Abstract
Objective
Undercarboxylated osteocalcin (ucOC) is a bone marker with potent metabolic effects. Leptin regulates Esp gene expression and osteocalcin carboxylation in animal models. We aim to elucidate day/night patterns of ucOC levels, whether short-term and/or chronic energy deprivation alter ucOC levels, and whether leptin may mediate these changes in humans.
Design/Methods
Twelve healthy males and females were studied for 72 hours in the fed state to study day/night pattern of ucOC. The six female subjects were also studied in a crossover interventional study in the fasting state for 72 hours with administration of either placebo or metreleptin in physiological doses. Blood samples were obtained hourly from 0800 a.m. on day 3 until 0800 a.m. on day 4. In a separate study, eleven obese subjects who underwent bariatric surgery were followed for 24 weeks to examine effects of post-surgery weight loss on ucOC levels.
Results
Males have higher ucOC levels compared to females. There is no day/night variation pattern of circulating ucOC in humans. Short-term and chronic energy deprivation or leptin administrations do not alter ucOC levels.
Conclusions
The hypothesis that ucOC plays a role in energy homeostasis or of leptin in regulating ucOC in humans is not supported.
Key terms: undercarboxylated osteocalcin, energy homeostasis, bariatric surgery, physiology
INTRODUCTION
Osteocalcin (OC), traditionally a marker of bone formation, is an osteoblast-secreted protein which undergoes gamma-carboxylation of glutamic acid residues to produce carboxylated OC [1]. Undercarboxylated OC (ucOC) includes all OC forms with carboxylation of less than three glutamic acid residues and is the biologically active form of osteocalcin. High serum ucOC levels are associated with increased risk of hip fracture [2], and various studies have suggested that carboxylation of OC may be related to bone quality [3, 4]. Recent studies have highlighted an association between bone health and metabolic regulation, underscoring the existence of a cross talk in the complex interactions between bone remodeling, energy expenditure, beta-cell regulation and adipocytes [5, 6]. Esp, a gene encoding an intracellular tyrosine phosphatase called OST-PTP, through its osteoblastic expression, exerts metabolic functions opposite to those of OC. Genetic and biochemical studies demonstrate that Esp acts upstream of OC to inhibit its metabolic function [7]. Lee et al. demonstrated that mice over expressing the Esp gene, leading to decreased osteoblast-secreted osteocalcin, displayed decreased β-cell proliferation, decreased adiponectin expression in adipocytes, and increased glucose intolerance and insulin resistance [7]. Administration of recombinant ucOC to wild-type mice on the other hand enhanced insulin production and sensitivity, and reduced the deleterious effect of a high fat diet on weight gain and glucose intolerance [8], demonstrating novel therapeutic potential of ucOC. In humans, a cross-sectional study in a cohort of men with type 2 diabetes mellitus found an inverse association between ucOC and various markers of metabolic profiles, including percent trunk fat, visceral to subcutaneous fat ratio, fasting plasma glucose, and glycated hemoglobin (HbA1c) [9]. A recent study investigating the changes in ucOC induced by osteoporosis treatment reported an association between changes in the ucOC levels and subsequent changes in various metabolic indices including body weight, fat mass and adiponectin [10]. These studies highlight a potential metabolic role of ucOC in humans.
Leptin, an adipocytokine with key roles in both signaling energy availability and bone remodeling [11], decreases quickly and out of proportion to changes in fat mass in energy deprivation [12]. Ducy et al. showed that leptin indirectly inhibits bone formation through a central pathway involving the hypothalamus and central nervous system [13]. Hinoi et al. demonstrated that leptin up-regulates the Esp gene, consequently leading to a decrease in ucOC levels via its central serotonin-mediated stimulation of sympathetic output, providing further evidence that adipose tissue, via the effect of leptin, regulates circulating levels of ucOC and bone metabolism [14].
Despite these studies demonstrating the role of ucOC in energy homeostasis and bone metabolism, the physiology of ucOC in energy homeostasis and its potential regulation by leptin in humans is unknown. In this study, we first investigated whether a biological rhythm of ucOC exist in both men and women and then we examined the physiology of ucOC in the energy-repleted and the energy-deprived state and also whether ucOC is regulated by leptin. We also investigated, using subjects with significant weight loss after bariatric surgery as a model of chronic energy deprivation, whether ucOC may be altered in response to post bariatric surgery weight loss.
SUBJECTS, MATERIALS AND METHODS
The study consisted of 3 arms as outlined in details below. Study A: Samples of 6 male non-diabetic subjects were recruited to evaluate biological rhythm of ucOC in the fed state. Study B: Samples of 6 female non-diabetic subjects were recruited to evaluate the biological rhythm of ucOC in 3 states, namely baseline fed state, fasting state with placebo administration and fasting state with leptin replacement. For both Study A and B, these were healthy individuals recruited from the Boston area using advertisement and postings through the Boston area Universities Students Offices. Study C: A chronic energy deprivation study with ucOC measured before and after significant weight loss with bariatric surgery in 11 obese subjects.
The study protocols were approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center (BIDMC), and written informed consent were obtained from all the subjects in all 3 components of the study. Clinical-quality r-metHuLeptin was supplied by Amgen, Inc. (Thousand Oaks, CA) and administered under an investigational new drug application submitted to the Food and Drug Administration (FDA).
Study A. UcOC Biological Rhythm in Males
Six healthy, lean men (mean age 21.8 ± 2.3 years; mean BMI 21.2 ± 1.6 kg/m2) were admitted to the BIDMC General Clinical Research Center (GCRC) and studied in the isocaloric fed state for 4 days. Subjects were given a standardized isocaloric diet with breakfast at 0800 a.m., lunch at 0100 p.m., dinner at 0600 p.m., and a snack at 1000 p.m. daily. Caloric intake was distributed with 20% of calories from breakfast, 35% from lunch, 35% from dinner, and 10% from the evening snack. Subjects were allowed ad libitum physical activities which were not different from their usual activities in the outpatient settings. All light/dark intervals and blood sampling schedule were standardized. On day 3, blood was drawn through an indwelling intravenous catheter, every 15 minutes from 0800 a.m. on Day 3 till 0800 a.m. on day 4, and then pooled every hour to meet the assays’ sample volume requirements.
Study B. UcOC Biological Rhythm and Effects of Short Term Energy Deprivation and Leptin Replacement in Females
Six healthy, lean and eumenorrheic women (mean age 22.8 ± 3.4 years; mean body mass index (BMI) 21.7 ± 2.2 kg/m2) were admitted to the BIDMC General Clinical Research Center (GCRC) and studied in the isocaloric fed state for 4 days in the first admission. In the following two admissions, the subjects were studied in the fasting state for 72 hours and were randomized to receive either placebo or metreleptin at replacement doses. A cross over to the opposite arm took place in the later admission so that all 6 subjects received both placebo and metreleptin. In the fasting studies, subjects received only caffeine-free and calorie-free liquids for 3 days, which included NaCl (500 mg), KCl (40 meq). The study admissions were separated by at least 8 weeks to enable adequate washout and recovery of metabolic status. All subjects had a regular menstrual cycle and were not on any medications including oral contraceptive pills. Study visits were standardized to occur between the 6th and 11th day of their menstrual cycle [15].
In the fed state study, dietary regimen was similar as for the male fed study. In the fasting with leptin admission, starting at 0800 a.m. on day 1 of the fasting/leptin admission, metreleptin was administered as a subcutaneous injection every 6 hours for 2 days, at a dose of 0.08 mg/kg/day on day 1 and 0.2 mg/kg/day on day 2 and 3, on the basis of prior pharmacokinetic studies [16–18]. During the fasting + placebo admission, a buffer solution of similar volume was administered subcutaneously every 6 hours, similar to the leptin arm. All activities, light/dark interval, and blood sampling schedule on day 3 to day 4 for all 3 studies were similar as for the male fed state study.
Study C. Chronic Energy Deprivation Study
11 obese subjects (6 males, 5 females, mean age 54.6 ± 7.1 years, mean BMI 50.95 ± 11.97 kg/m2) who were approved for and underwent bariatric surgery at BIDMC were prospectively enrolled in this study as a model of chronic energy deprivation. 4 out of 11 subjects were diabetics. 5 out of 11 subjects underwent a Roux-en-Y gastric bypass surgery (RYGB), while the remaining subjects underwent laparoscopic gastric banding (LGB). Subjects were assessed before surgery, at 8 weeks, 12 weeks and 24 weeks after surgery. Demographic characteristics and medical history review were obtained at the pre-surgery visit. Metabolic parameters, anthropometric and body composition measurements were obtained at the pre-surgery visit and at all subsequent follow up visits. Fasting bloods were drawn at baseline and at each visit for metabolic evaluations including glucose, lipid profile and ucOC measurement.
Assays
Serum ucOC was measured by ELISA (Takara Bio Inc, Shiga, Japan) with a sensitivity of 0.25 ng/ml, intra-assay CV of 4.58 – 6.66 % and inter-assay CV of 5.67 – 9.87 %, in accordance with the manufacturer’s instructions. Leptin and insulin levels were measured as previously reported [19]. Biochemical analysis for serum calcium was assayed on the Roche Cobas c311 (Roche Diagnostics, Indianapolis, IN). 25-hydroxyvitamin D was measured by radioimmunoassay (Diasorin, Stillwater, MN). All serum samples were stored at −80 °C as appropriate until analysis. All samples were analyzed in duplicate.
Statistical Analysis
Statistical analysis was performed with Stata version 12 (Stata Corp., College Station, TX) and trigonometric regression was performed with SigmaPlot version 12 (Systat Software, Inc., San Jose, CA). Variables are summarized as mean ± standard deviation. Normality of variables was evaluated visually with frequency histograms and the Shapiro-Wilks statistic.
Study A and B
To evaluate for potential day night variability, we performed trigonometric, 4-parameter, sine regression on ucOC levels across the day and the coefficient of determination (R2) was calculated. Comparison of ucOC levels between male and female subjects in the isocaloric fed state was performed using linear mixed-effect models. A similar approach was utilized for the comparison of ucOC levels in female subjects across the three conditions (isocaloric fed state, fasting + placebo and fasting + leptin replacement state). UcOC was modeled as a linear function of time and both fixed and random intercepts and slopes for “gender” and “condition” were introduced in the level-2 model. The final model was selected to minimize both Akaike’s and the Bayesian Information Criteria. 24-hour area under the curve (AUC) of ucOC was calculated with the trapezoidal rule. Comparison of ucOC AUC between the two genders was performed with Student’s t-test.
There are no human studies available to date, evaluating the changes of ucOC circulating levels during acute or chronic energy deprivation, thus there are no available data to perform exact power calculations. Nonetheless, this sample size has been demonstrated to be adequate in detecting periodic secretion patterns of other hormones [20–22]. In the short term energy deprivation study, the cross-over design used herein provided 80% power to detect a difference of 1.4 SD between fed and fasting plus placebo states with six subjects at the conventional alpha=0.05 level.
Study C
Baseline associations between total cholesterol, low-density lipoprotein (LDL), triglycerides (TG), high-density lipoprotein (HDL), fasting insulin, fasting glucose, HbA1c, body mass index (BMI), fat mass and ucOC were evaluated with Spearman’s correlation coefficient. Comparison of ucOC levels across the various time points after bariatric surgery was performed with repeated-measurements analysis of variance. In addition repeated measurements 2×2 factorial ANOVA was also performed to evaluate for potential differences between the different groups of bariatric surgery, diabetic status and the two genders. Results are presented as means ± standard deviation (SD). All tests were two-sided and the alpha-criterion was set to 0.05. In the bariatric surgery induced, chronic energy deprivation study, 11 subjects provided 80% power to detect a difference of 0.81 SD between baseline and six months after the surgery.
RESULTS
Study A. UcOC Biological Rhythm in Males
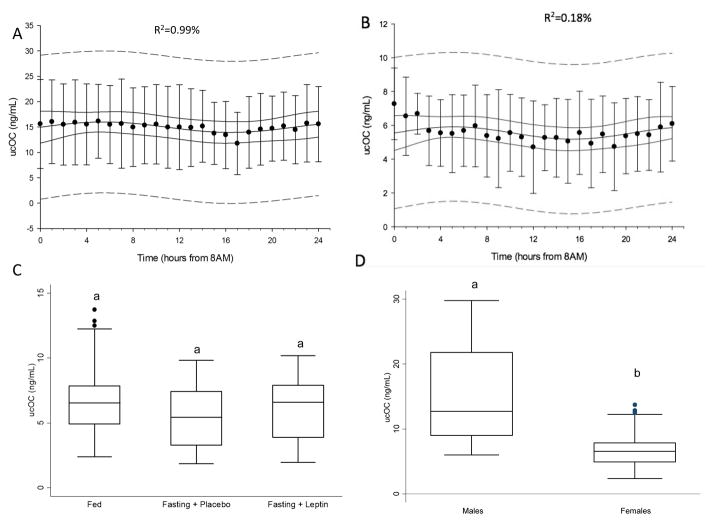
Trigonometric regression failed to demonstrate the existence of any statistically or clinically significant day/night variation in males (R2=0.99%) in the isocaloric fed state (Figure 1A). Clinically important day/night secretion patterns have traditionally been associated with adjusted coefficients of determination higher than 15%. Previous studies from our group and others have demonstrated that hormones with profound day/night secretion pattern, such as cortisol, yield an adjusted R2 of more than 30% in similar statistical models [23].
Figure 1.
A. Mean undercarboxylated osteocalcin (ucOC) levels (ng/mL) in the fed state demonstrating absence of day/night variation in males (n=6). Adjusted R2 is displayed at the top center. Solid line represents 95% confidence interval, interrupted line represents 95% prediction interval; B. Mean undercarboxylated osteocalcin (ucOC) levels (ng/mL) in the fed state demonstrating absence of day/night variation in females (n=6). Adjusted R2 is displayed at the top center. Solid line represents 95% confidence interval, interrupted line represents 95% prediction interval; C. Box plots of ucOC levels in all three states in females, demonstrating no change in levels in response to fasting and leptin replacement; no statistical significant difference was detected at the 0.05 level (n=6); D. Box plots of ucOC levels in fed state, signifying higher levels in males compared to females, different letters signify statistically significant difference at the 0.05 level.
Study B: UcOC Biological Rhythm and Effects of Short Term Energy Deprivation and Leptin Replacement in Females
Trigonometric regression failed to demonstrate the existence of any statistically or clinically significant day/night variation in the females (R2=0.18%) similar to the males, while the subjects were in the isocaloric fed state (Figure 1B). In the fasting + placebo and fasting + leptin study, ucOC again did not demonstrate any significant day/night variation. Mean serum leptin and insulin levels decreased significantly (14.66 to 2.78 ng/ml, p<0.001 and 6.06 to 1.22 uIU/ml, p=0.001 respectively) after 3 days of fasting. Leptin replacement restored leptin levels to normal physiological range as previously reported [19]. 24-hour mean ucOC levels in females were 6.99 ± 0.90 ng/ml. Neither short-term energy deprivation nor leptin administration had any significant effect on ucOC levels (p=0.47 and p=0.69 accordingly) (Figure 1C).
Comparison between male and female subjects in the fed state found that females had lower mean ucOC levels compared to males (−8.49±2.99 ng/mL, p=0.005) (Figure 1D). The slope of the trajectories of ucOC levels during the day was not different from zero, for both males and females meaning that the difference between male and female ucOC levels was, on average, constant throughout the day. Integrated daily ucOC secretion, as estimated by AUC, was also significantly lower in females compared to males (166.19 ± 65.74 vs. 376.26 ± 74.97, p=0.0345). These differences remained significant after adjusting for BMI (p<0.001). Both 24-hour mean ucOC levels and ucOC AUC were highly correlated with 0800 a.m. ucOC levels (beta=0.96, p<0.001 and beta=0.96, p<0.001 respectively).
Study C. Chronic Energy Deprivation Study
Baseline ucOC levels were positively associated with the baseline fasting glucose levels (rho = 0.88, p=0.004) but not with baseline BMI, fat mass, fasting insulin, total cholesterol, LDL, HDL or TG levels (data not shown). Baseline ucOC levels similarly showed no association with serum calcium levels (p=0.87) and 25-hydroxyvitamin D levels (p=0.97). Mean BMI of all 11 subjects decreased significantly 24 weeks after bariatric surgery (50.95 ± 11.97 to 40.80 ± 9.56 kg/m2, p<0.001). While mean serum leptin levels for these subjects decreased significantly 24 weeks post bariatric surgery (50.91 ± 28.06 to 27.26 ± 21.23, p=0.018), we failed to demonstrate any statistically or clinically significant change in ucOC levels any time point up to 24 weeks after bariatric surgery (Huynh Feldt corrected p-value = 0.23). In accordance to this, we demonstrated that changes in BMI, weight, fat mass, fasting insulin, and glucose levels after bariatric surgery were not related to any changes in ucOC levels (data not shown). Similarly there were no relationship between changes in ucOC levels after bariatric surgery and changes in calcium (p=0.42) or 25-hydroxyvitamin D (p=0.43) up to 24 weeks after surgery. There was no difference in BMI reduction between the two types of bariatric surgery or the two genders, or with their diabetic status, as evaluated with the factorial ANOVA models. Given these results and since our hypothesis was whether energy deprivation could affect ucOC levels, we did not expect surgery type, diabetic status and gender to affect ucOC levels. Indeed, the factorial ANOVA model evaluating for potential differential changes of ucOC levels between genders, diabetic status and both types of surgery confirmed no statistically significant differences.
DISCUSSION
Bone remodeling is an energy requiring process. In recent years, studies in animal models that have highlighted a promising role of ucOC in the cross talk between bone metabolism and energy homeostasis. Lee et al., using a genetically altered mouse model, demonstrated that deletion of Esp gene in mice, leading to higher levels of ucOC compared to wild type mice, is characterized by increased insulin sensitivity, greater islet cell mass and beta cell proliferation and these mice are hypoglycemic and protected from obesity [7]. Ferron et al. demonstrated that recombinant ucOC administered to mice increases beta cell proliferation, increases adiponectin release from fat cells and improves glucose tolerance in vivo [8], confirming the mechanistic role of ucOC in mediating these metabolic effects. The Esp gene expression is expressed in osteoblasts and Sertoli cells of the testes and is up-regulated in differentiating osteoblasts specifically by leptin through sympathetic signaling. These findings showed for the first time how the bone, via the action of leptin and its influence on ucOC regulation, exerts an effect on beta cell function and energy homeostasis. As promising as these animal data may be, there is no data currently to clarify if a similar relationship between ucOC, leptin and energy homeostasis exists in humans. Obesity, especially visceral adiposity, is an inflammatory disease, where the adipose tissue is able to produce inflammatory cytokines or collaborate with their production by other tissues. Hyperleptinemia, a state exhibited in obesity, is well known to correlate with inflammatory responses observed in obesity [24]. Similarly, serum osteocalcin has also been demonstrated to be inversely associated with glucose, insulin sensitivity, BMI and inflammatory markers such as C reactive protein and interleukin 6 [25]. Despite both their roles in obesity and chronic inflammation, there are again no studies investigating whether an interaction exists between leptin and ucOC in humans.
In this full physiology study, we report that there is no day/night variation in ucOC levels in both young, healthy males and females, and that energy deprivation of up to 72 hours in young healthy females resulting in hypoleptinemia, does not alter ucOC levels. Furthermore, despite the well-described effect of leptin on the Esp gene expression and ucOC levels in rodents, replacement of leptin to physiological levels in energy-deprived hypoleptinemia does not alter circulating ucOC levels, indicating a lack of interaction between ucOC and leptin in humans. Furthermore, in post-bariatric surgery subjects with significant weight loss leading to hypoleptinemia, there are no significant changes in ucOC levels up to 24 weeks post surgery. These results suggest that, contrary to animal models, circulating ucOC levels are not regulated by leptin and may not be playing a critical role in energy homeostasis in humans. We further observe that males have a higher circulating level of ucOC compared to females and that a single 0800 a.m. overnight fasting sample of ucOC is an accurate reflection of the overall circulating levels of ucOC over a 24-hour period. These novel physiological observations are critical for future research studies on ucOC in humans.
The lack of day/night variation of ucOC herein are in contrary to the study by Sokoll et al. [26], who reported in 1998 the presence of a diurnal variation of ucOC in both young healthy males and females. However Sokoll et al. utilized a radioimmunoassay developed in-house which had not gained widespread use in the measurement of ucOC, especially not in used with the recent wealth of human studies demonstrating an association of ucOC with various metabolic indices. This current study uses a well-validated assay which is similarly in extensive use by these recent human studies, enabling extrapolating of these observations to this study. Moreover, the Sokoll et al. study only has blood sampling every 4 hours which may not have adequate temporal resolution to detect day/night variation pattern of secretion. Furthermore, repeated measures analysis of variance (ANOVA), the statistical methods utilized in their study to assess day/night variation, may not be rigorous enough in ruling out a day/night variation pattern of secretion. In contrast, besides the higher temporal resolution of hourly blood sampling in this study, we utilized trigonometric regression, a rigorous and well-validated test [23], in assessing day/night variation pattern of ucOC, allowing us to confidently report the absence of day/night variation pattern of ucOC described herein.
The muted role of ucOC in energy homeostasis in humans as opposed to animal studies could be due to several factors. Firstly, the Esp gene is a pseudo gene in humans, and a functional homologue for the Esp gene has not been identified in humans thus far [27]. Therefore the effect of leptin on ucOC via its effect on Esp gene expression may not be similar in humans. Besides, studies in rodents often involved dramatically altered levels of ucOC, whereas physiological human studies such as the present study restrict fluctuations of the hormones within a narrow physiological range, and may not be able to replicate the findings demonstrated in knock-out mice and/or detect significantly altered levels of these hormones.
To test the hypothesis that a longer duration of energy deprivation might affect ucOC, we studied subjects who had undergone bariatric surgery with significant weight loss after surgery as a model of chronic energy deprivation. Prospective weight loss studies have demonstrated an association between weight loss, increased bone resorption and bone loss [28, 29], suggesting a link between chronic energy deprivation and bone metabolism. Weight loss is well associated with various hormonal adaptations including a significant reduction in circulating levels of leptin [30]. In our study, despite significant decrease in leptin levels following weight loss with bariatric surgery in these subjects over a 6-month period, this did not change ucOC levels. While other studies have found a significant increase in bone turnover markers (including urinary N-telopeptide, osteocalcin, beta-crosslaps, and N-terminal peptide of procollagen I) after surgery-induced weight loss [31, 32], the absence of changes in ucOC here suggests that its role in bone metabolism may not be similar to traditional bone turnover markers. Moreover the changes of ucOC levels before and after bariatric surgery in these subjects did not exhibit any significant correlation with the changes in BMI, weight, fat mass, insulin, and glucose levels before and after surgery. These results further propose that ucOC is unlikely to be playing a major role in the metabolic and neuroendocrine adaptations evident in post-bariatric individuals with significant weight loss.
The observation that males have a higher level of ucOC is interesting. A prior study has reported that serum ucOC concentration in women during hormone replacement therapy was significantly lower than that in women before treatment and that serum ucOC concentration showed a significant inverse correlation with estradiol concentration during hormone therapy [33], suggesting a possible role of the female sex hormone in suppressing ucOC levels. Another study from the same group showed that serum ucOC concentration rapidly increases in women after surgical menopause induced by bilateral oophorectomy in premenopausal women [34]. Lukacs et al. similarly reported a negative relationship with endogenous estrogen levels and ucOC levels [35]. Taken together, these observations suggest the female sex hormones might have a role in modulating the levels of ucOC. The implications of such an effect of the female sex hormone on ucOC and the mechanism underlying these observations need to be explored in future studies.
Our study has several strengths. This is the first human study to elucidate the physiology of ucOC in both males and females using an established assay widely utilized in recent human studies on ucOC. This is also the first randomized placebo-controlled study to examine the effect of fasting and leptin administration on circulating ucOC levels in humans. Moreover, this is the first study to report the effect of chronic energy deprivation via post bariatric surgery weight loss on circulating ucOC levels in humans. Together, these studies present a detailed study of the physiological properties of ucOC in energy homeostasis. The study conditions were highly standardized with all female subjects studied at the same phase of their menstrual cycle, all male and female subjects receiving similar isocaloric diet, activity level, with strictly uniform light/dark interval. Blood sampling was frequent, resulting in high temporal resolution, allowing us to conclude on the presence of a day/night pattern of ucOC with great confidence. The cross-over design in the short term energy deprivation arm and the several blood draws in the course of follow up in the chronic energy deprivation study serve to further strengthen the study observations.
Some limitations need to be acknowledged. The subjects in Study A and B consist of healthy young individuals who volunteered for the study and it remains to be studied whether the biological properties of ucOC in these subjects apply to older subjects with metabolic dysregulation. The apparently small sample size herein this study is to be acknowledged, although it is noteworthy that the sample size is no smaller than in previous similar studies and has been based on power calculations indicating that this sample size provides adequate power for this study and has provided adequate power in detecting periodic secretion patterns of other hormones [20–22]. Total osteocalcin was not measured in this study. However it is again noteworthy that this study is intended to evaluate the role of ucOC in energy homeostasis, which by itself is metabolically active, and the wealth of animal and human studies have correspondingly highlighted its metabolic role independent of total osteocalcin [6, 9, 10]. Lastly, the design of this study was not intended to elucidate the effect of ucOC on insulin sensitivity. Therefore the precise role of ucOC in mediating insulin resistance in humans remains to be clarified by further studies.
In summary, we demonstrate in this physiology study that ucOC does not display day/night variation in either male or female healthy subjects and therefore a single early morning determination of ucOC is an accurate reflection of the overall circulating levels of ucOC over a 24-hour period. We also show that ucOC is unaltered in both states of short term and chronic energy deprivation leading to significant weight loss, and that leptin administration to physiological replacement levels does not affect circulating levels of ucOC in humans. Females have a lower level of ucOC compared to males, possibly related to the differential action of sex hormones on ucOC, the underlying etiology of which remains to be studied further. Given the substantial interest of ucOC as a potential skeletal regulator of energy metabolism, the knowledge of these biological characteristics of ucOC is critical for future clinical studies not only in planning the timing and situations in which samples are collected to evaluate ucOC levels across different individuals or groups with comparable results, but furthermore serves to elucidate the role of ucOC in energy homeostasis and its potential regulation by leptin. Despite the promising data from recent animal studies and observational studies in humans, our findings do not support the hypothesis of a major role of ucOC in energy homeostasis or of leptin in regulating ucOC in humans.
Acknowledgments
Grant Support: The Mantzoros Laboratory is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants 58785, 79929 and 81913. The Mantzoros Laboratory is also supported by Award Number 1I01CX000422-01A1 from the Clinical Science Research and Development Service of the VA Office of Research and Development. Amylin Pharmaceuticals, Inc. supplied metreleptin for this study and approved the design of the study but had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. The project described was also supported by Grant Number UL1 RR025758- Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
Footnotes
Disclosure statement: All authors state that they have no conflicts of interest and have nothing to disclose.
Clinical trial registration: NCT00140231
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health
References
- 1.Burnier JP, et al. Gamma-carboxyglutamic acid. Mol Cell Biochem. 1981;39:191–207. doi: 10.1007/BF00232574. [DOI] [PubMed] [Google Scholar]
- 2.Vergnaud P, et al. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab. 1997;82(3):719–24. doi: 10.1210/jcem.82.3.3805. [DOI] [PubMed] [Google Scholar]
- 3.Sugiyama T, Kawai S. Carboxylation of osteocalcin may be related to bone quality: a possible mechanism of bone fracture prevention by vitamin K. J Bone Miner Metab. 2001;19(3):146–9. doi: 10.1007/s007740170034. [DOI] [PubMed] [Google Scholar]
- 4.Liu G, Peacock M. Age-related changes in serum undercarboxylated osteocalcin and its relationships with bone density, bone quality, and hip fracture. Calcif Tissue Int. 1998;62(4):286–9. doi: 10.1007/s002239900432. [DOI] [PubMed] [Google Scholar]
- 5.Ng KW. Regulation of glucose metabolism and the skeleton. Clin Endocrinol (Oxf) 2011;75(2):147–55. doi: 10.1111/j.1365-2265.2011.04133.x. [DOI] [PubMed] [Google Scholar]
- 6.Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011;54(6):1291–7. doi: 10.1007/s00125-011-2155-z. [DOI] [PubMed] [Google Scholar]
- 7.Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferron M, et al. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(13):5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanazawa I, et al. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22(1):187–94. doi: 10.1007/s00198-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 10.Schafer AL, et al. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study) J Clin Endocrinol Metab. 2011;96(12):E1982–9. doi: 10.1210/jc.2011-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei J, Ducy P. Co-dependence of bone and energy metabolisms. Arch Biochem Biophys. 2010;503(1):35–40. doi: 10.1016/j.abb.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan JL, et al. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. The Journal of clinical investigation. 2003;111(9):1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 14.Hinoi E, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183(7):1235–42. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou SH, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108(16):6585–90. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JL, et al. Pharmacokinetics of recombinant methionyl human leptin after subcutaneous administration: variation of concentration-dependent parameters according to assay. J Clin Endocrinol Metab. 2007;92(6):2307–11. doi: 10.1210/jc.2006-2864. [DOI] [PubMed] [Google Scholar]
- 17.Chan JL, Wong SL, Mantzoros CS. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clin Pharmacokinet. 2008;47(11):753–64. doi: 10.2165/00003088-200847110-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong SL, et al. Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab. 2004;89(6):2672–7. doi: 10.1210/jc.2003-031931. [DOI] [PubMed] [Google Scholar]
- 19.Chan JL, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006;103(22):8481–6. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shea SA, et al. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90(5):2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullington JM, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. Journal of neuroendocrinology. 2003;15(9):851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 22.Scheer FA, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20541–6. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vamvini MT, et al. Energy deprivation alters in a leptin- and cortisol-independent manner circulating levels of activin A and follistatin but not myostatin in healthy males. J Clin Endocrinol Metab. 2011;96(11):3416–23. doi: 10.1210/jc.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace AM, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104(25):3052–6. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 25.Pittas AG, et al. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94(3):827–32. doi: 10.1210/jc.2008-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokoll LJ, et al. Diurnal variation in total and undercarboxylated osteocalcin: influence of increased dietary phylloquinone. Calcif Tissue Int. 1998;62(5):447–52. doi: 10.1007/s002239900458. [DOI] [PubMed] [Google Scholar]
- 27.Cousin W, et al. Cloning of hOST-PTP: the only example of a protein-tyrosine-phosphatase the function of which has been lost between rodent and human. Biochemical and biophysical research communications. 2004;321(1):259–65. doi: 10.1016/j.bbrc.2004.06.137. [DOI] [PubMed] [Google Scholar]
- 28.Ricci TA, et al. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73(2):347–52. doi: 10.1093/ajcn/73.2.347. [DOI] [PubMed] [Google Scholar]
- 29.Jensen LB, Quaade F, Sorensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res. 1994;9(4):459–63. doi: 10.1002/jbmr.5650090404. [DOI] [PubMed] [Google Scholar]
- 30.Sumithran P, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 31.Granado-Lorencio F, et al. Time-course changes in bone turnover markers and fat-soluble vitamins after obesity surgery. Obes Surg. 2010;20(11):1524–9. doi: 10.1007/s11695-010-0257-1. [DOI] [PubMed] [Google Scholar]
- 32.Fleischer J, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasui T, et al. Undercarboxylated osteocalcin concentration in postmenopausal women receiving hormone therapy daily and on alternate days. Menopause. 2006;13(2):314–22. doi: 10.1097/01.gme.0000177908.40257.cf. [DOI] [PubMed] [Google Scholar]
- 34.Yasui T, et al. Change in serum undercarboxylated osteocalcin concentration in bilaterally oophorectomized women. Maturitas. 2007;56(3):288–96. doi: 10.1016/j.maturitas.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Lukacs JL, et al. Differential associations for menopause and age in measures of vitamin K, osteocalcin, and bone density: a cross-sectional exploratory study in healthy volunteers. Menopause. 2006;13(5):799–808. doi: 10.1097/01.gme.0000227023.89062.43. [DOI] [PubMed] [Google Scholar]