Abstract
Behaviors associated with breeding are seasonally modulated in a variety of species. These changes in behavior are mediated by sex steroids, levels of which likewise vary with season. The effects of androgens on behaviors associated with breeding may in turn be partly mediated by the nonapeptides vasopressin (VP) and oxytocin (OT) in mammals, and vasotocin (VT) in birds. The effects of testosterone (T) on production of these neuropeptides have been well-studied; however, the regulation of VT receptors by T is not well understood. In this study, we investigated steroid-dependent regulation of VT receptor (VTR) mRNA in a seasonally breeding songbird, the white-throated sparrow (Zonotrichia albicollis). We focused on VTR subtypes that have been most strongly implicated in social behavior: V1a and oxytocin-like receptor (OTR). Using in situ hybridization, we show that T-treatment of non-breeding males altered V1a and OTR mRNA expression in several regions associated with seasonal reproductive behaviors. For example, T-treatment increased V1a mRNA expression in the medial preoptic area, bed nucleus of the stria terminalis, and ventromedial hypothalamus. T-treatment also affected both V1a and OTR mRNA expression in nuclei of the song system; some of these effects depended on the presence or absence of a chromosomal rearrangement that affects singing behavior, plasma T, and VT immunolabeling in this species. Overall, our results strengthen evidence that VT helps mediate the behavioral effects of T in songbirds, and suggest that the chromosomal rearrangement in this species may affect the sensitivity of the VT system to seasonal changes in T.
Keywords: androgen, mesotocin, nonapeptide, oxytocin, song, testosterone, vasopressin, V1a, white-throated sparrow
INTRODUCTION
The neuropeptides vasopressin (VP) and oxytocin (OT) are widely understood to play important roles in a variety of social behaviors, including social recognition, pair bond formation, communication, and aggression (reviewed by Albers, 2012). The two nonapeptides likely evolved via gene duplication of a single molecule that was itself involved in social behavior in a common ancestor (Acher, 1980; van Kesteren et al.,1992; 1995). All vertebrates possess homologues of these peptides, which are expressed in a social behavior network in the brain (Goodson, 2005; Albers, 2012). In amphibians and birds, the vasopressin homologue vasotocin (VT) mediates behaviors such as social approach, courtship, and aggression (reviewed by Godwin & Thompson, 2012; Goodson et al., 2012a; Moore et al., 2005). The oxytocin homologue in these taxa, mesotocin (MT), is less well-studied but is thought to contribute to social behavior as well (e.g., Goodson et al., 2012a; Thayananuphat et al. 2011).
Many of the behaviors mediated by the VP/OT family are associated with reproduction, and vary according to the time of year in seasonally breeding species. The rates of these behaviors, which include territorial song in white-crowned sparrows (Zonotrichia leucophrys; Maney et al., 1997), flank-marking in Syrian hamsters (Mesocricetus auratus; Albers et al.,1986), and mate-calling in bullfrogs (Rana catesbeiana; Boyd, 1997), typically vary according to plasma levels of gonadal steroids. Hormones such as testosterone (T) and estradiol (E2) may therefore influence behavior by acting on VP/OT systems in the brain. VP/VT production is regulated by gonadal steroids in many species of rodents, birds, amphibians, and fish (reviewed by Goodson & Bass, 2001). In Japanese quail (Coturnix japonica) and canaries (Serinus canaria), for example, VT-immunoreactivity (IR) in the lateral septum (LS) and medial bed nucleus of the stria terminalis (BSTm) are reduced by castration and restored by subsequent treatment with T (Panzica et al., 2001; Voorhuis et al., 1988); T-treatment of adult female canaries increases VT-IR in the LS and BSTm to male-like levels (Voorhuis et al., 1988). Modulation of VT peptide synthesis thus represents one way that gonadal steroids might regulate reproductive behaviors.
In some cases, VP/OT and related neuropeptides do not seem to be regulated by gonadal hormones. In Syrian hamsters, for example, the distribution and number of VP cells is unaffected by T manipulation despite the fact that flank-marking, a territorial behavior induced by VP administration, is itself highly steroid-dependent (Albers et al. 1991). Further investigation has revealed that the relevant VP receptor, V1a, is regulated by T in the regions important for flank-marking (Johnson et al., 1995; Young et al., 2000). This and other findings have led to current thinking that regulation at the level of the receptor is an important route by which sex steroids may mediate seasonal changes in reproductive behavior.
Birds are well-known for dramatic changes in social behavior from one season, or life-history-stage, to the next (Jacobs & Wingfield, 2000). Despite the fact that VT modulates many seasonal behaviors, such as territorial aggression and song (Goodson, 1998; Maney et al., 1997), we know almost nothing about the seasonal regulation of VT receptors in birds. Studies of the effects of T manipulation on receptor density have been limited to females (Voorhuis et al., 1988; 1990). In female canaries, T-treatment increased the binding of radiolabeled VP in the ventral tegmental area (VTA) and the robust nucleus of the arcopallium (RA), a song control nucleus (Voorhuis et al., 1988). T-treatment did not alter binding of a mammalian OTR antagonist in any region (Voorhuis et al., 1990).
The above songbird studies tell us little about the regulation of V1a or oxytocin-like receptor (OTR) specifically, because the affinity of the ligands for avian VT1 receptors is unknown. In rodents, both of these receptors have been reported to be regulated by plasma gonadal steroids (e.g., Johnson et al., 1991; 1995; Young et al., 2000) and by photoperiod (Caldwell & Albers, 2003; Parker et al., 2001). In this study, we used in situ hybridization (ISH) to test whether the expression of OTR and V1a, formerly referred to in avian systems as VT3 and VT4, respectively, is regulated by gonadal steroids in a seasonally breeding songbird. Both receptors are expressed in the avian social behavior network and song system (see Goodson, 2005; Leung et al., 2011). In white-throated sparrows, for example, OTR mRNA is expressed in the LS, as well as in HVC, RA, and the tracheosyringeal portion of the hypoglossal nucleus (nXIIts; Leung et al., 2011). V1a mRNA is expressed throughout the hypothalamus, in the VTA, and in two nuclei in the anterior forebrain song pathway: the lateral and medial magnocellular nuclei of the anterior nidopallium (LMAN and MMAN, respectively; Leung et al. 2011). Here, we treated non-breeding males with T to mimic breeding-typical physiological levels, and quantified the effects on expression of both V1a and OTR in each of these regions.
White-throated sparrows occur in two morphs that exhibit alternative life-history strategies (Falls & Kopachena, 2010). Compared with males of the tan-striped (TS) morph, white-striped (WS) males have higher plasma T during the breeding season (Spinney et al. 2006; Swett & Breuner, 2009), and are more aggressive, provide less parental care, engage in more copulations outside of the pair-bond, and sing more (see Maney, 2008 for review). Males of the WS morph also have denser vasotocinergic innervation of the BSTm and ventrolateral portion of LS (LSc.vl) (Maney et al. 2005), regions thought to be involved in agonistic behavior and territoriality (Goodson 1998). Because the morphs differ in plasma T, social behavior, and the neural VT system, we balanced the number of WS and TS males in each treatment group and tested for effects of morph as well as treatment.
METHODS
Animal collection and housing
White-throated sparrows are abundant in Georgia during fall migration. For this study, we collected 20 males in November and December 2010. After all procedures were complete in that sample, in fall 2011 we collected 15 additional males and repeated the study to increase our sample size to 35 birds. All methods were identical for both years of the study except where noted below. Animal collection and housing were in accordance with guidelines for animal care established by Emory University, the Institutional Animal Care and Use Committee (IACUC), the National Institutes of Health, and federal and state laws. Blood samples were collected to confirm sex and morph via polymerase chain reaction (PCR) as described by Griffiths et al. (1998) and Thomas et al. (2008). After capture, the birds were housed in flight cages for about four months. At the start of the study, birds were transferred to individual cages in sound-attenuated booths.
Hormone manipulation and behavioral observation
Because we were interested in mimicking natural seasonal variation in plasma T, we did not perform castrations. Instead, we housed the birds from the day of capture on short photoperiod (8.5 hours of light) to ensure their gonads remained regressed (Wolfson 1952; Wingfield et al. 1997) and endogenous sex hormone levels remained low. To manipulate T, we used an established method that elevates plasma levels to those typical of breeding males (Maney et al., 2009; Spinney et al., 2006). Once in individual cages, half of the males (N=18) received subcutaneous silastic capsules (length 12 mm, I. D. 1.46 mm, O. D. 1.96 mm; Dow Corning, Midland, MI) filled with T (Steraloids, Newport, RI) and sealed at both ends with silicone adhesive. The remaining males (N=17) received empty capsules. Because the birds were maintained on short days, the control males experienced naturally low T levels typical of the non-breeding season. Each treatment group contained ten birds of the tan morph and seven or eight birds of the white morph. The design was balanced across year for treatment and morph. Males were housed in mixed-treatment groups, with four to six individual cages in each sound booth, for two weeks before tissue collection.
The evening before tissue collection, each male was relocated to a sound-attenuated booth equipped with a camera and microphone. Because we were interested in scoring singing behavior, we decided to present each male with a sexually receptive female for the behavioral observation. For the males collected in 2010, an E2-treated female was placed inside the booth, but outside visual contact with the male. We placed the female in the booth the night before the behavioral observation in order to prevent disturbance at lights-on the following morning when the observation began. Each male within a treatment group (T or control) was exposed to a unique female. Because males of this species mate nearly exclusively with females of opposite morph (Falls & Kopachena, 2010), and because behavioral responses to conspecifics depend on the morph of both individuals involved (Horton et al., 2012), all females presented were opposite in morph to the focal male. The camera began recording at lights-on the following morning, and recording proceeded for one hour. At that time, the female was placed in view of the male, and recording continued for another hour. Because the female’s presence seemed to inhibit, rather than stimulate, singing behavior, we repeated the study the following year without the females. For males collected in 2011, an experimenter briefly entered the booth after one hour of recording in order to control for disturbing the birds at that time point.
Tissue collection
After two hours of behavioral recording, each male was deeply anesthetized with isoflurane. A blood sample was collected from the jugular vein for assaying plasma T levels, and then the male was rapidly decapitated and the brain was removed and frozen on dry ice. All brains were subsequently stored at −80°C, and then sectioned at 20 μm in the coronal plane. Sections were mounted on Superfrost plus microscope slides (Fisher, Pittsburgh, PA) and kept at −80°C until ISH.
Hormone assays
Plasma was assayed for T, 5-alpha dihydrotestosterone (DHT), and estradiol (E2) according to the procedures of Stevenson et al (2012). Briefly, plasma samples (100μl) were fractionated by column chromatography to separate gonadal steroids and then analyzed by radioimmunoassay. Steroids were extracted from plasma by adding 5ml freshly distilled dichloromethane, vortexing, and allowing the plasma-solvent mixture to stand for a two-hour period, after which the extract (supernatant) was aspirated and evaporated under nitrogen gas at 40° C. The dried extract was reconstituted/dissolved in 0.5ml of 10% ethyl acetate in iso-octane, and this mixture was added to celite columns for chromatography.
To maximize detectability of the steroid hormones, the concentrations of which were expected to be low in the non-breeding birds, we did not split samples into duplicates. Standards (3 per assay) containing 250pg of each steroid (T, DHT, and E2) were used to calculate intra and interassay variation. Hormone concentrations were corrected for individual extraction efficiencies; average recoveries were 60% for T, 37% for DHT, and 58% for E2. Samples from years 1 and 2 of the study were run in two separate assays. Intra-assay variation for years 1 and 2 were 13% and 6% for T, 11% and 7% for DHT, and 3% and 18% for E2. Lower detectable limits were 0.05 and 0.09ng/mL for T, 0.10 and 0.14ng/mL for DHT and 0.04 and 0.06ng/mL for E2. Inter-assay variation was 6.8% for T, 18.0% for DHT, and 10.0% for E2.
In situ hybridization
ISH procedures have been previously described (Leung et al. 2011) and were adapted from Burmeister et al. (2008) and Wiemann et al. (1990). Slides containing sections were thawed, delipidated, acetylated and dehydrated. Complementary 35S-labeled riboprobes were transcribed from linearized pCRII plasmids containing 477 bp of V1a sequence or 677 bp of OTR sequence (Leung et al., 2011) by using a T7/SP6 Riboprobe In Vitro Transcription Kit (Promega, Madison, WI). The specificity of each riboprobe was previously demonstrated using sense riboprobes (Leung et al., 2011). Each probe was diluted to a concentration of 1 × 107 cpm/ml in hybridization buffer (Leung et al., 2011; Meddle et al., 2007; Wada et al., 2004), and 100 μl was applied to each slide. The slides were coverslipped and incubated overnight in a mineral oil bath at 67°C for V1a or 64°C for OTR. The next day, slides were subjected to stringency washes and RNase digestion, dehydrated, dried and placed onto Biomax film (see Leung et al., 2011). After five days of exposure, the films were developed and scanned at 1600 dpi with an Epson V700 scanner. All slides were then Nissl-stained with toluidine blue. Because the RNase step in the ISH destroys RNA, which is necessary for good cytoplasmic Nissl staining (Kadar et al., 2009), the sets of tissue that underwent ISH could not be used to delineate every cell group of interest. We therefore fixed and stained alternate sets from four of the birds (two from each treatment group) for clarification of cell groups. These slides were scanned at 20x resolution with an Olympus Nanozoomer system (Olympus, Center Valley, PA).
Quantification of signal
We chose regions of interest (ROIs) according to the following criteria: (1) labeled regions that are part of a vertebrate social behavior network (Newman et al., 2001; Goodson, 2005; Maney et al., 2008); (2) labeled regions of the vocal control system (Brenowitz et al., 1997); and (3) other regions with remarkably intense labeling. Examples of labeling in our regions of interest are shown in Fig. 1 (V1a) and Fig. 2 (OTR). Within the social behavior network, we quantified V1a mRNA expression in the medial preoptic area (POM), anterior hypothalamus (AH), bed nucleus of the stria terminalis (BSTm), ventromedial hypothalamus (VMH), and VTA. OTR expression was quantified in the medial amygdala (MeA; also called nucleus taeniae in birds) and the dorsal and ventral portions of the lateral septum (LSd and LSv). Within the vocal control system, we quantified V1a mRNA in LMAN and MMAN, as well as the intercollicular nucleus (ICo). We quantified OTR mRNA in HVC, RA, and the lingual and tracheosyringeal portions of the hypoglossal nucleus (nXIIl and nXIIts). Finally, we quantified V1a mRNA in a densely labeled cell group within the lateral forebrain bundle (LFB) and in the lateral striatum (LSt), and OTR mRNA in the medial septum (MS) and dorsal arcopallium (Ad). With the exception of the V1a mRNA labeling in AH, all of the labeling we quantified was previously described by Leung et al. (2011).
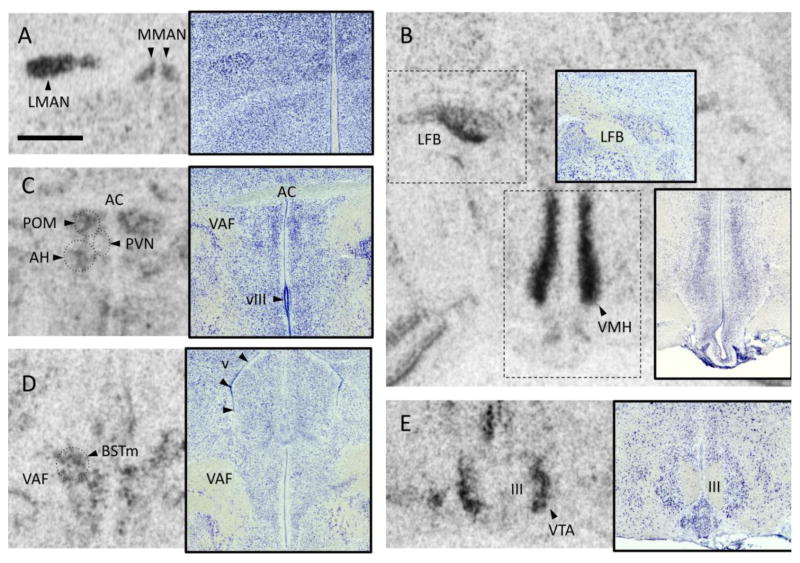
Fig. 1.
Examples of VT1 receptor mRNA labeling quantified in this study. Each panel shows a film autoradiogram along with an inset depicting a Nissl-stained alternate section from the same animal. All photos are from a T-treated male. Scale bar in A, 1mm, applies to all images. A. Medial and lateral portions of the magnocellular nucleus of the anterior nidopallium (MMAN and LMAN). The midline can be seen to the right. B. A cell group intermingled with fibers of the lateral forebrain bundle (LFB) is heavily labeled, as is the ventromedial hypothalamus (VMH). C. The dorsal hypothalamus showing the caudal portion of the medial preoptic area (POM), the paraventricular nucleus (PVN; seen in the Nissl stain), and the anterior hypothalamus (AH). The anterior commissure (AC) can be seen along the top of the photo and the third ventricle (vIII) at the bottom. The dotted circles show the approximate areas sampled for each region of interest. D. The medial portion of the bed nucleus of the stria terminalis (BSTm) as it appears just caudal to the AC, medial to the ventral amygdalofugal tract (VAF) and ventral to the lateral ventricle (v). The dotted circle shows the approximate area sampled. E. The ventral tegmental area (VTA) is seen on either side of the oculomotor nerve (III) as it exits the base of the brain.
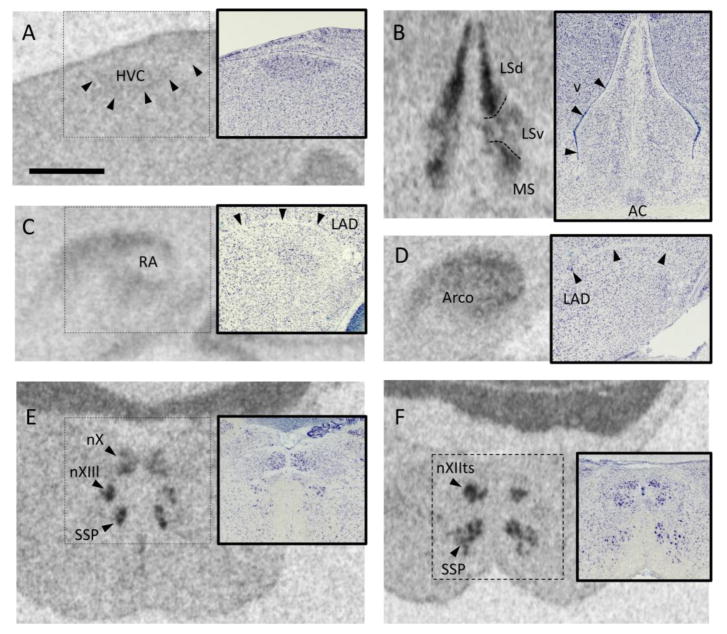
Fig. 2.
Examples of oxytocin-like receptor (OTR) mRNA signal quantified in this study. Each panel shows a film autoradiogram along with an inset depicting a Nissl-stained alternate section from the same animal. All photos are from a testosterone-treated male. Scale bar in A, 1mm, applies to all images. A. HVC (used as a proper name). Midline is to the right. B. The lateral septum, showing the dorsal (LSd) and ventral (LSv) subdivisions, and the medial septum (MS) Variation in the OTR mRNA signal was used to delineate the areas, which are not distinct in Nissl-stained sections (Goodson et al., 2004). v, lateral ventricle. C. The robust nucleus of the arcopallium (RA) was relatively unlabeled compared with the surrounding arcopallium. LAD, dorsal arcopallial lamina. Midline to the right. D. The arcopallium was examined in sections caudal to RA. Midline to the right. E. The lingual portion of the hypoglossal nucleus (nXIIl) can be seen ventral to the motor nucleus of the vagus (nX) and dorsal to the supraspinal nucleus (SSP). F. The tracheosyringeal portion of the hypoglossal nucleus (nXIIts) is positive for OTR mRNA.
Before quantifying the VTR signal in each region of interest, we first confirmed its anatomical location by superimposing digital images of scanned films and the corresponding images of Nissl-stained slides in Powerpoint (version 12.3.2). To quantify the area encompassed by the label and its optical density, the film images were opened with ImageJ (National Institutes of Health) and converted to 8-bit grayscale. For regions in which the label was clearly defined such that boundaries could be traced (LMAN, MMAN, MS, VMH, LFB, HVC, RA, nXIIl and nXIIts), the area covered by label was recorded as the summed area in mm2 from all sections. For AH, POM, PVN, BSTm, LSt, ICo, and MeA, we sampled within a circle (usually ~0.2mm2; see Figs. 1 and 2) placed in two to three consecutive sections in the largest part of the nucleus. POM was sampled both at its rostral extent ventral to the septomesencephalic tract and at its caudal extent ventral to the anterior commissure. All other regions were sampled by tracing their outlines. mRNA expression in the arcopallium was quantified only in sections caudal to RA to prevent sampling from RA. The mean gray value was recorded for each selection in all regions. To correct for variation in background labeling, we then subtracted the mean gray value of an area (0.2 mm2) near the sampled area but which contained no obvious label. The corrected mean gray value measurements were then averaged across the selections for each region of interest and converted to positive values for the statistical analysis. Those values are referred to hereafter as “density”. All regions were sampled bilaterally.
Statistics
Analysis of plasma hormone levels
To demonstrate that the hormone manipulation was equally effective in both morphs, we performed univariate ANOVAs on the plasma levels of T, DHT, and E2 with treatment and morph as between-subjects factors. Five birds were excluded from this analysis because of low recoveries. To show that T-treated WS and TS birds had similar levels of each of these hormones, we performed t-tests and calculated Cohen’s d for the T, DHT, and E2 data to look for effects of morph in the T-treated birds only.
Analysis of behavior
We assessed the effect of T-treatment on vocalizations (songs, tseets, chips, chip-ups, and trills; see Falls & Kopachena, 2010) and on eating, drinking, and preening behavior using Mann-Whitney U tests. Nonparametric tests were used because the data were not normally distributed, i.e. there were large numbers of zeros in the dataset.
Analysis of receptor mRNA labeling
Because investigating VTR mRNA signal in many brain regions predisposes us to type I error, we first performed two MANOVAs on data from all of the birds. One was performed on the area covered by signal and the other on the density values, with treatment (T or blank), morph (WS or TS), and year (2010 or 2011) as between-subjects factors and ROI as the within-subjects factor. Two missing LMAN values were replaced by the series mean for this analysis only (missing values were not replaced in the univariate tests, see below). HVC was analyzed separately because tissue damage during cryosectioning resulted in a high number of missing values for this region (the final N for HVC was 4 WS T-treated, 4 WS control, 6 TS T-treated and 6 TS control males).
Following these MANOVAs, we performed univariate ANOVAs for each receptor in each ROI, with treatment, morph, and year as between-subjects factors. When significant interactions between treatment and morph were found, we repeated the univariate ANOVAs within each morph with treatment and year as between-subjects factors to determine the effect of treatment within each morph.
To test whether variation in mRNA expression was predicted by variation in plasma T in the T-treated birds, we ran Pearson correlation tests between plasma T and the area or density values for each ROI in the T-treated birds only.
RESULTS
Plasma hormone levels
The results of the hormone assays are shown in Fig. 3. Plasma levels of all three hormones in the T-treated and blank-treated groups were typical of free-living breeding and non-breeding males, respectively (Spinney et al., 2006; Horton, Moore, and Maney, unpublished data). Birds that received implants filled with T had significantly higher plasma T (P < 0.0001), DHT (P < 0.0001) and E2 (P = 0.031) than blank-treated birds. T-treated WS and TS birds did not differ with respect to plasma T (F = 0.104, P = 0.752), DHT (F = 0.855, P = 0.373), or E2 (F = 0.000, P = 0.984). This result is consistent with our previous study showing that this dose of T produces identical plasma levels of T in the two morphs (Maney et al., 2009). In this study, however, despite the lack of a significant effect of morph, the effect size was large for T (Cohen’s d = 0.851) and medium for DHT (d = 0.211) and E2 (d = 0.346). Thus, we cannot completely rule out the possibility that T-treatment may have produced different levels of these hormones in the two morphs.
Fig. 3.
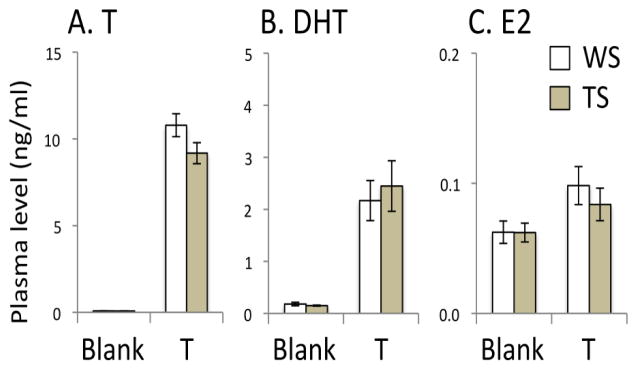
Effects of testosterone (T) -treatment on plasma levels of T, dihydrotestosterone (DHT) and estradiol (E2). No significant effects of morph were detected. WS, white-striped plumage morph. TS, tan-striped plumage morph.
Behavior
When data from both years were considered together, T-treatment increased the frequency of songs (P = 0.041), tseets (P = 0.001), chips (P = 0.015), and chip-ups (P = 0.010). Trills were very infrequent. There was no effect of T-treatment on eating (P = 0.117), drinking (P = 0.362), or preening (P = 0.251). Overall, the behavioral data show the same pattern of T-dependence that we showed in a previous study (Maney et al., 2009).
When the vocalization data were analyzed separately according to whether a female was present (2010) or absent (2011), the effect of T on vocalizations was seen only in the birds not exposed to females. Repeating this analysis within morph showed that in 2011, T-treatment did not increase singing significantly in the TS males (MWU = 3.000; P = 0.134) but there was a trend in the WS males (MWU = 1.500; P = 0.079). Although the 2010 birds were heard to sing before the behavioral test, when they were exposed to females they exhibited very little song (median number of songs per hr in the T-treated birds in 2010 was 0, range 0–1; median in 2011 was 14, range 0–101).
V1a and OTR mRNA
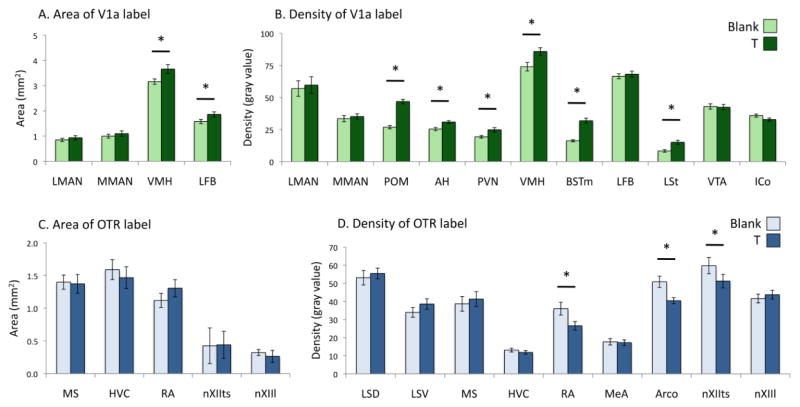
T-treatment altered VTR expression in regions implicated in social behaviors such as song. A MANOVA testing for effects of treatment and morph on the area covered by expression of OTR or V1a receptor mRNA showed a main effect of T treatment (F8,20 = 2.469, P = 0.048) but no main effect of morph (F8,20 = 1.042, P = 0.439). There was a significant treatment × morph interaction (F8,20 = 4.030, P = 0.005) on the area covered by expression. A MANOVA testing for effects of treatment and morph on the density of OTR or V1a mRNA expression showed a main effect of T treatment (F19,9 = 15.879, P < 0.001). There was no main effect of morph (F19,9 = 0.778, P = 0.693), nor was there a treatment × morph interaction (F19,9 = 1.253, P = 0.439) on the density of expression. There were no year x treatment, year x morph, or year x treatment x morph interactions for either the area or density measurements. Results specific to each region of interest are presented in Fig. 4 and significant effects are discussed below.
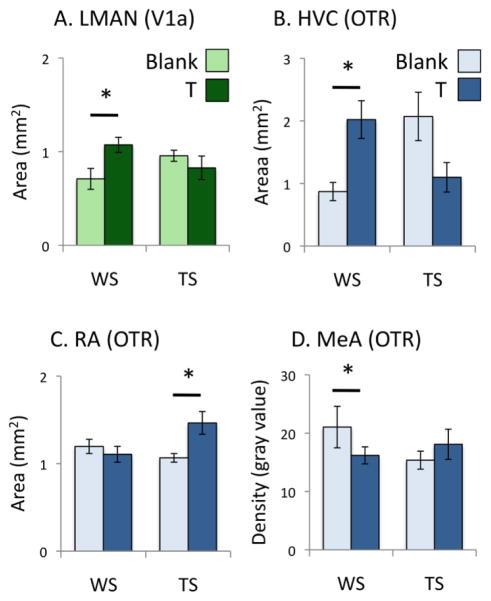
Fig. 4.
Effects of testosterone (T) -treatment on (A) the area covered by V1a receptor mRNA signal, (B) the density of V1a receptor mRNA signal, (C) the area covered by oxytocin-like receptor (OTR) mRNA signal, and (D) the density of OTR mRNA signal in regions of interest. “Density” refers to the gray value of the region of interest, minus the gray value of a nearby unlabeled region. Graphs show means ± SEM. *P < 0.05; see text for exact values. Arco, arcopallium. ICo, intercollicular nucleus. LSt, lateral striatum. MeA, medial amygdala. Other abbreviations, see Fig. 1 and 2.
Effects of T on V1a mRNA expression
The effects of T-treatment on V1a receptor mRNA expression are shown in Fig. 4A and 4B. T-treatment significantly increased the area covered by V1a signal in VMH (F1,34 = 7.202; P = 0.012) and in the LFB cell group (F1,34 = 7.901; P = 0.009), an area containing a population of vasotocinergic neurons interspersed with the fibers of the LFB (DL Maney, unpublished; see also Aste et al., 2013; Kiss et al., 1987; Panzica et al., 1999a). T-treatment increased the density of signal in POM (F1,34 = 78.008; P < 0.001), AH (F1,34 = 9.236; P = 0.005), VMH (F1,34 = 11.702; P = 0.002) PVN (F1,34 = 4.902; P = 0.035), BSTm (F1,34 = 43.985; P < 0.001), and LSt (F1,34 = 14.409; P = 0.001). The effects were particularly striking in POM and BSTm, for which the range of values in T- and blank-treated birds were nonoverlapping (see Fig. 5 for representative images). There were no significant main effects of T-treatment on V1a expression in the other regions of interest. Year did not interact with treatment or morph for any region.
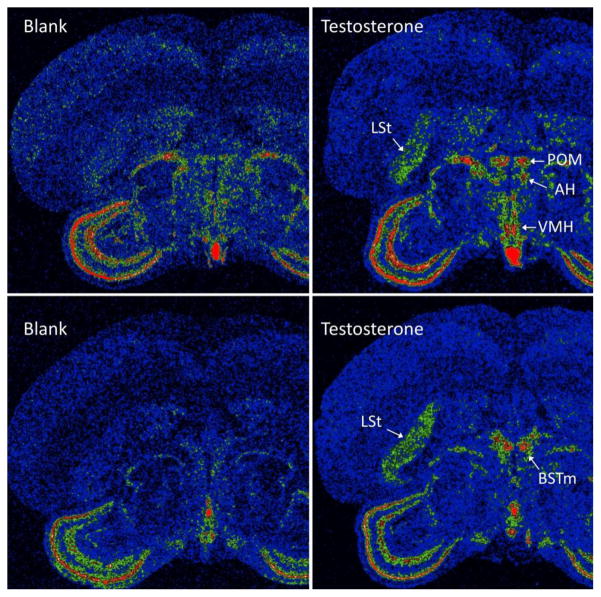
Fig. 5.
Pseudocolor images of representative sections showing V1a receptor mRNA labeling in blank-treated (A, C) or testosterone-treated birds (B, D). Warmer colors represent denser signal. The effect of testosterone treatment can be seen by comparing (A) with (B) and (C) with (D). Testosterone increased V1a receptor mRNA in the medial preoptic area (POM), anterior hypothalamus (AH), ventromedial hypothalamus (VMH), lateral striatum (LSt), and medial bed nucleus of the stria terminalis (BSTm).
Effects of T on OTR mRNA expression
The effects of T-treatment on OTR mRNA expression are shown in Fig. 4C and 4D. The density of the OTR signal was significantly reduced by T-treatment in the song nuclei RA (F1,34 = 8.017; P = 0.009) and nXIIts (F1,34 = 5.298; P = 0.029). The OTR signal was similarly reduced in the arcopallium, caudal to RA (F1,34 = 11.851; P = 0.002). There were no significant main effects of T-treatment on OTR expression in the other regions of interest. Year did not interact with treatment or morph for any region.
Interactions between treatment and morph
T-treatment affected the area covered by receptor mRNA in three song nuclei, but these effects depended on plumage morph (Fig. 5). In LMAN, the area covered by V1a mRNA increased only in the WS birds (treatment x morph interaction F1,34 = 4.637; P = 0.040; effect of T in WS birds F1,14 = 5.769; P = 0.035). The area covered by OTR mRNA increased in HVC only in the WS birds (treatment X morph interaction F1,34 = 6.738; P = 0.020; effect of T in WS birds F1,7 = 6.067; P = 0.049) and in RA only in the TS birds (treatment X morph interaction F1,34 = 9.482; P = 0.005; effect of T in TS birds F1,19 = 14.765; P = 0.001). In MeA, the effect of treatment likewise depended on morph; T-treatment decreased the density of OTR mRNA only in the WS birds (treatment x morph interaction F1,34 = 6.494; P = 0.017; effect of T in WS birds F1,14 = 6.374; P = 0.028).
Because we could not completely rule out a morph difference in plasma T in our T-treated birds (see above), we used Pearson correlations to investigate whether the interactions between morph and treatment in the song system could have been driven by morph differences in plasma sex steroids. We found that within the T-treated birds, neither T, DHT, nor E2 predicted the area covered by OTR signal in HVC or RA (all P ≥ 0.152). Neither did any of these steroid levels predict the area covered by V1a signal in LMAN (all P ≥ 0.689). This result suggests that differential effects of T–treatment on receptor mRNA in the song system (Fig. 5) are not likely be explained by one morph having slightly higher plasma T, DHT, or E2 than the other. There was, however, a significant positive relationship between the density of V1a signal in MeA and plasma DHT (R2 = 0.367; P = 0.013). It is thus possible that the morph-dependent effect of T-treatment in MeA was driven by a small difference in plasma DHT.
Relationship between ROI area and signal density
We noted that T-treatment decreased OTR mRNA density in RA (Fig. 4) while increasing the area covered by OTR mRNA expression in this nucleus, at least in TS birds (Fig. 6). Similarly, T-treatment reduced OTR mRNA density in nXIIts, a nucleus that is known to grow larger after T-treatment in canaries (DeVoogd et al. 1991). We therefore tested whether the decrease in signal density may have been caused simply by an increase in the size of the nucleus, which may have the overall effect of reducing apparent signal density because the cell density itself is reduced (see also Riters et al., 2002). Pearson correlations testing for relationships between the area covered by mRNA signal and its density showed that in nXIIts, the area covered by OTR signal was inversely related to the density of that signal (R2 = 0.135; P = 0.030). In RA, however, signal density was not related to the area of signal (R2 = 0.006; P = 0.650). We found no evidence that an increase in ROI area negatively impacts signal density in other ROIs; in fact, signal density was positively related to the area covered by signal both in HVC (R2 = 0.269; P = 0.007) and LMAN (R2 = 0.377; P < 0.001). In other words, birds with the darkest HVC and LMAN also had the largest area covered. Thus, with the exception of nXIIts, a decrease in signal intensity is more likely related to regulation of transcription than simply to the spacing of mRNA-positive cells.
Fig. 6.
Interactions between the effects of plumage morph and testosterone (T) -treatment on the area covered by V1a mRNA signal (A) or oxytocin-like receptor (OTR) signal (B, C, D). T-treatment increased the area covered by signal in LMAN (A) and HVC (B) only in white-striped (WS) birds, and increased the area covered by signal in RA (C) only in tan-striped (TS) birds. (D) The density of OTR mRNA in the medial amygdala (MeA) was reduced by T-treatment only in WS birds. Graphs show means ± SEM. *P < 0.05. Other abbreviations, see Figs. 1 and 2.
DISCUSSION
In this study, we have shown evidence that T regulates the transcription of OTR and V1a receptor mRNA in a seasonally breeding songbird. These receptors may thus help mediate many effects of T on seasonal reproductive behaviors, including song. Elevating plasma T from non-breeding to breeding-typical levels altered OTR and V1a mRNA expression in regions of the brain that play known roles in song and reproductive behavior, suggesting that T changes the dynamics of VT signaling in a way that is likely relevant to seasonal reproduction.
Our results contrast with previous reports of T-dependent regulation of VTR in songbirds. In female canaries, T administration increased the binding of radiolabeled VP in RA and VTA (Voorhuis et al., 1988a). T-treatment of female canaries also increased the binding of an OTR antagonist (OVTA) in the arcopallium (Voorhuis et al., 1990). We detected no increase in either OTR or V1a receptor in any of these regions; in fact T-treatment tended to reduce the area occupied by OTR mRNA in RA (P = 0.052; see Fig. 4). Our contrasting findings may be explained by the fact that we labeled mRNA instead of bound receptor, or by sex or species differences in the distribution of receptor subtypes. In zebra finches, for example, the dominant VTR in RA and VTA is the homologue of the V2b receptor (Leung et al., 2011), also called VT1 in birds (Tan et al., 2000; see also Ocampo-Daza et al., 2012). In contrast, white-throated sparrows of both sexes express very little of this receptor in those regions (Leung et al., 2011). Rather, in this species, the dominant receptors in RA and VTA are OTR and V1a, respectively (Leung et al., 2011). Species differences are not likely to explain discrepant findings in the arcopallium, however; the distribution of OVTA binding in this region is identical in canaries and white-throated sparrows (Leung et al., 2009; Voorhuis et al., 1990) and matches the distribution of OTR mRNA (Leung et al., 2011). Our findings may differ from those of Voorhuis et al. (1990) because we quantified the arcopallium signal only in sections caudal to RA.
The T we administered could have acted directly via androgen receptors (AR) or indirectly on estrogen receptors (ER) via aromatization. We hypothesize that both mechanisms play a role in the regulation of VTR density. In other songbirds, AR is expressed in several of the regions affected by T-treatment in this study, for example in LMAN, BSTm, HVC, RA, and nXIIts. Expressing AR was not sufficient, however, to drive a detectable change in VTR mRNA in this study; we noted no effects of T-treatment in MMAN, LS, or VTA, which express AR in related species (Gahr, 2001; Maney et al., 2001).
The ability of T to act via ER depends on the activity of aromatase (ARO). The significant increase in plasma E2 following T-treatment in this study indicates that these birds had at least a minimal level of ARO activity, but not enough to elevate plasma E2 to normal spring-like levels (~0.3ng/mL, BM Horton, unpublished results). Although ARO activity is present during the winter in seasonally breeding sparrows (Schlinger et al., 1992), it is much lower than in the spring (Soma et al., 2003). The seasonal increase in gonadal steroids is not what drives the increase in ARO activity to spring-like levels: actual photostimulation is necessary (Meitzen et al., 2007). Because the birds in this study were not photostimulated and presumably had relatively low ARO activity, exogenous T could act via ER only in brain regions that contain sufficient active ARO. Those regions include VMH and MeA, and possibly POM and BSTm (Balthazart et al., 1996; Wacker et al., 2010). The reported distributions of ERα, ERβ, and ARO mRNA in European starlings (Sturnus vulgaris) and song sparrows (Melospiza melodia) are remarkably similar to the distribution of V1a receptor mRNA in white-throated sparrows (Bernard et al., 1999; Leung et al., 2011; Wacker et al., 2010;). Aromatization followed by action at ERs therefore seems a likely mechanism by which V1a receptor expression was affected by T administration in VMH and nearby regions.
In this study, the areas that responded to T-treatment overlapped only partially with regions in which T is known to regulate VT peptide. In quail, castration reduces vasotocinergic innervation of BSTm and POM (Panzica et al., 2001). Here, we report that V1a receptor mRNA in the same regions was upregulated by T-treatment (Fig. 4B). Goodson et al. (2012) found that VT-IR increases during breeding in both of these regions as well as in AH and VMH, where we found effects of T-treatment on V1a mRNA levels. As has been suggested previously in mammals, however, nonapeptides and their receptors need not be regulated in parallel. For example, in rats, castration profoundly reduces VP-IR in the LS (de Vries et al. 1984; Gao et al., 1994) whereas VP binding in the LS seems unaffected (Gao et al., 1994). Similarly, whereas vasotocinergic innervation of LS is sensitive to T manipulation in canaries (Voorhuis et al., 1988b), VP binding in LS is not reduced by castration (Voorhuis et al., 1988a). In this study, we found no effect of T administration on VTR mRNA expression in the LS or in ICo, in which VT-IR was previously reported to be sensitive to T in dark-eyed juncos (Junco hyemalis; Plumari et al., 2004). We did, however, find effects in areas in which vasotocinergic innervation is thought to be relatively insensitive to T manipulation, for example in RA (Voorhuis et all 1988b; 1991) and the LFB cell group (Aste et al. 2013). Our findings thus provide additional evidence that the regulation of these peptides and their receptors do not always go hand-in-hand. In other words, depending on the region, sex steroids may regulate one but not the other.
White-throated sparrows occur in two plumage morphs that differ with respect to circulating T, T-dependent behaviors, and vasotocinergic innervation of LS and BSTm (Maney, 2008; Maney et al., 2005). In breeding populations, WS males sing more in response to simulated territorial intrusion and have higher plasma T than TS males (Falls & Kopachena, 2010; Spinney et al., 2006; Swett & Breuner, 2009). The more aggressive behavior of WS birds is thus thought to be mediated in part by T. In a previous study, however, when plasma T was experimentally equalized in laboratory-housed WS and TS males (as in the current study; see Fig. 3), WS males nonetheless sang more in response to playback than TS males (Maney et al., 2009). This result suggested that the sensitivity of T-responsive neural mechanisms underlying territorial behavior may differ according to morph. Here, we demonstrate that T-treatment does in fact affect the brain in a morph-dependent fashion. In the song system and in MeA, the effects of treatment depended on morph (Fig. 6). In the WS birds, T-treatment increased the area occupied by V1a receptor mRNA in LMAN and by OTR mRNA in HVC, and decreased the density of OTR mRNA in MeA. These effects were not seen in TS males. In contrast, expression of OTR mRNA increased in RA in birds of the TS morph but not the WS morph. Morph-dependent effects of T on the brain are consistent with the findings of Matragrano et al. (2013), who reported that T administration reduced serotonergic innervation of the auditory forebrain in WS but not TS males.
Morph-dependent sensitivity to T treatment likely originates in a chromosomal rearrangement that segregates absolutely with plumage morph (Thorneycroft, 1975). The rearrangement strongly suppresses recombination, causing genetic and behavioral differentiation between the WS and TS morphs (Thomas et al. 2008; Davis et al., 2011). Two genes located inside the rearrangement may play key roles in morph-dependent responses to T-treatment: ESR1, which encodes ERα (Thomas et al. 2008) and SRD5A2, which encodes 5α-reductase (J.W. Thomas, unpublished), an enzyme that converts T to the non-aromatizable androgen DHT. Although there were no morph differences in plasma DHT in the birds in this study (Fig. 3), expression of 5α-reductase may be morph-dependent in the brain, where it is strongly expressed in HVC (BM Horton, unpublished). We are currently mapping and characterizing the effects of morph on the expression of both genes in the brain, which may ultimately shed light on the mechanisms underlying morph-dependent effects of T-treatment on VTR expression. Our current results, together with the findings of Matragrano et al. (2013) on serotonin fiber density, demonstrate a clear mechanism by which hormones may interact with genotype to affect social behavior.
Because T treatment increases the frequency of vocalization behavior in this species (Maney et al., 2009 and this study), we cannot rule out the possibility that the effects shown here were caused by singing or other social interactions rather than direct effects of T on VTR mRNA expression. This caveat is especially important when considering the interactions between morph and treatment. We did not detect a morph difference in song rate, but this lack of effect was likely due to the presence of a female, which inhibited singing, during most of the 2 h behavioral observations. Other authors have demonstrated an inhibitory effect of a female on singing behavior in other species, for example in Cassin’s finches (Sockman et al., 2005) and great tits (Krebs et al., 1980). The inhibitory effect of a receptive female on song in white-throated sparrows is consistent with a territorial, rather than courtship, function for male song. In a previous study we demonstrated that in response to a playback of male song, T-treated WS males sing at higher rates than do their TS counterparts (Maney et al., 2009). In this study, outside of the 2 h observation, the birds were housed with other males and able to interact. Although we did not formally record behavior during that time, WS males likely dominated and sang more than TS (see Maney & Goodson, 2011). In hamsters, V1a can be regulated by social experience; dominant males express more V1a in the lateral VMH than subordinates (Cooper et al., 2005). In white-throated sparrows, rank in a hierarchy is unaffected by T-treatment if the hierarchy is already stable (reviewed by Maney & Goodson, 2011); nonetheless, we must consider that male-male social interactions before the behavioral observations played a role in T-dependent changes in VTR mRNA in this study.
The potential downstream effects of singing on VTR mRNA expression are especially relevant in the song system. DeVoogd et al. (1995) showed that in breeding, free-living white-throated sparrows, song nuclei such as LMAN and nXIIts were larger in WS birds than TS. They hypothesized that higher song rates in WS birds contributed to this effect. We found that in WS birds, T-treatment increased the area covered by V1a signal and by OTR signal in LMAN and HVC, respectively. Our finding that T-treatment increased the area covered by OTR signal in RA in TS birds seems paradoxical, however. Since we did not measure the volumes of the song nuclei themselves but rather the area covered by label, it is possible that the receptor signal and the nucleus size are independently regulated (see also Riters et al. 2002). Overall, our results suggest that seasonal changes in plasma T interact with both behavior and genotype to produce downstream effects on neurochemistry. Because of its well-understood genetic polymorphism that segregates with social behavior, this species represents an excellent model in which to explore these relationships.
Highlights.
We mimicked seasonal changes in testosterone (T) in male white-throated sparrows.
Expression of both V1a and oxytocin receptor homologues was affected by T.
T-treatment increased V1a receptor mRNA in hypothalamic areas and the bed nucleus.
Effects of T in the song system depended on plumage morph in this polymorphic species.
T may act via the vasotocin system to induce behaviors relevant to reproduction.
Acknowledgments
We thank Demesew Abebe, Ganapathy Bhat, Sarah Earp, Shuai Hao, Cary Leung, Jory Liang, Lisa Matragrano, Justin Michaud, David Nicholson, Sandra Shirk, and Emily Young for technical assistance, and the Emory University Department of Biology for the use of resources. We are grateful to Ignacio Moore for access to his RIA facility at Virginia Tech and for advice about the assays, and to David Gutman for access to the Nanozoomer scanner. This work was supported by IOS-0723805 and NIMH-R01MH082833 to DLM. Undergraduate research support was provided by the Behavioral Research Advancements in Neuroscience program (Center for Behavioral Neuroscience) and by the Summer Undergraduate Research at Emory and Scholarly Inquiry and Research at Emory programs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav. 2012;61:283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Albers HE, Pollock J, Simmons WH, Ferris CF. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J Neurosci. 1986;6:2085–2089. doi: 10.1523/JNEUROSCI.06-07-02085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996;31:129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Brain vasotocin pathways and the control of sexual behaviors in the bullfrog. Brain Res Bull. 1997;44:345–350. doi: 10.1016/s0361-9230(97)00213-x. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Mangiamele LA, Lebonville CL. Acoustic modulation of immediate early gene expression in the auditory midbrain of female túngara frogs. Brain Res. 2008;1190:105–114. doi: 10.1016/j.brainres.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Short-photoperiod exposure reduces vasopressin (V1a) receptor binding but not arginine-vasopressin-induced flank marking in male Syrian hamsters. J Neuroendocrinol. 2003;15:971–977. doi: 10.1046/j.1365-2826.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Karom M, Huhman KL, Albers HE. Repeated agonistic en- counters in hamsters modulate AVP V1a receptor binding. Horm Behav. 2005;48:545–551. doi: 10.1016/j.yhbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Davis JK, Mittel LB, Lowman JJ, Thomas PJ, Maney DL, Martin CL, Thomas JW NISC Comparative Sequencing Program. Haplotype-based genomic sequencing of a chromosomal polymorphism in the white-throated sparrow (Zonotrichia albicollis) J Heredity. 2011;102:380–390. doi: 10.1093/jhered/esr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoogd TJ, Houtman AM, Falls JB. White-throated sparrow morphs that differ in song production rate differ in the anatomy of some song-related brain areas. Dev Neurobiol. 1995;28:202–213. doi: 10.1002/neu.480280207. [DOI] [PubMed] [Google Scholar]
- DeVoogd TJ, Pyskaty DJ, Nottebohm F. Lateral asymmetries and testosterone-induced changes in the gross morphology of the hypoglossal nucleus in adult canaries. J Comp Neurol. 1991;307:65–76. doi: 10.1002/cne.903070107. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 1984;298:141–145. doi: 10.1016/0006-8993(84)91157-0. [DOI] [PubMed] [Google Scholar]
- Falls JB, Kopachena JG. White-throated Sparrow (Zonotrichia albicollis) In: Poole A, editor. The birds of North America online. Cornell Laboratory of Ornithology; Ithaca, NY: 2010. [Google Scholar]
- Gahr M. Distribution of sex steroid hormone receptors in the avian brain: functional implications for neural sex differences and sexual behavior. Micro Res Techn. 2001;55:1–11. doi: 10.1002/jemt.1151. [DOI] [PubMed] [Google Scholar]
- Gao X, Phillips P, Oldfield B, Trinder D, Risvanis J, Stephenson J, Johnston C. Androgen manipulation and vasopressin binding in the rat brain and peripheral organs. Eur J Endocrinol. 1994;130:291–296. doi: 10.1530/eje.0.1300291. [DOI] [PubMed] [Google Scholar]
- Godwin J, Thompson R. Nonapeptides and social behavior in fishes. Horm Behav. 2012;61:230–238. doi: 10.1016/j.yhbeh.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kelly AM, Kingsbury MA. Evolving nonapeptide mechanisms of gregariousness and social diversity in birds. Horm Behav. 2012a;61:239–250. doi: 10.1016/j.yhbeh.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wilson LC, Schrock SE. To flock or fight: neurochemical signatures of divergent life histories in sparrows. Proc Natl Acad Sci U S A. 2012b;109(Suppl 1):10685–10692. doi: 10.1073/pnas.1203394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Double MC, Orr K, Dawson RJ. A DNA test to sex most birds. Mol Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubrij KI, Chaturvedi CM, Ali N, Cornett LE, Kirby JD, Wilkerson J, Mikhailova M, Turner ML, Baeyens DA. Molecular cloning of an oxytocin-like receptor expressed in the chicken shell gland. Comp Biochem Physiol B Biochem Mol Biol. 2005;142:37–45. doi: 10.1016/j.cbpc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Jacobs JD, Wingfield JC. Endocrine control of life-cycle stages: a constraint on response to the environment? Condor. 2000;102:35–51. [Google Scholar]
- Johnson AE, Barberis C, Albers HE. Castration reduces vasopressin receptor binding in the hamster hypothalamus. Brain Res. 1995;674:153–158. doi: 10.1016/0006-8993(95)00010-n. [DOI] [PubMed] [Google Scholar]
- Johnson AE, Coirini H, Insel TR, McEwen BS. The regulation of oxytocin receptor binding in the ventromedial hypothalamic nucleus by testosterone and its metabolites. Endocrinology. 1991;128:891–896. doi: 10.1210/endo-128-2-891. [DOI] [PubMed] [Google Scholar]
- Kadar A, Wittmann G, Liposits Z, Fekete C. Improved method for combination of immunocytochemistry and Nissl staining. J Neurosci Meth. 2009;184:155–118. doi: 10.1016/j.jneumeth.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Okanoya K, Wada M. Effect of testosterone on the distribution of vasotocin immunoreactivity in the brain of the zebra finch, Taeniopygia guttata castanotis. Life Sciences. 1999;65:1663–1670. doi: 10.1016/s0024-3205(99)00415-4. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Voorhuis TA, van Eekelen JA, de Kloet ER, de Wied D. Organization of vasotocin-immunoreactive cells and fibers in the canary brain. J Comp Neurol. 1987;263:347–364. doi: 10.1002/cne.902630304. [DOI] [PubMed] [Google Scholar]
- Krebs JR, Avery M, Cowie RJ. Effect of removal of mate on the singing behaviour of great tits. Anim Behav. 1980;29:635–637. [Google Scholar]
- Leung CH, Abebe DF, Earp SE, Goode CT, Grozhik AV, Mididoddi P, Maney DL. Neural distribution of vasotocin receptor mRNA in two species of songbird. Endocrinology. 2011;152:4865–4881. doi: 10.1210/en.2011-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CH, Goode CT, Young LJ, Maney DL. Neural distribution of nonapeptide binding sites in two species of songbird. J Comp Neurol. 2009;513:197–208. doi: 10.1002/cne.21947. [DOI] [PubMed] [Google Scholar]
- Maney DL. Endocrine and genomic architecture of life history tradeoffs in an avian model of social behavior. Gen Comp Endocrinol. 2008;157:275–282. doi: 10.1016/j.ygcen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goodson JL. Neurogenomic mechanisms of aggression in songbirds. Adv Genetics. 2011;75:83–119. doi: 10.1016/B978-0-12-380858-5.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Lange HS, Raees MQ, Reid AE, Sanford SE. Behavioral phenotypes exist after gonadal steroid manipulation in white-throated sparrows. Horm Behav. 2009;55:113–120. doi: 10.1016/j.yhbeh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm Behav. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311:189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Wingfield JC. Intraventricular infusion of arginine vasotocin induces singing in a female songbird. J Neuroendocrinol. 1997;9:487–491. doi: 10.1046/j.1365-2826.1997.00635.x. [DOI] [PubMed] [Google Scholar]
- Matragrano LL, LeBlanc MM, Chitrapu A, Blanton ZE, Maney DL. Testosterone alters genomic responses to song and monoaminergic innervation of auditory areas in a seasonally breeding songbird. Dev Neurobiol. 2013;73:455–468. doi: 10.1002/dneu.22072. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27:12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FL, Boyd SK, Kelley DB. Historical perspective: hormonal regulation of behaviors in amphibians. Horm Behav. 2005;48:373–383. doi: 10.1016/j.yhbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Ocampo-Daza D, Lewicka M, Larhammar D. The oxytocin/vasopressin receptor family has at least five members in the gnathostome lineage, including two distinct V2 subtypes. Gen Comp Endocrinol. 2012;175:135–143. doi: 10.1016/j.ygcen.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Aste N, Castagna C, Viglietti-Panzica C, Balthazart J. Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: behavioral implications. Brain Res Rev. 2001;37:178–200. doi: 10.1016/s0165-0173(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Plumari L, García-Ojeda E, Deviche P. Central vasotocin-immunoreactive system in a male passerine bird (Junco hyemalis) J Comp Neurol. 1999;409:105–117. [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Kinney LF, Lee TM. Day length and sociosexual cohabitation alter central oxytocin receptor binding in female meadow voles (Microtus pennsylvanicus) Behav Neurosci. 115:1349–56. [PubMed] [Google Scholar]
- Plumari L, Plateroti S, Deviche P, Panzica GC. Region-specific testosterone modulation of the vasotocin-immunoreactive system in male dark-eyed junco, Junco hyemalis. Brain Res. 2004;999:1–8. doi: 10.1016/j.brainres.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of α2-noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. J Comp Neurol. 2002;444:63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Slotow RH, Arnold AP. Plasma estrogens and brain aromatase in winter White-crowned Sparrows. Ornis Scand. 1992;23:292–297. [Google Scholar]
- Sockman KW, Sewall KB, Ball GF, Hahn TP. Economy of mate attraction in the Cassin’s finch. Biol Lett. 2005;1:34–37. doi: 10.1098/rsbl.2004.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5-alpha reductase, and 5-beta reductase change seasonally in wild male song sparrows: Relationship to aggressive and sexual behavior. J Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- Spinney LH, Bentley GE, Hall M. Endocrine correlates of alternative phenotypes in the white-throated sparrow. Horm Behav. 2006;50:762–771. doi: 10.1016/j.yhbeh.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Small TW, Ball GF, Moore IT. Variation in the gonadotrophin-releasing hormone-1 and the song control system in the tropical breeding rufous-collared sparrow (Zonotrichia capensis) is dependent on sex and reproductive state. Gen Comp Endocrinol. 2012;178:1–7. doi: 10.1016/j.ygcen.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett MB, Breuner CW. Plasma testosterone correlates with morph type across breeding substages in male white-throated sparrows. Physiol Biochem Zool. 2009;82:572–579. doi: 10.1086/605392. [DOI] [PubMed] [Google Scholar]
- Tan FL, Lolait SJ, Brownstein MJ, Saito N, MacLeod V, Baeyens DA, Mayeux PR, Jones SM, Cornett LE. Molecular cloning and functional characterization of a vasotocin receptor subtype that is expressed in the shell gland and brain of the domestic chicken. Biol Reprod. 2000;62:8–15. doi: 10.1095/biolreprod62.1.8. [DOI] [PubMed] [Google Scholar]
- Thayananuphat A, Youngren OM, Kang SW, Bakken T, Kosonsiriluk S, Chaiseha Y, El Halawani ME. Dopamine and mesotocin neurotransmission during the transition from incubation to brooding in the turkey. Horm Behav. 2011;60:327–335. doi: 10.1016/j.yhbeh.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Thomas JW, Caceres M, Lowman JJ, Morehouse CB, Short ME, Baldwin EL, Maney DL, Martin CL. The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement and suppressor of recombination. Genetics. 2008;179:1455–1468. doi: 10.1534/genetics.108.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneycroft HB. A cytogenetic study of the white-throated sparrow, Zonotrichia albicollis. Evolution. 1975;29:611–621. doi: 10.1111/j.1558-5646.1975.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Balthazart J, Plumari L, Fratesi S, Absil P, Panzica GC. Estradiol mediates effects of testosterone on vasotocin immunoreactivity in the adult quail brain. Horm Behav. 2001;40:445–461. doi: 10.1006/hbeh.2001.1710. [DOI] [PubMed] [Google Scholar]
- Voorhuis TAM, de Kloet ER, de Wied D. The distribution and plasticity of [3H]vasopressin-labeled specific binding sites in the canary brain. Brain Res. 1988a;457:148–153. doi: 10.1016/0006-8993(88)90067-4. [DOI] [PubMed] [Google Scholar]
- Voorhuis TAM, Kiss JZ, de Kloet ER, de Wied D. Testosterone-sensitive vasotocin-immunoreactive cells and fibers in the canary brain. Brain Res. 1988b;442:139–146. doi: 10.1016/0006-8993(88)91441-2. [DOI] [PubMed] [Google Scholar]
- Voorhuis TA, Elands JP, Kloet ER. Vasotocin target sites in the capsular region surrounding the nucleus robustus archistriatalis of the canary brain. J Neuroendocrinol. 1990;2:653–7. doi: 10.1111/j.1365-2826.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Voorhuis TA, De Kloet ER, De Wied D. Ontogenetic and seasonal changes in immunoreactive vasotocin in the canary brain. Brain Res Dev Brain Res. 1991;61:23–31. doi: 10.1016/0165-3806(91)90110-5. [DOI] [PubMed] [Google Scholar]
- Wacker DW, Wingfield JC, Davis JE, Meddle SL. Seasonal changes in aromatase and androgen receptor, but not estrogen receptor mRNA expression in the brain of the free-living male song sparrow, Melospiza melodia morphna. J Comp Neurol. 2010;518:3819–3835. doi: 10.1002/cne.22426. [DOI] [PubMed] [Google Scholar]
- Wada K, Sakaguchi H, Jarvis ED, Hagiwara M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J Comp Neurol. 2004;476:44–64. doi: 10.1002/cne.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann JN, Clifton DK, Steiner RA. Gonadotropin-releasing hormone messenger ribonucleic acid levels are unaltered with changes in the gonadal hormone milieu of the adult male rat. Endocrinology. 1990;127:523–532. doi: 10.1210/endo-127-2-523. [DOI] [PubMed] [Google Scholar]
- Wolfson A. Day length, migration, and breeding cycles in birds. Scientific Monthly. 1952;7:191–200. [Google Scholar]
- Young LJ, Wang Z, Cooper TT, Albers HE. Vasopressin (V1a) receptor binding, mRNA expression and transcriptional regulation by androgen in the Syrian hamster brain. J Neuroendocrinol. 2000;12:1179–1185. doi: 10.1046/j.1365-2826.2000.00573.x. [DOI] [PubMed] [Google Scholar]