Abstract
The prevalence of unruptured intracranial aneurysms (UAIs) in the general population is up to 3%. Existing epidemiological data suggests that only a small fraction of UIAs progress towards rupture over the lifetime of an individual, but the surrogates for subsequent rupture and the natural history of UIAs are discussed very controversially at present. In case of rupture of an UIA, the case-fatality is up to 50%, which therefore continues to stimulate interest in the pathogenesis of cerebral aneurysm formation and progression. Actual data on the chronological development of cerebral aneurysm has been especially difficult to obtain and, until recently, the existing knowledge in this respect is mainly derived from animal or mathematical models or short-term observational studies. Here, we highlight the current data on cerebral aneurysm formation and progression as well as a novel approach to investigate the developmental chronology of cerebral aneurysms.
Keywords: Intracranial aneurysms, aneurysm progression, aneurysm formation, developmental chronology
Introduction
The prevalence of unruptured intracranial aneurysms (UAIs) is assumed to be up to 3% of the general population, with 20–30% of patients harboring more than one aneurysm. 1–3. While there is no data suggesting an increase of the prevalence of UIAs over the past decades, it seems that UIAs are increasingly detected, probably due to the higher distribution and technological improvement of imaging devices (i.e. magnet resonance imaging and computerized tomography) and also increasing scans in patients due to unspecific symptoms, such as headaches or dizziness. 4–7 UIAs can remain clinically silent, become symptomatic due to local mass effect or seizures or progress towards rupture. The case fatality of aneurysmal subarachnoid hemorrhage (SAH) due to a ruptured aneurysm remains 25–50%. The poor prognosis and moreover the existing uncertainty regarding the natural history of UIAs continues to motivate researchers to explore mechanisms of aneurysm formation and progression as well as to better understand chronological development of UIAs. Here, we review the existing knowledge in this respect.
Aneurysm formation
Under physiological conditions cerebral arteries consist of three layers (from inside to outside): A) the intima with the basal membrane and endothelial cells, B) the media, consisting of circumferentially oriented smooth muscles cells, embedded into a dense network of collagen and elastin fibers that enable compliance and C) the adventitia, which mainly consists of collagen, providing the structural integrity of the vessel wall. 8 Importantly, the intima and media are separated by the internal elastic lamina, which is believed to be the key structure that needs to degenerate in order to lead to aneurysm formation on the structural level. 8 Intracranial blood vessels are somewhat different, when compared to extracranial vessels, because of their thicker internal elastic lamina, decreased proportion of elastin fibers and smooth muscle cells in the media and the thinner adventitia. 9 This together with the lower amount of connective tissue within subarachnoid space itself may make cerebral arteries more prone to develop an aneurysm per se. Current hypotheses on cerebral aneurysm formation postulate the following mechanisms. First, initial apoptosis in vascular smooth muscle cells within the vessel wall and disruption of the internal elastic lamina. Second, collagen fiber reconstitution due to the resulting shift in tensile forces leads to subsequent collagen and/or elastin degradation as well as their break-down leading to vessel wall remodeling. 10, 11 Whether this remodeling will then result in aneurysm sack stabilization, progression or even rupture is assumed to be dependent on the degree of aneurysm wall inflammation, hemodynamic stress due to cardiovascular risk factors (e.g. hypertension or nicotine consumption) and remodeling/turnover of tissue and collagen in the aneurysm wall. 10, 12–14 The relevance of the inflammatory component in the pathogenesis of aneurysm progression and rupture is becoming increasingly evident in more recent studies. 15–21 The hypothesis that the incidence and degree of an inflammatory reaction may determine aneurysm rupture more strongly, as opposed to aneurysm size or location, is supported by the fact that the induction and progression of experimental cerebral aneurysms can be significantly reduced following administration of anti-inflammatory drugs in vivo. 22, 23 Additionally, there seems to be a protective effect of anti-inflammatory drugs with respect to cerebral aneurysm rupture in patients with UIAs. 24, 25
Predisposing factors to develop a cerebral aneurysm
A genetic predisposition to develop a CA must be assumed due to increased familial occurrence and association with hereditary conditions (e.g. autosomal dominant polycystic kidney disease) but a specific candidate gene linked to development of cerebral aneurysms has not yet been identified. 26 Genome-wide linkage studies in cohort with familial aneurysms have led to identification of several loci on chromosomes, which were strongly linked to CAs in patients with familial CAs: 1p34.3-p36.13, 19q13.3, Xp22 and 7q11. 4, 27, 28 Future studies on positional candidate genes from these regions may then help to identify people at increased risk of developing a CAs, within the general population. 26 The gene with robust evidence of linkage is located on 7q11, which is close to the elastin gene, and thus associated with structural integrity of the arterial wall. 29, 30 7q11 also contains the gene for collagen type 1 A2, which also fundamentally contributes to the integrity of the vascular wall. 30 Nevertheless, the present data remains to be validated with larger sample sizes in ethnicity diverse populations. 30, 31
Interestingly, about 20% of those patients harboring an UIA have a positive family history for a ruptured or unruptured CA. 32, 33 Conversely, the prevalence of UIAs in families with at least two members with cerebral aneurysms is as high as 19.1%, compared to 2–3% in the general population. 2, 34 Aside from patient age and duration of coexistent hypertension, the incidence of aneurysms in these patients is markedly associated with the amount of nicotine consumption and gender: Individuals with current nicotine consumption within families with a positive history for intracranial aneurysms, had 3-fold risk of harboring an UIA, compared to patients without nicotine consumption. Further, the odds for a positive UIA screening were twice as high in females (OR 2.46). 34 This underlines that in addition to a familial predisposition, modifiable risk factors are strong determinants for whether or not an aneurysm is formed throughout time and that serial radiological screening in such patient cohorts may be warranted. Nevertheless, the complex pathophysiology of cerebral aneurysm formation remains incompletely understood, since our current knowledge in this respect is mainly derived from in-vivo - or mathematical- models or observational studies. 12, 14, 35–40
Aneurysm growth and Progression intervals
De novo formation and growth or progression of cerebral aneurysm in serial imaging are important surrogates for instability of an UIA. 7, 41 Here, more knowledge is important to better understand the natural history of UIAs but somewhat difficult to obtain, as the majority of present data is derived from short-term follow-up studies, mostly in patients who already had a SAH from a different aneurysm; 14, 36, 37, 39, 40, 42–44 Additionally, this data is somewhat biased as a) patients with previous SAH are more prone to develop another aneurysm or even SAH, are b) usually younger and c) more likely to have hypertension or nicotine as a risk factors, compared to the general population. 36 Irrespective of this potential bias, the currently assumed annual rate of the novo aneurysm formation ranges from 0.3–1.8% in these populations. 36, 39, 40, 42, 44 The most relevant risk factors for de novo aneurysm formation in these cohorts were female gender, nicotine consumption, aneurysm multiplicity, patient age and longer follow-up duration. 43 The annual incidence of aneurysm growth in previous studies ranged from 1.51–22.7%. 43 In addition to aforementioned risk factors for aneurysm formation, an important risk factor for aneurysm growth is aneurysm size per se. Here, the cut-off diameter sizes for increased risk of aneurysm growth ranged from 5 to 10mm. 43 For UIAs there is data suggesting rather inconstant, non-linear aneurysm growth. Using population-based SAH incidence rates, different mathematical simulation models were applied to investigate aneurysm growth rate and it was concluded that aneurysms are unlikely to grow at constant, time-independent rates. Further, periods of aneurysm growth seem to be much shorter and less frequent than periods without such growth, as only 1 in 4 persons was likely to display aneurysm growth over 6.7 years. 37, 40 Nevertheless, the rate of de novo aneurysm formation and aneurysm growth in the general population may or may not be distinctly higher as in SAH patient cohorts but the chronological development of aneurysms has been difficult to estimate because of the lack of data from serial imaging in such populations. 36 However, more recently, we reported the feasibility to analyze chronological development and/or turn-over in human aneurysmal tissue in a pilot series using radiocarbon birth dating. 45
Accelerator mass spectrometry to measure chronological tissue turn-over
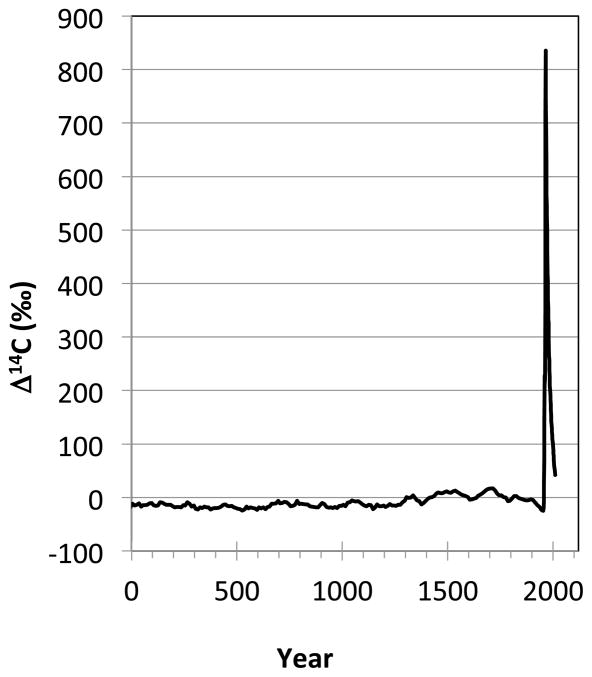
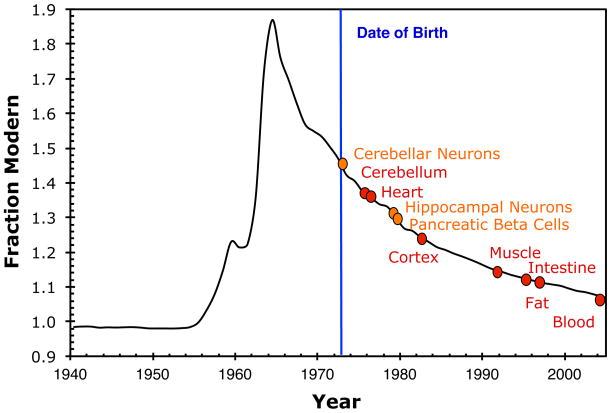
Accelerator mass spectrometry (AMS) is a technique for measuring part per trillion levels of rare long-lived radioisotopes such as 14C. 46, 47 AMS measures traces of anthropogenic and naturally occurring 14C in proteins to measure the time at which the protein was formed. 14C is produced naturally by the interaction of cosmic radiation and 14N in the atmosphere. The systematic radioactive decay of 14C (radioactive half-life T1/2=5730 y) is utilized in traditional radiocarbon dating. Natural 14C production has varied only slightly over the past 4000 years (FIGURE 1A). 48 Above ground nuclear testing produced a sharp and global increase of atmospheric 14C levels between 1955 and 1963. 49–52 This excess is often referred to as the radiocarbon bomb pulse. Whether a result of natural or anthropogenic processes, newly produced 14C in the atmosphere is rapidly oxidized to 14CO2 and enters the food chain as 14CO2 and is incorporated into the biosphere. After the ban on above ground nuclear testing in 1963, the atmospheric 14C levels have exponentially dropped, not because of radioactive decay but as a result of diffusion and equilibration of 14C with the biosphere and oceans. The consumption of plants and animals that live off plants leads to 14C levels in the human body parallel to those in the atmosphere. 53–55 14C can be measured to determine the age of any biomolecule using AMS, provided that the sample’s purity is high. The levels of 14C in proteins reflect the atmospheric 14C levels at the time at which the protein was formed, which corresponds to the protein age that is then used to calculate turnover rate that protein. 56, 57 Importantly, AMS counts atoms and not radioactive decay, which is more efficient and faster. 46 The measurement precision is 0.2–0.8%, which corresponds to a chronological uncertainty of ± 1–3 years in recent years. 57 Cell birth dating has been performed by measuring the 14C content of DNA for different human tissue types or cells, such as neurons, adipocytes, cardiomyocytes and beta cells (Figure 2). 58–63 Proteins and lipids have been dated to assess turnover and growth of pathological structures. 56, 57, 64
Figure 1.
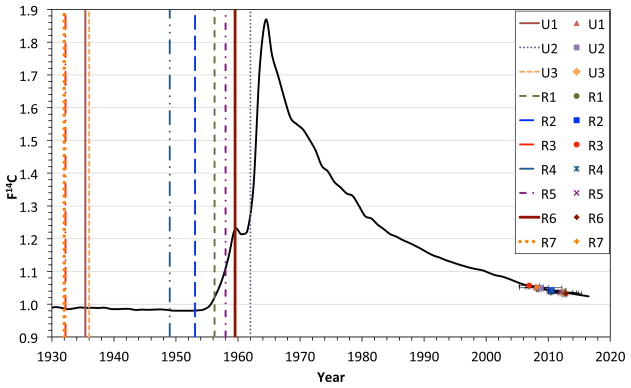
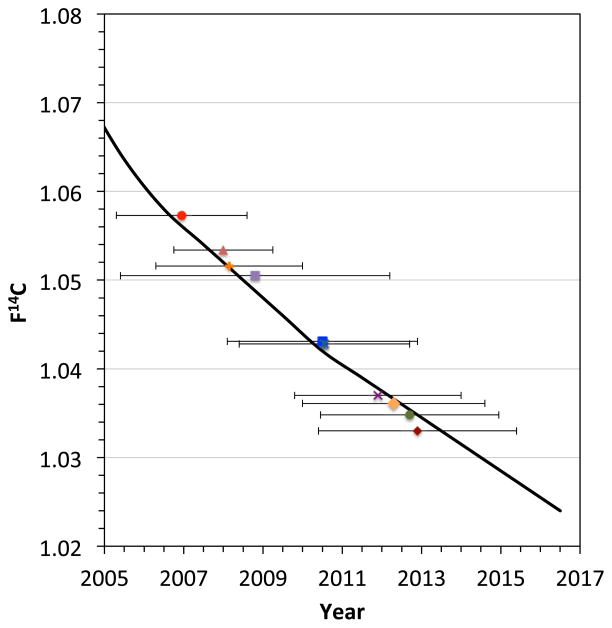
Original figure from the publication “Exploring the age of intracranial aneurysms using carbon birth dating: Preliminary Results” by Etminan et al. 45 A) Atmospheric 14CO2 levels have been essentially stable over the past 2000 years, except for a large increase between 1955–1963 due to above ground nuclear tests.. B, C) The collagen samples of each individual patient were placed on the 14C record with projected concentrations to 2017 expressed in the F14C nomenclature, which does not correct for radioactive decay and illustrates above ground 14C levels over the past 85 years. 48–50, 66 The patients’ birth dates (vertical lines) and the aneurysm collagen date ranges (horizontal lines) are illustrated for ruptured (R) and unruptured (U) samples.
Figure 2.
The average age of cells can be determined from the 14C/C content of the DNA of the cell population. The age of tissues (red) and specific cell types (orange) are depicted for a human subject who was born in 1973 (vertical line) and died in 2005 based on published turnover rates..59, 61–63, 67, 68
14C birth dating of cerebral aneurysms
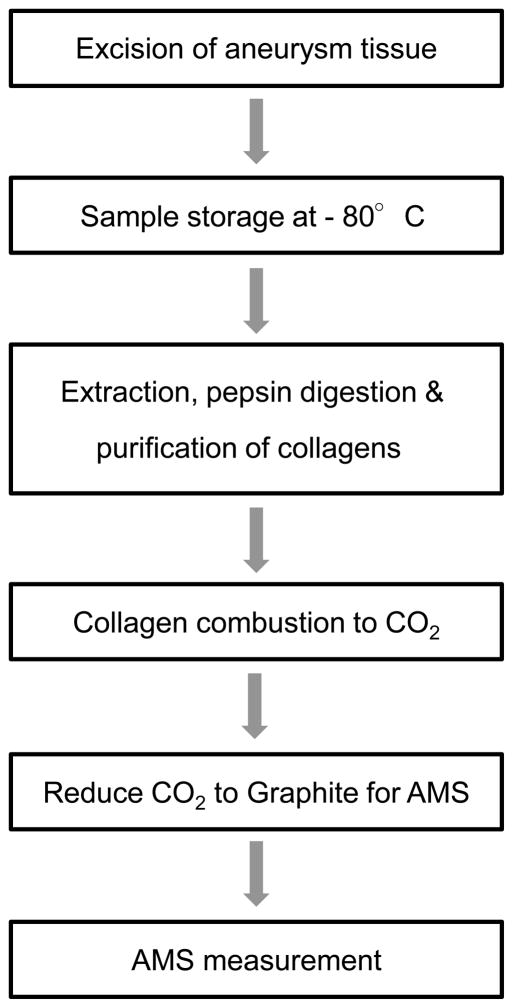
Radiocarbon birth dating of cerebral aneurysms has the following prerequisites: A) sufficient amount of aneurysm tissue, B) a specific molecule representative of aneurysm age, and C) a specific biomolecule or class of molecules that can be separated to high purity with robust yield.45 Based on these prerequisites, collagen is the best molecule for aneurysm dating since it constitutes the main component of the aneurysmal mass and can be isolated and purified after limited digestion of tissues with pepsin. Furthermore, collagen is routinely harvested from bone for traditional radiocarbon dating, so techniques to produce high purity collagen free of carbon contamination suitable for AMS analyses are known. 65 Following surgical repair of ruptured or unruptured aneurysms, we excised aneurysm domes and froze them at −80°C (see Figure 3). The aneurysms were on average larger than 4–5mm because it is difficult to harvest tissue from smaller aneurysms after aneurysm clipping and also in order to ensure sufficient amounts of collagen. For the 14C birth dating of the aneurysm tissue using AMS, we isolated and purified mixtures of collagen types I and V from the aneurysmal sack based on the modified Longin method. 65 In a first step, we analyzed the samples for content and purity using SDS-PAGE electrophoresis and verified that the main proteins after pepsin digestion and purification of the aneurysms were collagen types I and V (not shown). In a second step, collagen samples were lyophillized. Collagens were also isolated from tendons from new born mice and served as controls to ensure that significant carbon contamination was not added during this procedure.
Figure 3.
Sequence of sample processing for birth dating on aneurysm collagen.
AMS sample preparation and measurement procedures vary slightly among AMS facilities but all employ the same general process of combustion to CO2 followed by reduction to elemental carbon (graphite) when measuring solid samples. Dried collagen samples are transferred to quartz combustion tubes with excess copper oxide, the tubes evacuated and sealed using a H2/O2 torch. Sealed quartz tubes are placed in an oven at 900°C for 3.5 h to combust carbon to CO2. The CO2 is cryogenically purified to remove water and reduced to elemental carbon on Fe or Co metal powder catalyst with H2. Graphite samples and sputtered with Cs+ ions and negative ions are extracted at generally 40–60 keV. Samples, isotopic standards, and controls are measured to better than 1% precision.
In a pilot study 7 ruptured and 3 unruptured aneurysms were retrieved from 9 patients following surgical clipping. The yield of collagen from the aneurysm tissue was sufficient for further analysis (mean 0.46 ± 0.31 mg) in all samples. As illustrated in figure 1, the 14C concentrations of collagen were placed on the 14C record with the projected concentrations to 2017 to determine intercept age ranges. The preliminary data suggested that aneurysm collagen was distinctly younger than the individual patient, which harbored the aneurysm, since we did not find an aneurysm sample, which was older than 5 years. If verified in a larger patient cohort, these findings suggest, that there is constant collagen turn-over in cerebral aneurysms. However, due to the small sample size, we have no knowledge whether factors such as patient age, aneurysm size and location or rupture status might determine the turnover of aneurysm. Additionally, the current method holds several limitations, as the measurement precision corresponds to chronological uncertainty up to 3 years and since we cannot estimate whether sample closer to the aneurysm neck, i.e. where aneurysms form, may actually be older. Further, we do not have any knowledge on the relationship of collagen turn-over in aneurysm and their parent arteries, since parent arteries cannot be removed in the surgeries that removed the aneurysms. Future 14C birth dating of cerebral arteries may provide some additional insight in this respect.
Nevertheless, the existing and preliminary human data on developmental chronology of aneurysms suggests that they undergo permanent structural change. This may challenge hypotheses on aneurysm development. However, at present, it remains unknown which factors may determine aneurysm turnover and how this would translate into the management of patients with unruptured intracranial aneurysms.
CONCLUSION
The pathogenesis of cerebral aneurysm formation is a multifactorial and incompletely understood process. At present, it remains unknown what the actual cause for the initial disruption of the elastic internal lamina in the arterial vessel wall is and whether or not this process occurs early or later throughout the patients lifetime. However, the current knowledge on chronological aneurysm development suggests that the progression of aneurysms and remodeling of aneurysm tissue is a discontinuous but ongoing process and that cerebral aneurysm cannot be generally assumed to be stable lesions. To understand the true natural history of cerebral aneurysms, future studies should investigate determinants for their chronological formation and growth.
Acknowledgments
NE and RLM receive grant support from the Physicians Services Incorporated Foundation. RLM receives grant support from the Brain Aneurysm Foundation, Canadian Institutes of Health Research and the Heart and Stroke Foundation of Ontario. RLM is a consultant for Actelion Pharmaceuticals and Chief Scientific Officer of Edge Therapeutics, Inc. NE, DH and RLM are scientific advisors/officers for Edge Therapeutics, Inc.
Support was also provided by NIH/NIGMS 8P41GM103483. This work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344
References
- 1.Weir B. Unruptured intracranial aneurysms: A review. J Neurosurg. 2002;96:3–42. doi: 10.3171/jns.2002.96.1.0003. [DOI] [PubMed] [Google Scholar]
- 2.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, et al. Incidental findings on brain mri in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 3.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet neurology. 2011;10:626–636. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 4.Krischek B, Inoue I. The genetics of intracranial aneurysms. Journal of human genetics. 2006;51:587–594. doi: 10.1007/s10038-006-0407-4. [DOI] [PubMed] [Google Scholar]
- 5.Morita A, Fujiwara S, Hashi K, Ohtsu H, Kirino T. Risk of rupture associated with intact cerebral aneurysms in the japanese population: A systematic review of the literature from japan. J Neurosurg. 2005;102:601–606. doi: 10.3171/jns.2005.102.4.0601. [DOI] [PubMed] [Google Scholar]
- 6.Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, et al. The natural course of unruptured cerebral aneurysms in a japanese cohort. N Engl J Med. 2012;366:2474–2482. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 7.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 8.Lasheras JC. The biomechanics of arterial aneurysms. Annu Rev Fluid Mech. 2007;39:293–319. [Google Scholar]
- 9.Fang H. A comparison of blood vessels of the brain and peripheral blood vessels. In: Wright IS, Millikan CH, editors. Cerebrovascular diseases. Grune and Stratton; New York: 1958. pp. 17–22. [Google Scholar]
- 10.Chatziprodromou I, Tricoli A, Poulikakos D, Ventikos Y. Haemodynamics and wall remodelling of a growing cerebral aneurysm: A computational model. Journal of biomechanics. 2007;40:412–426. doi: 10.1016/j.jbiomech.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Steiger HJ. Pathophysiology of development and rupture of cerebral aneurysms. Acta neurochirurgica Supplementum. 1990;48:1–57. [PubMed] [Google Scholar]
- 12.Chang HS. Simulation of the natural history of cerebral aneurysms based on data from the international study of unruptured intracranial aneurysms. J Neurosurg. 2006;104:188–194. doi: 10.3171/jns.2006.104.2.188. [DOI] [PubMed] [Google Scholar]
- 13.Chatziprodromou I, Poulikakos D, Ventikos Y. On the influence of variation in haemodynamic conditions on the generation and growth of cerebral aneurysms and atherogenesis: A computational model. Journal of biomechanics. 2007;40:3626–3640. doi: 10.1016/j.jbiomech.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Watton PN, Ventikos Y, Holzapfel GA. Modelling the growth and stabilization of cerebral aneurysms. Mathematical medicine and biology: a journal of the IMA. 2009;26:133–164. doi: 10.1093/imammb/dqp001. [DOI] [PubMed] [Google Scholar]
- 15.Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, et al. Nf-kappab is a key mediator of cerebral aneurysm formation. Circulation. 2007;116:2830–2840. doi: 10.1161/CIRCULATIONAHA.107.728303. [DOI] [PubMed] [Google Scholar]
- 16.Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, Hasan D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke. 2013 doi: 10.1161/STROKEAHA.113.002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starke RM, Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, et al. The role of oxidative stress in cerebral aneurysm formation and rupture. Current neurovascular research. 2013;10:247–255. doi: 10.2174/15672026113109990003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang HF, Zhao MG, Liang GB, Song ZQ, Li ZQ. Expression of pro-inflammatory cytokines and the risk of intracranial aneurysm. Inflammation. 2013 doi: 10.1007/s10753-013-9655-6. [DOI] [PubMed] [Google Scholar]
- 20.Hasan D, Chalouhi N, Jabbour P, Hashimoto T. Macrophage imbalance (m1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: Preliminary results. Journal of neuroinflammation. 2012;9:222. doi: 10.1186/1742-2094-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoh BL, Hosaka K, Downes DP, Nowicki KW, Fernandez CE, Batich CD, et al. Monocyte chemotactic protein-1 promotes inflammatory vascular repair of murine carotid aneurysms via a macrophage inflammatory protein-1alpha and macrophage inflammatory protein-2-dependent pathway. Circulation. 2011;124:2243–2252. doi: 10.1161/CIRCULATIONAHA.111.036061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenzel T, Lee CZ, Kim H, Quinnine NJ, Hashimoto T, Lawton MT, et al. Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: Pilot study of adverse events and tolerance. Cerebrovasc Dis. 2008;25:157–163. doi: 10.1159/000113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, et al. Pharmacological stabilization of intracranial aneurysms in mice: A feasibility study. Stroke. 2012;43:2450–2456. doi: 10.1161/STROKEAHA.112.659821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan DM, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, et al. Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: Preliminary results. Journal of the American Heart Association. 2013;2:e000019. doi: 10.1161/JAHA.112.000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasan DM, Mahaney KB, Brown RD, Jr, Meissner I, Piepgras DG, Huston J, et al. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. doi: 10.1161/STROKEAHA.111.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caranci F, Briganti F, Cirillo L, Leonardi M, Muto M. Epidemiology and genetics of intracranial aneurysms. European journal of radiology. 2013 doi: 10.1016/j.ejrad.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Grobelny TJ. Brain aneurysms: Epidemiology, treatment options, and milestones of endovascular treatment evolution. Disease-a-month: DM. 2011;57:647–655. doi: 10.1016/j.disamonth.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Krischek B, Tatagiba M. The influence of genetics on intracranial aneurysm formation and rupture: Current knowledge and its possible impact on future treatment. Advances and technical standards in neurosurgery. 2008;33:131–147. doi: 10.1007/978-3-211-72283-1_3. [DOI] [PubMed] [Google Scholar]
- 29.Onda H, Kasuya H, Yoneyama T, Takakura K, Hori T, Takeda J, et al. Genomewide-linkage and haplotype-association studies map intracranial aneurysm to chromosome 7q11. American journal of human genetics. 2001;69:804–819. doi: 10.1086/323614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruigrok YM, Rinkel GJ. Genetics of intracranial aneurysms. Stroke. 2008;39:1049–1055. doi: 10.1161/STROKEAHA.107.497305. [DOI] [PubMed] [Google Scholar]
- 31.Ruigrok YM, Rinkel GJ, Wijmenga C, Kasuya H, Tajima A, Takahashi T, et al. Association analysis of genes involved in the maintenance of the integrity of the extracellular matrix with intracranial aneurysms in a japanese cohort. Cerebrovasc Dis. 2009;28:131–134. doi: 10.1159/000223438. [DOI] [PubMed] [Google Scholar]
- 32.Kissela BM, Sauerbeck L, Woo D, Khoury J, Carrozzella J, Pancioli A, et al. Subarachnoid hemorrhage: A preventable disease with a heritable component. Stroke. 2002;33:1321–1326. doi: 10.1161/01.str.0000014773.57733.3e. [DOI] [PubMed] [Google Scholar]
- 33.Schievink WI, Schaid DJ, Michels VV, Piepgras DG. Familial aneurysmal subarachnoid hemorrhage: A community-based study. J Neurosurg. 1995;83:426–429. doi: 10.3171/jns.1995.83.3.0426. [DOI] [PubMed] [Google Scholar]
- 34.Brown RD, Jr, Huston J, Hornung R, Foroud T, Kallmes DF, Kleindorfer D, et al. Screening for brain aneurysm in the familial intracranial aneurysm study: Frequency and predictors of lesion detection. J Neurosurg. 2008;108:1132–1138. doi: 10.3171/JNS/2008/108/6/1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. Reduced collagen biosynthesis is the hallmark of cerebral aneurysm: Contribution of interleukin-1beta and nuclear factor-kappab. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1080–1086. doi: 10.1161/ATVBAHA.108.180760. [DOI] [PubMed] [Google Scholar]
- 36.Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: A long-term follow-up study. Stroke. 2001;32:485–491. doi: 10.1161/01.str.32.2.485. [DOI] [PubMed] [Google Scholar]
- 37.Koffijberg H, Buskens E, Algra A, Wermer MJ, Rinkel GJ. Growth rates of intracranial aneurysms: Exploring constancy. J Neurosurg. 2008;109:176–185. doi: 10.3171/JNS/2008/109/8/0176. [DOI] [PubMed] [Google Scholar]
- 38.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprengers ME, van Rooij WJ, Sluzewski M, Rinkel GJ, Velthuis BK, de Kort GA, et al. Mr angiography follow-up 5 years after coiling: Frequency of new aneurysms and enlargement of untreated aneurysms. AJNR American journal of neuroradiology. 2009;30:303–307. doi: 10.3174/ajnr.A1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wermer MJ, van der Schaaf IC, Velthuis BK, Algra A, Buskens E, Rinkel GJ. Follow-up screening after subarachnoid haemorrhage: Frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain: a journal of neurology. 2005;128:2421–2429. doi: 10.1093/brain/awh587. [DOI] [PubMed] [Google Scholar]
- 41.Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: Probability of and risk factors for aneurysm rupture. J Neurosurg. 2000;93:379–387. doi: 10.3171/jns.2000.93.3.0379. [DOI] [PubMed] [Google Scholar]
- 42.David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT, Partovi S. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg. 1999;91:396–401. doi: 10.3171/jns.1999.91.3.0396. [DOI] [PubMed] [Google Scholar]
- 43.Ferns SP, Sprengers ME, van Rooij WJ, van den Berg R, Velthuis BK, de Kort GA, et al. De novo aneurysm formation and growth of untreated aneurysms: A 5-year mra follow-up in a large cohort of patients with coiled aneurysms and review of the literature. Stroke. 2011;42:313–318. doi: 10.1161/STROKEAHA.110.591594. [DOI] [PubMed] [Google Scholar]
- 44.Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: Results of long-term follow-up angiography. Stroke. 2001;32:1191–1194. doi: 10.1161/01.str.32.5.1191. [DOI] [PubMed] [Google Scholar]
- 45.Etminan N, Dreier R, Buchholz BA, Bruckner P, Steiger HJ, Hanggi D, et al. Exploring the age of intracranial aneurysms using carbon birth dating: Preliminary results. Stroke. 2013;44:799–802. doi: 10.1161/STROKEAHA.112.673806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogel JS, Love AH. Quantitating isotopic molecular labels with accelerator mass spectrometry. Methods in enzymology. 2005;402:402–422. doi: 10.1016/S0076-6879(05)02013-6. [DOI] [PubMed] [Google Scholar]
- 47.Vogel JS, Turteltaub KW, Finkel R, Nelson DE. Accelerator mass spectrometry. Analytical chemistry. 1995;67:353A–359A. doi: 10.1021/ac00107a001. [DOI] [PubMed] [Google Scholar]
- 48.Stuiver M, Reimer PJ, Braziunas TF. High-precision radiocarbon age calibration for terrestrial and marine samples. Radiocarbon. 1998;40:1127–1151. [Google Scholar]
- 49.Graven HD, Guilderson TP, Keeling RF. Observations of radiocarbon in co2 at la jolla, california, USA 1992–2007: Analysis of the long-term trend. J Geophys Res-Atmos. 2012:117. [Google Scholar]
- 50.Hua Q, Barbetti M. Review of tropospheric bomb c-14 data for carbon cycle modeling and age calibration purposes. Radiocarbon. 2004;46:1273–1298. [Google Scholar]
- 51.Levin I, Hammer S, Kromer B, Meinhardt F. Radiocarbon observations in atmospheric co2: Determining fossil fuel co2 over europe using jungfraujoch observations as background. The Science of the total environment. 2008;391:211–216. doi: 10.1016/j.scitotenv.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Levin I, Naegler T, Kromer B, Diehl M, Francey RJ, Gomez-Pelaez AJ, et al. Observations and modelling of the global distribution and long-term trend of atmospheric 14co2. Tellus. 2010:26–46. [Google Scholar]
- 53.Harkness DD. Further investigations of the transfer of bomb 14 c to man. Nature. 1972;240:302–303. doi: 10.1038/240302a0. [DOI] [PubMed] [Google Scholar]
- 54.Harkness DD, Walton A. Carbon-14 in the biosphere and humans. Nature. 1969;223:1216–1218. doi: 10.1038/2231216a0. [DOI] [PubMed] [Google Scholar]
- 55.Libby WF, Berger R, Mead JF, Alexander GV, Ross JF. Replacement rates for human tissue from atmospheric radiocarbon. Science. 1964;146:1170–1172. doi: 10.1126/science.146.3648.1170. [DOI] [PubMed] [Google Scholar]
- 56.Lovell MA, Robertson JD, Buchholz BA, Xie C, Markesbery WR. Use of bomb pulse carbon-14 to age senile plaques and neurofibrillary tangles in alzheimer’s disease. Neurobiology of aging. 2002;23:179–186. doi: 10.1016/s0197-4580(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 57.Stewart DN, Lango J, Nambiar KP, Falso MJ, FitzGerald PG, Rocke DM, et al. Carbon turnover in the water-soluble protein of the adult human lens. Molecular vision. 2013;19:463–475. [PMC free article] [PubMed] [Google Scholar]
- 58.Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478:110–113. doi: 10.1038/nature10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P, et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 61.Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. The Journal of clinical endocrinology and metabolism. 2010;95:E234–239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 63.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 64.Hagg S, Salehpour M, Noori P, Lundstrom J, Possnert G, Takolander R, et al. Carotid plaque age is a feature of plaque stability inversely related to levels of plasma insulin. PloS one. 2011;6:e18248. doi: 10.1371/journal.pone.0018248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown TA, Nelson DE, Vogel JS, Southon JR. Improved collagen extraction by modified longin method. Radiocarbon. 1988;30:171–177. [Google Scholar]
- 66.Reimer PJ, Brown TA, Reimer RW. Discussion: Reporting and calibration of post-bomb c-14 data. Radiocarbon. 2004;46:1299–1304. [Google Scholar]
- 67.Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]