Abstract
High angular resolution diffusion imaging (HARDI) tractography has provided insight into major white matter pathways and cortical development in the human fetal cerebrum. Our objective in this study was to further apply HARDI tracography to the developing human cerebellum ranging in fetal and adult stages, to outline in broad strokes the 3-dimensional development of white matter and local gray matter organization in the cerebellum. We imaged intact fixed fetal cerebellum specimens at 17 gestational weeks (W), 21W, 31W, 36W, and 38W along with an adult cerebellum for comparison. At the studied earliest gestational age (17W), coherent pathways that formed the superior, middle, inferior cerebellar peduncles were already detected, but pathways between deep cerebellar nuclei and the cortex were not observed until after 38W. At 36–38W, we identified emerging regional specification of the middle cerebellar peduncle. In the cerebellar cortex, we observed disappearance of radial organization in the sagittal orientation during the studied developmental stages similar to our previous observations in developing cerebral cortex. In contrast, in the axial orientation, cerebellar cortical pathways emerged first sparsely (31W) and then with increased prominence at 36–38W with pathways detected both in the radial and tangential directions to the cortical surface. The cerebellar vermis first contained only tangential pathways to the long axes of folia (17–21W), but pathways parallel to the long axes of folia emerged between 21–31W. Our results show the potential for tractography to image developing cerebellar connectivity using HARDI tractography.
Keywords: Development, Brain, Cerebellum, Human Fetus, Diffusion Imaging, Tractography
Introduction
The maturation of the human cerebellum is more protracted than that of the cerebrum and continues through the first postnatal year (Altman and Bayer, 1997; Wang and Zoghbi, 2001; Saksena et al., 2008). Like the cerebral cortex, the morphogenesis of the cerebellar cortex is characterized by phases of neuronal proliferation, migration, differentiation, axon growth, synaptogenesis, and pruning (Sidman and Rakic, 1973; Wang and Zoghbi, 2001; Lavezzi et al., 2006; Catz et al., 2008). Cerebellar development is distinct, however, in that granule cell precursors migrate in the reverse direction from the external granular layer inward past the molecular layer and Purkinje dendrites and somas, and the human cerebellar cortex is composed at first of two layers, then three, five, four, and finally after birth acquires the adult three layered pattern (Rakic and Sidman, 1970). In the cerebellar cortex, Bergmann glial fibers provide the radial guidance for this migration (Sidman and Rakic, 1973).
Coincident with the formation of these fiber pathways is the process of axonal myelination which starts well before birth in humans, and, depending on location in the brain, continues into adulthood (Yakovlev and Lecours, 1967; Brody et al., 1987). Cerebellar white matter does not myelinate uniformly, but along a temporal gradient commencing with the archicerebellum, and followed by the white matter of the paleocerebellum and neocerebellum (Gilles et al., 1976; Brody et al., 1987). Myelination in cerebellar white matter pathways begins during the third trimester and continues after birth. Myelination in the middle cerebellar peduncle (MCP) begins some weeks later, around the time of birth (Yakovlev and Lecours, 1967; Brody et al., 1987).
These myelination processes have been demonstrated in pathological specimens derived from human fetuses (Chong et al., 1997; Triulzi et al., 2005, 2006). They have also been shown using conventional MRI techniques (Barkovich et al., 1988; Van der Knaap and Valk, 1990; for review Paus et al., 2001). Diffusion tensor imaging (DTI), based on measurement of the directional bias water molecule diffusion in brain tissue (Basser et al., 1994) and associated post-processing data reconstruction using tractography techniques (Mori et al., 1999; Jones et al., 1999; Conturo et al., 1999), permits examination of white matter axonal organization running in many directions throughout the entire brain in vivo. Our understanding of developing human cerebellar organization lags behind the cerebrum, however, because with DTI tractography it is particularly difficult to resolve the 3-dimensional geometry of the cerebellar folia and associated connectivity.
Recently, high-angular resolution diffusion imaging (HARDI) has been shown to improve the characterization of complex tissue coherence compared to DTI, by defining a fiber orientation distribution function. This approach improves the ability to resolve different diffusion directions within the same voxel that result from crossing axonal bundles (Tuch et al., 2003, Leergaard et al., 2010). HARDI has been effective for delineating tissue coherence associated with the structural changes that occur in developing fetal (preterm) brains, in which the process of migration and myelination is incomplete.
A number of DTI studies on fetal and newborn human cerebrum (Rutherford et al., 1991; Sakuma et al., 1991; Huppi et al., 1998; Neil et al., 1998; Baratti et al., 1999; for review Neil et al., 2002; Gupta et al., 2005; Prayer et al., 2006; Rollins, 2007; Huang et al., 2009) as well as cerebellum (Saksena et al., 2008; Huang et al., 2009) have reported that FA values increase in the white matter with age. Some investigators have also performed DTI tractography in developing human brains including fetal and preterm brains (Berman et al., 2005; Bui et al., 2006; Huang et al., 2006; Bassi et al., 2008; Kasprian et al., 2008; Saksena et al., 2008; Huang et al., 2009; Tam et al., 2009) showing the development of major white matter pathways. We have applied HARDI tractography to immature cat and human cerebrum to provide whole-brain 3-dimensional visualization of developing fiber systems (Takahashi et al., 2010, Takahashi et al., 2011, 2012). However, to date there has been no HARDI tractography study of the developing human cerebellum. Our objective in this study was to apply HARDI tractography to developing human cerebellum during fetal and adult ages, to explore the 3-dimensional development of white matter and local gray matter pathways in the cerebellum.
Experimental Procedures
Datasets
We used human fetal cerebella at 17 gestational week (W), 21W, 31W, 36W, 38W (Engle 2004), as well as adult cerebella (eight samples in total; two samples at 17W and in adult). The cerebella were obtained from the Brigham and Women's Hospital Department of Pathology, under protocols approved by the hospital's institutional review board for human research. They include specimens from terminations, stillbirths, and neonatal deaths, submitted for pathologic examination after consent of parent(s) or guardian(s). A perinatal neuropathologist studied each sample at the time of post-mortem examination, and only those tissues not needed for immediate diagnosis were fixed in 4% paraformaldehyde and submitted for coded (de-identified) specimen scanning (mean fixation period was around 2–3 months). Any cases with known or suspected malformations, disruptions, or other lesions (on basis of in utero ultrasonography, or post-mortem findings) were excluded from this study. Cerebella were removed from the cranium and fixed in a 4% paraformaldehyde solution containing 1 mM gadolinium (Gd-DTPA) MRI contrast agent for at least 1 week to reduce the T1 relaxation time while ensuring sufficient T2-weighted signal remains. During image acquisition, the brains were placed in Fomblin solution (Ausimont, Thorofare, NJ).
Scanning parameters
The pulse sequence used for HARDI acquisition was a 3D diffusion-weighted spin-echo echo-planar imaging (EPI) sequence (Takahashi et al., 2012; Xu et al., 2012; Takahashi et al., 2013), TR/TE 1000/40 ms, with an imaging matrix of 112 × 112 × 128 pixels. Sixty diffusion-weighted measurements (b = 8,000 sec/mm2) and one non-diffusion-weighted measurement (b = 0 sec/mm2) were acquired at a 4.7T Bruker Biospec MR system with δ = 12.0 ms, Δ = 24.2 ms. Spatial resolution was 415 × 500 × 550µm for 17–22W, 525 × 525 × 600µm for 31W, 700 × 830 × 860µm for 36–40W, and 525 × 525 × 600µm for adult specimens. The adult specimens were scanned after being dissected in half in the midline. The total acquisition time was approximately 1 hour and 50 minutes for each imaging session.
Diffusion Data Analyses
Diffusion Toolkit and TrackVis (http://trackvis.org) were used to reconstruct and visualize tractography pathways. The color-coding of tractography pathways is based on a standard RGB code, applied to the vector between the end-points of each fiber. We used a streamline algorithm for diffusion tractography (Mori et al., 1999) described in previous publications (Takahashi et al., 2011, 2012). The term “streamline” refers to the fact that we connect tractography pathways using local maximum or maxima. This is true for both DTI and HARDI. The streamline technique is limited in its ability to resolve crossing pathways when used with the traditional DTI technique, because one simply connects the direction of the principal eigenvector on a tensor to produce the DTI tractography pathways. This is a recognized limitation of DTI, as discussed in the DTI paper of Mori (1999). For this reason, in the current study, we used HARDI, which detects multiple local maxima on an ODF (orientation distribution function). We used all the local maxima to produce HARDI tractography pathways, thus enabling us to identify crossing pathways within a voxel.
Trajectories were propagated by consistently pursuing the orientation vector of least curvature. We terminated tracking when the angle between two consecutive orientation vectors was greater than the given threshold (40°). As in previous studies, no FA threshold was applied (Vishwas 2009, Takahashi 2010). In many tractography studies, fractional anisotropy (FA) values are used to terminate fibers in the gray matter, which in adults has lower FA values than the white matter. However, as one of the objectives of our study was to detect fibers in low FA areas where myelination is not complete, we used brain mask volumes to terminate tractography fibers instead of the FA threshold (Takahashi et al., 2010). This method has been used previously (Takahashi et al., 2011) and is an acceptable alternate method (Schmahmann et al., 2007; Wedeen et al., 2008).
Results
Improved tractography results using a size-optimized MR coil for the cerebellum
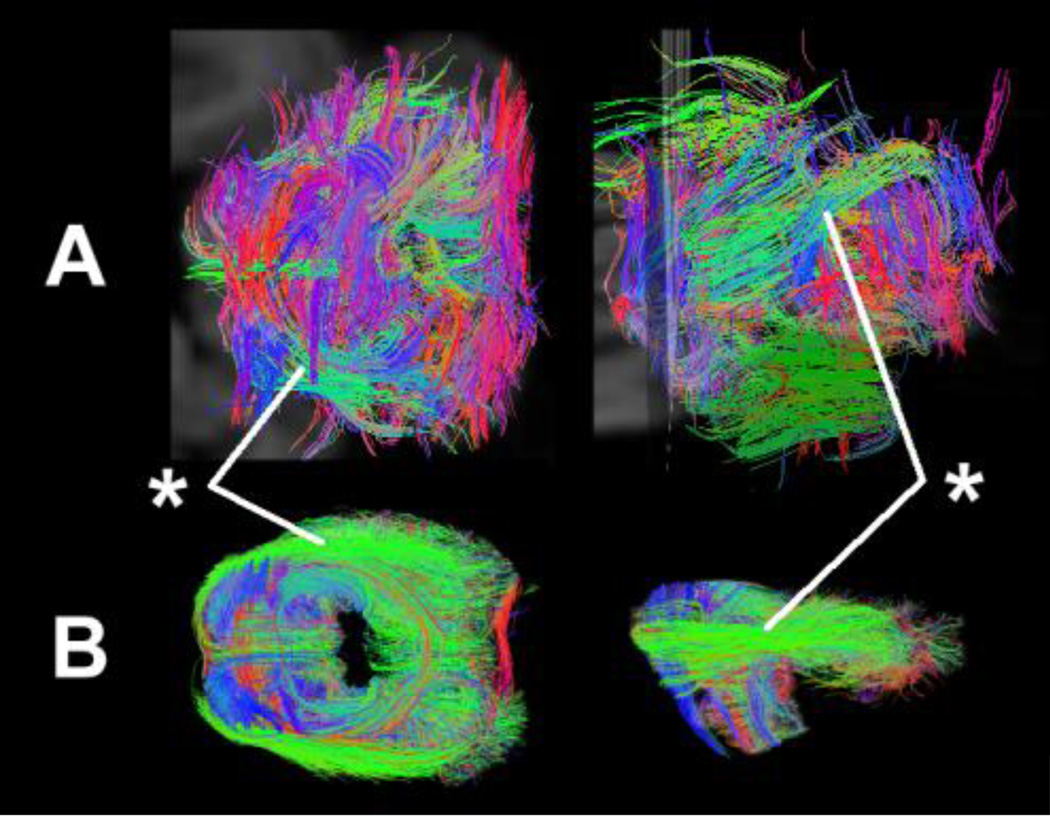
Signal-to-noise ratio and tractography outcomes significantly improved using a size-optimized RF coil for each sample (Fig. 1). The cerebellum with the cerebrum attached was scanned using a large coil for a whole brain (Fig. 1, upper row), which produced many short irregular non-anatomic tractography pathways that are inconsistent with out knowledge of cerebellar anatomy. We then used a size-optimized coil for the cerebellum after dissecting the cerebellum from the specimen, and obtained longer more coherent tractography pathways due to the higher signal-to-noise ratio and spatial resolution (Fig. 1, lower row).
Figure 1.
Comparison of tractography pathways using different RF coil. Upper row: Overview of cerebellar pathways using a large coil for a whole brain. Lower row: Overview of cerebellar pathways using a size-optimized coil for the cerebellum after dissecting the cerebellum from the specimen. Asterisks: the middle cerebellar peduncle.
Cerebellar white matter pathways development
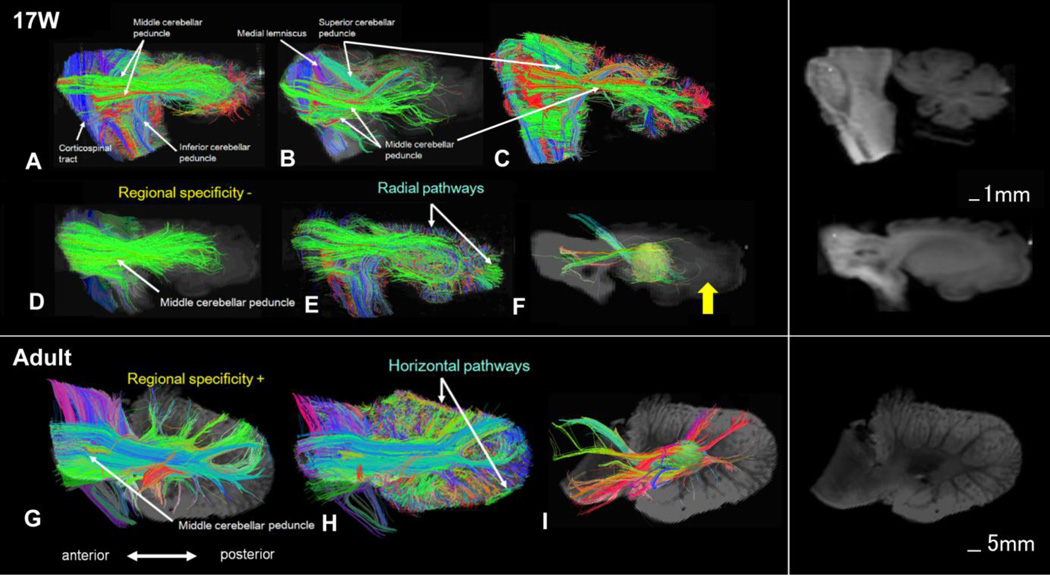
The corticospinal tracts and medial lemniscus were readily identified along with the superior, middle, inferior cerebellar peduncles at 17W (Fig. 2A–C). The tracks passing through the MCP did not reach the cerebellar cortex, but projected to broad areas in the cerebellar hemisphere, terminating deep to the cerebellar cortex (Fig. 2D, E). Pathways between deep cerebellar nuclei and the cerebellar cortex were not detected (Fig. 2F). In the adult cerebellum, the tracks passing through the MCP reached and projected to specific areas in the cerebellar cortex (Fig. 2G, H), and pathways between deep cerebellar nuclei and the cerebellar cortex were clearly observed (Fig. 2I).
Figure 2.
Sagittal views of cerebellar pathways at W17 (Fig. 2A–F) and in adults (Fig. 2G–I). Mean diffusion-weighted images in the background of tractography pathways are separately shown on the right side without tractography pathways.
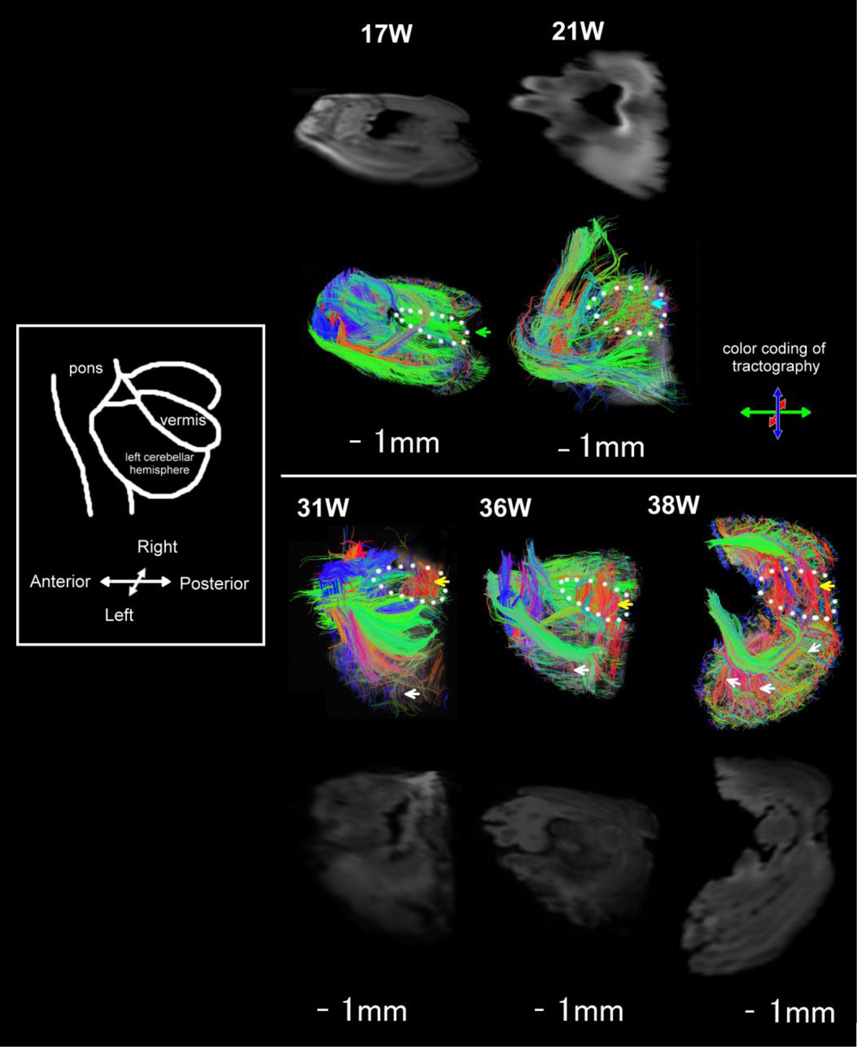
We then focused the analyses on the MCP to study the emerging regional specificity of the tracts passing through this peduncle and their relationships to the cerebellar cortex. As the cerebellar folia developed, the course of the MCP became deeper to the cerebellar surface at 17W. Gradually the main body of the MCP tract took a deeper course from the surface of the cerebellum at and after 31W (Fig. 3, arrowheads). The MCP fibers did not reach the cerebellar cortex in 17W to 21W. At and after 31W, tracts passing through the MCP penetrating into the cerebellar folia were observed (Fig. 3, asterisks). Regional specificity of the fibers conveyed in the MCP also became more evident at 31–38W.
Figure 3.
Evolution of the middle cerebellar peduncle (MCP) and its relationships to the cerebellar cortex at 17W– 38W. Mean diffusion-weighted images in the background of tractography pathways are separately shown side-by-side without tractography pathways. The main body of the MCP tract took a deeper course from the surface of the cerebellum at and after 31W (arrowheads). At and after 31W, tracts passing through the MCP penetrating into the cerebellar folia were observed (asterisks). Note that the reason why the MCP is not crossing the midsagittal line in 31W, 36W, and 38W is that the most regions of the pons in 31W and 36W, and the entire pons in 38W were removed during craniotomy, so the MCP pathways appeared to be cut off before reaching the midline.
Cerebellar cortical pathways development
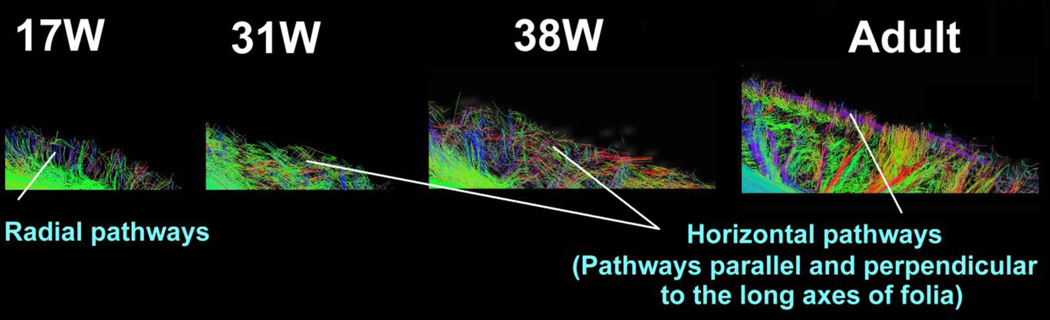
The cerebellar cortex contained dominant radial pathways at 17W in the sagittal plane (Fig. 2E, Fig. 4). Horizontal pathways (pathways parallel and perpendicular to the long axes of folia) emerged at or before 31W in the cerebellar cortex (Fig. 4). Radial pathways were not detected in the axial planes at 17W (Fig. 5). Only a few cortical pathways were identified in axial planes at 17–21W. At 31–36W, cortical pathways gradually emerged mainly in a direction tangential to the pial surface in axial planes, and only a few pathways were observed in the radial direction (Fig. 5, white arrows). At 38W, radial pathways in the cerebellar cortex increased their lengths coincident with increased thickness of the cerebellar cortex (Fig. 5, white arrows for example).
Figure 4.
Sagittal views of pathways in the cerebellar cortex. Tractography from brains that were damaged in the corresponding cortical region is not shown.
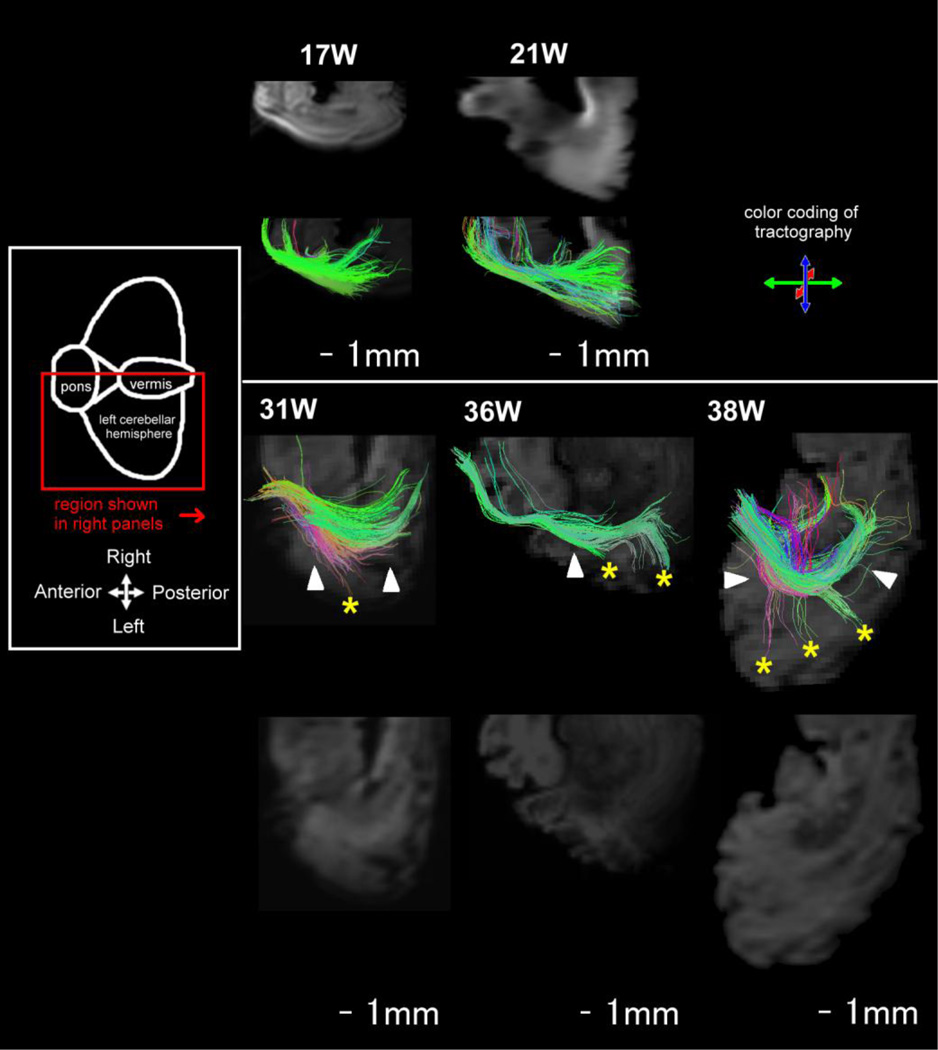
Figure 5.
Oblique views of cerebellar tractography pathways at 17W–38W. Mean diffusion-weighted images in the background of tractography pathways are separately shown side-by-side without tractography pathways. White arrows: a few pathways observed in the radial direction from the deep cerebellar white matter to the cerebellar cortex at 31W–38W. Pathways in the cerebellar vermis are indicated by a green arrow at 17W, by a blue arrow at 21W, and by yellow arrows at 31W–38W. Dotted lines indicate the location of he cerebellar vermis.
In the cerebellar vermis (Fig. 5, dotted lines), only superficial pathways perpendicular to the long axes of folia running anterior to posterior were evident at 17W (green pathways) (Fig. 5, green arrow). At 21W, in addition to the perpendicular pathways at 17W, pathways parallel to the long axes of folia were vaguely detected, and the parallel and perpendicular pathways were crossing one another (Fig. 5, blue arrow). Pathways parallel to the long axes of folia (red pathways) became obvious from 31W, and at 36–38W, the cerebellar vermis contained distinct parallel pathways (red pathways) (Fig. 5, yellow arrows).
Changes in FA values during development
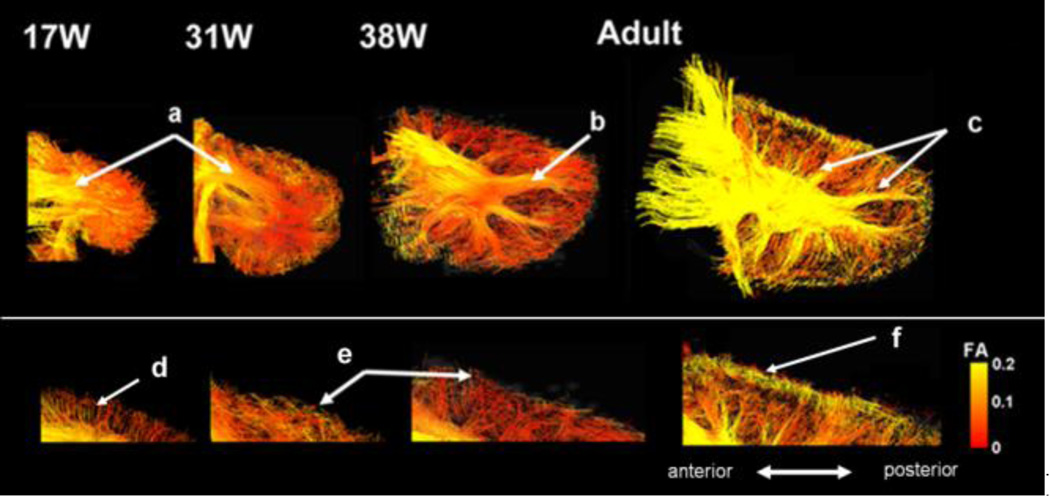
In the white matter, only deep white matter showed high FA values at 17–31W (Fig. 6a). At 38W, branching fiber pathways to the cerebellar cortex started to show high FA values (Fig. 6b). In the cerebellar cortex, abundant radial pathways with low FA values were detected at 17–22W (Fig. 6d) but not horizontal pathways (pathways parallel and perpendicular to the long axes of folia) as observed in later stages. Between 31W and 38W, parallel pathways were emerging in the cerebellar cortex (Fig. 6e, see also Fig 4). FA values of those horizontal pathways were relatively low until 38W (Fig. 6e), but clearly high in adult (Fig. 6f).
Figure 6.
Sagittal views of cerebellar pathways at 17W–38W and in the adult color-coded by FA values. a)–c): cerebellar white matter pathways. d)–f): cerebellar cortex pathways. Tractography from brains that were damaged in the corresponding cortical region is not shown.
Discussion
Our results show the usefulness of HARDI tractography to image developing cerebellar connectivity. We observed disappearance of radial organization in the cerebellar cortex and the emergence of regional specificity of cerebellar peduncles that were similar to our previous observations on the development of cerebral cortex. Our results demonstrated the potential for HARDI tractography to improve our understanding of neuronal circuitry and connectivity in both white and gray matter in the developing cerebellum.
Development of cerebellar cortex - Changes of FA values
Consistent with our previous tractography studies on the human fetal and cat newborn cerebral cortex (Takahashi et al., 2012, 2011), pathways in the cerebellar cortex were dominantly radial at an early gestational stage (17W). However, FA values of such radial pathways were relatively low, unlike in the previous studies on the developing cerebral cortex (Takahashi et al., 2012, 2011). The FA values in the cerebellar cortex stayed low throughout the gestational ages before birth in this study. In the cerebellar cortex, Bergmann’s glial fibers serve as a scaffolding structure for neurons migrating radially to/from the cerebellar surface (Qu and Smith, 2005). Our findings of dominant radial tractography pathways at 17W is in good agreement with the previous reports: at 17W, radial migration of immature Purkinje cells from the ventricular zone to the Purkinje cell layer is almost complete, and radial migration of granular cells from the surface of the cerebellar cortex to internal layers as well as radial migration of neurons from the ventricular zone to deep cerebellar nuclei begins around this fetal stage (Sidman and Rakic, 1973). Most of the radial tractography pathways at 17W probably reflected all the three types of radial migration, because the tractography pathways were mostly continuous throughout the developing cerebellar cortex at this stage (Fig. 4).
The low FA values in the cerebellar cortex between 31–38W could be explained by crossing pathways as observed in Figures 4 and 5: radial pathways may represent Bergmann’s glial fibers along which granule cells migrate, and tangential pathways may represent emerging dendrites of Purkinje cells. However, there were only few crossing tractography pathways found in the cerebellar cortex at 17W. Given that the cerebellar cortex at this stage shows a high amount of non-axonal cellular or molecular components that may also influence the local diffusion properties and lead to local anisotropy visualized as trajectory (see Kostovic and Jovanov-Milosevic, 2006), detailed correlation studies with histology will help refine the interpretation of diffusion tractography results at this developmental stage.
Development of cerebellar cortex - Timing of the emergence of parallel fibers
Emerging pathways running parallel to the long axes of folia in the cerebellar vermis were first imaged at 21W along with pathways running perpendicular to the folia that existed from the earliest studied developmental stage (17W), and the pathways parallel to the folia became evident and dominant in the cerebellar vermis around 31W (Fig. 5). Given that the dominant component of fiber pathways running parallel to the long axes of folia is the parallel fibers, the emerging tractography pathways parallel to the folia likely reflect the emergence of these parallel fibers. In the mouse cerebellum, parallel fibers develop after birth: postmitotic granule cells differentiate and migrate around birth, and parallel fiber growth occurs and they become almost fully matured within a few days of cell birth at postnatal day (P)3–P5 (Soha et al., 1997), which corresponds to about 21–24W in humans (Clancy et al., 2007). Our results are in good agreement with the report from Soha et al. (1997) in terms of the timing of parallel fiber development, although the tractography pathways did not yet seem to be fully mature at 21W. The study of more specimens between 21W and 31W should make it possible to define the time of maturation of human parallel fibers with greater accuracy.
Development of cerebellar white matter - Changes of FA values in the white matter
Myelination in cerebellar white matter pathways begins during the third trimester and continues post-natally. Myelination in the MCP begins some weeks later, around the time of birth (Yakovlev and Lecours, 1967; Brody et al., 1987). It has been reported that the clear increase in FA values in the cerebellar white matter, which partly reflects the degree of myelination, occurs during the first 3 years (Saksena et al., 2008). Our present results agree with these previous findings: the clear increase of FA values in the cerebellar white matter occured sometime after 38W. Interestingly, we identified in the current study the main stems of the MCP at the earliest studied developmental stage (17W), but branched white matter pathways from the main stems were only identified after 31W. Given that the MCP is not myelinated at these early gestational stages, and that we have been able to identify tissue coherence with tractography even in areas with low myelination (Takahashi et al., 2010, 2011), the timing of the identification of the branched MCP tractography pathways may correspond to the timing of axonal penetration into the cerebellar folia.
Development of cerebellar white matter - Development of fibers from deep cerebellar nuclei
Continuous tractography pathways from deep cerebellar nuclei to/from the cerebellar cortex were not detected in the fetal stages that we studied. We did, however detect such pathways in the adult cerebellum. Our results are in well agreement with the known fiber development between deep cerebellar nuclei and cerebellar cortex. For example, neurons in the dentate nucleus begin to proliferate and maturate before 30W (Mihajlovic and Zecevic, 1986; Hayaran et al., 1992; Milosević et al., 2010), but Purkinje cell corticonuclear fibers, and reciprocal nucleocortical and nuclear efferent fibers to brainstem and thalamus begin to be formed around or after birth and continue for about 6 months postnatally.
Limitations of the current study
We are aware that the results may be sensitive to data quality. We therefore excluded cerebellum specimens that were grossly damaged or were not in good condition. Hence we ended up with six prenatal samples. The cerebellum is more difficult to obtain intact than the cerebrum samples, because it is easily damaged when the cerebrum is taken out, and by the time when pathologists reach the cerebellum, usually more postmortem time passes. The postmortem interval could also affect the data quality, but it was not recorded by pathologists. We will try to collect the information in our future study.
We also admit that a presentation of histology data would be necessary to validate our current results. However, the focus of the current study is to show gross and global anatomical changes during development, and histological correlation is beyond our scope. We have previously done a similar sequence of research studies in the cerebrum. One of our papers (Takahashi et al., 2012) presented only cerebrum tractography pathways without quantification, and we validated key tractography findings with histology in a follow-up paper (Xu et al., 2013). Moreover, as shown in the Xu’s paper, correlation between tractography and histology is not always straight-forward with having a significant scaling difference between macro (tractography) and micro (histology). Such work would be a whole other project.
Our current study is descriptive and some quantification might help to improve the work. However, we only have 1 or 2 samples at each time-point, and at some time-points, one of the samples was not in good condition across all cerebellar regions. In such regions, quantification will not be reliable. Moreover, measures from 2 samples at each time-point still might not be enough to quantitatively conclude measures across ages. We therefore have minimized confusing quantitative-like wordings unless it is obvious. We believe that Figure 6 is a kind of quantification, showing FA maps of tractography pathways with an FA scale bar, which would be a solution for not extracting one value from each sample with uncertain reproducibility. In this study we used a threshold of 40° for tractography. Although the choice of angle thresholds to terminate tractography fibers in many tractography studies is somewhat arbitrary (ranging from 35°–60°), such arbitrary selection has the potential to cause erroneous findings. According to the complexity of the visualized fibers and the fact that regional differences in maturation of gray matter (e.g. thickness and contents of the developing cerebellar cortex) and white matter (e.g. degrees of myelination, cell migration) depend on developmental stages, it may be necessary in future studies to utilize different tractography settings for different brain areas in different developmental stages. Further, optimal angle threshold for each brain area may depend on spatial resolutions that are partly dependent on brain size.
In fetal and newborn brains, cerebellar cortex is characterized by low FA values which have been difficult to image. In this study, we successfully imaged developing pathways in the cerebellar cortex by increasing the sensitivity of the technique in areas with low FA values. This approach is potentially disadvantageous in regions with high FA such as in the white matter. In these very high FA regions, however, ODFs tend to resemble the ellipsoids obtained using DTI, rendering them less problematic.
Acknowledgments
This work was supported the Eunice Shriver Kennedy National Institute of Child Health and Development (NICHD) (R01HD078561, R21HD069001) (ET) and National Institute of Mental Health (R01MH06044) (JDS). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41RR14075, a P41 Regional Resource supported by the Biomedical Technology Program of the National Center for Research Resources (NCRR), National Institutes of Health. This work also involved the use of instrumentation supported by the NCRR Shared Instrumentation Grant Program (1S10RR023401, 1S10RR019307, and 1S10RR023043) and High-End Instrumentation Grant Program (S10RR016811). The brain specimens were provided by Dr. Rebecca D. Folkerth, Brigham and Women’s Hospital, Boston Children’s Hospital, Harvard Medical School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Bayer SA. Development of the Cerebellar System in Relation to Its Evolution, Structure, and Function. Boca Raton, FL: CRC Press, Inc.; 1997. [Google Scholar]
- Baratti C, Barnett AS, Pierpaoli C. Comparative MR imaging study of brain maturation in kittens with T1, T2, and the trace of the diffusion tensor. Radiology. 1999;210:133–142. doi: 10.1148/radiology.210.1.r99ja09133. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166:173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Bassi L, Ricci D, Volzone A, Allsop JM, Srinivasan L, Pai A, Ribes C, Ramenghi LA, Mercuri E, Mosca F, Edwards AD, Cowan FM, Rutherford MA, Counsell SJ. Probabilistic diffusion tractography of the optic radiations and visual function in preterm infants at term equivalent age. Brain. 2008;131:573–582. doi: 10.1093/brain/awm327. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, Vigneron DB, Henry RG. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27:862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J. Neuropathol. Exp. Neurol. 1987;46:283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Bui T, Daire J-L, Chalard F, Zaccaria I, Alberti C, Elmaleh M, Garel C, Luton D, Blanc N, Sebag G. Microstructural development of human brain assessed in utero by diffusion tensor imaging. Peatr. Radiol. 2006;36:1133–1140. doi: 10.1007/s00247-006-0266-3. [DOI] [PubMed] [Google Scholar]
- Catz N, Dicke PW, Their P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci U S A. 2008;105:7309–7314. doi: 10.1073/pnas.0706032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong BW, Babcook CJ, Pang D, Ellis WG. A magnetic resonance template for normal cerebellar development in the human fetus. Neurosurgery. 1997;41:924–928. doi: 10.1097/00006123-199710000-00029. discussion 928–929. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich RB, Bradley WG, Zaragoza EJ, I11V, Otto RJ, Taira RK, Wilson GH, et al. MR evaluation of early myelination patterns in normal and developmentally delayed infants. AJR Am J Roentgenol. 1988;150:889–896. doi: 10.2214/ajr.150.4.889. [DOI] [PubMed] [Google Scholar]
- Engle WA. Age terminology during the perinatal period. American Academy of Pediatrics Committee on Fetus and Newborn. Pediatrics. 2004 Nov;114(5):1362–1364. doi: 10.1542/peds.2004-1915. [DOI] [PubMed] [Google Scholar]
- Gilles FH, Dooling E, Fulchicro A. Sequence of myelination in the human fetus. Tran Am Neurol Assoc. 1976;101:244–246. [PubMed] [Google Scholar]
- Hayaran A, Wadhwa S, Bijlani V. Cytoarchitectural development of the human dentate nucleus: a Golgi study. Dev Neurosci. 1992;14:181–194. doi: 10.1159/000111662. [DOI] [PubMed] [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards L, Yarowsky P, Miller MI, Mori S. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J. Neurosci. 2009;29:4263–4273. doi: 10.1523/JNEUROSCI.2769-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards L, Yarowsky P, Donohue P, Graham E, van Zjil PCM, et al. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Jones DK, Simmons A, Williams SC, Horsfield MA. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn. Reson. Med. 1999;42:37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonat Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Terni L, Matturri L. Histological and biological developmental characterization of the human cerebellar cortex. Int J Dev Neurosci. 2006;24:365–371. doi: 10.1016/j.ijdevneu.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Leergaard TB, White NS, de Crespigny A, Bolstad I, D’Arceuil H, Bjaalie JG, Dale AM. Quantitative histological validation of diffusion MRI fiber orientation distributions in the rat brain. PLoS ONE. 2010;5(1):e8595. doi: 10.1371/journal.pone.0008595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihajlovic P, Zecevic N. Development of the human dentate nucleus. Hum Neurobiol. Hum Neurobiol. 1986;5:189–197. [PubMed] [Google Scholar]
- Milosević NT, Ristanović D, Marić DL, Rajković K. Morphology and cell classification of large neurons in the adult human dentate nucleus: a quantitative study. Neurosci Lett. 2010;468:59–63. doi: 10.1016/j.neulet.2009.10.063. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zjl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CF, Akbudak E, Aronovitz JA, Miller JP, Lee BC, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;15:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- Neil JJ, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain—a technical review. NMR Biomed. 2002;15:543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res. Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Prayer D, Kasprian G, Krampl E, Ulm B, Witzani L, Prayer L, Brugger PC. MRI of normal fetal brain development. Eur. J. Radiol. 2006;57:199–216. doi: 10.1016/j.ejrad.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Qu Q, Smith FI. Neuronal migration defects in cerebellum of the Largemyd mouse are associated with disruptions in Bergmann glia organization and delayed migration of granule neurons. Cerebellum. 2005;4:261–270. doi: 10.1080/14734220500358351. [DOI] [PubMed] [Google Scholar]
- Rakic P, Sidman RL. Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J Comp Neurol. 1970;139:473–500. doi: 10.1002/cne.901390407. [DOI] [PubMed] [Google Scholar]
- Rollins NK. Clinical applications of diffusion tensor imaging and tractography in children. Pediatr. Radiol. 2007;37:769–780. doi: 10.1007/s00247-007-0524-z. [DOI] [PubMed] [Google Scholar]
- Rutherford MA, Cowan FM, Manzur AY, Dubowitz LM, Pennock JM, Hajnal JV, Young IR, Bydder GM. MR imaging of anisotropically restricted diffusion in the brain of neonates and infants. J Comput Assist Tomogr. 1991;15:188–198. doi: 10.1097/00004728-199103000-00002. [DOI] [PubMed] [Google Scholar]
- Sakuma H, Nomura Y, Takeda K, Tagami T, Nakagawa T, Tamagawa Y, Ishii Y, Tsukamoto T. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion weighted MR imaging. Radiology. 1991;180:229–233. doi: 10.1148/radiology.180.1.2052700. [DOI] [PubMed] [Google Scholar]
- Saksena S, Husain N, Das V, Pradhan M, Trivedi R, Srivastava S, Malik GK, Rathore RKS, Sarma M, Pandey CM, Gupta RK. Diffusion tensor imaging in the developing human cerebellum with histologic correlation. Int. J. Devl Neurosci. 2008;26:705–711. doi: 10.1016/j.ijdevneu.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schneider JF, Il’yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Soha JM, Kim S, Crandall JE, Vogel MW. Rapid growth of parallel fibers in the cerebella of normal and staggerer mutant mice. J Comp Neurol. 1997;389:642–654. doi: 10.1002/(sici)1096-9861(19971229)389:4<642::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Folkerth RD, Galaburda A, Grant PE. Emerging cerebral connectivity in the human fetal brain: An MR tractography study. Cereb Cortex. 2012;22:455–464. doi: 10.1093/cercor/bhr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Dai G, Wang R, Ohki K, Rosen GD, Galaburda A, Grant PE, Wedeen VJ. Development of cerebral fiber pathways in cats revealed by diffusion spectrum imaging. Neuroimage. 2010;49:1231–1240. doi: 10.1016/j.neuroimage.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Dai G, Rosen GD, Wang R, Ohki K, Folkerth RD, Galaburda A, Wedeen VJ, Grant PE. Developing neocortex organization and connectivity in cats revealed by direct correlation of diffusion tractography and histology. Cereb Cortex. 2011;21:200–211. doi: 10.1093/cercor/bhq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Song JW, Folkerth RD, Grant PE, Schmahmann JD. Detection of cerebellar cortex and white matter pathways using high angular resolution diffusion tractography. Neuroimage. 2013;68:105–111. doi: 10.1016/j.neuroimage.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam EYW, Ferriero DM, Xu D, Berman JI, Vigneron DB, Barkovich AJ, Miller SP. Cerebellar Development in the Preterm Neonate: Effect of Supratentorial Brain Injury. Pediatr Res. 2009;66:102–106. doi: 10.1203/PDR.0b013e3181a1fb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triulzi F, Parazzini C, Righini A. MRI of fetal and neonatal cerebellar development. Semin Fetal Neonatal Med. 2005;10:411–420. doi: 10.1016/j.siny.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Triulzi F, Parazzini C, Righini A. Magnetic resonance imaging of fetal cerebellar development. Cerebellum. 2006;5:199–205. doi: 10.1080/14734220600589210. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Wedeen VJ. Diffusion MRI of complex neural architecture. Neuron. 2003;40:885–895. doi: 10.1016/s0896-6273(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Van der Knaap MS, Valk J. MR imaging of the various stages of normal myelination during the first year of life. Neuroradiology. 1990;31:459–470. doi: 10.1007/BF00340123. [DOI] [PubMed] [Google Scholar]
- Vishwas M, Chitnis T, Pienaar R, Healy BC, Grant PE. Tract based analysis of callosal, projection and association pathways in pediatric patients with multiple sclerosis: A preliminary study. Am J Neuroradiol. 2010;31:121–128. doi: 10.3174/ajnr.A1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D’Arceuil H, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation in the brain. In: Minowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell; 1967. pp. 3–69. [Google Scholar]