Abstract
Background
In neonatal rodents, serotonin (5-HT) neurons are critical for successful autoresuscitation. We hypothesized that caffeine, a respiratory stimulant, would hasten the onset of gasping and improve autoresuscitation in 5-HT-deficient, Pet-1−/− mice.
Methods
Using a head-out system and electrocardiogram, we measured respiratory and heart rate (HR) responses of Pet-1−/− rodents and their littermates during episodic asphyxia at postnatal days 8–9 (P8–9). After a baseline recording, we injected either vehicle or caffeine (i.p.) at doses of 1, 5, or 10 mg/ kg. We then induced 10 brief (~30 s) episodes of asphyxia, each interspersed with 5 min of room air to allow autoresuscitation. In addition to measuring survival, we measured the duration of hypoxic apnea (time to initiate gasping) and time to recover eupnea and HR.
Results
Caffeine had a dose-dependent effect of hastening the onset of gasping, recovery of breathing, and restoration of HR in Pet-1−/−(but not in wild-type) rodents, thereby improving survival across asphyxic episodes. Increased survival was strongly correlated with hastened onset of gasping.
Conclusion
Our data suggest that caffeine reduces mortality relating to asphyxia and 5-HT deficiency. These findings may be relevant for efforts to reduce the incidence of sudden infant death syndrome (SIDS), given that SIDS is associated with failed autoresuscitation and reduced brainstem 5-HT.
When confronted with severe hypoxia (low oxygen (O2)) or asphyxia (low O2 and high carbon dioxide (CO2)), young mammals, including human infants, sustain life through a protective process termed “autoresuscitation.” Severe hypoxia and asphyxia induce apnea and bradycardia as a result of tissue hypoxia within the brainstem respiratory centers and sino-atrial node, as well as adjustments in autonomic tone (1). Severe hypoxia of brainstem tissue elicits gasping, a respiratory behavior characterized by a slower frequency, greater inspiratory neuronal activity, and reduced or absent expiratory activity (2,3). If the animal is allowed to breathe normal air during autoresuscitation, gasping and the associated increase in sympathetic activity are adequate to restore oxygenation, normal breathing (eupnea), and heart rate (HR), allowing the animal to recover (4,5).
There is mounting evidence to indicate that the serotonin (5-HT) system in the brainstem is critical for successful autoresuscitation. Neonatal mice (Pet-1−/−) and rats treated with 5,7-dihydroxytryptamine, having lost most of their 5-HT neurons, have impaired autoresuscitation and consequently high mortality rates when exposed to episodic asphyxia (6–8). A characteristic feature of failed autoresuscitation in these animals is prolonged hypoxic apnea (i.e., delay in the initiation of gasping) in response to asphyxia, and a delay in the recovery of HR and eupnea after returning to room air. In Pet-1−/− mice this phenotype is most evident during a critical window in postnatal development, i.e., postnatal days 7–10 (P7–10).
Caffeine is a widely used, effective drug for treating apnea of prematurity in infants (9–11). Caffeine has also been shown to increase overall ventilation (V̇E) and reduce apnea frequency in newborn rat pups (12). These effects were not brought about by an increase in the ventilatory response to hypoxia; rather, they were attributable to the ability of caffeine to antagonize the action of adenosine. Adenosine, a neuromodulator released during hypoxia, potently inhibits brainstem respiratory neurons including those in the pre-Bötzinger complex, a group of intrinsically bursting neurons that are important for inducing gasping (13–15). Indeed, the antagonizing of adenosine receptors has been shown to improve gasping in neonatal rat pups (16).
In the current study, we tested the hypothesis that caffeine can reverse the delay in initiation of gasping and improve the ability of Pet-1−/− mice to withstand episodic asphyxia in the developmental period during which they display failure of autoresuscitation.
Results
As compared with their littermates, Pet-1−/− pups had lower resting HRs (Δ 38 beats/min; P = 0.02; Table 1) and metabolic rate (V̇O2) (Δ 5.8 ml/min/kg; P = 0.03; Table 1), in line with our previous findings (17). As compared with their wild-type littermates, Pet-1−/− pups had similar resting V̇E but hyperventilated (i.e., a greater V̇E relative to the underlying V̇O2 (P < 0.001; Table 1)), as previously described (18). Neither vehicle nor treatment with 1 or 5 mg/kg caffeine had any significant effects on these baseline variables (Table 2). The highest dose (10 mg/kg) of caffeine resulted in a mild (10%) increase in HR both in wild-type pups and in Pet-1−/− pups (P = 0.02; Table 2).
Table 1.
Resting parameters of all wild-type (WT, n = 54) and Pet-1−/− pups (n = 38) studied
| Genotype | HR (beats/min) | V̇E (ml/min/kg) | V̇O2 (ml/min/kg) | V̇E/V̇O2 |
|---|---|---|---|---|
| WT | 538 ± 19 | 1,025 ± 77 | 48.4 ± 3.5 | 19.8 ± 2.0 |
| Pet-1−/− | 500* ± 23 | 1,098 ± 69 | 42.6* ± 3.3 | 26.8* ± 2.8 |
Shown are mean data ± 95% confidence interval. HR, heart rate; V̇E, ventilation; V̇O2, metabolic rate.
Significantly different than WT, P < 0.05.
Table 2.
Effect of drug or saline injection on baseline cardio-respiratory parameters
| Genotype | Treatment | HRpre- | HRpost- | V̇Epre- | V̇Epost- | V̇O2pre- | V̇O2post- | V̇E/V̇O2pre- | V̇E/V̇O2post- |
|---|---|---|---|---|---|---|---|---|---|
| WT (n = 17) | Veh | 539 ± 36 | 553 ± 18 | 1,005 ± 179 | 996 ± 158 | 51.3 ± 9.0 | 50.4 ± 7.5 | 19.8 ± 2.0 | 19.2 ± 2.0 |
| Pet-1−/− (n = 10) | 496 ± 27 | 518 ± 21 | 1,100 ± 152 | 1,057 ± 71 | 41.6 ± 6.0 | 41.2 ± 6.2 | 26.8 ± 2.8 | 26.6 ± 3.2 | |
| WT (n = 9) | 1 mg/kg | 487 ± 45 | 519 ± 34 | 833 ± 106 | 922 ± 112 | 44.3 ± 5.2 | 50.3 ± 7.1 | 18.9 ± 1.5 | 22.0 ± 1.0 |
| Pet-1−/− (n = 9) | 484 ± 39 | 480 ± 30 | 1,026 ± 135 | 993 ±133 | 45.4 ± 7.5 | 44.1 ± 8.3 | 23.2 ± 3.4 | 23.1 ± 2.4 | |
| WT (n = 13) | 5 mg/kg | 583 ± 32 | 588 ± 37 | 1,264 ± 143 | 1,174 ± 108 | 54.9 ± 7.4 | 52.2 ± 5.9 | 23.7 ± 2.4 | 23.4 ± 3.5 |
| Pet-1−/− (n = 12) | 548 ± 49 | 554 ± 43 | 1,235 ± 115 | 1,239 ± 130 | 44.4 ± 6.5 | 47.0 ± 5.9 | 28.7± 3.0 | 26.9 ± 24 | |
| WT (n = 16) | 10mg/kg | 527 ± 32 | 582* ± 23 | 958 ± 79 | 1,050 ± 86 | 42.7 ± 3.1 | 44.9 ± 4.1 | 22.6 ± 1.7 | 24.1 ± 2.7 |
| Pet-1−/− (n = 8) | 444 ± 28 | 487* ± 54 | 953 ± 72 | 1,043 ± 125 | 37.3 ± 4.6 | 47.0 ± 12.0 | 26.1 ± 3.1 | 23.5 ± 3.7 |
Values for heart rate (HR, beats/min), ventilation (V̇E, ml/min/kg), metabolic rate (V̇O2, ml/min/kg), and the ventilatory equivalent (V̇E/V̇O2) during room air conditions in wild-type (WT) and Pet-1−/− pups before (pre-) and following (post-) treatment with either vehicle (Veh) or 1, 5, and 10 mg/kg caffeine.
Significant effect of caffeine or vehicle, P < 0.05. Shown are mean data ± 95% confidence interval.
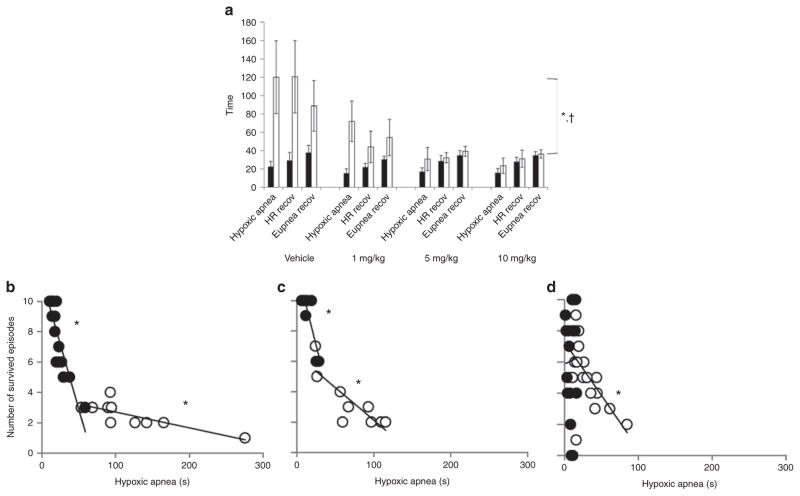
Wild-type pups demonstrated a higher survival over the course of the 10 asphyxia challenges, irrespective of caffeine treatment (P < 0.0001; Figure 1a). The median numbers of asphyxic episodes survived by untreated wild-type and Pet- 1−/− animals were 9 and 2, respectively (Figure 1a,b). Caffeine had a strong, genotype-dependent effect on survival (genotype–caffeine interaction: P < 0.001). The median number of asphyxic episodes survived by Pet-1−/− animals was 3 after treatment with 1 mg/kg caffeine (not significantly different from the group receiving vehicle; post hoc: P = 0.07), 5 after treatment with 5 mg/kg caffeine (post hoc: P = 0.0004), and 5.5 after treatment with 10 mg/kg caffeine (post hoc: P = 0.0006) (Figure 1b). The beneficial effect of caffeine in Pet-1−/− animals was most apparent during the first few asphyxia challenges. Treatment with 5 mg/kg caffeine increased median survival from 0 to ~70% after the fourth episode of asphyxia (Figure 1a). There was no significant change in the number of asphyxic episodes survived by wild-type animals at any dose of caffeine as compared with the group receiving vehicle (post hoc P = 0.56, 0.49, and 0.06 for 1, 5, and 10 mg/kg, respectively) (Figure 1b). The marginal difference in survival rates between wild-type pups treated with 10 mg/kg caffeine and those receiving vehicle is attributable largely to the fact that 2 of the 17 treated subjects died immediately, consequent to a complete failure to initiate gasping after the first asphyxic episode. This was not observed in any other animals at any dose of caffeine.
Figure 1.
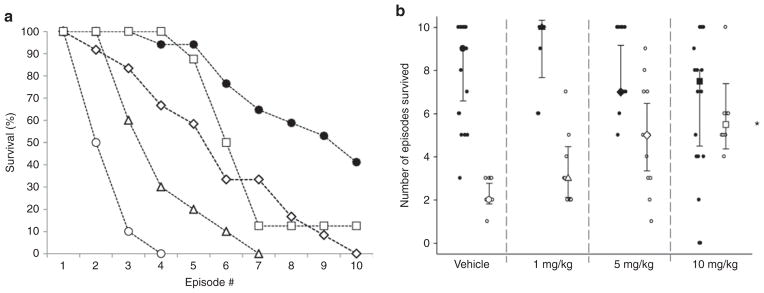
Caffeine treatment significantly increased the number of episodes of asphyxia withstood by neonatal Pet-1−/− mice. (a) Survival curves for vehicle-treated wild-type (n = 17; solid circles) and Pet-1−/− littermates (open symbols) given an i.p. injection of vehicle (n = 10; circles) or caffeine at doses of 1 mg/kg (n = 9; triangles), 5 mg/kg (n = 12; diamonds), or 10 mg/kg (n = 8; squares). Survival curves for wild-type animals treated with caffeine are not significantly different from those treated with vehicle; for the purposes of clarity of the figure, these data are not shown. (b) Individual animals (small circles) and median number of episodes survived (large symbols ± 95% confidence interval) for wild-type animals (solid symbols) and Pet-1−/− littermates (open circles, doses represented by same symbols as are used in (a). *Significant interaction between caffeine dose and genotype, P < 0.001. Note that asymmetric data distribution, given our paradigm, uses a maximum of 10 hypoxic episodes.
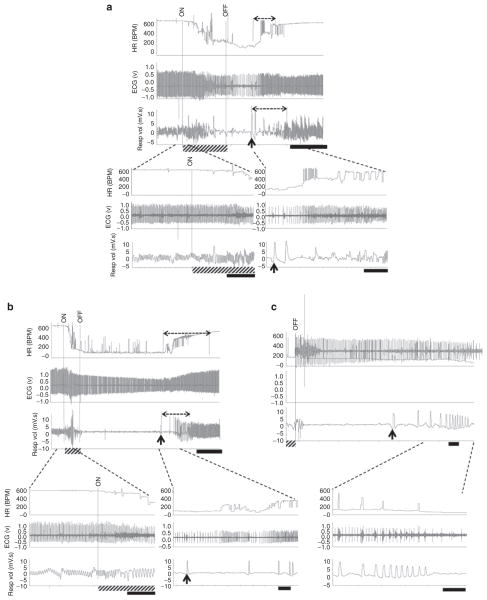
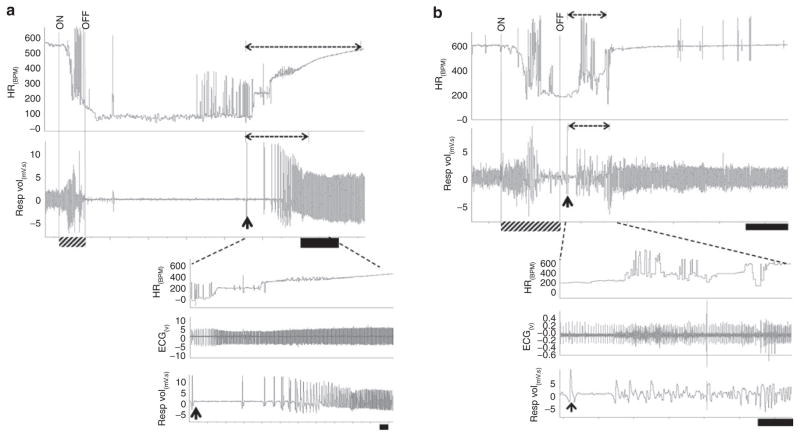
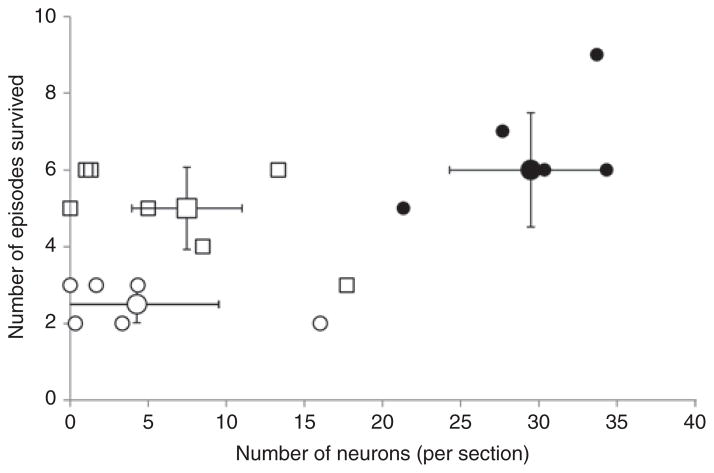
The failure in autoresuscitation displayed by Pet-1−/− animals was preceded by prolonged hypoxic apnea (i.e., a delay in the initiation of gasping) and a delay in the recovery of HR and eupnea (Figure 2). On average, hypoxic apneas were fivefold longer in untreated Pet-1−/− animals as compared with wild-type ones, and were accompanied by a fivefold and twofold greater delay in the recovery of HR and eupnea, respectively (P < 0.001 for all; Figures 2 and 3a). Failed autoresuscitation of Pet-1−/− animals was associated with a “run-down” of gasping (Figure 2c), but this was also observed in wild-type animals with increasing episodes of asphyxia (data not shown). Caffeine, even at the lowest dose used in the study, improved autoresuscitation in Pet-1−/− animals (caffeine × genotype effect: P < 0.001 for all variables; Figures 3a and 4). The quicker initiation of gasping was strongly associated with the ability of caffeine-treated Pet-1−/− animals to survive more episodes of asphyxia (Figure 3b). There was no difference between vehicle- treated and caffeine-treated Pet-1−/− pups with respect to the number of 5-HT-positive neurons identified post mortem in the raphe magnus, a major component of the medullary 5-HT system (Figure 5).
Figure 2.
Autoresuscitation failure in Pet-1−/− mice. Raw heart rate (HR, beats/min (bpm)), electrocardiogram (ECG), and respiratory (Resp vol) responses of wild-type mice and Pet-1−/− littermates to asphyxia. For all traces, the period of asphyxia is denoted by “ON” and “OFF” and by the hatched bar on the x-axis. Broken-line horizontal arrows over the HR and respiratory traces denote the period required for the recovery of HR and eupnea. The onset of gasping (first gasp taken) is denoted by an upward arrow. (a, top) A 2-min trace showing cardio-respiratory responses of a wild-type pup to the first episode of asphyxia. Black scale bar = 30 s. (a, bottom) Close-up traces showing HR and respiratory activity at the transition from normoxia to asphyxia (left) and recovery from asphyxia (right). Black scale bars = 5 s. (b, top) A ~6 min trace showing responses of a Pet-1−/− animal to the first episode of asphyxia. Black scale bar = 1 min. (b, bottom) Close-up traces showing HR and respiratory activity at the transition from normoxia to asphyxia (left) and partial recovery (right) (black scale bars = 5 s). (c, top) Autoresuscitation failure in the Pet-1−/− animal shown in (b) after the third episode of asphyxia. Black scale bar = 5 s. (c, bottom) Close-up traces showing “run-down” of gasping, persistent bradycardia, and death of a Pet-1−/− animal (black scale bars = 5 s).
Figure 3.
Effects of Pet-1-deficiency and caffeine on autoresuscitation. (a) Summary of autoresuscitation variables in wild-type mouse pups and their Pet-1−/− littermates. Group data showing the average duration of hypoxic apnea and time required to recover heart rate (HR recov) and eupnea (Eupnea recov) across all episodes of environmental asphyxia for wild-type (black bars) and Pet-1−/− littermates (open bars) receiving an i.p. injection of either vehicle or 1, 5, or 10 mg/kg of caffeine. *Significant effect of genotype (P < 0.001 for all variables); †significant genotype × caffeine interaction (P < 0.001 for all variables). The data shown are mean values ± 95% confidence intervals. (b–d) Relationship between number of asphyxic episodes survived and the duration of apnea in wild-type mice (closed circles) and Pet-1−/− mice (open circles), treated with vehicle (b; R2= 0.76 (wild-type) and 0.78 (Pet-1−/−)), 1 mg/kg caffeine (c; R2 = 0.75 (wild-type) and 0.70 (Pet-1−/−)), or 5 or 10 mg/kg caffeine (grouped) (d; R2 < 0.001 (wild-type) and 0.34 (Pet-1−/−)). *Significant relationships, P < 0.05. Data points represent individual animals.
Figure 4.
Normalization of autoresuscitation in Pet-1−/− animals after administration of caffeine. In all traces, the period of asphyxia is denoted by “ON” and “OFF” and as a hatched bar on the x-axis. Broken-line horizontal arrows over heart rate (HR) and respiratory traces denote the period required for the recovery of HR and eupnea. The onset of gasping (first gasp taken) is denoted by an upward arrow. (a, top) ~8 min raw trace showing a vehicle-treated Pet-1−/− animal’s cardio-respiratory responses to asphyxia. Black scale bar = 1 min. (a, bottom) Close-up traces showing prolonged recovery of HR and eupnea following the initiation of gasping. Black scale bar = 5 s. (b, top) ~3 min tracing showing cardio-respiratory responses of a Pet-1−/− animal treated with 5 mg/kg of caffeine. Black scale bar = 30 s. (b, bottom) Close-up traces showing hastened recovery of HR and eupnea, as compared with vehicle-treated Pet-1−/− animal. Black scale bar = 5 s.
Figure 5.
Relationship between number of episodes of environmental asphyxia survived and the number of 5-HT neurons in the raphe magnus in wild-type animals receiving an intracerebroventricular injection of vehicle (black circles) and in Pet-1−/− animals receiving an injection of either vehicle (open circles) or 10 mg/kg caffeine (open squares). Also shown are the mean data (large symbols) ± 95% confidence intervals.
Discussion
The novel finding in this study was that a systemically administered low dose of caffeine (5 mg/kg) restores proper autoresuscitation in Pet-1−/− mice that have a major loss of brainstem 5-HT neurons, thereby extending the number of asphyxic episodes they can withstand. Our data indicate that the enhanced survival of Pet-1−/− pups after caffeine treatment is related to a more prompt initiation of gasping. In this way, caffeine helps re-oxygenate cardiac and brain tissue to restore HR and breathing in 5-HT-deficient Pet-1−/− mice that are subjected to asphyxic conditions.
Untreated Pet-1−/− mice have severe autoresuscitation defects at a critical period of postnatal life (6,8). No untreated Pet-1−/− animals survived past the fourth episode of asphyxia. In contrast, 50% of wild-type animals were alive after the 10th episode. For both wild-type and Pet-1−/− mice—irrespective of caffeine treatment—death is immediately preceded by a progressive run-down of gasping, with an associated failure to reverse bradycardia. In the raw tracings there was no evidence of atrio-ventricular block before the run-down in gasping (Figure 2c; however, note that the detection of an atrio-ventricular block may have been precluded by background noise). If atrio-ventricular block is indeed absent, the autoresuscitation failure of neonatal wild-type and Pet-1−/− mice at P8–9 is similar to the failure observed in P10–11 neonatal rats (19), but different from that described in different strains of wild-type weanling mice (20).
The cellular mechanism(s) through which caffeine restores autoresuscitation and promotes survival in Pet-1−/− animals is a focus for future research. Given its ability to hasten the onset of gasping, caffeine possibly exerts its effects by increasing the activity of Pet-1-deficient respiratory neurons responsible for the induction of gasping (e.g., preBotzinger Complex) (15,21,22). In adult rats, caffeine has been shown to increase brainstem 5-HT content (23) and promote increase in V̇E after repetitive hypoxia (i.e., long-term facilitation)—a process known to be dependent on 5-HT signaling at phrenic motoneurons (12,24). In a similar way, caffeine may also enhance excitatory drive to inspiratory neurons in Pet-1−/− mice exposed to repetitive asphyxia, acting through the Pet-1- independent subset of 5-HT neurons retained by these animals (25,26). However, the rescue of Pet-1-deficient mice need not be mediated via 5-HT neurons. However, caffeine’s effect on the remaining 5-HT neurons is not its only possible mechanism of action; caffeine is a potent antagonist of adenosine receptors, some of which (A1) are expressed by, and reduce the activity of, neurons in the preBötzinger complex during hypoxia-induced release of adenosine (13,14). Adenosine can also suppress respiratory drive through adenosine receptors (A2A) expressed on GABAergic interneurons (27). It is possible that caffeine forestalls adenosine-induced suppression of respiratory activity during asphyxia by blocking both types of adenosine receptors, with no involvement of the 5-HT system. Caffeine also has the ability to directly modulate intracellular calcium and cyclic adenosine monophosphate levels, both of which are second messengers utilized by 5-HT receptors and may play a role in the modulation of respiratory rhythm (28–30).
Our data demonstrate that the positive effects of caffeine on autoresuscitation are limited to 5-HT deficient animals; in wild-type animals, neither autoresuscitation nor survival was significantly improved by caffeine. Indeed, 2 of the 17 wild-type animals may have been negatively affected by caffeine, failing to initiate gasping even after the highest dose. However, because this latter effect did not reach statistical significance in our sample, we are not certain that the deaths of these two animals were attributable specifically to caffeine administration.
Our findings may have relevance for reducing the incidence of sudden infant death syndrome (SIDS), a syndrome associated with reduced 5-HT (31) and failed autoresuscitation (32,33). As has been found in Pet-1−/− mice (6), evidence from rare electrophysiological tracings obtained immediately before death from SIDS cases indicates failed autoresuscitation, with gasping being ineffective at restoring normal heart rate and normal breathing (32,33). However, the cellular phenotype of Pet-1−/− mice is distinct from any of the 5-HT system defects that have been identified in SIDS cases. Pet-1−/− mice show an ~60–70% loss of brainstem 5-HT neurons (25,26), leading to a brainstem 5-HT content of only ~80–90% (18). In contrast, infants who experience SIDS have a ~30% reduction in 5-HT content within the medullary raphe and extra-raphe nuclei, associated with other brainstem abnormalities that are yet to be identified in Pet-1−/− mice, namely, increased density of immature 5-HT neurons, a relative reduction in 5-HT transporter binding, and decreased 5-HT1A and GABAA receptor binding in the medullary 5-HT network (31,34–38). Nevertheless, the ability of small doses of caffeine to hasten the onset of gasping and improve autoresuscitation raises the intriguing possibility that caffeine may be beneficial for infants in whom deficiency of 5-HT is a key component of their neuropathology.
Ultimately, the treatment of the complex neurotransmitter defects in affected infants who are at risk of SIDS may, as with other diseases involving respiratory dysfunction (e.g., Rett syndrome) (39), require a combination of pharmacologic agents. Nevertheless, our results suggest that small doses of caffeine are effective at reversing major defects in autoresuscitation resulting from severe brainstem 5-HT deficiency. Caffeine could reduce the risk of sudden death in infants with 5-HT deficiency if they were to experience severe hypoxia from a drop in environmental oxygen content or from defects in respiratory, cardiovascular, or arousal systems that maintain oxygen homeostasis.
Materials and Methods
Animals
We tested pups from heterozygous breeders (i.e., Pet-1+/−: possessing only one Pet-1 allele) maintained on a genetic background composed of both C57Bl/6 and 129Sv mouse strains. All the animals were provided food and water ad libitum and were housed with a 12 h light–dark cycle and a room temperature of 21–23°C. Autoresuscitation was tested in a total of 54 wild-type and 38 Pet-1−/− pups, from a total of 30 litters from 18 breeding pairs. Pups were chosen from each litter randomly, with genotype unknown (experimenter blinded). Two to four pups were selected from each litter; also, each litter was randomly assigned to either vehicle or a specific dose of caffeine (i.e., all the pups belonging to a given litter were given the same treatment). Data from “wild-type” animals represent both Pet-1+/+ and Pet-1+/− pups, given that the loss of one Pet-1 allele has no effect on the number of 5-HT-positive neurons or on any other phenotype studied to date (25). The animals were studied between P8–9, within the period when Pet-1−/− animals display autoresuscitation failure (6). All experimental protocols were approved by the Institutional Animal Care and Use Committee of Dartmouth College.
Genotyping
Genotyping was performed from isolated DNA, using a procedure published earlier (25), using primers: 5′-CGC ACT TGG GGG GTC ATT ATC AC-3′, 5′-CGG TGG ATG TGG AAT GTG TGC-3′ and 5′-GCC TGA TGT TCA AGG AAG ACC TCG G-3′. PCR was performed using an initial 5-min denaturing step at 95 °C, followed by 35 cycles of 94 °C for 1 min, 62 °C for 30 s, and 72 °C for 50 s. The PCR products generated were a WT allele and a Pet-1−/− allele, containing 209 and 361 base pairs, respectively.
Experimental Setup
Measurements were made using a mask-pneumotachograph system with the animal in a head-out configuration (Figure 6) (40). The animal chamber (volume ~ 40 ml) was constructed from a water-jacketed glass cylinder. Body temperature, measured with a thermocouple, was maintained at 36 ± 0.5°C in all animals throughout the experiment, and was controlled by perfusing the chamber with warmed water from a bath. The ambient temperature was adjusted from ~32–34 °C to ~34–36 °C throughout the experiment to counteract the effects of hypoxia on thermogenesis. The head chamber was made by fitting a section of vinyl over the end of a syringe tube (volume ~3 ml) held in place with another rubber gasket inside the anterior end of the chamber. The snout of the animal was placed into a small hole in the vinyl and sealed with polyether material (Impregum F, Polyether Impression material, 3M, St. Paul, MN).
Figure 6.
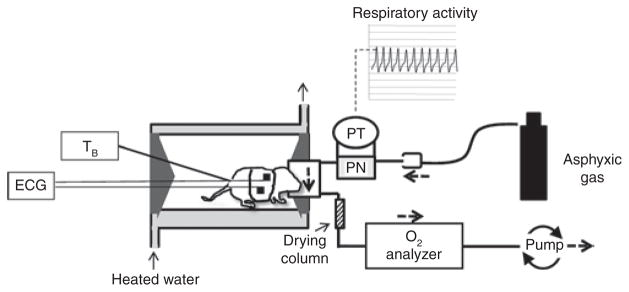
Schematic of experimental setup used to measure cardio- respiratory responses to episodic asphyxia in Pet-1−/− mice and littermates. The animals were placed into a glass chamber perfused with warmed water. Respiratory activity was measured using a pneumotach (PN) attached to a pressure transducer (PT), with the faces of the animals sealed into small masks (volume = ~3 ml). The flow of gas (75 ml/min) through the mask originated from a downstream pump and permitted a rapid (~3 s) wash-in of asphyxic gas, delivered via a tank, to the surrounds of the mask and pneumotach. ECG signals were obtained using surface electrodes embedded in a small vest. V̇O2was determined in room air by sending the effluent gas through a drying column and O2 analyzer.
A pump (AEI Technologies, Naperville, IL) pulled air through the pneumotach and mask at 75 ml/min. Effluent gas was drawn through a column of Drierite (W. A. Hammond Drierite Co. Ltd., Xenia, OH) to remove moisture, followed by an O2 analyzer (AEI Technologies, Pittsburgh, PA) to determine metabolic rate (V̇O2). Asphyxia (97% N2/3% CO2) was induced directly from a tank using a 50 c.c. syringe mounted on a stand that could be rotated to deliver gas to the pneumotach. A two-lead surface electrocardiogram was used to measure HR.
Inspiratory and expiratory airflows were detected by connecting both side-arms of the pneumotachograph to a differential pressure transducer (Validyne Engineering, Northridge, CA). Integration of the flow trace provided respiratory volume, calibrated by injecting and withdrawing known volumes of air (0.025 and 0.05 ml) at the end of each experiment. The pneumotachograph responded in a linear fashion to these volumes. All analog signals were recorded and analyzed in Labchart 6 (ADInstruments, Colorado Springs, CO) using Powerlab data acquisition system (ADInstruments).
Experimental Protocol
The experiments were performed with the investigator blinded as to genotype. The animals were connected to electrocardiogram leads (contained in a small vest) and a rectal thermocouple was inserted (~0.5–1 cm). The snout of the animal was then sealed into the mask, and the animal was allowed to warm to a body temperature of ~36.0 °C (~20 min). After a 10-min baseline recording, the animal was removed from the chamber and given an intra-peritoneal, 50 ul injection of either vehicle (saline) or one of 3 doses of caffeine (1, 5, or 10 mg/kg). The animal was then returned to the chamber for another 20 min to allow for drug absorption and the restoration of body temperature to 36.0 °C. The animals were then exposed to a maximum of 10 brief (~30 s) episodes of asphyxia (by inspiring a gas mixture containing 97% N2 and 3% CO2) to elicit hypoxic apnea and gasping. Each episode of asphyxia was followed by 5 min of exposure to room air, allowing autoresuscitation. Given the high flow rate and low mask volume, the wash-in time for gases was ~3 s. In some vehicle-treated Pet-1−/− animals, the first episode of asphyxia elicited hypoxic apnea of >3 min. For these animals we allowed 10 min of exposure to room air for cardio-respiratory variables to return to pre-challenge levels. Asphyxia was induced only until apnea ensued (~30 s); thereafter the syringe delivering the asphyxiainducing gas to the pneumotachograph was removed, and the mask was immediately flushed with room air.
Data Analysis
Mean data are expressed as ± 95% confidence intervals. Values measured were HR, tidal volume (VT), respiratory frequency (fB), V̇E (product of VT and fB), V̇O2 (V̇O2 = (0.21 − fractional O2 exhausted from mask) × flow (ml.min−1)/ mass (kg)), and the respiratory equivalent (V̇E/V̇O2). HR and breathing were analyzed using peak detection on the respiratory and electrocardiogram traces. After each challenge, we measured (i) the duration of hypoxic apnea (i.e., the delay in initiation of gasping), (ii) the time required to restore HR to 90% of baseline, and (iii) the total time required to restore a regular breathing pattern (eupnea). We plotted the number of episodes survived by each animal, as well as the median number of survived episodes for each genotype at each dose of caffeine (± the 95% confidence interval).
Statistical Analyses
Student’s t-tests were used to compare resting variables (HR, V̇E, V̇O2, and V̇E/V̇O2) between wild-type and Pet-1−/− pups. Two-factor, repeatedmeasures analysis of variance (ANOVA) was used to test for any effects of caffeine on cardio-respiratory variables in room air. A Cox proportional hazards survival model was fitted to test for differences in the number of episodes survived (censored at 10 episodes or death) by genotype (wild-type vs. Pet-1−/−), treatment (vehicle and 1, 5, and 10 mg/kg caffeine), and their interaction (41). The interaction term was used to test whether the effect of caffeine varied by genotype; in the presence of a significant interaction, post hoc tests were used to compare specific groups (e.g., wild-type vehicle receivers vs. Pet-1−/− vehicle receivers, Pet-1−/− vehicle receivers vs. Pet-1−/− 10 mg/kg caffeine receivers, and wild-type vehicle receivers vs. wild-type 10 mg/kg caffeine receivers). ANOVA was used to test for differences in autoresuscitation variables (duration of hypoxic apnea and time required to recover HR and eupnea) by genotype, treatment, and their interaction. The correlations between the number of episodes survived and the autoresuscitation variables were assessed using regression analyses.
Acknowledgments
Statement of Financial Support
Funding for this study was provided by National Institutes of Health grants HD36379 (to H.C.K. and E.E.N.) and HL28066 (primary investigator, E.E.N.), the Parker B. Francis Family and its Foundation (fellowship to K.C.), HD20991 award (to H.C.K.), the Marley Jaye Cherella Foundation (to H.C.K.), the CJ Foundation for SIDS (to H.C.K.), and the First Candle/SIDS Alliance (to H.C.K.).
We thank Evan Deneris at Case Western University School of Medicine, Cleveland, Ohio, for generously supplying the Pet-1−/− mice. We thank Donald Bartlett (Dartmouth Medical School, Lebanon, NH) and Holcombe R. Grier (Harvard Medical School, Boston, MA) for their critical review of the manuscript. All experiments were performed at Dartmouth Medical School.
References
- 1.Fewell JE. Protective responses of the newborn to hypoxia. Respir Physiol Neurobiol. 2005;149:243–55. doi: 10.1016/j.resp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 2.St-John WM, Paton JF. Respiratory-modulated neuronal activities of the rostral medulla which may generate gasping. Respir Physiol Neurobiol. 2003;135:97–101. doi: 10.1016/s1569-9048(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 3.St John WM. Medullary regions for neurogenesis of gasping: noeud vital or noeuds vitals? J Appl Physiol. 1996;81:1865–77. doi: 10.1152/jappl.1996.81.5.1865. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey S. Respiratory and cardiovascular changes during asphyxia and resuscitation of foetal and newborn rabbits. Q J Exp Physiol Cogn Med Sci. 1968;53:97–118. doi: 10.1113/expphysiol.1968.sp001965. [DOI] [PubMed] [Google Scholar]
- 5.Guntheroth WG, Kawabori I. Hypoxic apnea and gasping. J Clin Invest. 1975;56:1371–7. doi: 10.1172/JCI108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings KJ, Commons KG, Hewitt JC, et al. Failed heart rate recovery at a critical age in 5-HT-deficient mice exposed to episodic anoxia: implications for SIDS. J Appl Physiol. 2011;111:825–33. doi: 10.1152/japplphysiol.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol (Lond) 2011;589(Pt 21):5247–56. doi: 10.1113/jphysiol.2011.214445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson JT, Sposato BC. Autoresuscitation responses to hypoxia-induced apnea are delayed in newborn 5-HT-deficient Pet-1 homozygous mice. J Appl Physiol. 2009;106:1785–92. doi: 10.1152/japplphysiol.90729.2008. [DOI] [PubMed] [Google Scholar]
- 9.Aranda JV, Beharry K, Valencia GB, Natarajan G, Davis J. Caffeine impact on neonatal morbidities. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):20–3. doi: 10.3109/14767058.2010.517704. [DOI] [PubMed] [Google Scholar]
- 10.Gray PH, Flenady VJ, Charles BG, Steer PA Caffeine Collaborative Study Group. Caffeine citrate for very preterm infants: Effects on development, temperament and behaviour. J Paediatr Child Health. 2011;47:167–72. doi: 10.1111/j.1440-1754.2010.01943.x. [DOI] [PubMed] [Google Scholar]
- 11.Poets CF. Interventions for apnoea of prematurity: a personal view. Acta Paediatr. 2010;99:172–7. doi: 10.1111/j.1651-2227.2009.01604.x. [DOI] [PubMed] [Google Scholar]
- 12.Julien CA, Joseph V, Bairam A. Caffeine reduces apnea frequency and enhances ventilatory long-term facilitation in rat pups raised in chronic intermittent hypoxia. Pediatr Res. 2010;68:105–11. doi: 10.1203/PDR.0b013e3181e5bc78. [DOI] [PubMed] [Google Scholar]
- 13.Vandam RJ, Shields EJ, Kelty JD. Rhythm generation by the pre-Bötzinger complex in medullary slice and island preparations: effects of adenosine A(1) receptor activation. BMC Neurosci. 2008;9:95. doi: 10.1186/1471-2202-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–42. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung ML, Wang W, St John WM. Medullary loci critical for expression of gasping in adult rats. J Physiol (Lond) 1994;480 (Pt 3):597–611. doi: 10.1113/jphysiol.1994.sp020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fewell JE, Zhang C, Gillis AM. Influence of adenosine A(1)-receptor blockade and vagotomy on the gasping and heart rate response to hypoxia in rats during early postnatal maturation. J Appl Physiol. 2007;103:1234–41. doi: 10.1152/japplphysiol.01421.2006. [DOI] [PubMed] [Google Scholar]
- 17.Cummings KJ, Li A, Deneris ES, Nattie EE. Bradycardia in serotonin- deficient Pet-1−/− mice: influence of respiratory dysfunction and hyperthermia over the first 2 postnatal weeks. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1333–42. doi: 10.1152/ajpregu.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings KJ, Li A, Nattie EE. Brainstem serotonin deficiency in the neonatal period: autonomic dysregulation during mild cold stress. J Physiol (Lond) 2011;589(Pt 8):2055–64. doi: 10.1113/jphysiol.2010.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fewell JE, Smith FG, Ng VK, Wong VH, Wang Y. Postnatal age influences the ability of rats to autoresuscitate from hypoxic-induced apnea. Am J Physiol Regul Integr Comp Physiol. 2000;279:R39–46. doi: 10.1152/ajpregu.2000.279.1.R39. [DOI] [PubMed] [Google Scholar]
- 20.Jacobi MS, Gershan WM, Thach BT. Mechanism of failure of recovery from hypoxic apnea by gasping in 17- to 23-day-old mice. J Appl Physiol. 1991;71:1098–105. doi: 10.1152/jappl.1991.71.3.1098. [DOI] [PubMed] [Google Scholar]
- 21.Solomon IC. Ionotropic excitatory amino acid receptors in pre-Botzinger complex play a modulatory role in hypoxia-induced gasping in vivo. J Appl Physiol. 2004;96:1643–50. doi: 10.1152/japplphysiol.01133.2003. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. J Physiol (Lond) 1998;507 (Pt 3):895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkowitz BA, Spector S. The effect of caffeine and theophylline on the disposition of brain serotonin in the rat. Eur J Pharmacol. 1971;16:322–5. doi: 10.1016/0014-2999(71)90034-3. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GS, Baker TL, Nanda SA, et al. Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–75. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 25.Hendricks TJ, Fyodorov DV, Wegman LJ, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–47. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 26.Kiyasova V, Fernandez SP, Laine J, et al. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J Neurosci. 2011;31:2756–68. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaidi SI, Jafri A, Martin RJ, Haxhiu MA. Adenosine A2A receptors are expressed by GABAergic neurons of medulla oblongata in developing rat. Brain Res. 2006;1071:42–53. doi: 10.1016/j.brainres.2005.11.077. [DOI] [PubMed] [Google Scholar]
- 28.Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–32. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 30.Shao XM, Ge Q, Feldman JL. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBötzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J Physiol (Lond) 2003;547(Pt 2):543–53. doi: 10.1113/jphysiol.2002.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan JR, Paterson DS, Hoffman JM, et al. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–7. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res. 1999;45:350–4. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol. 2003;36:113–22. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- 34.Broadbelt KG, Paterson DS, Belliveau RA, et al. Decreased GABAA receptor binding in the medullary serotonergic system in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2011;70:799–810. doi: 10.1097/NEN.0b013e31822c09bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol. 2009;117:257–65. doi: 10.1007/s00401-008-0468-x. [DOI] [PubMed] [Google Scholar]
- 37.Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–9. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- 38.Paterson DS, Trachtenberg FL, Thompson EG, et al. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–32. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 39.Abdala AP, Dutschmann M, Bissonnette JM, Paton JF. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2010;107:18208–13. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings KJ, Frappell PB. Breath-to-breath hypercapnic response in neonatal rats: temperature dependency of the chemoreflexes and potential implications for breathing stability. Am J Physiol Regul Integr Comp Physiol. 2009;297:R124–34. doi: 10.1152/ajpregu.91011.2008. [DOI] [PubMed] [Google Scholar]
- 41.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Stat Methodol. 1972;34:187–220. [Google Scholar]