Abstract
This article examines changes from 2005 to 2011 in the use of an evidence-based clinical innovation, buprenorphine use, among a nationally representative sample of opioid treatment programs and identifies characteristics associated with its adoption. We apply a model of the adoption of clinical innovations that focuses on the work needs and characteristics of staff; organizations’ technical and social support for the innovation; local market dynamics and competition; and state policies governing the innovation. Results indicate that buprenorphine use increased 24% for detoxification and 47% for maintenance therapy between 2005 and 2011. Buprenorphine use was positively related to reliance on private insurance and availability of state subsidies to cover its cost and inversely related to the percentage of clients who injected opiates, county size, and local availability of methadone. The results indicate that financial incentives and market factors play important roles in opioid treatment programs’ decisions to adopt evidence-based clinical innovations such as buprenorphine use.
Keywords: buprenorphine, adoption, evidence-based practice, opioid treatment, innovation
Introduction
Science focused on developing evidence-based practices (EBPs) has advanced substantially in the past few decades. Yet the science of ensuring that new treatments and services actually reach patients or populations for whom they are intended, and are implemented correctly, has lagged (Eccles & Mittman, 2006; Feldstein & Glasgow, 2008; Fixsen, Naoom, Blase, Friedman, & Wallace, 2005; Institute of Medicine, 2005; Lamb, Greenlick, & McCarty, 1998; Woolf, 2008; Zerhouni, 2003).
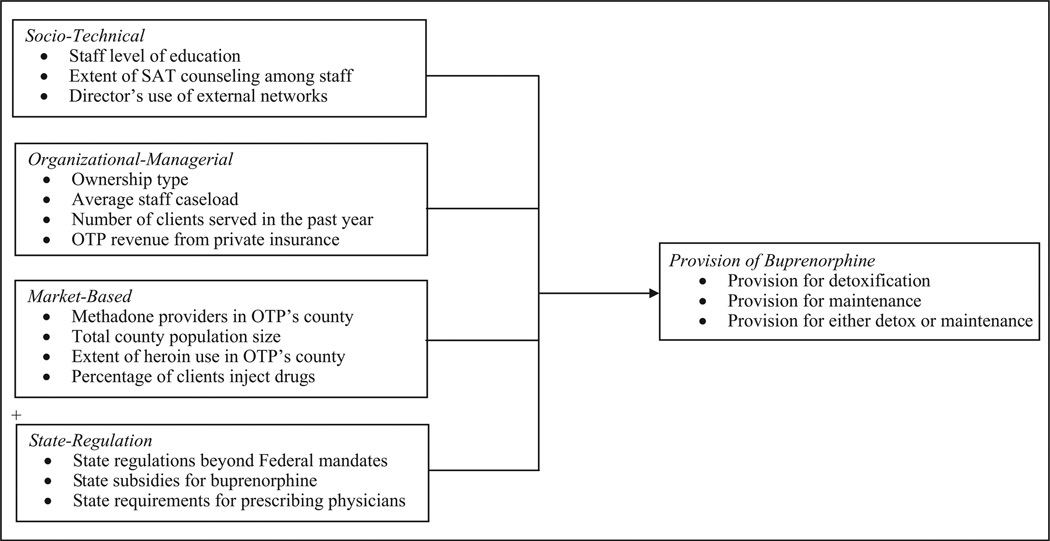
This article aims to understand factors that promote the delivery of EBPs in health care organizations. We identify and test four distinct conceptual models that account for variation in the diffusion and implementation of EBPs among health care organizations: (a) a sociotechnical model that emphasizes how well the innovation matches the work needs and characteristics of its intended users; (b) an organizational-managerial model that emphasizes technical and social support for the innovation within its host organization; (c) a market model that focuses on the dynamics of local competition and social networks; and (d) a state regulation model that emphasizes the role of government rules. We examine these models in a national study of the uptake of an important clinical practice among opioid treatment programs (OTPs): the prescription of buprenorphine.
Buprenorphine is an innovative and effective treatment for opioid dependence. In detoxification, buprenorphine decreases withdrawal symptoms and short-term craving (Kleber, 2007) and attenuates reinforcing effects of opioids (Mattick, Kimber, Breen, & Davoli, 2008). It is also effective as an opioid maintenance therapy, having been demonstrated to decrease opioid use and relapse risk (Mattick et al., 2008). Buprenorphine is widely recognized as a viable alternative to methadone, the predominant medication-assisted treatment for opioid dependence.
While methadone remains the recommended treatment for individuals with more severe opiate dependence, research shows that buprenorphine has fewer withdrawal symptoms and lower risk of abuse (Mattick et al., 2008). First approved by the Food and Drug Administration (FDA) for the treatment of opioid dependence in 2002, buprenorphine is accessible in two ways. First, physicians in office-based settings can provide buprenorphine, contingent on training in its use or certification in the addictions, and a government waiver. Second, OTPs are permitted to provide buprenorphine under the same federal regulations that govern their dispensing of methadone.
Despite extensive research that documents buprenorphine’s effectiveness—and despite public policies that have broadened the range of providers permitted to administer this medication—availability remains limited. While studies indicate that buprenorphine adoption has increased modestly over the past decade, less than one third of specialty substance abuse treatment programs in the United States offer it (Knudsen, Abraham, & Roman, 2011; Knudsen, Roman, & Oser, 2010; Roman, Abraham, & Knudsen, 2011). Buprenorphine use for maintenance therapy lags behind its use for detoxification. A nationally representative study of substance abuse treatment programs conducted in 2005 found that only 9% offered buprenorphine for maintenance (Friedmann, Jiang, & Alexander, 2010). A study of physicians that prescribe buprenorphine found that they were reluctant to prescribe buprenorphine for a period of maintenance longer than 1 year (Reif, Thomas, & Wallack, 2007).
Background: Prior Research on Adoption of Buprenorphine
Over the past decade, a broad base of research suggests that organizations that adopt buprenorphine serve a more affluent population than providers of methadone. Buprenorphine adopters are more likely to be for-profit (Ducharme & Roman, 2009; Knudsen, Abraham, Johnson, & Roman, 2009; Knudsen, Ducharme, & Roman, 2006), privately funded (Roman et al., 2011), office-based (Stein et al., 2011), and accredited (Knudsen et al., 2006; Koch, Arfken, & Schuster, 2006). They are also likely to report more reliance on revenue from private insurance and client self-pay (Knudsen et al., 2006). On average, clients served by substance abuse treatment programs that offer buprenorphine are also more like likely to be white (Knudsen et al., 2010; Wallack, Thomas, Martin, Chilingerian, & Rief, 2008) and to use opiates other than heroin (Ducharme, Knudsen, Roman, & Johnson, 2007).
Prior studies also highlight the importance of organizational infrastructure. Adopting programs are more likely to report preexisting infrastructure enabling provision of the medication, and to offer a scope of services compatible with its use. For example, early adopters of buprenorphine were more likely to employ physicians (Ducharme et al., 2007; Knudsen et al., 2009; Knudsen, Abraham, & Roman, 2011) and nurses (Knudsen et al., 2010; Knudsen, Abraham, & Oser, 2011). Substance abuse treatment programs that already provide detoxification services also are more likely to adopt buprenorphine (Ducharme et al., 2007; Ducharme & Abraham, 2008; Knudsen et al., 2006; Knudsen et al., 2009), serve a proportionately greater number of opiate-dependent clients (Knudsen et al., 2006), and offer other medication-oriented treatments, such as Naltrexone (Knudsen et al., 2006; Koch et al., 2006).
New Contribution
The present study extends prior work in two significant ways. First, it remains unclear how the broader environment in which OTPs operate may influence decisions about whether to offer buprenorphine. Recent literature reviews support the need for more integrated and comprehensive conceptual models of the adoption and implementation of innovations in health care and highlight the importance of accounting for the broader environmental context in which potential adopters operate (e.g., Damschroder et al., 2009; Rye & Kimberly, 2007).
In a comprehensive review of studies examining adoption of innovations in health care organizations, Rye and Kimberly (2007) identified “environmental influences” and “organizational connectedness” as key components. Specifically, they identify several environmental factors that influence adoption: demand, including need in the local population, the overall size of the market, and the potential supply of providers; competition; geography, including region and urbanicity; network structure, including directors’ professional networks and programs’ service collaborations; and, finally, government regulation.
Despite the importance of these factors in influencing the adoption of innovations in health care settings, few studies examining predictors of adoption of clinical innovation have examined these factors simultaneously. In the case of buprenorphine specifically, little attention has been paid to role of market and state policy factors in influencing adoption. A few notable exceptions have examined the role of government regulation. Ducharme and Abraham (2008) found that treatment programs located in states that provided public insurance coverage for buprenorphine were more likely to provide it. Similarly, Knudsen and Abraham (2012), using data from provider self-reports, found that treatment programs were more likely to have adopted any medication for addiction, including but not limited to buprenorphine, if they perceived greater support for medications by the Single State Agency overseeing publicly funded substance abuse treatment services. They also find that the odds of adoption were significantly greater if program managers were aware that public funding was available to support at least one medication for treatment of addiction.
Second, while existing literature lends insight into the organizational attributes of programs that offer buprenorphine, it remains unclear whether these factors may differentially influence its use for detoxification versus maintenance purposes. This represents a significant gap in the literature, as prior research indicates that OTPs are more likely to adopt buprenorphine for detoxification purposes than for maintenance (Friedmann et al., 2010; Stein & Friedmann, 2007). Moreover, a study of a national sample of specialty outpatient substance abuse treatment programs in the United States found that different factors were important in driving OTP adoption of buprenorphine for detoxification and maintenance (Friedmann et al., 2010). Both an emphasis on abstinence-only treatment approaches and employment of medical staff were associated with adoption of buprenorphine for maintenance, but not for detoxification. Despite evidence suggesting the need to analyze these outcomes separately, no other multivariate analyses of adoption of buprenorphine have considered detoxification and maintenance individually.
Conceptual Framework
The present study broadens the scope of prior work by considering not only the organizational attributes of treatment programs but also the characteristics of the market and policy environments in which they are situated. Our conceptual framework, depicted in Figure 1, includes four distinct models of adoption of EBPs among OTPs. We identified these models from prior research on adoption of innovations in substance abuse treatment, as well as broader reviews of diffusion of innovation in health care organizations (e.g., Damschroder et al., 2009; Rye & Kimberly, 2007). They include (a) a sociotechnical model that emphasizes how well the innovation matches the work needs and characteristics of its intended users; (b) an organizational-managerial model that emphasizes social and material support for the innovation within its host organization; (c) a market model that focuses on the dynamics of market supply, demand, and competition; and (d) a state regulation model that emphasizes the role of government regulations that may hinder or support adoption of innovation.
Figure 1.
Conceptual framework.
Drawing on the conceptual framework described above, and data from a nationally representative, longitudinal survey of OTPs, we examine two questions. First, has provision of buprenorphine by OTPs changed between 2005 and 2011—overall, and separately for detoxification and maintenance purposes? And second, how are each of the four models identified in our conceptual framework (sociotechnical, organizational-managerial, market, and state regulation) associated with adoption of this clinical innovation— overall, and separately for detoxification and maintenance purposes?
Understanding the role of the four conceptual models in the adoption and use of evidence-based clinical innovations is important for health services researchers, policymakers, and managers who seek to increase use of EBPs more generally, and those who seek to promote buprenorphine use in particular. That is, we aim to develop new insights into the respective contributions of organizational, market, and policy level factors in influencing health care providers’ decisions to adopt evidence-based clinical innovations. At the same time, information about the influence of market and policy factors on the adoption of clinical innovations can help point to policy levers and incentives that policy makers and managers can use to influence rates of use over time.
Method
The study draws from two nationally representative surveys of OTPs. The first survey includes data from 170 OTPs collected as part of the 2005 National Drug Abuse Treatment System Survey (NDATSS). This survey was one of five survey waves conducted as part of the broader NDATSS study, beginning in 1988 and ending in 2005 (D’Aunno, Foltz-Murphy, & Lin, 1999; D’Aunno & Pollack, 2002; D’Aunno & Vaughn, 1992; Pollack & D’Aunno, 2008). The second was a follow-up survey conducted in 2011 by Cornell University’s Survey Research Institute. This survey included OTPs that participated in the 2005 wave of NDATSS as well as a subsample of replacement OTPs. The study also includes data on state policies regarding financing and regulation of buprenorphine, drawn from surveys of state substance abuse treatment agency officials conducted in 2006 and 2011. Each survey is described in detail below.
Survey of Opioid Treatment Programs
Sampling Frame and Sample
The NDATSS study defines an OTP as a physical facility that dedicates more than half of its resources to treatment of opiate dependence using methadone or buprenorphine (D’Aunno & Pollack, 2008). The sampling frame included all 1,459 OTPs in the country licensed by the Substance Abuse and Mental Health Services Administration (SAMHSA) in 2011. Together, these OTPs are estimated to treat approximately one quarter of all patients receiving substance abuse treatment in specialty settings nationally on any given day. The current study uses data from the nationally representative sample of 170 OTPs that participated in the 2005 wave of NDATSS, which yielded a response rate of 88% (Friedmann et al., 2010; Pollack & D’Aunno, 2010). For the 2011 sample, we contacted OTPs that participated in 2005. We also contacted randomly selected OTPs from SAMHSA’s 2011 list to ensure that the 2011 sample was nationally representative, with the goal of obtaining a total sample size of 200 OTPs.
Of all the 2005 and newly selected OTPs contacted in 2011, 200 completed surveys and 22 refused to participate, resulting in a final sample of 200 OTPs for 2011 (a response rate of 86.6%). To assess possible nonresponse bias stemming from the 22 OTPs that refused to participate in 2011, we compared OTPs that participated in the study with these nonresponding OTPs, by size (as measured by staff, clients, and budget), location, specialization in buprenorphine or methadone, organizational age, and ownership (public, private for-profit, private nonprofit). The analysis yielded no significant differences across any of these variables.
Data and Measures
In both the 2005 and 2011 waves, administrative directors and clinical supervisors completed a telephone survey about their organization. We used this approach for several reasons: it reduces common-method variance in data analyses that stems from having a single survey respondent; it facilitates tailoring of each survey to the respondent’s responsibilities; and it allows for reliability checks (by cross-checking clinical supervisor responses with those of the administrative director for selected survey items). Administrative directors were asked about ownership, therapy activities, environment, finances, organizational structure, and managed care arrangements. Clinical supervisors provided information about staff composition, client characteristics, and ancillary services.
The phone survey data were merged with two additional data sources that capture features of the broader market environment in which the study OTPs are located. First, SAMSHA’s National Survey of Substance Abuse Treatment Services survey (2005, 2010) provided measures of the structure and organization of substance abuse treatment services and systems in the counties in which our OTPs were situated. Second, the Treatment Episode Dataset (2005, 2010), an administrative data set on publicly funded substance abuse treatment episodes, permitted calculation of the proportion of state admissions with a primary diagnosis of opiate dependence.
Data Reliability and Validity
We followed established methods that maximize reliability and validity in phone surveys (Groves, 1988). These methods include pretesting the survey with a random sample of 10 programs; providing training about our study for telephone interviewers who already have been trained at Cornell’s Survey Research Institute (which collected the 2011 data); sending each program director a cover letter explaining the study, along with web-based work-sheets that inform participants of the requested data and enable them to consult financial and administrative records prior to the call; and making a brief phone call to follow-up on the letter.
Furthermore, as data are collected, we performed extensive computer reliability checks to signal interviewers of inconsistent or infeasible responses (e.g., percentage of clients with various demographic characteristics should sum to 100%). Interviewers then work with respondents to resolve inconsistencies. Results are further scrutinized for reliability and validity. Reliability checks include comparisons of reported totals (e.g., total revenue) with the sum of reported detail (e.g., revenues by source); comparison of responses to related questions; comparison of responses between director and supervisor; and, for panel programs, comparison of responses over time. Results from several analyses provide support for NDATSS data reliability and validity (Pollack & D’Aunno, 2010).
Survey of Single State Agencies
Telephone surveys were conducted in 2006 and 2011 with at least one state official employed within the agency that has primary oversight for the provision of buprenorphine to gather information on state government regulations and financing for buprenorphine. Respondents were asked about state policies governing buprenorphine, including whether the state imposed regulations on its provision beyond those mandated by federal law; provided incentives to encourage adoption of buprenorphine; covered buprenorphine under its state Medicaid program; and provided non-Medicaid funds to subsidize it. In addition, state officials were asked to rate their overall stance on the use of buprenorphine for treatment of opiate dependence. Forty-nine of 50 states participated in 2006 (all except for South Carolina), and 49 of 50 states participated in 2011 (all except for Colorado).
Study Measures
Dependent Variables
We assessed adoption of buprenorphine using three primary outcomes: any buprenorphine use; buprenorphine use for opioid detoxification; and buprenorphine use for opioid maintenance therapy. Clinical supervisors were asked the percentage of the program’s clients receiving buprenorphine for opioid detoxification and the percentage of clients receiving buprenorphine for opioid maintenance therapy. Programs reporting at least one client receiving buprenorphine for less than 3 months were coded affirmatively for buprenorphine use for detoxification; programs reporting at least one client receiving buprenorphine for 3 months or longer were coded affirmatively for buprenorphine use for maintenance. Programs were coded affirmatively for any adoption of buprenorphine if the program had at least one substance abuse treatment client receiving buprenorphine for either of the abovementioned purposes.
Explanatory Variables
The four conceptual models were operationalized using program-, county-, and state-level variables. At the program level, the sociotechnical model examined how well the innovation matches the work needs and characteristics of staff and clients. Variables in this model included percentage of program staff holding a professional degree; percentage of staff with certification in substance abuse counseling; and director’s use of external networks and connections.
The organizational-managerial model examines infrastructure and capacity to adopt buprenorphine within OTPs. Variables included average staff caseload; ownership type (public, private for-profit, private nonprofit); number of clients served in the past year; and organizational auspices (hospital-based, mental health center-based, freestanding, other); and percentage of revenue from private insurance.
The market model includes variables measured at the provider and county levels that examine the influence of market supply, demand, and competition on provision of buprenorphine. Variables in this model included county population size (logged); percentage of county substance abuse treatment admissions with a primary diagnosis of heroin abuse or dependence; percentage of program clients that clinical supervisors reported injecting drugs at admission; and the percentage of substance abuse treatment programs in the county in which the OTP was located that provided methadone (using data from the National Survey of Substance Abuse Treatment Services, 2005 and 2010).
The state regulation model emphasizes the role of government regulations that might hinder or support adoption of buprenorphine. Variables include whether the state imposed regulations on buprenorphine provision beyond those mandated by federal law; provided subsidies to support its use; and imposed special requirements on prescribing physicians above and beyond federal guidelines. To measure time trends, we included a dichotomous variable identifying survey year.
Data Analysis
Descriptive statistics were weighted to adjust for organizational size. Two-tailed t tests were used to examine change in rates of adoption of buprenorphine for each of the study’s three outcome variables: any adoption of buprenorphine, adoption for opiate detoxification, and adoption for opioid maintenance therapy. Population averaged logit models were developed using STATA’s xtlogit function to account for correlated error structure among OTPs sampled in both 2005 and 2011 (Diggle, Liang, & Zeger, 1994). We chose not to restrict our analysis to OTPs that participated in both survey waves, as such nonrandom exclusion appears to foster bias (Little & Rubin, 2002). Huber–White robust estimation was used to calculate standard errors (Wooldridge, 2002).
Multiple imputation was conducted to fill in missing values by assuming the data were missing at random (Rubin, 1987). Some independent variables from the survey of OTPs (e.g., percentage of clients requiring preauthorization, profit margin in most recent fiscal year) displayed missing observations in particular survey waves. Each missing value was replaced with five plausible values using the Markov Chain Monte Carlo (MCMC) method (Schaefer, 1997). The resulting five imputed data sets were merged for further statistical analysis. This imputation had no substantive impact on our point estimates but increased our sample size in multivariate regressions from 350 to 394 programs. The process was repeated for each of three outcome variables included in the study: any adoption of buprenorphine, adoption for opiate detoxification, and adoption for opioid maintenance therapy. All analyses were conducted in STATA MP 11.
Results
In 2005, buprenorphine use among the sample OTPs for detoxification and maintenance purposes was equally common (Table 1). Provision of buprenorphine increased for both opioid detoxification and opioid maintenance therapy between 2005 and 2011. During this period, buprenorphine use for opioid detoxification increased from 37% to 46%, and use for opioid maintenance therapy increased from 36% to 53%. Overall, the percentage of OTPs providing buprenorphine for any purpose increased from 49% in 2005 to 57% in 2011. Taken together, these findings show an increase in buprenorphine use among OTPs in the United States between 2005 and 2011.
Table 1.
| Variable | 2005 | 2011 |
|---|---|---|
| Outcome Variables | ||
| Provides buprenorphine for any purpose | 98 (49%) | 107 (57%) |
| Provides buprenorphine for detoxification | 76 (37%) | 87 (46%) |
| Provides buprenorphine for maintenance | 73 (36%) | 99 (53%) |
| Sociotechnical | ||
| Percentage of OTP staff who are professionals | 35.41 (23.41) | 26.57 (18.67) |
| Percentage of staff who possess SATS’ credentialing | 37.17 (27.23) | 37.72 (22.86) |
| OTP director has extensive professional networks | 8.71 (8.74) | 7.49 (4.95) |
| Organizational-Managerial | ||
| Ownership type | ||
| Publicly owned | 40 (20%) | 17 (9%) |
| Private nonprofit | 93 (47%) | 109 (59%) |
| OTP director rates staff caseload as “high or “too high” | 78 (39%) | 60 (32%) |
| Number of clients served during past year (log) | 6.75 (1.11) | 6.59 (0.79) |
| Percentage of OTP revenue from private insurance | 8.27 (17.47) | 8.81 (17.23) |
| Market-Based | ||
| Percentage programs in OTP’s county offer methadone | 68.42 (25.36) | 53.19 (26.96) |
| Population in OTP’s county (log) | 13.27 (1.33) | 13.34 (1.28) |
| Percentage of SATS’ admits for heroin in OTP’s state | 19.62 (11.30) | 23.08 (8.88) |
| Percentage of OTP’s clients in past year injected drugs | 38.59 (29.21) | 38.49 (27.10) |
| State Regulation | ||
| State imposes special regulations on OTPs | 21 (10%) | 71 (38%) |
| State funds available to subsidize buprenorphine | 42 (20%) | 129 (71%) |
| State imposes special requirements on prescribing physicians | 30 (15%) | 8 (5%) |
| N | 202 | 187 |
Note. OTP = opioid treatment program; SATS = substance abuse treatment.
. Weighted by the total number of clients served in the past year by the OTP.
. Counts and percentages (the latter in parentheses) are reported for dichotomous variables and means and standard deviations (the latter in parentheses) are reported for continuous variables.
Overall, multivariate models showed little support for the role of organizational-level factors in predicting adoption of buprenorphine (Table 2). None of the variables from the sociotechnical model were associated with any of the study outcomes in our multivariate model results. Of the four variables from the organizational-managerial model, only one was associated with adoption: the percentage of OTP clients with private insurance. OTPs that had a higher percentage of clients with private insurance were more likely to provide buprenorphine. The percentage of programs’ total revenue from private insurance was associated with greater odds of adoption across all three study outcomes.
Table 2.
| Any use | For detoxification | For maintenance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AOR | LCI | UCI | AOR | LCI | UCI | AOR | LCI | UCI | |
| Sociotechnical | |||||||||
| Percentage of OTP staff who are professionals | 1.01 | 0.99 | 1.02 | 1.00 | 0.98 | 1.01 | 1.01 | 1.00 | 1.02 |
| Percentage of staff who possess SATS’ credentialing | 1.01 | 1.00 | 1.02 | 1.01 | 1.00 | 1.02 | 1.01 | 0.99 | 1.02 |
| Director’s use of external networks | 1.02 | 0.98 | 1.06 | 1.02 | 0.97 | 1.07 | 0.99 | 0.95 | 1.04 |
| Organizational-Managerial | |||||||||
| OTP director rates staff caseload as “high” or “too high” | 0.72 | 0.41 | 1.26 | 0.65 | 0.37 | 1.14 | 0.74 | 0.43 | 1.27 |
| Program ownership | |||||||||
| Private for-profit | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Private nonprofit | 1.28 | 0.72 | 2.26 | 1.47 | 0.82 | 2.61 | 0.82 | 0.47 | 1.43 |
| Public | 0.77 | 0.33 | 1.83 | 1.01 | 0.40 | 2.57 | 0.52 | 0.22 | 1.25 |
| Number of clients served during past year (log) | 1.28 | 0.95 | 1.73 | 1.28 | 0.95 | 1.72 | 1.27 | 0.95 | 1.70 |
| Percentage of OTP revenue from private insurance | *1.03 | 1.01 | 1.06 | *1.02 | 1.01 | 1.04 | *1.02 | 1.00 | 1.03 |
| Market-Based | |||||||||
| Percentage of programs in OTP’s county offer methadone | *0.98 | 0.97 | 0.99 | *0.97 | 0.96 | 0.99 | *0.99 | 0.98 | 1.00 |
| Population in OTP’s county (log) | *0.70 | 0.57 | 0.87 | 0.85 | 0.69 | 1.06 | *0.73 | 0.59 | 0.90 |
| Percentage of SATS admits for heroin in OTP’s state | *0.97 | 0.94 | 0.99 | *0.97 | 0.95 | 1.00 | 0.99 | 0.97 | 1.01 |
| Percentage of OTP’s clients in past year injected drugs | *0.98 | 0.97 | 0.99 | *0.98 | 0.97 | 0.99 | *0.98 | 0.98 | 0.99 |
| State-Regulatory | |||||||||
| State regulates buprenorphine beyond federal standards | 1.23 | 0.64 | 2.38 | 0.69 | 0.33 | 1.42 | 1.06 | 0.57 | 1.94 |
| State funds available to subsidize buprenorphine | *2.06 | 1.13 | 3.78 | *2.51 | 1.40 | 4.45 | *1.81 | 1.02 | 3.19 |
| State imposes special requirements on prescribing physicians | 1.94 | 0.83 | 4.55 | 1.13 | 0.42 | 3.01 | *2.88 | 1.22 | 6.77 |
| Wave 2005 | 0.80 | 0.45 | 1.41 | 1.01 | 0.56 | 1.84 | 0.38 | 0.21 | 0.67 |
| N | 394 | 394 | 394 | ||||||
Note. OTP = opioid treatment program; SATS = substance abuse treatment.
. Dependent variables include three dichotomous outcomes: buprenorphine use for any treatment purpose (Any Adoption), buprenorphine use for opioid detoxification (For Detoxification), and buprenorphine use for maintenance (For Maintenance).
. Adjusted odds ratios (AOR) with an asterisk are significant at p < .05. LCI and UCI refer to lower-bound and upper-bound confidence intervals, respectively.
In contrast, the results of the study demonstrated substantial support for the market model, which assessed the role of local supply and demand of buprenorphine and its substitute, methadone. Indeed, all of the variables used to measure dimensions of this model were significantly associated with one or more of our study outcomes. The percentage of substance abuse treatment programs providing methadone in the county in which the OTP was located was inversely associated with provision of buprenorphine for detoxification, maintenance, and overall, suggesting that buprenorphine is less likely to offered in areas in which there is more ample supply of methadone. Additionally, the total population of the county was also associated with buprenorphine use by OTPs; controlling for other variables in the model, programs located in larger counties were less likely to offer buprenorphine overall, and specifically for maintenance therapy.
The characteristics of the local market and its users were also important in predicting provision of buprenorphine by OTPs. The percentage of program clients that clinical supervisors reported as injection drug users was associated with significantly lower odds of buprenorphine provision across all three study outcomes. Similarly, the percentage of public substance abuse treatment admissions with a primary diagnosis of heroin abuse or dependence was associated with lower odds of any adoption of buprenorphine. Taken together, these findings suggest that OTPs may be less likely to offer buprenorphine when they are embedded within local markets in which there is greater heroin and injection drug use, as opposed to use of prescription opioids.
From the state regulation model, two factors were important. First, programs in states that offered such subsidies had nearly the twice the odds of offering buprenorphine as programs located in states without such subsidies across all three study outcomes Second, programs located in states with special requirements for buprenorphine-prescribing physicians had more than twice the odds of providing buprenorphine for opioid maintenance therapy.
Discussion
The overall rate of buprenorphine use increased by 16% between 2005 and 2011. This finding is consistent with prior research examining trends in buprenorphine use since its introduction in 2003. Using nationally representative samples of private and public substance abuse treatment programs in the United States, Roman et al. (2011) found that buprenorphine use increased significantly across two data collection periods, spanning from 2002 to 2004 and 2007 to 2010. Buprenorphine use increased from 13% to 44% among private programs, and from 4% to 24% among public programs. These findings indicate lower overall rates of adoption than our study, but this is likely because of the fact that our sample was limited to OTPs. However, both studies show substantial increases in buprenorphine use over a similar period.
Between 2005 and 2011, buprenorphine use increased by 24% for detoxification, and 47% for maintenance. These results suggest that some OTPs that may have already provided buprenorphine for detoxification only or maintenance only, and expanded their use to include both purposes between 2005 and 2011. Moreover, OTPs were not more likely to provide buprenorphine for detoxification than for maintenance. This finding is contrary to our expectations and prior research, which have documented lower adoption of buprenorphine for maintenance. Drawing from the 2005 NDATSS survey used in this study, Friedmann et al. (2010) found lower adoption for maintenance. However, their study was not limited to OTPs, suggesting that reluctance to adopt buprenorphine for maintenance therapy may be more common in non-OTPs, which do not have the same history and experience using medication-assisted treatments.
Results of multivariate analyses highlight the importance of the market and policy environment in shaping OTPs’ decisions to provide buprenorphine. Unlike prior research, our results yielded little support for the sociotechnical and organizational-managerial models. Only the percentage of OTPs’ revenue from private insurance was significantly associated with the odds of buprenorphine use after accounting for market and policy characteristics, and this finding was consistent across all three study outcomes: any use, use for detoxification, and use for maintenance. Broader coverage for medication-assisted treatments for addiction and higher overall rates of reimbursement by private insurance may explain broader uptake of buprenorphine among programs that focus on the privately insured population. However, no other organizational characteristics of OTPs—in either the sociotechnical or organizational-managerial models—were associated with provision of buprenorphine.
Our study highlights the importance of market-based factors. The client profile in communities in which OTPs were located was significantly associated with buprenorphine use. The percentage of clients with injection drug use was negatively associated with buprenorphine use. This finding has been previously reported (Ducharme et al., 2007) and may reflect social and economic differences in the clients of methadone-versus buprenorphine-dispensing programs. The proportion of admissions because of heroin use among publicly funded substance abuse treatment facilities was also negatively associated with adoption of buprenorphine.
We speculate that the reason for this finding is that urban OTPs that serve more indigent heroin injectors are long-standing methadone providers, while prescription opioid users, many of whom are insured, prefer buprenorphine. Indeed, buprenorphine was less likely to be adopted in large urban areas, even when accounting for differences in the availability of methadone. Stein et al. (2011) reported higher uptake of buprenorphine in rural areas that have had an epidemic of prescription opioid misuse. These findings are consistent with SAMHSA data that show that the expansion of opioid treatment with buprenorphine has benefited communities with a predominance of prescription opioid abuse.
The broader treatment market environment in which OTPs are situated also appears to influence buprenorphine use. Programs located in counties in which methadone was widely available were less likely to offer buprenorphine. OTPs may be more likely to enter the buprenorphine market when demand for medication-assisted treatment is not already being addressed by another product—in this case, methadone. Programs in areas without a significant methadone presence may perceive better potential for growth resulting from unmet demand. Another related possibility is that programs in certain markets may find that buprenorphine is more acceptable to their clients than methadone.
Factors from the state regulation model also proved to be significant correlates of buprenorphine provision among OTPs. Programs located in states that offered such subsidies had roughly twice the odds of providing buprenorphine, suggesting that such financial incentives may be an important policy lever though which to increase the availability of EBPs. This finding is salient in light of the dramatic rise in the availability of these subsidies during the study time period: between 2005 and 2011, the percentage of states offering subsidies more than tripled from 20% to 71%. This finding is consistent with recent findings that the odds of adoption were significantly greater if the program was aware that state-contract funding permitted the purchase of medication (Knudsen & Abraham, 2012).
Finally, states that impose requirements on physicians that prescribe buprenorphine, in addition to federal requirements established under the Drug Addiction and Treatment Act of 2000, were more likely to report buprenorphine use for maintenance. Because it is unlikely that greater regulation would result in greater buprenorphine use, we suggest that this may be a case of reverse causation: states that have witnessed large increases in buprenorphine use for maintenance may have responded by increasing regulations on prescribers. Results from our study indicate that some states, such as Washington and Mississippi, have instituted regulations on prescribing physicians that limit dose levels and treatment duration because of concerns about diversion and costs of buprenorphine. More research is needed to explore the potential connection between state requirements for prescribing physicians and buprenorphine use among OTPs.
This study has several limitations. First, our analyses did not include data on physicians licensed to prescribe buprenorphine, and consequently, our findings are generalizable only to OTPs that prescribe buprenorphine. This is of particular significance because physicians constitute an estimated 64% of all providers of buprenorphine (Stein et al., 2011). There is also the risk of omitted variable bias stemming from our inability to measure prescription of buprenorphine by physicians in office-based practices.
Second, our analyses draw from program-level data and, as a consequence, cannot directly explore client characteristics associated with receipt of buprenorphine or the impact of receipt on health and social outcomes. Although organizational-level reports in NDATSS have been compared against client-level responses for some measures, and indicate high validity of NDATSS data, reports of buprenorphine adoption have not been specifically validated in the 2005 wave or the follow-up survey conducted in 2011. A related limitation is that our data are based on responses provided by administrative directors and clinical supervisors based within OTPs. These responses may be susceptible to desirability bias. Finally, the present study does not examine the percentage of clients who receive buprenorphine and, consequently, cannot provide insight as to the extent of implementation of buprenorphine within OTPs.
In spite of these limitations, the study offers several findings of interest to policymakers and other stakeholders working to increase adoption of evidence-based clinical innovations, in general, and buprenorphine, in particular. Overall, buprenorphine use increased between 2005 and 2011 among OTPs in the United States. Despite increased buprenorphine use during this time period, more than 40% of OTPs still do not offer buprenorphine for any purpose to any of their clients. High-quality OTPs should offer a range of treatment options to their clients. Continued efforts will be needed to sustain this upward trajectory in adoption of buprenorphine.
More broadly, our findings suggest that health care provider decisions regarding adoption of clinical innovations may be particularly sensitive to the availability financing, as well as the structure of supply and demand for the innovation within their communities. Independent of other factors we considered, market-based environmental characteristics emerged as the strongest and most consistent correlates of buprenorphine use. Further research examining adoption of clinical innovations should consider the potential role of market factors and financial incentives in health care providers’ decisions to adopt evidence-based clinical innovations such as buprenorphine.
Acknowledgments
The authors would like to thank Lan Jiang, MS, for assistance with statistical analysis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Institute on Drug Abuse (R01DA030459-02).
Footnotes
Aspects of this analysis were presented at the Addiction Health Services Research Conference in New York, NY, in October 2012.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- D’Aunno T, Foltz-Murphy N, Lin X. Changes in methadone treatment practices: Results from a panel study, 1988–1995. American Journal of Drug and Alcohol Abuse. 1999;25:681–699. doi: 10.1081/ada-100101886. [DOI] [PubMed] [Google Scholar]
- D’Aunno T, Pollack HA. Changes in methadone treatment practices: Results from a national panel study, 1988–2000. Journal of the American Medical Association. 2002;288:850–856. doi: 10.1001/jama.288.7.850. [DOI] [PubMed] [Google Scholar]
- D’Aunno T, Vaughn TE. Variations in methadone treatment practices: Results from a national study. Journal of the American Medical Association. 1992;267:253–258. [PubMed] [Google Scholar]
- Damschroder L, Aron D, Keith R, Kirsh S, Alexander J, Lowery J. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implementation Science. 2009 Aug;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford, England: Oxford University Press; 1994. [Google Scholar]
- Ducharme LJ, Abraham AJ. State policy influence on the early diffusion of buprenorphine in community treatment programs. Substance Abuse Treatment, Prevention, and Policy. 2008;3(1):17. doi: 10.1186/1747-597X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Knudsen HK, Roman PM, Johnson JA. Innovation adoption in substance abuse treatment: Exposure, trialability, and the clinical trials network. Journal of Substance Abuse Treatment. 2007;32:321–329. doi: 10.1016/j.jsat.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Roman PM. Opioid treatment programs in the Clinical Trials Network: Representativeness and buprenorphine adoption. Journal of Substance Abuse Treatment. 2009;37:90–94. doi: 10.1016/j.jsat.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles MP, Mittman BS. Welcome to implementation science. Implementation Science. 2006;1(1):1. [Google Scholar]
- Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) Joint Commission Journal on Quality and Patient Safety. 2008;34:228–243. doi: 10.1016/s1553-7250(08)34030-6. [DOI] [PubMed] [Google Scholar]
- Fixsen DL, Naoom SF, Blase KA, Friedman RM, Wallace F. Implementation research: A synthesis of the literature. Tampa: University of South Florida, Louis de la Parte Florida Mental Health Institute, The National Implementation Research Network; 2005. [Google Scholar]
- Friedmann PD, Jiang L, Alexander JA. Top manager effects on buprenorphine adoption in outpatient substance abuse treatment programs. Journal of Behavioral Health Services and Research. 2010;37:322–336. doi: 10.1007/s11414-009-9169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves RM. Theories and methods of telephone surveys. Annual Review of Sociology. 1990;16:221–240. [Google Scholar]
- Institute of Medicine. Crossing the quality chasm in mental and substance use treatment. Washington, DC: National Academies Press; 2005. [Google Scholar]
- Kleber HD. Pharmacologic treatments for opioid dependence: Detoxification and maintenance options. Dialogues in Clinical Neuroscience. 2007;9:455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ. Perceptions of the state policy environment and adoption of medications in the treatment of substance use disorders. Psychiatric Services. 2012;63(1):19–25. doi: 10.1176/appi.ps.201100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ, Johnson JA, Roman PM. Buprenorphine adoption in the National Drug Abuse Treatment Clinical Trials Network. Journal of Substance Abuse Treatment. 2009;37:307–312. doi: 10.1016/j.jsat.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ, Oser CB. Barriers to the implementation of medication- assisted treatment for substance use disorders: The importance of funding policies and medical infrastructure. Evaluation and Program Planning. 2011;34:375–381. doi: 10.1016/j.evalprogplan.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. Journal of Addiction Medicine. 2011;5(1):21–27. doi: 10.1097/ADM.0b013e3181d41ddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Ducharme LJ, Roman PM. Early buprenorphine adoption in substance abuse treatment centers: Data from the private and public sectors. Journal of Substance Abuse Treatment. 2006;30:363–373. doi: 10.1016/j.jsat.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM, Oser CB. Facilitating factors and barriers to the use of medications in publicly funded addiction treatment organizations. Journal of Addiction Medicine. 2010;4(2):99–107. doi: 10.1097/ADM.0b013e3181b41a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AL, Arfken CL, Schuster CR. Characteristics of U.S. substance abuse treatment facilities adopting buprenorphine in its initial stage of availability. Drug and Alcohol Dependence. 2006;83:274–278. doi: 10.1016/j.drugalcdep.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Lamb SJ, Greenlick MR, McCarty D. Bridging the gap between practice and research: Forging partnerships with community-based drug and alcohol treatment. Washington, DC: National Academies Press; 1998. [PubMed] [Google Scholar]
- Little RA, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley-Interscience; 2002. [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Oxford, England: Cochrane Collaboration; 2008. [DOI] [PubMed] [Google Scholar]
- Pollack HA, D’Aunno T. Dosage patterns in methadone treatment: Results from a national survey, 1988–2005. Health Services Research. 2008;43:2143–2163. doi: 10.1111/j.1475-6773.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack HA, D’Aunno T. HIV testing and counseling in the nation’s outpatient substance abuse treatment system, 1995–2005. Journal of Substance Abuse Treatment. 2010;38:307–316. doi: 10.1016/j.jsat.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Reif S, Thomas CP, Wallack SS. Factors determining how early adopter physicians use buprenorphine in treatment. Journal of Addiction Medicine. 2007;1:205–212. doi: 10.1097/ADM.0b013e31814c3fa8. [DOI] [PubMed] [Google Scholar]
- Roman PM, Abraham AJ, Knudsen HK. Using medication-assisted treatment for substance use disorders: Evidence of barriers and facilitators of implementation. Addictive Behaviors. 2011;36:584–589. doi: 10.1016/j.addbeh.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: Wiley; 1987. [Google Scholar]
- Rye CB, Kimberly JR. The adoption of innovations by provider organizations in health care. Medical Care Research and Review. 2007;64:235–278. doi: 10.1177/1077558707299865. [DOI] [PubMed] [Google Scholar]
- Schaefer JL. Analysis of incomplete multivariate data. New York, NY: Chapman & Hall; 1997. [Google Scholar]
- Stein MD, Friedmann PD. Optimizing opioid detoxification: Rearranging deck chairs on the Titanic. Journal of Addictive Diseases. 2007;26(2):1–2. doi: 10.1300/J069v26n02_01. [DOI] [PubMed] [Google Scholar]
- Stein BD, Gordon AJ, Sorbero M, Dick AW, Schuster J, Farmer C. The impact of buprenorphine on treatment of opioid dependence in a Medicaid population: Recent service utilization trends in the buprenorphine use and methadone. Drug and Alcohol Dependence. 2011;123:72–78. doi: 10.1016/j.drugalcdep.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Wallack SS, Thomas CP, Martin TC, Chilingerian J, Reif S. Substance abuse treatment organizations as mediators of social policy: Slowing the adoption of a congressionally approved medication. Journal of Behavioral Health Services and Research. 2010;37(1):64–78. doi: 10.1007/s11414-008-9132-4. [DOI] [PubMed] [Google Scholar]
- Wooldridge JM. Econometric analysis of cross section and panel data. Cambridge: MIT Press; 2002. [Google Scholar]
- Woolf SH. The meaning of translational research and why it matters. Journal of the American Medical Association. 2008;299:211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- Zerhouni E. The NIH roadmap. Science. 2003;302:63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]