into plasmablasts10,11. Recent evidence also suggests that some of the T cell-selected B cells differentiate into unswitched memory B cells12. Finally, of the subset of B cells that enter the GC pathway, only those with the highest relative affinity within a pool of antigen-specific B cells gain access to the GC reaction13,14, and this has recently been attributed to interclonal competition for T cell signals15. Thus, the characteristic oligoclonality of the GC1,5 is determined days before the GC begins to form. It was known that B cells that are destined to undergo the GC pathway change their migratory properties, enabling them to localize to the centre of the follicle16,17. Recent studies using two-photon intravital microscopy have provided new insights into the phenotypic changes and migratory properties of GC precursor B cells and T cells over time and defined the earliest time points of GC commitment18,19.
GC B cell and TFH cell differentiation begins outside follicles
Two independent groups have used intravital microscopy to determine the movements of antigen-specific B cells and T cells in lymph nodes during the immune response to a model antigen. These studies collectively established that 1 day after immunization of a model antigen, antigen-specific B cells and T cells have moved to and started to interact within the interfollicular region of the lymph node18,19 (FIG. 1). T cells that are committed to become T follicular helper cells (TFH cells), through priming by dendritic cells20, at this time begin to upregulate their expression of B cell lymphoma 6 (BCL6)18,21 — the master regulator of both the TFH cell and GC B cell programme. By contrast, in interacting B cells, BCL-6 expression is detectable at low levels only 2 days after immunization (FIG. 2). By day 2 the majority of T cells have acquired a TFH cell phenotype (expressing CXC-chemokine receptor 5 (CXCR5), programmed cell death protein 1 (PD1) and the activation marker GL7), and by day 3, they have migrated from the interfollicular region into the follicle. GC precursor B cells move towards the centre of the follicle to form an early GC 1 day later, at day 4 (FIG. 1). Kerfoot et al.18 further report that antigen-specific B cells that have moved to a region adjacent to the subcapsular sinus of the follicles at day 3 remained BCL-6-negative and acquired features of plasmablasts18. A word of caution is needed regarding the generalization of results from studies of immune responses to model antigens, as different types of antigen and the particular lymphoid tissue under investigation may be inherently associated with differences in the kinetics and the organization of the GC response. In this regard, a study using a different model antigen showed that in the spleen, GC precursor B cells undergo initial clonal expansion at the perimeter of the follicles adjacent to the marginal zone before entering the follicles22. Despite differences in certain specific details, the recent studies collectively show that commitment to the GC reaction by antigen-specific B cells and T cells is rapidly initiated and occurs outside of the follicle by upregulating the expression of GC-associated proteins, most importantly BCL-6. GC commitment seems to occur in T cells before it does in B cells. In the future, it will be interesting to identify the specific signals that instruct an antigen-activated B cell to follow an extrafollicular or GC pathway.
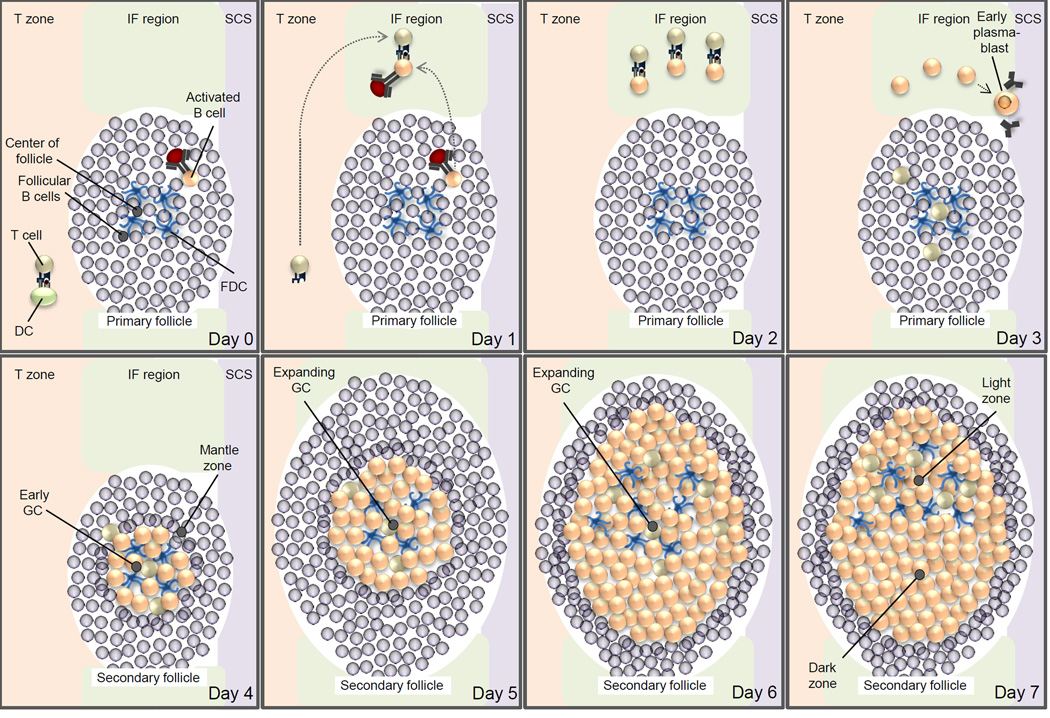
Figure 1. Initiation of the GC reaction and the formation of the mature GC in the lymph node.
This schematic figure is primarily based on two recent studies that determined the movements of antigen-specific B cells and T cells in lymph nodes during the immune response to a model antigen18,19. On day 0, B cells and T cells are activated by recognition of their cognate antigen in the primary follicle and T cell zone, respectively. On day 1, the activated B cells and T cells migrate to the interfollicular region and start to interact. On day 2, B cells and T cells form long-lived interactions, resulting in the full activation of B cells. T cells acquire the characteristic T follicular helper cell (TFH cell) phenotype. On day 3, TFH cells migrate from the interfollicular region into the follicle. Some antigen-activated B cells differentiate into antibody-secreting cells or early plasmablasts that move to a region adjacent to the subcapsular sinus (SCS). On day 4, B cells migrate from the interfollicular region into the centre of the follicle — which is characterized by a network of follicular dendritic cells (FDCs) — begin to proliferate and, as a result, push the resident follicular B cells aside to form the early germinal centre (GC), which consists of B cell blasts surrounded by the mantle zone. This structure is also referred to as the secondary follicle, thus distinguishing it from the ‘GC-less’ primary follicle. On days 5–6, the GC rapidly expands as a result of the fast proliferation of the B cell blasts. On day 7, dark zones and light zones form, which results in the establishment of the mature GC. The dark zone mainly consists of densely packed B cell blasts, whereas the light zone contains TFH cells and FDCs. CD40L, CD40 ligand; DC, dendritic cell; TCR, T cell receptor.
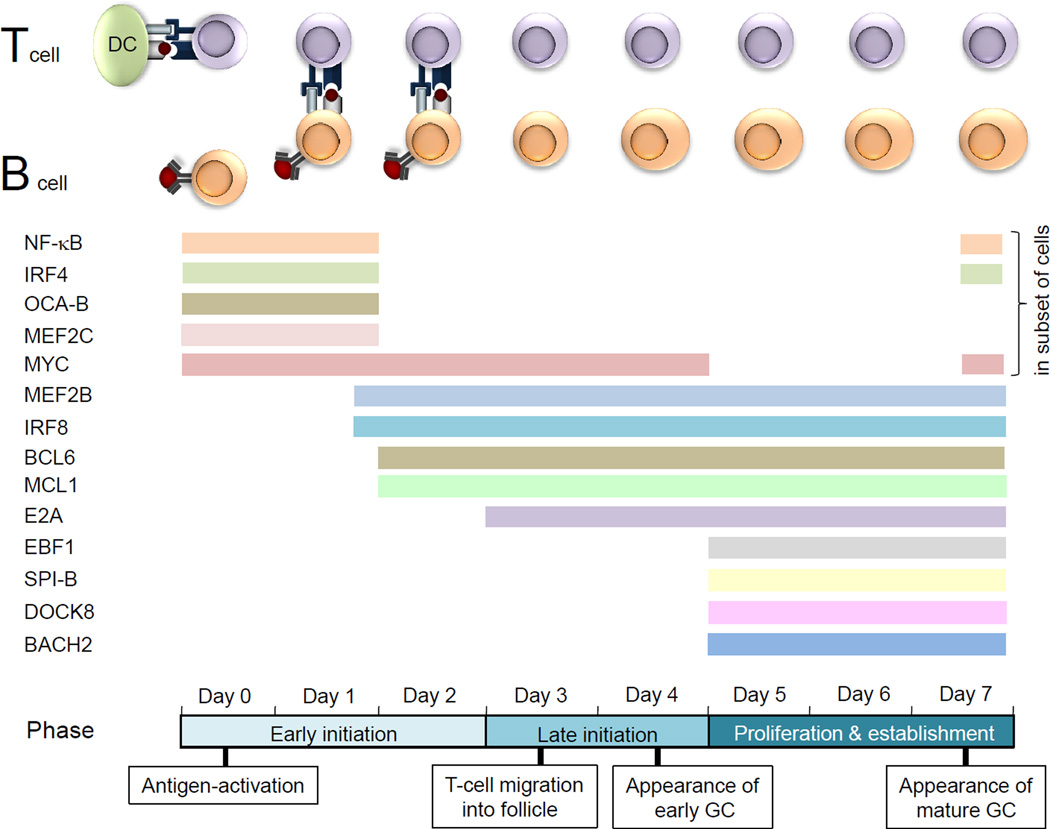
Figure 2. Expression pattern and/or functional requirement of molecules during the initiation and formation of the mature GC.
Published findings are consistent with the existence of three separate phases during the first 7 days of the germinal centre (GC) reaction. An early initiation phase (days 0–2) encompasses the activation of the B cells and T cells by their cognate antigen and the long-lived interactions of B cells and T cells in the interfollicular region. A late initiation phase (days 3–4) is characterized by the migration of the T cells first and 1 day later the migration of the B cells out of the interfollicular region and the appearance of the early GC. A proliferation and establishment phase (days 5–7) occurs in which the GC grows and eventually forms the characteristic dark and light zone patterning of the mature GC. Studies of the GC reaction following the deletion of molecules in antigen-activated or GC B cells have revealed a functional requirement for: nuclear factor-κB (NF-κB); interferon-regulatory factor 4 (IRF4); OCA-B; myocyte-specific enhancer factor 2C (MEF2C); MYC; B cell lymphoma 6 (BCL-6); myeloid cell leukaemia 1 (MCL1); E2A; early B cell factor 1 (EBF1); SPIB; and dedicator of cytokinesis protein 8 (DOCK8). A functional role has also been suggested for several other factors: MEF2B, on the basis of its expression pattern in antigen-activated B cells in vivo; IRF8, on the basis of its high expression in GC B cells and its role in the induction of BCL-6 expression; and BACH2 (BTB and CNC homologue 2), on the basis of its expression in established GC B cells (earlier time points have not been analysed). The exact time point at which BACH2 deficiency affects the formation of GCs in not known. As BACH2 cooperates with BCL-6 in the suppression of genes in GC B cells65, we propose that BACH2 is required in the proliferation and establishment phase, but note that it could also be required in an earlier phase. CD40L, CD40 ligand; DC, dendritic cell; TCR, T cell receptor.
GC formation and establishment of dark and light zones
Early GCs within follicles can first be histologically identified at day 4 after immunization. At this time, B cells grow and differentiate into B cell blasts that rapidly divide and begin to populate the network of FDCs in the centre of the follicle1. During this process, the B cell blasts displace the IgM+IgD+ B cells, which results in the formation of the mantle zone around the GC (FIG. 1). From day 5–6 post immunization, the GC increases in size because of the rapid proliferation of the B cell blasts, which continues until day 7, the point at which the GC has been fully established and is polarized into two microenvironments known as the dark zone and the light zone1,5. The dark zone is named for its histological appearance and consists of densely packed blasts that proliferate within a recently identified interconnected network of reticular cells that express CXC-chemokine ligand 12 (REF.23); this cell type is morphologically similar to the FDCs of the light zone. The light zone is more sparsely populated by B cells than the dark zone and is characterized by the presence of several cell types, including TFH cells, FDCs and macrophages5. In human lymphoid tissue, the B cells of the dark zone and light zone were defined (on the basis of morphological criteria) as centroblasts and centrocytes, respectively, with the centrocytes appearing smaller in size. However, intravital microscopy studies in mice failed to identify a difference in size and morphology between B cells in the dark zone and the light zone24, which led to the suggestion that GC B cells should instead be defined by their location, functional characteristics or immunophenotype5,24, as discussed below.
Molecular control of GC initiation
BCL-6
The upregulation of BCL-6 expression is essential for initiation of the GC reaction as BCL-6-deficient GC precursor B cells fail to enter the follicle19. Of note, a particular region within the BCL-6 protein — repression domain 2 (RD2) — was found to be specifically required for the migration of GC precursor B cells towards the inner follicle25. GC precursor B cells deficient for BCL-6 or with an inactivating mutation in the BCL-6 RD2 domain did not cease expression of G protein-coupled receptor 183 (GPR183; also known as EBI2)19,25, which must be downregulated by GC precursor B cells to allow their migration towards the centre of the follicle16,17. In addition, it was found that BCL-6-deficient GC precursor B cells did not upregulate expression of CXCR4 (REF.19), which is a chemokine receptor that is expressed by dark zone B cells in mice and humans26,27. Also, Bcl6RD2mut GC precursor B cells failed to downregulate expression of sphingosine-1-phosphate receptor type 1 (S1PR1)25, which — in contrast to S1PR2, which promotes confinement of B cells in the GC28 — is minimally expressed by GC B cells28; this is consistent with the role of S1PR1 in mediating B cell trafficking out of the follicle29. These findings indicate that in GC precursor B cells, BCL-6 controls a transcriptional programme that facilitates the migration of these cells to the centre of the follicle.
Upregulation of BCL-6 expression by GC precursor B cells seems to enhance integrin-dependent conjugation of B cells and T cells19, which is a defining feature of their interaction30. Finally, it is interesting to note that naive B cells express BCL6 mRNA in the absence of protein31,32, which suggests a role for post-transcriptional regulation of BCL-6 expression in B cells32 and T cells33, perhaps allowing for the rapid upregulation of BCL-6 protein expression upon antigen activation.
MEF2B
Myocyte-specific enhancer factor 2B (MEF2B) and MEF2C have distinct roles during B cell activation. MEF2C seems to be required for GC formation as a result of its role in B cell proliferation immediately after antigen stimulation34,35, whereas MEF2B — which, similarly to BCL-6, is highly expressed by GC B cells36 — was shown to directly activate the transcription of BCL6 (REF.36). MEF2B expression by antigen-activated B cells slightly precedes the upregulation of BCL-6 expression, which suggests that MEF2B induces BCL-6 expression in GC precursor B cells36.
IRF4
Interferon-regulatory factor 4 (IRF4) has diverse, context-dependent functions in late GC B cell development37,38 and was recently found to be required for the formation of GCs in both a B cell- and a T cell-autonomous manner39–41. This role is supported by the findings that IRF4 regulates the differentiation of TFH cells39 and Irf4 deletion in B cells prevents the formation of GCs following immunization40,41. On the basis of a chromatin immunoprecipitation followed by sequencing (ChIP–seq) analysis of in vitro antigen-activated B cells, one of the studies suggested that IRF4 induces Bcl6 expression by binding to a region that is 25 kB upstream of the transcriptional start site40, which differs from the region associated with the repression of Bcl6 by IRF4 that occurs towards the end of the GC reaction42. The observation that IRF4 can both activate40 and repress42 Bcl6 transcription may be a result of the context dependency of IRF4 function: an issue that could be resolved by mutating the respective activating and repressive IRF4-binding sites in the Bcl6 locus in transgenic mouse systems.
Interestingly, as opposed to B cell deletion using Cd19–Cre40,41, Ighg1–Cre-mediated deletion of Irf4 (which abolishes Irf4 expression only in cells expressing the immunoglobulin heavy chain γ1 constant region), which occurs shortly after immunization (at day 2), does not affect the proper formation of GCs43. This suggests a requirement for IRF4 in GC precursor B cell development only within a limited time window (possibly days 0–1) immediately after antigen activation, when the B cell still resides in the outer follicle. Indeed, as IRF4 expression is known to be rapidly activated by B cell receptor (BCR) stimulation44, IRF4 may have a crucial role in this early phase of GC development. This finding is in contrast to the observation that BCL-6 and MYC (see below) are continuously required throughout early GC development (days 0–4).
MYC
MYC has a crucial role in almost all proliferating cells. Through both transcriptional and non-transcriptional mechanisms, MYC supports robust proliferation by regulating diverse processes including cell cycle progression, metabolism and telomere maintenance45. As BCL-6 is known to repress MYC transcription in the rapidly proliferating dark zone B cells46, the absence of MYC expression in those cells had been a point of confusion47,48. With this in mind, it was surprising that the early GCs that arise at day 4 post immunization are characterized by the appearance of a sizeable proportion of MYC+BCL-6+ blasts49, which implicates a role for MYC in GC formation. Indeed, mice in which Myc is deleted from activated B cells shortly after immunization (day 2) do not form GCs49, which shows that MYC has an essential function in GC precursor B cell development. At day 4, the MYC+BCL-6+ GC B cells, in contrast to their MYC−BCL-6+ counterparts, transcribed the MYC target gene cyclin D2 (Ccnd2) in addition to Ccnd3 (REF.49), which is the dark zone B cell-specific D-type cyclin50,51 (see below). As MYC+BCL-6+ cells seem to be the precursors of MYC−BCL-6+ GC B cells49, this finding suggests that MYC establishes a CCND2-dependent proliferation programme in GC precursor B cells, which is replaced by the typical CCND3-dependent proliferation programme of dark zone B cells. So, how can BCL-6 and MYC be jointly required during initiation of the GC reaction, given that they have mutually exclusive functions in the fully established GC? One possible explanation for this discrepancy is that in GC precursor B cells, BCL-6 predominantly represses its target genes through RD2-dependent interactions, whereas in dark zone B cells repression mainly occurs through the BCL-6 amino-terminal BTB domain52. The use of different BCL-6 protein domains may result in the control of different target genes. After the establishment of the early GC at day 4, MYC expression is almost entirely absent in the proliferating B cell blasts until it is re-expressed in a subpopulation of light zone B cells at day 7 (FIG. 2), as discussed below.
MCL1
Somewhat mirroring the finding that one specific D-type cyclin, CCND3, regulates proliferation in dark zone B cells50,51, the anti-apoptotic protein myeloid cell leukaemia 1 (MCL1) has recently been identified as the principal regulator of B cell survival in GC formation53. By contrast, BCL-XL was dispensable in this context53. The conditional deletion of Mcl1 early after immunization (at day 2) prevented the formation of GCs, which suggests a continuous requirement for MCL1 throughout the entire GC initiation phase53, which is similar to the requirement for MYC. It will be interesting to determine the mechanism underlying the specific dependency of GC B cells on MCL1.
Other factors
Several other factors have been reported to participate in the initiation of the GC reaction (FIG. 2), including molecules downstream of BCR activation. The canonical nuclear factor-κB (NF-κB) pathway has an important role in B cell activation54,55, and it was recently shown in vivo that the inability to activate this pathway in antigen-activated B cells impaired GC formation56. Another transcription factor involved in this process is OCA-B (also known as POU2AF1 and OBF1)57,58.
IRF8 is expressed by GC B cells and contributes to the induction of BCL6 expression59, which suggests that IRF8 has a role in the early initiation phase before the upregulation of BCL-6 expression. The transcription factor E2A was found to be dispensable during the antigen-activation phase60; however, E2A deficiency markedly impaired the development of early GCs, which suggests that E2A has a specific role in a later phase of initiation60.
The RHO–RAC GTP-exchange factor family member dedicator of cytokinesis protein 8 (DOCK8) was found to be required for GC formation only after the early GC has formed61. Similarly, GCs developed at normal frequencies in mice with B cell-specific ablation of the transcription factor early B cell factor 1 (EBF1)62; however, mature GCs were considerably smaller at day 7, which indicates a requirement for EBF1 in the proliferating B cell blasts. Mice deficient for the ETS protein SPIB formed GCs at the expected frequency, but these were smaller in size than those in wild-type mice63. Finally, mice lacking the transcriptional repressor BACH2 (BTB and CNC homologue 2) lack GCs64, but the exact time point at which BACH2 deficiency impairs GC formation has not been determined. As BACH2 and BCL-6 cooperate in the suppression of genes in GC B cells65, BACH2 may be functionally active during the proliferation and establishment phase; although an earlier role cannot be excluded.
Dynamics of the GC reaction
For many years, there has been a general understanding of antigen-based affinity maturation within the GC. GC B cells rapidly proliferate within the dark zone and undergo SHM to further diversify the rearranged IgV genes. In a process that is reminiscent of Darwinian evolution, SHM produces mutant GC B cell clones that have a large range of affinities for the immunizing antigen. Upon transit into the light zone, GC B cells that express high-affinity antigen receptors are positively selected. Recirculation between the two zones facilitates several iterative rounds of mutation and selection. Within a short time, this process results in the generation of memory B cells and plasma cells that secrete high-affinity antibodies that effectively neutralize the invading pathogen.
Understanding the intricate mechanics of affinity maturation is of great importance for the development of improved vaccine strategies. Within the past several years, technological advances focused on the use of intravital microscopy and photoactivation have allowed the dynamics of the GC reaction to be resolved in greater detail than ever before24,66–68. These initial studies have been thoroughly reviewed elsewhere5,69,70 and we therefore only briefly recount the major findings within the context of more recent advances.
Interzonal movements within the GC
The dark zone and light zone of the GC are organized by the expression of the chemokine receptors CXCR4 and CXCR5, respectively26. An important advance in the field has been the ability to distinguish between dark zone and light zone B cells phenotypically by using the expression of CXCR4 (REFS26,27) and the activation markers CD83 and CD86 (REF.68). Dark zone B cells can be identified as CXCR4hiCD83lowCD86low cells and light zone B cells as CXCR4lowCD83hiCD86hi cells.
Classic models of the GC define the existence of two distinct GC B cell differentiation states1. Centroblasts were thought to reside within the dark zone and to differentiate into smaller centrocytes upon entry into the light zone. The rapid flux of GC B cells observed between the dark zone and the light zone challenged this original definition24,66,68. GC B cells in the dark zone and the light zone were also found to have similar morphology24,68, and to have a considerable overlap in gene expression68. Therefore, these cell states are perhaps more accurately described as transient states that are associated with positioning in the dark zone and the light zone5,69. This issue was recently addressed by the study of CXCR4-deficient GC B cells, which are restricted to the light zone26. Surprisingly, the transition from the dark zone to the light zone, as defined by CD83 and CD86 expression, was unhindered in the absence of dark zone cues and cell cycle progression proceeded normally23. Therefore, it seems that these phenotypic states are not promoted by environmental cues within the dark zone and the light zone but are instead the result of a timed, cell-intrinsic programme. This model suggests the initiation of a cellular programme in which reduced expression of CXCR4 and dark zone-associated genes is accompanied by increased expression of genes that impart a light zone-associated phenotype, and this expression programme enables the cell to transit to the light zone microenvironment.
However, light zone-confined CXCR4-deficient GC B cells were found to be less somatically mutated than their wild-type counterparts and were gradually outcompeted over time23. Therefore, GC polarization into dark and light zones is crucial for optimal GC function. It is likely that spatial separation of SHM and the selection of high-affinity GC B cells is required for efficient rounds of affinity maturation (FIG. 3). In the absence of this polarization, inappropriate or premature selection may occur. The importance of the unique zonal structure of GCs is perhaps most strongly indicted by the fact that it is evolutionarily conserved across species, including humans71. The propagation of high-affinity GC B cells, which is supported by the zonal structure of GCs, is discussed in detail below.
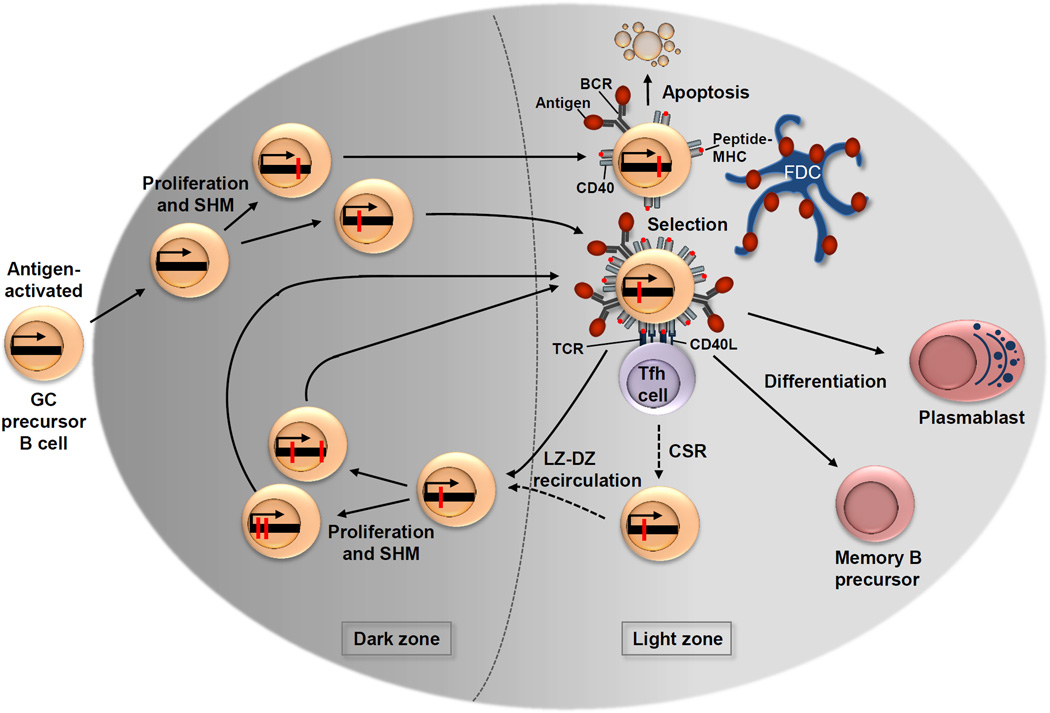
Figure 3. Dynamics of the GC reaction and selection of high-affinity antibody mutants.
Antigen-activated germinal centre (GC) precursor B cells form the early GC at day 4, in which they differentiate into blasts that over the following days undergo clonal expansion until the mature GC that is characterized by the dark zone and the light zone forms at day 7. During proliferation, the process of somatic hypermutation (SHM) introduces base-pair changes into the V(D)J region of the rearranged immunoglobulin variable region (IgV) genes (red dots); some of these base-pair mutations lead to a change in the amino acid sequence. Dark zone B cells then move to the light zone, where the modified B cell receptor (BCR) of the light zone B cell, with help from immune cells, including T follicular helper cells (TFH cells) and follicular dendritic cells (FDCs), is selected for improved binding to the immunizing antigen. Among the newly generated light zone B cells that express BCR mutants resulting from SHM in the dark zone, higher BCR affinity is directly associated with greater antigen capture and leads to a higher density of peptide–MHC complexes presented on the surface of the B cell. This results in the greatest share of T cell help, which in turn drives positive selection. Therefore, newly generated light zone B cells that produce an unfavourable antibody are rendered unable to capture sufficient antigen and undergo apoptosis. Following positive selection, a subset of light zone B cells is instructed to recirculate to the dark zone. Light zone B cells may undergo immunoglobulin class-switch recombination (CSR) before light zone–dark zone recirculation, whereas other cells switch and directly differentiate (not depicted). Back in the dark zone, these cells undergo further proliferation and SHM, thus potentially generating antibody mutants with an improved affinity. Recirculation between the dark zone and the light zone facilitates several iterative rounds of mutation and selection, and within a short time, leads to the generation of high-affinity memory B cells and plasma cells. Antigen-selected light zone B cells eventually differentiate into memory B cell precursor cells and plasmablasts, which are the precursors of plasma cells. CD40L, CD40 ligand; TCR, T cell receptor.
Dominant role of TFH cells in the selection of high-affinity B cells
Following SHM within the dark zone, high-affinity GC B cell mutants must be selected in the light zone before undergoing further rounds of hypermutation in the dark zone. It was originally believed that the selection of high-affinity GC B cells was mainly driven by a process of direct competition for antigen acquired from FDCs1. High-affinity GC B cells would sequester all available antigen and thereby deprive lower affinity competitors of survival signals that are triggered by BCR ligation. An alternative hypothesis suggested that competition for T cell help is the major factor determining the selection of antibody mutants (reviewed in REF.5).
Using an experimental system in which antigen is delivered directly to GC B cells independent of the BCR, it was recently shown that TFH cells promote selection within the light zone68. GC B cells capture antigen via the BCR and present the processed antigen on MHC complexes to TFH cells. Higher BCR affinity is directly associated with greater antigen capture and leads to a higher density of peptide–MHC presentation on the surface of the B cell68. This results in the greatest share of T cell help, which in turn drives selection (FIG. 3). Visualization of these processes in vivo showed that TFH cells patrol GC B cells and form the largest and the longest contacts with those cells that present the highest density of antigen on MHC complexes72. These contacts are transient, thereby allowing TFH cells to continuously survey many different light zone B cells, which results in the selection of the antibody mutants with the highest affinity on a population level.
These findings are complemented by a study that addressed the role of BCR signalling within the GC73. Surprisingly, it was found that both spontaneous and antigen-induced BCR signal transduction is inhibited by increased phosphatase activity in GC B cells. SH2 domain-containing phosphatase 1 (SHP1; also known as PTPN6) was found to be necessary for GC maintenance, which implies that quiescent BCR signalling is required to maintain the GC reaction. Therefore, it seems that within the context of the GC, the role of the BCR is primarily to capture and internalize antigen rather than to induce BCR signalling. However, active BCR signalling could be detected specifically in the G2–M phase of the cell cycle, which suggests that the signalling capacity of mutant BCRs can potentially be tested within the GC. How and at which time point during the GC reaction such a signal would integrate with TFH cell-mediated selection remains to be determined.
Acquisition of T cell help has been identified as the dominant force behind the selection of high-affinity GC B cells. However, the question remained — what is the underlying mechanism by which GC B cells with the highest antigen affinity are selected from the large pool of antigen-specific GC B cells with heterogeneous affinities? This question could not be formally addressed as a result of the lack of tools required to track the minor population of GC B cells undergoing selection within the dynamic GC microenvironment. However, the recent development of a method that allowed cell division to be tracked within the GC showed that B cells that receive the greatest magnitude of T cell help via robust antigen capture are programmed to divide the greatest number of times upon re-entry into the dark zone74. SHM and proliferation are intimately linked; the SHM process introduces a single nucleotide exchange per 1,000 bases into the rearranged IgV gene per division within the GC75. With this in mind, it is not surprising that the most proliferative GC B cells had considerably more affinity-enhancing mutations in their IgV genes compared with their less proliferative competitors. This indicates a mechanism by which affinity maturation is achieved following several rounds of interzonal cycles. In a ‘feedforward’ loop, the GC B cells with the highest affinity are repeatedly instructed to recirculate to the dark zone to undergo further rounds of division and SHM. As a result of this iterative process, GC B cells with the highest affinity dominate the population of B cells participating in the GC reaction within only a few days following the initiation of the GC reaction.
The findings described above show that TFH cells provide crucial signals to high-affinity GC B cells to facilitate their selection, and their transit back to and division in the dark zone (TFH cells have been thoroughly reviewed elsewhere76,77). The exact nature of these signals remains unclear and is being actively investigated in the field. Light zone B cells were found to be enriched for several early activation signatures, including CD40 (also known as TNFRSF5), NF-κB, BCR and MYC signatures68. Recent studies have provided new insights into the putative function of these pathways during the selection process, as is discussed below. Contact between TFH cells and GC B cells has also been shown to induce increased calcium signalling and the co-expression of interleukin-4 and interleukin-21 in TFH cells72. Moreover, inducible T cell co-stimulator ligand (ICOSL) expression by GC B cells has been shown to promote the transient but expansive ‘entanglement’ with TFH cells that express the surface receptor ICOS. This interaction stimulates the increased expression of CD40L (also known as TNFSF5) on the surface of TFH cells, thereby providing GC B cells with CD40 stimulation78. In turn, CD40 stimulation leads to the upregulation of ICOSL expression by GC B cells, which results in a feedforward loop that enables high-affinity B cells to reiteratively acquire a greater share of T cell help than their lower affinity competitors78. In summary, these findings show that the integration of several different signals provided by T cells controls affinity maturation over time.
Other factors in the selection of high-affinity B cells
A recent study provided evidence that, besides the crucial role of TFH cells in the selection of high-affinity antibody clones, positive selection of B cells within the GC is fine-tuned by limiting access to antigen deposited on FDCs79. This process of antigen masking via antibodies secreted from plasma cells that are generated early in the response enables only those GC B cells that express BCRs with high affinity to successfully compete for antigen acquisition. As antibodies with increasing affinity are progressively produced, the stringency of GC B cell selection increases over time.
Constraining antigen access by antibody masking is also likely to have a role in inter-GC communication79, as antibodies also infiltrate neighbouring GCs. Indeed, communication between GCs operates on several levels. TFH cells move freely between GCs and the invasion of mature GCs by newly activated TFH cells has been shown in vivo80. Furthermore, newly activated B cells can reuse pre-existing GCs81. Therefore, although GCs have evolved to clonally restrict GC B cells to prevent inter-GC competition and the development of an immune response that depends on a single dominant clone, coordination between several GCs can still be achieved. This communication promotes the common goal of producing antibodies of the highest possible affinity for the large range of epitopes that may be present on invading pathogens. These mechanisms may be particularly relevant during immune responses against pathogens that are capable of antigenic variation.
As SHM is a mostly random process, the benefit of SHM to affinity maturation is tempered by the inevitable generation of GC B cell clones that express self-reactive BCRs. In contrast to tolerance mechanisms that control early B cell development, the means by which self-reactive GC B cells are eliminated is poorly understood. The development of an experimental system in which a transgenic BCR gains cross-reactivity to a self-antigen following SHM has shed new light on the factors that regulate negative selection82. It was found that self-reactive GC B cells can be successfully cleared when self-antigen is expressed ubiquitously or near to the GC microenvironment. By contrast, GC B cells that are reactive to rare or tissue-specific antigens can escape clearance and are positively selected. The exact mechanism behind these divergent outcomes is yet to be determined.
Molecular control of the GC reaction
The GC reaction is a highly complex process involving several distinct, timed events that are topographically segregated within the GC microenvironment. This complex cascade of events must be coordinated with precise molecular control. Much has been learnt over the years about the major regulators within the GC that determine the specialized physiology of the dark zone B cells, facilitating rapid proliferation and SHM, and preventing their terminal differentiation. This topic has been reviewed elsewhere83. As outlined in the previous section, the depth with which we understand GC dynamics has increased considerably in recent years to encompass elastic cellular states within the GC, T cell-based selection and light zone–dark zone recirculation. These processes are carried out in a tiny subpopulation of GC B cells, and identifying the fine molecular events that control these processes is currently an important area of inquiry. In this section, we discuss recent developments regarding the transcription factors, epigenetic mediators and post-transcriptional mechanisms that coordinate GC dynamics.
Maintenance of the GC reaction: light zone–dark zone recirculation
As outlined above, MYC is required throughout the early and late initiation phases of GC formation but it is not expressed in the proliferating dark zone B cells. Interestingly, recent studies showed that MYC is expressed by a small subset of light zone B cells within mature GCs that have segregated into dark and light zones at day 7 post immunization46,49. This pattern of expression coincides with the point at which the selection of high-affinity mutants is thought to begin4,84. Moreover, MYC+ GC B cells were found to have an activated phenotype similar to that described by gene expression profiling of light zone B cells46,49,68. These MYC+ cells were also actively progressing through the cell cycle and had a greater number of affinity-enhancing mutations than MYC− light zone B cells46,49. Finally, T cell help was found to induce MYC expression in light zone B cells46. These findings collectively suggest that MYC+ B cells within the light zone are undergoing affinity-based positive selection.
As described in a previous section, positive selection of a high-affinity B cell within the light zone programmes re-entry to and division within the dark zone. This suggests that cyclic re-entry sustains the population of cells that proliferate within the dark zone and maintains the GC reaction over time. Therefore, it would be predicted that genetic ablation of an essential regulator of positive selection and cyclic re-entry would lead to the collapse of established GCs. As expected, the inhibition of MYC function within GC B cells led to the dissolution of established GCs46,49. These findings collectively show that MYC has essential roles in GC selection, cyclic re-entry and GC maintenance (FIG. 4a).
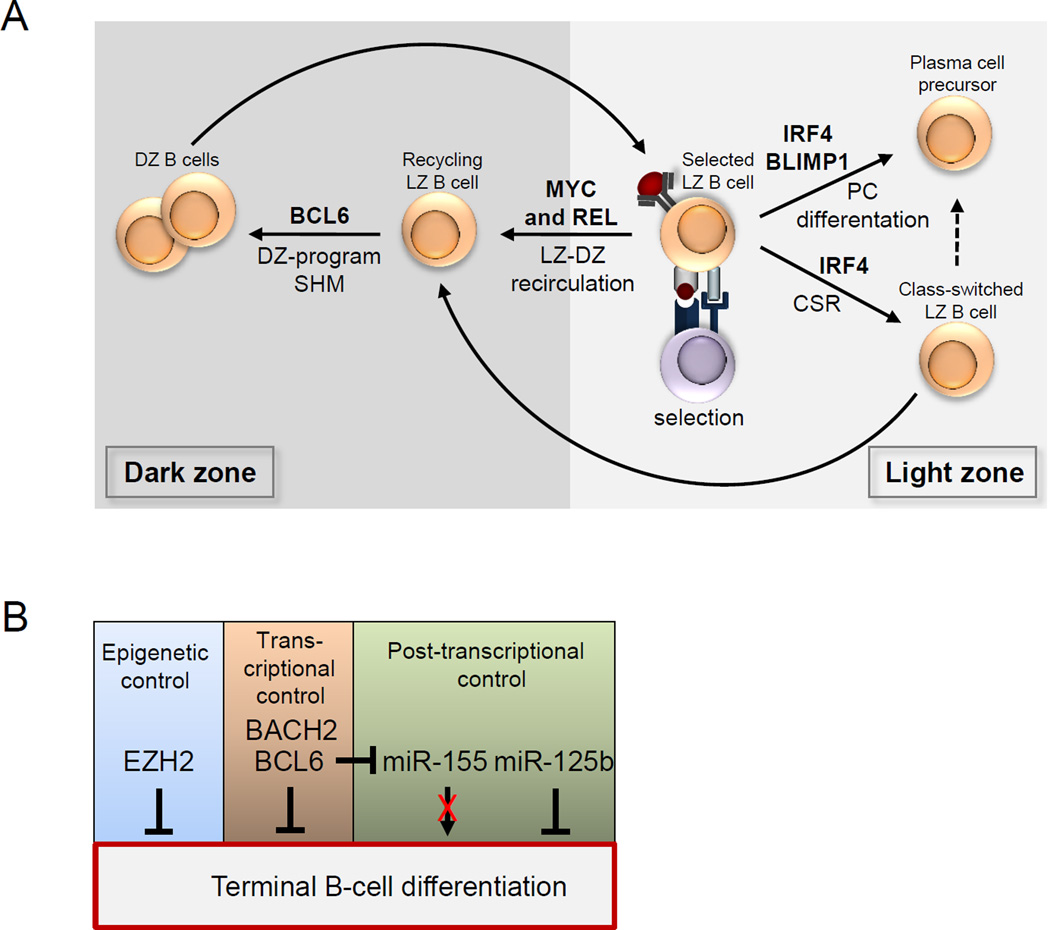
Figure 4. Molecular control of GC maintenance.
a Roles of transcriptional regulators in stages of germinal centre (GC) B cell development, as identified by genetic analysis, are shown. B cell lymphoma 6 (BCL-6) is required for the establishment of the specialized dark zone-associated gene expression programme that allows for somatic hypermutation (SHM) of the B cell receptor (BCR). MYC and REL are required for recirculation between the light zone and the dark zone, and thus for the maintenance of the GC reaction. Interferon-regulatory factor 4 (IRF4), although expressed in light zone B cells and associated with the downregulation of the dark zone-associated programme, is not required for GC maintenance. Instead, IRF4 is required for immunoglobulin class-switch recombination (CSR)43,114 and thus the frequency of isotype-switched B cells exiting the GC, and also for the production of GC-derived plasma cells. B lymphocyte-induced maturation protein 1 (BLIMP1) is also required for plasma cell differentiation115. b Different genetic mechanisms maintain the dark zone phenotype by inhibiting terminal differentiation. The core catalytic component of polycomb repressive complex 2, enhancer of zeste homologue 2 (EZH2), inhibits terminal differentiation by silencing the expression of the plasma cell regulators Irf4 and Prdm1 (which encodes BLIMP1). BACH2 (BTB and CNC homologue 2) cooperates with BCL-6 and represses genes involved in post-GC differentiation116. BCL-6 transcriptionally represses plasma cell-associated genes including Prdm1, directly and indirectly via downregulation of the microRNA miR-155 that is associated with terminating the dark zone-associated programme. miR-125b downregulates the expression of IRF4 and BLIMP1; as this microRNA is highly expressed in dark zone B cells, it probably contributes to the repression of expression of Irf4 and Prdm1. CD40L, CD40 ligand; TCR, T cell receptor.
As NF-κB is commonly associated with cellular proliferation, it was paradoxical to find that NF-κB activation is silenced in most GC B cells that are proliferating at an extremely high rate85. Interestingly, nuclear translocation of canonical NF-κB subunits was instead observed in a subset of light zone B cells85. Indeed, NF-κB-associated gene signatures were identified in flow cytometry-sorted light zone B cells68, which suggests an important role for NF-κB activation in these cells. Evidence from the analysis of human cell lines suggested that in GC B cells, CD40 stimulation leads to the NF-κB-mediated upregulation of IRF4 expression, which in turn represses BCL6 transcription, thus terminating the dark zone-associated transcription programme42.
The downstream effectors of the NF-κB pathway are REL, RELA (also known as p65) and p50 for the canonical pathway, and RELB and p52 for the alternative pathway. Conditional deletion of Rel in GC B cells was recently found to induce the collapse of established GCs after day 7 of the GC reaction86. This collapse was not caused by defective delivery of pro-survival signals in the absence of REL; instead, REL seems to be required for the establishment of a metabolic programme that enables cell growth before cell division86. Dark and light zone B cell fractions were equally lost upon GC-specific deletion of Rel86, which suggests that REL is essential for the maintenance of the GC reaction as it ‘licenses’ antigen-selected light zone B cells to recirculate back to the dark zone (FIG. 4a).
Many parallels are evident when comparing the activity of MYC and NF-κB. Both factors have a biphasic pattern of activation during the GC reaction and are curiously absent from most highly proliferative GC B cells. Both NF-κB- and MYC-associated gene signatures are enriched in light zone B cells68, and NF-κB-associated gene signatures are also specifically enriched in MYC+ GC B cells46,49. Most importantly, the ablation of either MYC or REL was shown to impair GC maintenance46,49,86. These findings suggest some level of interplay between these factors that function during GC B cell selection and that permit recirculation and GC maintenance.
Finally, several other factors have been reported to participate in the maintenance of mature GCs. Reminiscent of what has been observed for REL ablation in GC B cells86, deficiency of the transmembrane protein tyrosine phosphatase CD45 in B cells is associated with the disappearance of GC B cells specifically in the time period after day 7 following immunization87. As discussed earlier, the deficiency of EBF1 or SPIB in B cells affects the proliferation phase (at day 5–7) of GC initiation. In addition, a dramatic loss of GC B cells was observed at day 14 (REFS62,63), which suggests that EBF1 and SPIB have an additional role in GC maintenance.
Epigenetic regulation of the dark zone phenotype
Epigenetic regulation provides an additional layer of control that is crucially important in a range of biological contexts88. Epigenetic mechanisms were recently found to have a major role in maintaining the phenotype of dark zone B cells89,90. Polycomb repressive complex 1 (PRC1) and PRC2 are epigenetic regulators that work together to modulate gene repression. The core catalytic component of PRC2 is the enhancer of zeste homologue 2 (EZH2), which is a histone methyltransferase that is responsible for trimethylating lysine 27 of histone 3. This histone mark — H3K27me3 — correlates with closed chromatin and, therefore, silenced gene expression. The expression of EZH2 is induced in GC B cells and high expression is maintained until they exit the GC91,92. Conditional ablation of EZH2 early at day 2 following antigen activation markedly impairs the GC reaction and consequently abolishes affinity maturation89,90. Of note, EZH2 deficiency in GC B cells was shown to lead to the upregulated expression of plasma cell regulators, including IRF4 and B lymphocyte-induced maturation protein 1 (BLIMP1; which is encoded by Prdm1) (FIG. 4b).
Interestingly, the promoters of many genes involved in GC exit and terminal differentiation in both mouse and human GC B cells showed the activating H3K4me3 mark in addition to the repressive H3K27me3 mark. Therefore, these genes, which included Irf4 and Prdm1, have bivalent chromatin domains. In embryonic and tissue-specific stem cells, EZH2 contributes to the establishment of bivalent chromatin domains at several gene loci93. These domains maintain the potential of key lineage-commitment genes to be repressed or activated as required until a final decision has been made for the cell to differentiate. The presence of bivalent chromatin domains in GC B cells, which — in contrast to pluripotent cells — are a committed cell type, is unusual and could suggest a system in which key developmental factors are poised for activation or repression. Such a system would fit well into the dynamic environment of the GC, in which rapid shifts in cellular phenotype are observed before an eventual decision to exit the GC reaction has been made. This system would facilitate transient or reversible suppression of factors that direct terminal differentiation, such as IRF4 and BLIMP1. Upon the receipt of signals to exit the GC reaction, removal of H3K27me3 would allow commitment towards terminal differentiation.
BCL-6-mediated non-transcriptional regulation of the dark zone phenotype
BCL-6 is known to recruit several co-repressors. Transcriptional repression can be achieved through various mechanisms. In one such mechanism, it was found that the enhancers of certain BCL-6 target genes remain poised for activation or repression94. This poised configuration is achieved by the selective recruitment of histone deacetylase 3 (HDAC3)-containing SMRT (silencing mediator for retinoic acid receptor and thyroid hormone receptor; also known as NCOR2) co-repressor complexes by BCL-6 to certain enhancers to achieve repression via H3K27 deacetylation. This effect is counterbalanced by p300 complexes that enable H3K27 acetylation and enhancer activation. Competition between these two events thereby allows reversible ‘toggling’ of the expression of key GC genes and, similarly to EZH2, allows dynamic regulation of BCL-6 target genes as the GC reaction proceeds and cells transition into diverse phenotypic fates.
MicroRNA-mediated regulation of the dark zone phenotype
An important role of microRNAs in GC development is implied by the severe impairment of GC formation upon deletion of the RNaseIII enzyme Dicer, which is a component of the microRNA-processing machinery, in antigen-activated B cells95. MicroRNA-155 (miR-155) has been identified as a microRNA that has roles during the GC reaction. Mice deficient for miR-155 have deficiencies in GC formation and in affinity maturation96,97. Perhaps surprisingly, it was later found that BCL-6 represses mir-155 transcription in GC B cells and thereby indirectly modulates the expression of genes that are associated with establishing or maintaining the dark zone phenotype98. As the precursor of miR-155 is expressed in a small subset of GC B cells99 and its expression is upregulated upon CD40 and BCR stimulation in vitro99, it is possible that miR-155 expression in light zone B cells contributes to the termination of the dark zone programme (FIG. 4b). Conversely, evidence suggests that miR-125b, which is highly expressed by dark zone B cells, may directly inhibit the differentiation of GC B cells into plasmablasts by downregulating the expression of BLIMP1 and IRF4 in dark zone B cells100. The recent identification of several other microRNAs with roles in GC B cell development98,101,102 highlights the important role for post-transcriptional regulation in the control of GC differentiation.
Transcriptional regulation of the dark zone phenotype
Transcriptional regulation of the dark zone phenotype is complex and has been extensively reviewed elsewhere83. In this section, we update this picture with new information regarding IRF8. A function for IRF8 in the physiology of dark zone B cells has long been assumed on the basis of its high expression by GC B cells and its role in the induction of BCL-6 expression59. However, deletion of IRF8 in B cells did not affect GC formation103. A recent study showed that the combined ablation of IRF8 and the transcription factor PU.1 led to increased plasma cell differentiation104, which suggests that the IRF8–PU.1 complex has a crucial role in the inhibition of terminal B cell differentiation.
Conclusions and perspectives
Considerable progress has been made in understanding the GC reaction. We now have a greatly enhanced picture of the events that follow initial antigen encounter and culminate in the formation of a mature GC that is optimally structured for the selection of high-affinity antibody mutants. Nevertheless, we have only begun to dissect the complexity of the GC reaction. GC B cells rapidly transition between different cellular states within the GC; this flux in phenotype is punctuated by decisions to commit to post-GC differentiation. This dynamic system must be instructed by intricate mechanisms of control, which are probably achieved through the integration of transcriptional, post-transcriptional and epigenetic programmes. Several important questions remain to be answered regarding the fate of a selected light zone B cell. What are the molecular cues that are coordinated to drive a cell to recirculate into the dark zone? Light zone B cells expressing MYC and/or activated NF-κB do not express BCL-6; however, it is clear that these cells must have a mechanism by which the BCL-6-dependent dark zone-associated gene expression programme is reactivated upon cyclic re-entry. How and when is the decision to undergo class-switch recombination (CSR) to one of several isotypes determined? What sequence of events signals a GC B cell to finally cease cyclic re-entry and to commit to memory B cell or plasma cell differentiation105–108?
It is evident from these considerations that the population of antigen-selected light zone B cells must be extremely heterogeneous. It will be interesting to determine what role asymmetric cell division109 or stochastic events110 have in this coordinated process of selection and cellular differentiation. With every new detail that is derived from experimental observations, improved computational models111 can be developed that provide new hypotheses for future research. Importantly, identifying the nature and the temporal progression of the molecular changes that occur within selected light zone B cells may give us the opportunity to manipulate the GC reaction for improved vaccine efficacy. Finally, unravelling these intricacies of the GC reaction may also improve our understanding of the transforming mechanisms that drive the development of GC-derived B cell malignancies112,113.
Key Points.
The germinal centre (GC) of lymphoid organs is the microenvironment in which antigen-activated B cells diversify their immunoglobulin genes by somatic hypermutation (SHM) to generate high-affinity antibodies. A subset of the cells also undergoes class-switch recombination to generate antibodies with specialized effector functions.
Early in an immune response, antigen-stimulated B cells form long-lived interactions with antigen-specific T cells at the border between the B cell zone and the T cell zone or the interfollicular region to become fully activated. Antigen-activated B cells and T cells are committed to differentiate into GC B cells and T follicular helper cells (TFH cells), respectively, outside of the follicle. Migration into the follicle is facilitated by B cell lymphoma 6 (BCL-6), which is the master transcriptional regulator of GC B cells.
One day after TFH cells have moved into the follicle, GC precursor B cells migrate from the border between the B cell zone and the T cell zone or the interfollicular region into the centre of the follicle to form an early GC. The B cells differentiate into blasts and, over the next several days, rapidly divide and begin to fill the centre of the follicle until they have formed a mature GC that is polarized into two microenvironments known as the dark and light zones.
Dark zone B cells, which are GC B cells that undergo active SHM, are programmed to proliferate extremely rapidly and thereby to generate a large number of immunoglobulin mutations in a short time. Dark zone B cells differentiate into light zone B cells, at which stage mutants expressing high-affinity antibodies are selected and instructed to either recirculate to the dark zone to undergo further rounds of SHM or to differentiate into memory B cells or plasma cells.
Light zone B cells capture antigen via the B cell receptor (BCR) and present the processed antigen on MHC complexes to TFH cells. Higher BCR affinity is directly associated with greater antigen capture and leads to a higher density of peptide–MHC complex presentation on the surface of the B cell. This results in the greatest share of T cell help, which in turn drives selection.
Evidence suggests that the transcription factors MYC and the nuclear factor-κB subunit REL are essential for the maintenance of the GC reaction as they 'license' antigen-selected light zone B cells to recirculate to the dark zone. Inhibition of the terminal differentiation of GC B cells is controlled by multiple mechanisms that include both transcriptional and non-transcriptional regulation.
Acknowledgements
The authors thank the members of the Klein laboratory and in particular N. Heise for discussions. This work was supported by National Cancer Institute (NCI) and US National Institutes of Health (NIH) grant R01-CA157660 to U.K. and a Cancer Biology Training Program fellowship (NCI and NIH grant 5T32-CA009503-26) to N.S.D..
Glossary terms
- Plasma cells
Non-dividing, terminally differentiated antibody-secreting cells of the B cell lineage.
- Memory B cells
Antigen-experienced B cells that express high-affinity antibodies and that quickly differentiate into plasma cells in antigen-recall responses.
- Somatic hypermutation
(SHM). The process by which point mutations are introduced in the heavy or light chain variable region gene segments, resulting in a change in the expressed protein, which may alter affinity or specificity for antigen.
- Affinity maturation
The somatic mutation process by which B cells are selected for survival and proliferation on the basis of their increased affinity for antigen.
- Follicular dendritic cells
(FDCs). Specialized non-haematopoietic stromal cells that reside in lymphoid follicles and germinal centres. These cells have long dendrites and carry intact antigens on their surface. They are crucial for the optimal selection of B cells that produce antigen-binding antibodies.
- Plasmablasts
The B cell lineage precursors of non-dividing plasma cells, which have the capacity to divide and have migratory potential.
- Two-photon intravital microscopy
Laser-scanning microscopy that uses pulsed infrared laser light for the excitation of conventional fluorophores or fluorescent proteins. The main advantage is deep tissue penetration of the infrared light owing to the low level of light scattering within the tissue.
- T follicular helper cells
(TFH cells). A specialized subset of T cells that guide the selection of high-affinity germinal centre (GC) B cells within the light zone of the GC.
- B cell lymphoma 6
(BCL-6). A transcriptional repressor and the master regulator of the germinal centre B cell reaction. Together with cofactors, BCL-6 represses genes involved in B cell activation, negative cell cycle regulation, the response to genotoxic stress, and differentiation into memory B cells and plasma cells.
- Early GC
(Early germinal centre). The earliest GC structure that arises from the coalescence of antigen-activated B cell blasts within the network of follicular dendritic cells in the centre of the B cell follicle.
- Dark zone B cells
Proliferating germinal centre B cells localized in the dark zone with rearranged variable region immunoglobulin genes that are undergoing somatic hypermutation.
- Interferon-regulatory factor 4
(IRF4). A member of the interferon-regulatory factor family of transcription factors. IRF4 exerts its function through heteromerization with cofactors and can either activate or repress gene expression. IRF4 is required for germinal centre formation and for plasma cell differentiation.
- Light zone B cells
The progeny of dark zone B cells. These cells need to be selected on the basis of their affinity for antigen, following interaction with immune complexes that are associated with follicular dendritic cells and their ability to elicit help from T follicular helper cells. A subset of light zone B cells undergoes class-switch recombination or differentiates into memory B cells or plasma cells.
- Nuclear factor-κB
(NF-κB). Activation of the NF-κB signalling pathway via stimulation of cell-surface receptors can occur via two different routes, the canonical and the alternative pathways, and it induces the transcription of genes that are involved in cellular activation, cell growth and proliferation.
- Class-switch recombination
(CSR). The process by which B cells change their immunoglobulin isotype to generate antibodies with different effector functions.
Biographies
Nilushi S. De Silva is a graduate student in the Department of Microbiology and Immunology at Columbia University, New York, USA. She obtained a B.Sc. in Biomedical Science at the University of Melbourne, Australia. In her Ph.D. thesis, she studied the role of nuclear factor-κB in the development of germinal centre B cells and plasma cells.
Ulf Klein is an assistant professor of Pathology and Cell Biology, and Microbiology and Immunology, in the Herbert Irving Comprehensive Cancer Center, Columbia University, New York, USA. He obtained a Ph.D. in genetics at the University of Cologne, Germany. Research in his laboratory focuses on the molecular mechanisms of mature B cell differentiation.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.MacLennan IC. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 2.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 3.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 4.Blink EJ, et al. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J. Exp. Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Victora GD, Nussenzweig MC. Germinal centers. Annu. Rev. Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 6.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nature Rev. Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 7.Okada T, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J. Exp. Med. 1992;176:679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paus D, et al. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J. Exp. Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor BP, et al. Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor. J. Immunol. 2006;177:7723–7732. doi: 10.4049/jimmunol.177.11.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J. Exp. Med. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dal Porto JM, Haberman AM, Kelsoe G, Shlomchik MJ. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J. Exp. Med. 2002;195:1215–1221. doi: 10.1084/jem.20011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih TA, Meffre E, Roederer M, Nussenzweig MC. Role of BCR affinity in T cell dependent antibody responses in vivo. Nature Immunol. 2002;3:570–575. doi: 10.1038/ni803. [DOI] [PubMed] [Google Scholar]
- 15.Schwickert TA, et al. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J. Exp. Med. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31:259–269. doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460:1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerfoot SM, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitano M, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct waves of BCL6 expression during T follicular helper cell development. J. Immunol. 2011;187:2089–2092. doi: 10.4049/jimmunol.1101393. References 18–21 collectively show that antigen-activated B cells and T cells are committed to differentiate into GC B cells and TFH cells outside of the follicle.
- 22.Coffey F, Alabyev B, Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009;30:599–609. doi: 10.1016/j.immuni.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bannard O, et al. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 2013;39:912–924. doi: 10.1016/j.immuni.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, et al. The BCL6 RD2 domain governs commitment of activated B cells to form germinal centers. Cell Rep. 2014;8:1497–1508. doi: 10.1016/j.celrep.2014.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen CD, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nature Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 27.Caron G, Le Gallou S, Lamy T, Tarte K, Fest T. CXCR4 expression functionally discriminates centroblasts versus centrocytes within human germinal center B cells. J. Immunol. 2009;182:7595–7602. doi: 10.4049/jimmunol.0804272. [DOI] [PubMed] [Google Scholar]
- 28.Green JA, et al. The sphingosine 1-phosphate receptor S1P2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nature Immunol. 2011;12:672–680. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cinamon G, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nature Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 30.Cannons JL, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allman D, et al. BCL-6 expression during B-cell activation. Blood. 1996;87:5257–5268. [PubMed] [Google Scholar]
- 32.Basso K, Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B cells. Immunol. Rev. 2012;247:172–183. doi: 10.1111/j.1600-065X.2012.01112.x. [DOI] [PubMed] [Google Scholar]
- 33.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nature Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khiem D, Cyster JG, Schwarz JJ, Black BL. A p38 MAPK–MEF2C pathway regulates B-cell proliferation. Proc. Acad. Natl Sci. USA. 2008;105:17067–17072. doi: 10.1073/pnas.0804868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilker PR, et al. Transcription factor Mef2c is required for B cell proliferation and survival after antigen receptor stimulation. Nature Immunol. 2008;9:603–612. doi: 10.1038/ni.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying CY, et al. MEF2B mutations lead to deregulated expression of the oncogene BCL6 in diffuse large B cell lymphoma. Nature Immunol. 2013;14:1084–1092. doi: 10.1038/ni.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Silva NS, Simonetti G, Heise N, Klein U. The diverse roles of IRF4 in late germinal center B-cell differentiation. Immunol. Rev. 2012;247:73–92. doi: 10.1111/j.1600-065X.2012.01113.x. [DOI] [PubMed] [Google Scholar]
- 38.Shaffer AL, Emre NC, Romesser PB, Staudt LM. IRF4: Immunity. Malignancy! Therapy? Clin. Cancer Res. 2009;15:2954–2961. doi: 10.1158/1078-0432.CCR-08-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bollig N, et al. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proc. Acad. Natl Sci. USA. 2012;109:8664–8669. doi: 10.1073/pnas.1205834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ochiai K, et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity. 2013;38:918–929. doi: 10.1016/j.immuni.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willis SN, et al. Transcription factor IRF4 regulates germinal center cell formation through a B cell-intrinsic mechanism. J. Immunol. 2014;192:3200–3206. doi: 10.4049/jimmunol.1303216. [DOI] [PubMed] [Google Scholar]
- 42.Saito M, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Klein U, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nature Immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 44.Sciammas R, et al. An incoherent regulatory network architecture that orchestrates B cell diversification in response to antigen signaling. Mol. Sys Biol. 2011;7:495. doi: 10.1038/msb.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominguez-Sola D, et al. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nature Immunol. 2012;13:1083–1091. doi: 10.1038/ni.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaffer AL, et al. Signatures of the immune response. Immunity. 2001;15:375–385. doi: 10.1016/s1074-7613(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 48.Klein U, et al. Transcriptional analysis of the B cell germinal center reaction. Proc. Acad. Natl Sci. USA. 2003;100:2639–2644. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Calado DP, et al. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nature Immunol. 2012;13:1092–1100. doi: 10.1038/ni.2418. References 46 and 49 define the long unknown role of the MYC proto-oncogene in GC formation and in the maintenance of the GC reaction.
- 50.Peled JU, et al. Requirement for cyclin D3 in germinal center formation and function. Cell Res. 2010;20:631–646. doi: 10.1038/cr.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cato MH, Chintalapati SK, Yau IW, Omori SA, Rickert RC. Cyclin D3 is selectively required for proliferative expansion of germinal center B cells. Mol. Cell. Biol. 2011;31:127–137. doi: 10.1128/MCB.00650-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatzi K, Melnick A. Breaking bad in the germinal center: how deregulation of BCL6 contributes to lymphomagenesis. Trends Mol. Med. 2014;20:343–352. doi: 10.1016/j.molmed.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vikstrom I, et al. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330:1095–1099. doi: 10.1126/science.1191793. This study shows that MCL1 is the main anti-apoptotic regulator in GC B cells.
- 54.Kaileh M, Sen R. NF-κB function in B lymphocytes. Immunol. Rev. 2012;246:254–271. doi: 10.1111/j.1600-065X.2012.01106.x. [DOI] [PubMed] [Google Scholar]
- 55.Gerondakis S, Siebenlist U. Roles of the NF-κB pathway in lymphocyte development and function. Cold Spring Harb. Perspect. Biol. 2010;2:a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacque E, et al. IKK-induced NF-κB1 p105 proteolysis is critical for B cell antibody responses to T cell-dependent antigen. J. Exp. Med. 2014;211:2085–2101. doi: 10.1084/jem.20132019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim U, et al. The B-cell-specific transcription coactivator OCA-B/OBF-1/Bob-1 is essential for normal production of immunoglobulin isotypes. Nature. 1996;383:542–547. doi: 10.1038/383542a0. [DOI] [PubMed] [Google Scholar]
- 58.Schubart DB, Rolink A, Kosco-Vilbois MH, Botteri F, Matthias P. B-cell-specific coactivator OBF-1/OCA-B/Bob1 required for immune response and germinal centre formation. Nature. 1996;383:538–542. doi: 10.1038/383538a0. [DOI] [PubMed] [Google Scholar]
- 59.Lee CH, et al. Regulation of the germinal center gene program by interferon (IFN) regulatory factor 8/IFN consensus sequence-binding protein. J. Exp. Med. 2006;203:63–72. doi: 10.1084/jem.20051450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon K, et al. Instructive role of the transcription factor E2A in early B lymphopoiesis and germinal center B cell development. Immunity. 2008;28:751–762. doi: 10.1016/j.immuni.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Randall KL, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nature Immunol. 2009;10:1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vilagos B, et al. Essential role of EBF1 in the generation and function of distinct mature B cell types. J. Exp. Med. 2012;209:775–792. doi: 10.1084/jem.20112422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su GH, et al. Defective B cell receptor-mediated responses in mice lacking the Ets protein, Spi-B. EMBO J. 1997;16:7118–7129. doi: 10.1093/emboj/16.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muto A, et al. The transcriptional programme of antibody class switching involves the repressor Bach2. Nature. 2004;429:566–571. doi: 10.1038/nature02596. [DOI] [PubMed] [Google Scholar]
- 65.Huang C, Geng H, Boss I, Wang L, Melnick A. Cooperative transcriptional repression by BCL6 and BACH2 in germinal center B-cell differentiation. Blood. 2014;123:1012–1020. doi: 10.1182/blood-2013-07-518605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwickert TA, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 67.Hauser AE, et al. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007;26:655–667. doi: 10.1016/j.immuni.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 68. Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. This study provides evidence that T cell help is the major factor promoting the selection of high-affinity antibodies in the light zone. Reference 24 and references 66–68 are an elegant series of studies that dissect the dynamics of GC B cell differentiation using intravital microscopy.
- 69.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hauser AE, Shlomchik MJ, Haberman AM. In vivo imaging studies shed light on germinal-centre development. Nature Rev. Immunol. 2007;7:499–504. doi: 10.1038/nri2120. [DOI] [PubMed] [Google Scholar]
- 71.Victora GD, et al. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood. 2012;120:2240–2248. doi: 10.1182/blood-2012-03-415380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shulman Z, et al. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345:1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336:1178–1181. doi: 10.1126/science.1213368. This work provides evidence that in GC B cells, BCR signalling is quiescent; rather, it seems that the role of the BCR is primarily to capture and to internalize antigen.
- 74. Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509:637–640. doi: 10.1038/nature13300. This study identifies the mechanism by which GC B cell clones with the highest affinity for the immunizing antigen are selectively expanded.
- 75.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu. Rev. Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 76.Crotty S. Follicular helper CD4 T cells (TFH) Annu. Rev. Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 77.Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol. Rev. 2013;252:146–155. doi: 10.1111/imr.12031. [DOI] [PubMed] [Google Scholar]
- 78. Liu D, et al. T–B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015;517:214–218. doi: 10.1038/nature13803. References 72 and 78 show that interactions between TFH cells and GC B cells are transient but extensive. These contacts initiate changes within TFH cells that guide the selection of high-affinity GC B cells.
- 79.Zhang Y, et al. Germinal center B cells govern their own fate via antibody feedback. J. Exp. Med. 2013;210:457–464. doi: 10.1084/jem.20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shulman Z, et al. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwickert TA, Alabyev B, Manser T, Nussenzweig MC. Germinal center reutilization by newly activated B cells. J. Exp. Med. 2009;206:2907–2914. doi: 10.1084/jem.20091225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan TD, et al. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37:893–904. doi: 10.1016/j.immuni.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 83.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nature Rev. Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 84.Jacob J, Przylepa J, Miller C, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J. Exp. Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Basso K, et al. Tracking CD40 signaling during germinal center development. Blood. 2004;104:4088–4096. doi: 10.1182/blood-2003-12-4291. [DOI] [PubMed] [Google Scholar]
- 86.Heise N, et al. Germinal center B cell maintenance and differentiation are controlled by distinct NF-κB transcription factor subunits. J. Exp. Med. 2014;211:2103–2118. doi: 10.1084/jem.20132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huntington ND, et al. CD45 links the B cell receptor with cell survival and is required for the persistence of germinal centers. Nature Immunol. 2006;7:190–198. doi: 10.1038/ni1292. [DOI] [PubMed] [Google Scholar]
- 88.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 89.Beguelin W, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23:677–692. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Caganova M, et al. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J. Clin. Invest. 2013;123:5009–5022. doi: 10.1172/JCI70626. References 89 and 90 show an important role for epigenetic regulation in the maintenance of the dark zone B cell phenotype.
- 91.Raaphorst FM, et al. Cutting edge: polycomb gene expression patterns reflect distinct B cell differentiation stages in human germinal centers. J. Immunol. 2000;164:1–4. doi: 10.4049/jimmunol.164.1.1. [DOI] [PubMed] [Google Scholar]
- 92.Velichutina I, et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood. 2010;116:5247–5255. doi: 10.1182/blood-2010-04-280149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 94.Hatzi K, et al. A hybrid mechanism of action for BCL6 in B cells defined by formation of functionally distinct complexes at enhancers and promoters. Cell Rep. 2013;4:578–588. doi: 10.1016/j.celrep.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu S, Guo K, Zeng Q, Huo J, Lam KP. The RNase III enzyme Dicer is essential for germinal center B-cell formation. Blood. 2012;119:767–776. doi: 10.1182/blood-2011-05-355412. [DOI] [PubMed] [Google Scholar]
- 96.Thai TH, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 97.Vigorito E, et al. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Basso K, et al. BCL6 positively regulates AID and germinal center gene expression via repression of miR-155. J. Exp. Med. 2012;209:2455–2465. doi: 10.1084/jem.20121387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van den Berg A, et al. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer. 2003;37:20–28. doi: 10.1002/gcc.10186. [DOI] [PubMed] [Google Scholar]
- 100.Gururajan M, et al. MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int. Immunol. 2010;22:583–592. doi: 10.1093/intimm/dxq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Yebenes VG, et al. miR-217 is an oncogene that enhances the germinal center reaction. Blood. 2014;124:229–239. doi: 10.1182/blood-2013-12-543611. [DOI] [PubMed] [Google Scholar]
- 102.Schneider C, et al. MicroRNA 28 controls cell proliferation and is down-regulated in B-cell lymphomas. Proc. Acad. Natl Sci. USA. 2014;111:8185–8190. doi: 10.1073/pnas.1322466111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng J, et al. IFN regulatory factor 8 restricts the size of the marginal zone and follicular B cell pools. J. Immunol. 2011;186:1458–1466. doi: 10.4049/jimmunol.1001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carotta S, et al. The transcription factors IRF8 and PU.1 negatively regulate plasma cell differentiation. J. Exp. Med. 2014;211:2169–2181. doi: 10.1084/jem.20140425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nature Rev. Immunol. 2012;12:24–34. doi: 10.1038/nri3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zotos D, Tarlinton DM. Determining germinal centre B cell fate. Trends Immunol. 2012;33:281–288. doi: 10.1016/j.it.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 107.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 2012;247:52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 108.Nutt SL, Taubenheim N, Hasbold J, Corcoran LM, Hodgkin PD. The genetic network controlling plasma cell differentiation. Semin. Immunol. 2011;23:341–349. doi: 10.1016/j.smim.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 109.Barnett BE, et al. Asymmetric B cell division in the germinal center reaction. Science. 2012;335:342–344. doi: 10.1126/science.1213495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duffy KR, et al. Activation-induced B cell fates are selected by intracellular stochastic competition. Science. 2012;335:338–341. doi: 10.1126/science.1213230. [DOI] [PubMed] [Google Scholar]
- 111.Meyer-Hermann M, et al. A theory of germinal center B cell selection, division, and exit. Cell Rep. 2012;2:162–174. doi: 10.1016/j.celrep.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 112.Shaffer AL, 3rd, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu. Rev. Immunol. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nature Rev. Immunol. doi: 10.1038/nri3814. (in the press). [DOI] [PubMed] [Google Scholar]
- 114.Sciammas R, et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 115.Shapiro-Shelef M, et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 116.Igarashi K, Ochiai K, Itoh-Nakadai A, Muto A. Orchestration of plasma cell differentiation by Bach2 and its gene regulatory network. Immunol. Rev. 2014;261:116–125. doi: 10.1111/imr.12201. [DOI] [PubMed] [Google Scholar]