Abstract
Purpose
To examine relationships following adjuvant chemotherapy between circulating proinflammatory cytokines, regional cerebral metabolism, and cognitive complaints in early stage breast cancer patients.
Patients and Methods
33 breast cancer patients who had completed initial treatment (surgery, ± radiation, 23 chemotherapy, 10 no chemotherapy) obtained resting (18)F-FDG PET/CT brain imaging at baseline and one year later. Pro-inflammatory cytokine markers (IL-1ra, sTNF-RII, CRP, and IL-6) and cognitive complaints were also assessed at both time points.
Results
At baseline, consistent correlations were seen between the left medial frontal and right inferior lateral anterior temporal cortices and inflammatory markers within the chemotherapy group, and not in the group who did not receive chemotherapy. After one year, correlations persisted in the medial frontal cortex and the temporal cortex, the latter shifting superiorly. Both of these regional correlations demonstrated the highest levels of significance when looking across the one year time frame (IL-1ra: peak voxel p<0.0005; cluster size p<0.0005, p=0.001 after correction (medial prefrontal), p<0.0005; cluster size p=0.001, p=0.029 corr. (anterior temporal), sTNF-RII: p<0.0005; cluster size p=0.001, p=0.040 corr. (medial prefrontal)). Positive correlations were also seen within the chemotherapy group between baseline memory complaints and the medial frontal (p<0.0005; cluster size p<0.0005, p<0.0005 corr.) and anterior temporal (p<0.0005; cluster size p<0.0005, p=0.002 corr.) cortices at baseline and one year later.
Conclusion
Metabolism in the medial prefrontal cortex and anterior temporal cortex was found to correlate with both memory complaints and cytokine marker levels in chemotherapy patients.
Keywords: Positron emission tomography, Brain imaging, Breast cancer, Cognitive complaints, Chemotherapy, Pro-inflammatory cytokines
1 Introduction
Cancer is a leading cause of death worldwide, accounting for nearly 8 million deaths per year (WHO 2012). In the United States, the American Cancer Society estimates an incidence of 1.6 million new cases of cancer annually. With advances in diagnosis and treatment, more people are surviving cancer and living long after initial diagnosis and therapy. For example, of the more than 0.2 million people who are diagnosed with breast cancer each year, 90% survive at least 5 years (ACS 2011). This increased survival has shed light on a sizable portion of patients who complain of cognitive dysfunction following cancer treatment, sometimes for years or even decades, after treatment had been completed.
There has been a large increase in research on this subject over the past few years, focusing mainly on breast cancer patients. Most early studies were cross-sectional and examined breast cancer survivors (Ahles et al. 2002; Brezden et al. 2000; Castellon et al. 2004; Schagen et al. 1999; Tchen et al. 2003; van Dam et al. 1998). Many found lower neuropsychological (NP) performance in chemotherapy treated patients, demonstrating that the scope of neurocognitive alterations is wide and can include problems with attention, learning, retrieval, language, visuoperception, construction abilities, and motor skills (Argyriou et al. 2011). Executive function, processing speed, and memory seem to be especially affected (Correa and Ahles 2008; Dietrich et al. 2008; Wefel et al. 2004; Wefel and Schagen 2012; Wefel et al. 2008). Early imaging studies with functional magnetic resonance imaging (fMRI), structural MRI, and positron emission tomography (PET) also displayed alterations in frontal and cerebellar regions in chemotherapy treated patients (Ferguson et al. 2007; Silverman et al. 2007; McDonald et al. 2010). Recently, many prospective longitudinal studies have been conducted showing similar neuropsychological dysfunction and alterations in brain structure and function including both white and grey matter (Ahles et al. 2010; Biglia et al. 2012; Collins et al. 2009; Deprez et al. 2012; Deprez et al. 2011; Jansen et al. 2011; McDonald et al. 2012; Wefel et al. 2010; McDonald et al. 2010).
A possible mechanism for these changes seen in chemotherapy treated patients involves the association between pro-inflammatory cytokines and decline in cognitive function (Barber 2011; Fritz-French and Tyor 2012; Kuo et al. 2005; O’Bryant et al. 2010; Teunissen et al. 2003; Tilvis et al. 2004). Cytokines can cross the blood brain barrier through active transport, a possible humoral route, and in regions where the blood brain barrier is weaker, including circumventricular areas (Myers 2010). Both administration and production of cytokines has been associated with a type of sickness behavior that includes inability to conce1ntrate and impaired learning (Myers 2010). In addition, animal studies have demonstrated that cytokines can produce specific neuropathologic changes and functional impairment (Lee et al. 2004). Data are also available in humans. In healthy individuals, an inverse relationship was found between circulating IL-6 levels and performance on executive function, attention and working memory, and auditory recognition memory tests (Marsland et al. 2006). Recent studies of breast cancer patients have also shown reduced memory performance and elevated IL-6 and TNFα concentrations in chemotherapy treated patients (Kesler et al. 2012), and we recently reported higher sTNF-RII concentrations associated with increased memory complaints in chemotherapyexposed patients (P. A. Ganz et al. 2012). Additionally, studies have shown an association between chemotherapy and elevated inflammatory cytokines (Pusztai et al. 2004; Torres et al. 2013; Wang et al. 2012).
In order to further investigate the associations between cytokines, cognitive function, and chemotherapy, we began a prospective, longitudinal, observational cohort study in 2007 (the UCLA Mind Body Study [MBS]) of early stage, newly-diagnosed breast cancer patients, described in greater detail previously (P. A. Ganz et al. 2012). Participants were recruited after the completion of primary treatment (surgery, radiation treatment, adjuvant chemotherapy treatment), and before the start of endocrine treatment. With this design, we aimed to compare patients who underwent chemotherapy vs. those who did not undergo chemotherapy, with assessment both before and after endocrine treatment if indicated. We also wanted to investigate the potential role of reproductive factors in the presenting cognitive dysfunction.
The MBS evaluated self-report cognitive complaints, standardized neuropsychological test performance, brain metabolic data (a sub-study population), behavioral symptoms (fatigue, depression, insomnia), and immune alterations (pro-inflammatory cytokines), at baseline (T1) and one year later (T3) (All measures except brain imaging were measured at a 6-month interval as well (T2). They will not be reported in this paper focusing on the brain imaging). Additionally, findings concerning many of the variables measured in the large MBS study have been reported elsewhere (Bower et al. 2011; Bower et al. 2013; P. A. Ganz et al. 2012; P. Ganz et al. (in press)). Over all, this larger study was designed to examine the possible effects of chemotherapy, endocrine therapy, radiation therapy, fatigue, depression, insomnia, levels of pro-inflammatory cytokines, and endogenous hormones on cognitive function.
In this report, we present data on the PET sub-study population and their relationships between cerebral metabolic data, a battery of four inflammatory markers, and self-reported cognitive complaints, with both cross-sectional analysis at baseline (post-chemotherapy, prior to endocrine therapy) and one year later, and evaluating correlations longitudinally. Based on the previous associations seen between cytokines and cognitive changes (Barber 2011; Fritz-French and Tyor 2012; Kuo et al. 2005; O’Bryant et al. 2010; Teunissen et al. 2003; Tilvis et al. 2004), we hypothesized to also see changes in regional brain metabolism associated with cytokine levels, and also with cognitive complaints. In the present paper, we report on correlations with self-reported cognitive complaints rather than standardized neuropsychological test performance due to more significant differences between the chemotherapy group and no chemotherapy group in self-reported complaints.
Methods
2.1 Patients
This observational cohort study recruited women with early stage breast cancer from the Los Angeles community. Women entering the study could have undergone chemotherapy prior to the study or not. The study inclusion criteria were: (1) women aged 21-65 years; (2) newly diagnosed with Stage 0, I, II, or IIIA breast cancer; (3) completion of primary treatment (surgery, radiation treatment, and/or adjuvant chemotherapy treatment); (4) prior to start of endocrine therapy if planned; (5) geographically accessible for 1-year follow-up; (6) English language proficient; (7) able to provide informed consent. Exclusion criteria included: (1) evidence of current or past disorder/disease of the central nervous system or any medical condition that might be expected to impact cognitive functioning (e.g. multiple sclerosis, thyroid dysfunction); (2) history of head trauma with loss of consciousness greater than 30 min; (3) epilepsy, dementia, or severe learning disability; (4) current psychotic-spectrum disorder (e.g. schizophrenia, bipolar disorder, major depressive disorder) or current substance abuse or dependence; (5) history of whole brain irradiation or surgery; (6) history of past cancer treatment with chemotherapy; (7) active diagnosis of autoimmune and/or inflammatory disorder (e.g., systemic lupus erythematosis, rheumatoid arthritis, vasculitis) or disorders that may influence inflammatory processes (e.g. uncontrolled allergic condition or asthma); (8) chronic use of oral steroid medication; (9) hormone therapy (estrogen, progestin compounds) other than vaginal estrogen. The medical conditions noted above and women older than 65 were excluded because of the known potential impact on cognitive function or inflammation. A total of 190 patients were recruited into the MBS, and 33 participated in the PET sub-study. Table 1 displays the demographics of this sub-population. One of the 33 participants was excluded from baseline analyses including the pro-inflammatory cytokines because of lack of inflammatory measures at baseline and one year later, 3 other participants did not return for the follow-up PET scan, and 2 additional participants did not have inflammatory measures at T3 because of technical difficulty with drawing or processing of blood samples. Specifics of recruitment have been previously described (Bower et al. 2011; P. A. Ganz et al. 2012).
Table 1.
Patient demographic and medical characteristics
| Total (n=33) |
Chemo (n=23) |
No Chemo (n=10) |
p-valuea | ||||
|---|---|---|---|---|---|---|---|
| Percent | N | Percent | N | Percent | N | ||
| or Mean (SD) | or Mean (SD) | or Mean (SD) | |||||
| Age at baseline | 52.2 (9.0) | 50.7 (9.5) | 55.7 (6.8) | .14 | |||
| Race: White | 70% | 23 | 61% | 14 | 90% | 9 | .12 |
| Marital status: Married | 58% | 19 | 52% | 12 | 70% | 7 | .46 |
| Education Post college College Post high school |
45% 33% 21% |
15 11 7 |
52% 30% 17% |
12 7 4 |
30% 40% 30% |
3 4 3 |
.44 |
| WTAR | 116.0 (7.8) | 116.3 (7.8) | 115.2 (8.0) | .71 | |||
| Employment: Full- or Part-time | 61% | 20 | 70% | 16 | 40% | 4 | .14 |
| Annual Household income: >$100,000 |
66% |
21 |
61% |
14 |
78% |
7 |
.44 |
| BMI | 26.7 (5.0) | 27.4 (5.2) | 25.1 (4.5) | .24 | |||
| Surgery Mastectomy Lumpectomy |
18% 82% |
6 27 |
17% 83% |
4 19 |
20% 80% |
2 8 |
1.00 |
| Radiation: Yes | 85% | 28 | 87% | 20 | 80% | 8 | .63 |
| History of Hormone Replacement Therapy: Yes |
30% |
10 |
26% |
6 |
40% |
4 |
.44 |
| Change in period after dx/treatment Post-menopausal No change Became irregular Stopped but resumed Amenorrhea |
52% 9% 3% 0% 36% |
17 3 1 0 12 |
48% 4% 0% 0% 48% |
11 1 0 0 11 |
60% 20% 10% 0% 10% |
6 2 1 0 1 |
.05 |
| Chemotherapy regimen: Anthracycline containing |
35% |
8 |
35% |
8 |
n/a |
n/a |
n/a |
| Received endocrine therapy after baseline Type of endocrine therapy if yes Tamoxifen Aromatase inhibitor |
61% 65% 35% |
20 13 7 |
61% 64% 36% |
14 9 5 |
60% 67% 33% |
6 4 2 |
1.00 |
| Stage at diagnosis 0 1 2 3 |
9% 38% 44% 9% |
3 12 14 3 |
0% 27% 59% 14% |
0 6 13 3 |
30% 60% 10% 0% |
3 6 1 0 |
.002b |
P-values are the result of t-tests for continuous variables, or Fisher’s Exact test for categorical variables
This p-value reflects a comparison of stage 0/1 vs. stage 2/3
WTAR=Wechsler Test of Adult Reading; BMI=Body Mass Index
2.2 Measures
Demographic and clinical information was obtained from self-report and medical record abstraction. Cognitive complaints were assessed with the Patient’s Assessment of Own Functioning Inventory (PAOFI), where higher scores indicate more cognitive complaints (Chelune et al. 1986). PAOFI has four sub-scores: memory, high level cognition, sensory motor, and language/communication. Participants may answer on a scale of 1-6 for each question. The combined scores were tabulated in the direction that higher scores corresponded to more complaints and the total scores (Rourke et al. 1999) were used to examine the relationship between cognitive complaints and patterns in brain metabolism.
Blood samples for circulating inflammatory markers were collected by venipuncture into EDTA tubes, placed on ice, centrifuged for acquisition of plasma, and stored at −80°C for subsequent batch testing. We evaluated four inflammatory markers that have been examined previously by our group, and also reported to be altered following cancer diagnosis and/or treatment: IL-1 receptor antagonist (IL-1ra) and soluble TNF receptor type II (sTNF-RII), surrogate markers for IL-1 and TNF-α activity, respectively, as well as IL-6 and C reactive protein (CRP) (Alexander et al. 2009; Bower et al. 2002; Bower et al. 2007; Bower et al. 2009; Collado-Hidalgo et al. 2006; Orre et al. 2009; Wang et al. 2010; Wang et al. 2012). Specifics for determining the plasma levels have been previously described (P. A. Ganz et al. 2012). Tables within the manuscript display raw plasma cytokine levels; however the statistical analyses were conducted and graphical presentations with made using the natural log of the plasma values in order to produce a more statistically normal distribution.
Acquisition and analysis of PET scans, as well as the complete PET protocol have been detailed previously (Silverman et al. 2007; P. A. Ganz et al. 2012). Briefly, PET was performed with 3D acquisitions, using a 64-slice PET/CT scanner (Siemens). Low-dose CT scans were used for attenuation correction. FDG was used to assess regional cerebral metabolism during mental rest. Subjects were scanned in the supine position, 40 min following injection of 185 MBq FDG in a dimly lit room having low ambient noise, with eyes and ears unoccluded. Effects on resting metabolism were evaluated by both standardized volume of interest (sVOI) and statistical parametric methods. In sVOI analyses, mean activities in 47 standardized volumes of interest were quantified for each scan, and normalized to mean global activity, using a commercially available display-and-analysis software package (NeuroQ; Syntermed Inc.). For statistical parametric mapping (SPM) analyses, SPM8 software was used by methods previously described (Silverman et al. 2011). In brief, a p<0.01 (uncorrected) height threshold, a 0 voxel extent threshold, and both family wise error (FWE) and false discovery rate (FDR) multiple comparison corrections were used. Images were coregistered and reoriented into a standardized Talairach Tournoux based coordinate system (Talairach and Tournoux 1988) to the MNI template, using the nonlinear spatial transformation package in SPM8 (Friston et al. 2007), smoothed three-dimensionally at a full-width half-maximum of 8 mm, and normalized to mean global activity. Pooled data were then statistically assessed to identify the voxels which significantly differed between treatment groups, or within treatment groups at different points of time, or which significantly correlated with a specified neuropsychologic parameter or peripheral cytokine measure. Results were reported in terms of locations of the most significant effects (regionally and/or in x, y, z Talairach style millimeter coordinates) with corresponding t and p values, along with the statistical significance of the size of the region in some cases.
Results
3.1 Between group analyses
Between baseline and one year later, neither the chemotherapy nor no chemotherapy groups differed significantly in regional resting brain metabolism or circulating inflammatory cytokine marker levels. At baseline, the chemotherapy group demonstrated higher mean values of IL-1ra, sTNF-RII, and CRP, and at one year, had higher CRP compared to the no chemotherapy group. The groups did report significantly different levels of memory complaints at baseline (p = 0.004) and one year later (p= 0.01).
3.2 Relationships between inflammatory markers and brain metabolism at baseline
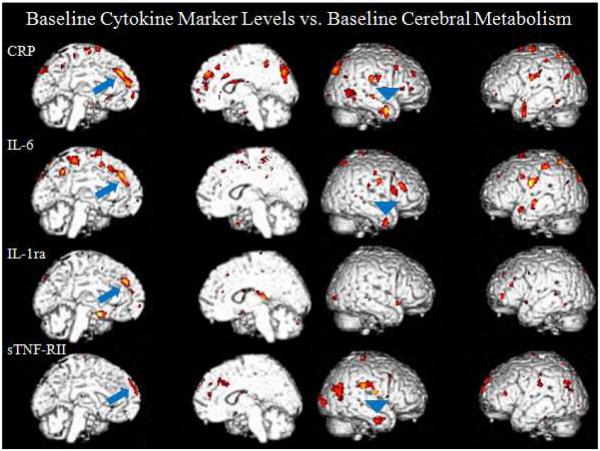
When correlating baseline cytokine marker levels to baseline cerebral metabolism (raw values displayed in Table 2), within the chemotherapy group (n=23), scattered correlations were seen most consistently in the left medial frontal and right inferior lateral anterior temporal cortices. In the chemotherapy group, positive correlations were seen with SPM analyses between the left medial prefrontal cortex and the four cytokine markers measured (CRP (t=5.30, p<0.0005, peak voxel [−10, 42, 34]), IL-1ra (t=4.82, p<0.0005, [−14, 42, 36]), IL-6 (t=4.65, p<0.0005, [−10, 40, 38]), sTNF-RII (t=4.02, p<0.0005, [−6, 60, 36]). Also in the chemotherapy group, positive correlations were seen in the inferior lateral anterior temporal cortex in three of the cytokine markers (CRP (t=3.76, p=0.001, peak voxel [44, 2, −30]), IL-6 (t=3.63, p=0.001, [40, 4, −36], sTNF-RII (t=3.60, p=0.001, [46, −6, −30])) Figure 1. Consistent negative correlations of comparable statistical significance were not seen within the chemotherapy group. Again these correlations were not seen for the non-chemotherapy group.
Table 2.
Circulating Inflammatory Cytokine Level and FDG-PET sVOI levels in Chemotherapy Subjects at Baseline and One Year Later
| Circulating Inflammatory Markers | |||||
| Baseline Chemo (n=23) | One-Year Chemo (n=22) | p-Value | |||
| Mean | SD | Mean | SD | ||
| Il-1ra (pg/mL) | 283 | 146 | 272 | 165 | 0.8231 |
| IL-6 (pg/mL) | 1.7 | 1.3 | 1.7 | 1.3 | 0.8110 |
| CRP (mg/L) | 4.1 | 7.4 | 2.6 | 3.7 | 0.4016 |
| sTNF-RII (pg/mL) | 2480 | 669 | 2040 | 580 | 0.0230 |
| FDG-PET sVOI Values | |||||
| Baseline Chemo (n=23) | One-Year Chemo (n=22) | p-Value | |||
| Mean | SD | Mean | SD | ||
| rGFd | 1.085 | 0.023 | 1.081 | 0.024 | 0.5883 |
| lGFd | 1.080 | 0.048 | 1.074 | 0.050 | 0.6808 |
| riLAT | 0.885 | 0.019 | 0.875 | 0.018 | 0.0761 |
| liLAT | 0.900 | 0.025 | 0.878 | 0.020 | 0.0023 |
Fig. 1.
Medial and lateral surface renders of correlations between baseline cytokine levels and baseline cerebral metabolism. The chemotherapy group demonstrates positive correlations in the left medial prefrontal cortex (arrows) and the right inferior lateral anterior temporal cortex (arrow heads). These correlations were absent in the no chemotherapy group. *For all figures: The color scale represents increasing significance in yellow (a higher t score) and decreasing significance in red (lower t score).
3.3 Relationships between one year inflammatory markers and one year brain metabolism
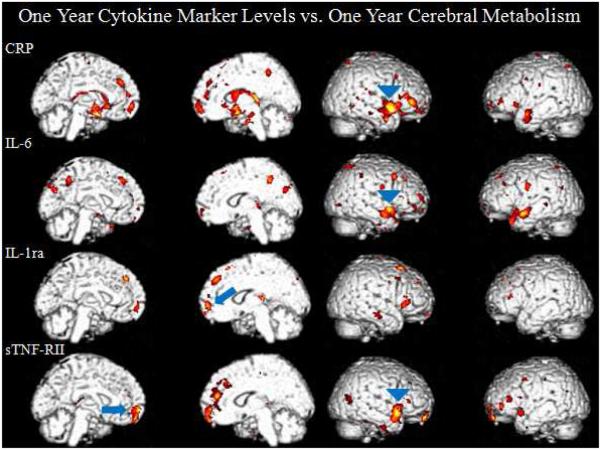
At the one year time point, the chemotherapy group (n=21) continued to demonstrate positive correlations in the medial frontal cortex and the right temporal cortex, the latter shifting superiorly. Positive correlations were seen with SPM analyses between the medial prefrontal cortex and inflammatory markers (sTNF-RII (t=3.85, p=0.001, [−4, 58, −16]), IL-1ra (t=3.20, p=0.002, [10, 60, 0]). Positive correlations were also seen in the anterior temporal cortex (IL-6 (left t=5.59, p<0.0005, [−46, 18, −30], right t=4.38, p<0.0005, [58, 12, −14]), sTNF-RII (t=5.01, p<0.0005, [54, 14, −12], CRP (t=4.48, p<0.0005, [46, −26, −14])) Figure 2. Consistent negative correlations were not seen in the chemotherapy group. And again, these correlations were not seen in the non-chemotherapy group (n=6). Additionally, when comparing change in metabolism to change in cytokine levels over one year within the chemotherapy group, two regions significantly (P<0.01) correlated; including a positive correlation between CRP and medial prefrontal cortex, as well as between sTNF-RII and the left associative visual cortex.
Fig. 2.
Medial and lateral surface renders of correlations between one-year cytokine levels and one-year cerebral metabolism. The chemotherapy group demonstrates positive correlations in the medial prefrontal cortex and the anterior temporal cortex. These correlations were absent in the no chemotherapy group.
3.4 Relationships between baseline inflammatory markers and brain metabolism one year later
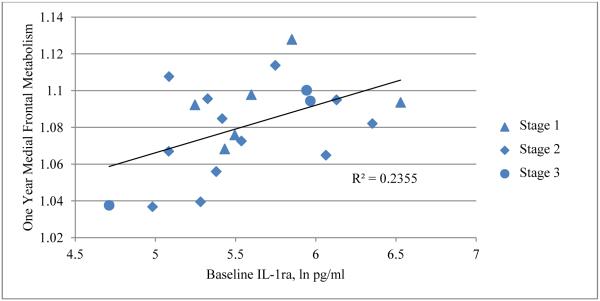
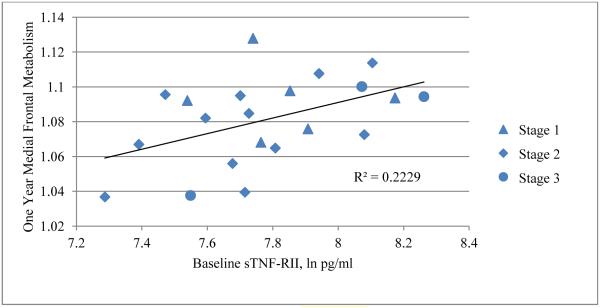
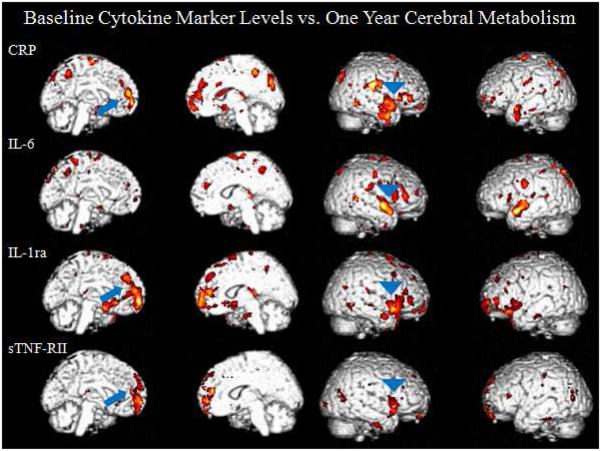
Within the chemotherapy group (n=21), correlations in both the medial prefrontal cortex and anterior temporal cortex showed the highest levels of significance when looking across the one year time frame, comparing baseline inflammatory marker levels to one year regional metabolism. The most significant were between IL-1ra and sTNF-RII and the bilateral medial frontal cortex (GFd), and between IL-1ra and the anterior temporal cortex. A positive correlation was seen between both IL-1ra and sTNF-RII and the bilateral GFd (IL-1ra: sVOI: rGFd r=0.4853, t=2.4195, p=0.0257, lGFd r= 0.4960, t=2.4902, p=0.0222, Figure 3, SPM: t=5.69, p<0.0005, peak voxel [−22, 60, −4]; cluster size = 2,246 voxels, p<0.0005, p=0.001 FDR and FWE corr., sTNF-RII: sVOI: rGFd r= 0.4721, t=2.3343, p=0.0307, lGFd r= 0.4345, t=2.1028, p=0.0490, Figure 4, SPM: t=4.54, p<0.0005, peak voxel [−4, 58, −2]); cluster size = 1,061 voxels, p=0.001, p=0.040 FDR corr., p=0.046 FWE corr. Figure 5). Metabolism in the anterior temporal cortex correlated positively with IL-1ra (t=4.93, p<0.0005, peak voxel [60, 26, 8]; cluster size = 1,034 voxels, p=0.001, p=0.029 FDR corr., p=0.048 FWE corr.). Comparable negative correlations were not present in the chemotherapy group. Additionally, these correlations were absent in the group who did not undergo chemotherapy. A comparison of the correlations described above is listed in Table 3.
Fig. 3.
Positive correlation between baseline IL-1ra and one-year medial frontal resting metabolism in the chemotherapy group (n=21).
Fig. 4.
Positive correlation between baseline sTNF-RII and one-year medial frontal resting metabolism in the chemotherapy group (n=21).
Fig. 5.
Medial and lateral surface renders of correlations between baseline cytokine levels and one-year cerebral metabolism. The chemotherapy group demonstrates positive correlations in the medial prefrontal cortex and the anterior temporal cortex. These correlations were absent in the no chemotherapy group.
Table 3.
Size and significance of cytokine marker vs. regional brain metabolism correlations
| Correlation Between | Peak Voxel | No. of Voxels | P-value | Z-value |
|---|---|---|---|---|
| baseline CRP and baseline left medial prefrontal metabolism |
[−10, 42, 34] | 352 | p<0.0005 | 418 |
| baseline IL-lra and baseline left medial prefrontal metabolism |
[−14, 42, 36] | 147 | p<0.0005 | 3.91 |
| baseline IL-6 and baseline left medial prefrontal metabolism |
[−10, 40, 38] | 276 | p<0.0005 | 3.81 |
| baseline sTNF-RII and baseline left medial prefrontal metabolism |
[−6, 60, 36] | 73 | p<0.0005 | 3.42 |
| baseline CRP and baseline inferior lateral anterior temporal metabolism |
[44, 2, −30] | 287 | p=0.001 | 3.25 |
| baseline IL-6 and baseline inferior lateral anterior temporal metabolism |
[40, 4, −36] | 84 | p=0.001 | 3.16 |
| baseline sTNF-RII and baseline inferior lateral anterior temporal metabolism |
[46, −6, −30] | 213 | p=0.001 | 3.28 |
| one-year IL- Ira and one-vear medial prefrontal metabolism |
[10, 60, 0] | 78 | p=0.002 | 2.83 |
| one-year sTNF-RII and one-year medial prefrontal metabolism |
[−4, 58, −16] | 589 | p=0.001 | 3.27 |
| one-year CRP and one-year right anterior temporal metabolism |
[46, −26, −14] | 1,458 | p<0.0005 | 3.65 |
| one-year IL-6 and one-year right anterior temporal metabolism |
[58, 12, −14] | 563 | p<0.0005 | 3.6 |
| one-year IL-6 and one-year left anterior temporal metabolism |
[−46, 18, −30] | 673 | p<0.0005 | 4.25 |
| one-year sTNF-RII and one-year right anterior temporal metabolism |
[54, 14, −12] | 633 | p<0.0005 | 3.95 |
| baseline IL-lra and one-year medial frontal metabolism |
[−22, 60, −4] | 2,246 | p<0.0005 (p=0.001, FDR corr.) |
429 |
| baseline sTNF-RII and one-year medial frontal metabolism |
[−4, 58, −2] | 1,061 | p=0.001 (p=0.040, FDR corr.) |
3.69 |
| baseline IL-1ra and one-year anterior temporal metabolism |
[60, 26, 8] | 1,034 | p=0.001 (p=0.029, FDR corr.) |
3.91 |
3.5 Relationships between brain metabolism and memory complaints
Strong correlations in the anterior temporal cortex and the medial frontal cortex were also seen when comparing to memory complaints in the chemotherapy group (n=23 at T1, n=21 at T3). At baseline, the bilateral anterior temporal cortex and the bilateral medial frontal cortex correlated positively with total severity scores on the PAOFI memory subscale. The largest region to correlate was located in the bilateral medial frontal cortex and was corroborated by SPM and sVOI methods (sVOI: rGFd r=0.5425, t=2.9595, p=0.0075, lGFd r= 0.4278, t=2.1689, p=0.0417, SPM: t=5.98, p<0.0005, peak voxel [−16, 44, 24]; cluster size = 3,283 voxels p<0.0005, p<0.0005 FDR and FWE corr.). The bilateral anterior temporal cortex also positively correlated (right: t=6.92, p<0.0005, peak voxel [52, 12, −14]; cluster size = 1,773 voxels, p<0.0005, p=0.002 FDR corr., p=0.004 FWE corr., left: t=5.49, p<0.0005, peak voxel [−46, −8, −14], cluster size = 1,852 voxels, p<0.0005, p=0.002 FDR corr., p=0.003 FWE corr.). Interestingly, baseline total severity scores on the PAOFI memory subscale also positively correlated with baseline IL-6 values (p=0.0287), suggesting a link between cognitive complaints and plasma IL-6 levels, in addition to their correlation with specific regions of brain metabolism. When correlating baseline memory complaints to metabolism one year later the relationship persisted, however was less robust (anterior temporal: left t=6.36, p<0.0005, peak voxel [−46, 16, −28], t=5.65, p<0.0005, peak voxel [54, 12, −18], medial frontal: t=5.42, p<0.0005, peak voxel [−10, 32, 50]) Figure 6. Additionally, a comparison of the correlations described above is listed in Table 4.
Fig. 6.
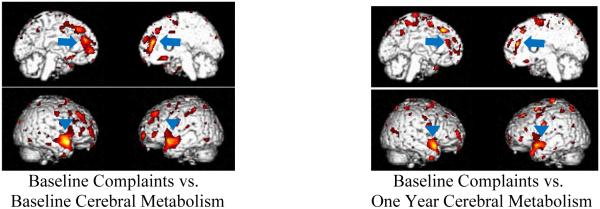
Positive correlations between memory complaints and cerebral metabolism within the chemotherapy group.
Table 4.
Size and significance of memory complaints vs. regional brain metabolism correlations
| Correlation Between | Peak Voxel | No. of Voxels | P-value | Z-value |
|---|---|---|---|---|
| baseline PAOFI memory subscale and baseline left medial frontal metabolism |
[−16, 44, 24] | 3,283 | p<0.0005 (p<0.0005, FDR corr.) |
452 |
| baseline PAOFI memory subscale and baseline right anterior temporal metabolism |
[52, 12, −14] | 1,773 | p<0.0005 (p=0.002, FDR corr.) |
4.94 |
| baseline PAOFI memory subscale and baseline left anterior temporal metabolism |
[−46, −8, −14] | 1,852 | p<0.0005 (p=0.002, FDR corr.) |
428 |
4 Discussion
As described above, the most consistent correlations between cerebral metabolism and inflammatory markers were seen in the left medial frontal and right lateral anterior temporal cortices. Because this analysis was done on an exploratory basis, we currently have no a priori basis for why these regions may be more influenced by cytokines. We focus on these findings because both of these regional correlations showed the highest levels of significance when looking across the one-year time frame, comparing baseline inflammatory marker levels to one-year regional metabolism. This is consistent with the possibility that an initial inflammatory response could set up a cascade that has long-term impact on brain metabolism. Future studies may address the duration of this effect with long-term follow-up. The positive correlation between inflammatory cytokines and resting medial frontal and anterior temporal metabolism could represent increased resting metabolism as a compensatory mechanism for diminished function mediated by other areas. It may also represent a loss of diffuse inhibitory input into those regions, among other things. Interestingly, positive correlations were also seen within the chemotherapy group between baseline memory complaints and the medial frontal and anterior temporal cortices at baseline and one year later, which could represent a neurologic substrate for persistent sequelae of the acute treatment setting.
Perception of cognitive abilities and levels of circulating cytokines both vary widely across individuals before and after being diagnosed with cancer, as well as before and after undergoing chemotherapy. It is thus unsurprising to see no large or significant differences in these measures between chemotherapy-treated and untreated patients. In fact, it would be rather remarkable if group based differences along these lines were detected in this investigation given the natural heterogeneity before and after the cancer diagnosis, let alone the further considerable inter-individual variability that may occur in response to chemotherapy, with respect to a wide array of clinical parameters, particularly as the longitudinal aspect of this study does not come into play until after initial therapy is complete. The most we might thus reasonably expect to observe (and which in fact was observed) is correlation between parameters that are biologically inter-related such as, putatively, responses to chemotherapy exposure with respect to levels of cytokines, cerebral metabolism, and perceived cognition. This is also in line with our original observations of brain metabolism patterns in adjuvant chemotherapy-treated breast cancer patients (Silverman et al. 2007), in which we did not report group-based differences in regional cerebral metabolism between chemotherapy-exposed and unexposed subjects, but rather a significant correlation between whatever level of cognitive impairment was present after chemotherapy and the degree of hypometabolism measured.
Similarly, in the present study, though when taken as groups, the chemotherapy-exposed and unexposed subjects do not significantly differ in regional resting brain metabolism or circulating inflammatory cytokine marker levels, the significance of the correlations described here nevertheless demonstrates that individuals within the chemotherapy group do possess metabolism in certain regions of the brain that co-vary with inflammatory cytokine marker levels, both concurrently and over time. The absence of such a relationship in the group unexposed to chemotherapy may reflect the absence of this common perturbing factor. The relationship that emerges in the chemotherapy-exposed group does not appear to be related to a special subset of patients, as both cytokine levels and regional metabolism levels are distributed relatively uniformly across their respective ranges, and throughout the scatter surrounding the line of regression relating them to each other. In fact, though there are no significant between-group differences in the cytokine markers described, there was a weak trend observed in the direction of the chemotherapy-treated group having higher levels of medial frontal metabolism at the one year time point, and when these patients with the higher levels were censored from the analysis, the significance of the cytokine correlation with metabolism became even stronger. In contrast, as stated above, memory complaint levels did differ between groups, with a substantially greater number of high complainers among those exposed to chemotherapy, and the significant correlation observed between complaint scores and medial frontal metabolism in the brains of chemotherapy-treated patients did depend upon inclusion of the high-complaining subgroup.
Cytokines have been discussed as a candidate mechanism for chemotherapy associated cognitive changes (Ganz et al. 2012). The rationale behind this includes the idea that cancer and chemotherapy can stimulate the release of peripheral cytokines that can cross the blood brain barrier. Cytokines are involved in neuronal and glial cell functioning, neuronal regeneration and neurodegeneration, and cholinergic and dopaminergic pathways. If chemotherapy deregulates cytokine levels, and in turn interferes with their important functions in the brain, this could lead to cognitive impairment (Ahles and Saykin 2007). Although the effects of cytokines on cognitive functioning in the breast cancer patient population is little explored, we recently found that among chemotherapy patients, higher levels of sTNF-RII were significantly correlated with greater memory complaints after controlling for age, BMI, radiation, depression, and time since last chemotherapy treatment. The memory complaints were assessed with the Squire Memory Questionnaire (P. A. Ganz et al. 2012).
Another recent study also found that an administration of endotoxin to nine healthy individuals was associated with increased levels of inflammatory cytokines (serum levels of tumor necrosis factor-α and interleukin-6 were measured) and higher normalized glucose metabolism as measured by FDG-PET in the insula and a trend toward lower normalized glucose metabolism in the cingulate (Hannestad et al. 2012). Because of the consistency of the metabolic changes seen across all subjects, the authors believe their data suggest that, “systemic inflammation induces fundamental physiologic changes in regional brain glucose metabolism.” Additionally, alterations in fMRI have been associated with intravenous injection of low-dose endotoxin (Eisenberger et al. 2009). In a study conducted by Eisenberger and colleagues, 20 subjects received endotoxin while 16 received placebo. The subjects underwent fMRI while completing the Cyberball social exclusion task two hours post endotoxin injection and IL-6 levels were measured hourly for 6 hours. Within the group that received endotoxin, the subjects that had greater increases in IL-6 levels prior to scanning, demonstrated greater activity in the medial and dorsomedial prefrontal cortex, similar to findings in our study, as well as demonstrated greater activity in the posterior superior temporal cortex, the temporal pole, the posterior cingulate cortex, and the precuneus. Although these studies involve directly induced systemic inflammation and not chemotherapy, it is interesting to note changes seen in brain activity associated with serum cytokine levels.
As is generally the case for PET imaging studies of this kind, limitations to our study include the relatively small number of subjects. Secondly, lack of a healthy female comparison limits the study. Differences between the chemotherapy and non-chemotherapy groups potentially could be confounded by disease burden. It is also important to consider depression, fatigue, and menopausal status as possible confounds. To eliminate depression as a possible confound, patients with clinical depression were excluded from the study. As for fatigue and menopausal status, we found no relationship between menopausal status or fatigue and self-reported cognitive complaints within the full MBS sample (P. Ganz et al. (in press)). Additionally, after controlling for menopausal status within our PET sub-population, the significance of the correlations discussed above did not change. It is also important to note that although we are identifying a chain of correlations that would be consistent with a plausible previously proposed mechanism for cognitive effects of chemotherapy; this cannot be concluded to demonstrate causality. The data presented here however, do provide a window through which these relationships have been able to be systematically examined for the first time in cancer patients who have been exposed to chemotherapy.
Acknowledgments
Supported by grant NIH/NCI R01 CA 109650 and the Breast Cancer Research Foundation (PAG); R01-AG034588; R01-AG026364; R01 CA160245-01; R01-CA119159; R01 HL095799; R01 DA032922-01; P30-AG028748 to MRI; and UCLA CTSI UL1TR000124, the Cousins Center for Psychoneuroimmunology (MRI).
Footnotes
The authors declare that they have no conflict of interest.
References
- ACS . Cancer Facts & Figures 2011. GA, USA: 2011. [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. Research Support, N.I.H., Extramural Review. doi:10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20(2):485–493. doi: 10.1200/JCO.2002.20.2.485. Comparative Study Research Support, U.S. Gov't, P.H.S. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. Clinical Trial Research Support, N.I.H., Extramural. doi:10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45(3):384–392. doi: 10.1016/j.ejca.2008.09.010. Research Support, Non-U.S. Gov't. doi:10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou A, Assimakopoulous K, Iconomou G, Giannakopoulou F, Kalofonos H. Either called "Chemobrain" or "Chemofog" the long-term chemotherapy-induced cognitive decline in cancer survivors is real. Journal of Pain Symptom Management. 2011;41(1):126–139. doi: 10.1016/j.jpainsymman.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Barber R. Inflammatory signaling in Alzheimer disease and depression. Cleve Clin J Med. 2011;78(Suppl 1):S47–49. doi: 10.3949/ccjm.78.s1.08. Review. doi:10.3949/ccjm.78.s1.08. [DOI] [PubMed] [Google Scholar]
- Biglia N, Bounous VE, Malabaila A, Palmisano D, Torta DM, D'Alonzo M, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur J Cancer Care (Engl) 2012;21(4):485–492. doi: 10.1111/j.1365-2354.2011.01320.x. doi:10.1111/j.1365-2354.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun. 2007;21(3):251–258. doi: 10.1016/j.bbi.2006.08.001. Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. doi:10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW. Cytokine Genetic Variations and Fatigue Among Patients With Breast Cancer. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.46.2143. doi:10.1200/JCO.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154. Research Support, N.I.H., Extramural. doi:10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. doi:10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18(14):2695–2701. doi: 10.1200/JCO.2000.18.14.2695. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26(7):955–969. doi: 10.1080/13803390490510905. Clinical Trial Comparative Study Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. doi:10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- Chelune G, Heaton R, Lehman R. Neuropsychological and personality correlates of patients' complaints of disability. Advances in clinical neuropsychology. 1986;3:95–126. R.E.T. Gerald Goldstein. [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. doi:10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18(2):134–143. doi: 10.1002/pon.1379. Research Support, Non-U.S. Gov't. doi:10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14(6):396–400. doi: 10.1097/PPO.0b013e31818d8769. Review. doi:10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30(3):274–281. doi: 10.1200/JCO.2011.36.8571. Research Support, Non-U.S. Gov't. doi:10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32(3):480–493. doi: 10.1002/hbm.21033. Research Support, Non-U.S. Gov't. doi:10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13(12):1285–1295. doi: 10.1634/theoncologist.2008-0130. Research Support, Non-U.S. Gov't Review. doi:10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47(3):881–890. doi: 10.1016/j.neuroimage.2009.04.040. Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. doi:10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25(25):3866–3870. doi: 10.1200/JCO.2007.10.8639. Case Reports Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Twin Study. doi:10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Kiebel S, Nichols T, Penny W. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; Burlington, MA: 2007. [Google Scholar]
- Fritz-French C, Tyor W. Interferon-alpha (IFNalpha) neurotoxicity. Cytokine Growth Factor Rev. 2012;23(1-2):7–14. doi: 10.1016/j.cytogfr.2012.01.001. Review. doi:10.1016/j.cytogfr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Ganz P, Kwan L, Castellon SA, Oppenheim A, Bower JE, Silverman D, et al. Cognitive complaints after breast cancer treatments: Is there a relationship to neuropsychological test performance? Journal of the National Cancer Institute. doi: 10.1093/jnci/djt073. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.015. doi:10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, et al. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med. 2012;53(4):601–607. doi: 10.2967/jnumed.111.097014. Randomized Controlled Trial Research Support, N.I.H., Extramural. doi:10.2967/jnumed.111.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19(10):1647–1656. doi: 10.1007/s00520-010-0997-4. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. doi:10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.05.017. doi:10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol. 2005;4(6):371–380. doi: 10.1016/S1474-4422(05)70099-5. Meta-Analysis Review. doi:10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279–292. doi: 10.1159/000079408. Research Support, Non-U.S. Gov't Review. doi:10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, et al. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom Med. 2006;68(6):895–903. doi: 10.1097/01.psy.0000238451.22174.92. Research Support, N.I.H., Extramural. doi:10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123(3):819–828. doi: 10.1007/s10549-010-1088-4. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. doi:10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. doi:10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JS. The possible role of cytokines in chemotherapy-induced cognitive deficits. Adv Exp Med Biol. 2010;678:119–123. doi: 10.1007/978-1-4419-6306-2_15. [DOI] [PubMed] [Google Scholar]
- O'Bryant SE, Xiao G, Barber R, Reisch J, Doody R, Fairchild T, et al. A serum protein-based algorithm for the detection of Alzheimer disease. Arch Neurol. 2010;67(9):1077–1081. doi: 10.1001/archneurol.2010.215. Research Support, N.I.H., Extramural. doi:10.1001/archneurol.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fossa SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav Immun. 2009;23(6):868–874. doi: 10.1016/j.bbi.2009.04.003. Research Support, Non-U.S. Gov't. doi:10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25(3):94–102. doi: 10.1016/j.cyto.2003.10.004. Clinical Trial Comparative Study Controlled Clinical Trial Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Halman MH, Bassel C. Neuropsychiatric correlates of memory-metamemory dissociations in HIV-infection. J Clin Exp Neuropsychol. 1999;21(6):757–768. doi: 10.1076/jcen.21.6.757.852. Comparative Study Research Support, Non-U.S. Gov't. doi:10.1076/jcen.21.6.757.852. [DOI] [PubMed] [Google Scholar]
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85(3):640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. Clinical Trial Controlled Clinical Trial Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat. 2007;103(3):303–311. doi: 10.1007/s10549-006-9380-z. Research Support, Non-U.S. Gov't. doi:10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Geist CL, Kenna HA, Williams K, Wroolie T, Powers B, et al. Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology. 2011;36(4):502–513. doi: 10.1016/j.psyneuen.2010.08.002. Randomized Controlled Trial Research Support, N.I.H., Extramural. doi:10.1016/j.psyneuen.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme; New York: 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Tchen N, Juffs HG, Downie FP, Yi QL, Hu H, Chemerynsky I, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21(22):4175–4183. doi: 10.1200/JCO.2003.01.119. Comparative Study Research Support, Non-U.S. Gov't. doi:10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- Teunissen CE, van Boxtel MP, Bosma H, Bosmans E, Delanghe J, De Bruijn C, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134(1-2):142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59(3):268–274. doi: 10.1093/gerona/59.3.m268. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Torres MA, Pace TW, Liu T, Felger JC, Mister D, Doho GH, et al. Predictors of depression in breast cancer patients treated with radiation: Role of prior chemotherapy and nuclear factor kappa B. Cancer. 2013 doi: 10.1002/cncr.28003. doi:10.1002/cncr.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90(3):210–218. doi: 10.1093/jnci/90.3.210. Clinical Trial Randomized Controlled Trial. [DOI] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24(6):968–974. doi: 10.1016/j.bbi.2010.03.009. Research Support, N.I.H., Extramural. doi:10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Williams LA, Krishnan S, Liao Z, Liu P, Mao L, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun. 2012;26(5):699–705. doi: 10.1016/j.bbi.2011.12.007. Research Support, N.I.H., Extramural. doi:10.1016/j.bbi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Kayl AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer. 2004;90(9):1691–1696. doi: 10.1038/sj.bjc.6601772. Review. doi:10.1038/sj.bjc.6601772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. doi: 10.1002/cncr.25098. Clinical Trial, Phase III Randomized Controlled Trial. doi:10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12(3):267–275. doi: 10.1007/s11910-012-0264-9. Review. doi:10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Witgert ME, Meyers CA. Neuropsychological sequelae of non-central nervous system cancer and cancer therapy. Neuropsychol Rev. 2008;18(2):121–131. doi: 10.1007/s11065-008-9058-x. Review. doi:10.1007/s11065-008-9058-x. [DOI] [PubMed] [Google Scholar]
- WHO Cancer fact sheet No. 297. 2012 http://www.who.int/mediacentre/factsheets/fs297/en/