INTRODUCTION
Cognitive complaints in elderly patients may arise from the patient or from family, friends, or caregivers. Any cognitive complaint, regardless of the source, should be investigated rather than attributed to aging. Although current recommendations do not recommend for or against routine screening for cognitive impairment in geriatric patients,1 many geriatric practitioners routinely assess cognitive function, especially as the Medicare Annual Wellness Visit now requires a cognitive assessment.2
Despite increased awareness and education about the most common type of dementia, Alzheimer disease (AD), many patients and families are unsure which cognitive changes are normal and which may signal a more serious problem. Age-associated memory impairment (AAMI) is a term used to broadly define normal age-related cognitive changes, including difficulty recalling specific details or dates of past events (episodic memory), difficulty multitasking, and slower processing speed for new learning or “working” memory. AAMI may occur as early as age 50 years and is not associated with progression to dementia or an underlying disease state. Most importantly, changes resulting from AAMI should not affect functional abilities.
Subjective cognitive impairment is a relatively new construct whereby patients perceive deficits that are not detectable with objective measures. However, some data suggest that patients with subjective cognitive impairment are at increased risk of developing objective deficits over time.3,4 Mild cognitive impairment (MCI) is defined as objective cognitive impairment with preserved function.5 MCI has historically been a controversial entity with significant clinical heterogeneity. Estimates of the conversion rate to dementia (usually AD) in patients with MCI vary, but appear to be approximately 10% per year, compared with 1% to 3% per year in cognitively normal older adults.6–8 However, many patients with MCI remain stable over time, and some studies have shown improvement in a substantial proportion of patients.8–10 Whether MCI is in fact prodromal AD has thus been debated. Recent efforts have focused on distinguishing MCI due to AD from that attributable to other causes to facilitate clinical utility.11 The feature that separates dementia from MCI is the effect of the impairment on daily function. In dementia the impairments affect daily function, whereas in MCI they do not.12
EVALUATION
Patient History
As with many geriatric conditions, cognitive impairment is frequently multifactorial; thus, a detailed history is critical (Table 1). Even though truly reversible causes of dementia are exceedingly rare, multiple factors such as medications, depression, delirium, infections, alcohol use, or metabolic disorders may exacerbate underlying cognitive impairment. Addressing possible contributing factors may significantly improve cognitive symptoms even though the underlying disease process cannot be treated.
Table 1.
Key elements of cognitive impairment history
| History of present illness | Details, timing, and progression of complaints Corroboration from a reliable informant Functional status (basic and instrumental ADLs) Safety (driving, appliances, firearms, wandering, finances) |
| Medical history | Cardiovascular diseases or risk factors Chronic neurologic diseases History of head trauma or concussions Recent illness or hospitalizations |
| Social history | Current living situation and support network Past or present substance use/abuse Recent relocation, life events, losses |
| Medication review | Benzodiazepines Anticholinergic/antimuscarinic agents Sedative hypnotics Tricyclic antidepressants Opioids Anticonvulsants |
| Review of systems | Mood or behavioral disturbances, personality changes Focal neurologic symptoms (sensory or motor complaints, headaches, seizure activity, tremor, gait impairment) Incontinence Sleep disturbance |
Abbreviation: ADLs, activities of daily livings.
In addition to the timing and progression of symptoms, specific examples of deficits should be elicited from the patient and a reliable informant. Ideally the patient and informant are interviewed separately, as informants may hesitate to contradict the patient or describe episodes or symptoms that may be embarrassing to the patient. Directed questions about changes in handling finances, participation in hobbies or activities, and driving abilities can help assess the severity of complaints. Specific inquiries about safety are essential, as these require immediate action. As older adults with cognitive impairment are at high risk for physical abuse, neglect, and financial exploitation, knowledge of the patient’s living situation, care providers, and the caregiver’s capabilities and support network is imperative.
Traditional cardiometabolic risk factors such as diabetes, hypertension, obesity, and dyslipidemia are strongly linked to the development of cognitive impairment and dementia, including AD.13 A history of cerebrovascular disease or neurologic disorder (eg, multiple sclerosis, amyotrophic lateral sclerosis, Parkinson disease) may suggest an underlying cause in other cases. As with any geriatric syndrome, a complete review of all medications, including over-the-counter products, vitamins, supplements, and herbal remedies, should be part of the evaluation.
Delirium should be considered, especially in patients who have recently been hospitalized. Delirium is distinguished from other causes of cognitive impairment by the acute time course, association with a specific stressor such as an infection or medication effect, and marked inattention. However, it is well recognized that delirium may persist for weeks to months, and that an episode of delirium may unmask previously unrecognized dementia.14
Patients with cognitive complaints should be asked about a history of depression and about current depressive symptoms. The relation between depression and dementia is complex; a history of depression increases the risk of developing dementia, and depression itself can cause significant cognitive impairment that may mimic dementia. Furthermore, depression is a common feature of dementia, present in approximately 50% of patients with AD,15 especially in early-stage disease when significant insight into the disease process may exist.
Pathophysiology
The wide clinical spectrum of cognitive impairment and dementia reflects the interplay of neuropathology, cerebral metabolism, synaptic failure, and inflammation that result in temporary or permanent cognitive decline. Regardless of the specific underlying pathology, which is often poorly understood, the final common pathway in dementia is neuronal death and cell loss, as evidenced by correlations between atrophy and dementia across all ages.16 The clinical presentation reflects the affected regions of the brain. Cognitive complaints tend to stem from damage to the cerebral cortex; subcortical injury can also cause cognitive impairment, but is often associated with psychiatric or motor symptoms.
The pathophysiology of AD has been the most intensively studied to date. There are likely multiple mechanisms and pathways leading to the initiation and progression of AD, but most research to date has centered on the neuropathologic hallmarks required for definitive diagnosis, amyloid plaques, and neurofibrillary tangles. Though controversial, the amyloid hypothesis of AD has dominated AD research since it was proposed in the early 1990s.17 According to the amyloid hypothesis, it is the accumulation and aggregation of misfolded β-amyloid peptide (Aβ) that initiates and perpetuates neurodegeneration in AD. Cleavage of the amyloid precursor protein produces Aβ, which aggregates into toxic oligomers.18 Over time these oligomers merge into insoluble fibrils and, eventually, the characteristic plaques of AD.
Neurofibrillary tangles consist of aggregations of abnormally hyperphosphorylated tau proteins, which self-aggregate to form paired helical filaments and, eventually, tangles.18 This process destabilizes microtubules, impairing axonal transport and resulting in neuronal dysfunction and degeneration.18 Tau accumulation, or tauopathy, is also a feature of frontotemporal and subcortical dementias.19
Vascular contributions to AD are an active area of research. Approximately 60% to 90% of patients with AD have ischemic disease, and up to one-third of presumed cases of vascular dementia exhibit the neuropathologic features of AD.18 Some have suggested that better management of modifiable cardiovascular risk factors may be partly responsible for the recently observed decrease in the prevalence and incidence of age-specific dementia.20
Physical Examination
The physical examination may be completely normal in many patients with cognitive complaints. The patient’s general appearance may offer some clues as to possibility of the cause and severity of the cognitive complaint. For example, delirious patients may show signs of either psychomotor agitation or slowing. Patients with either dementia or depression may show signs of self-neglect or poor hygiene. A thorough neurologic examination should be performed to detect any focal deficits, Parkinsonian signs, upper motor neuron signs, or gait disturbance that may indicate a potential underlying process and guide further evaluation and testing. Frontal release signs (eg, grasp, palmomental, snout, glabellar reflexes) are typically present only in advanced dementia.
Cognitive Testing
Administration of a structured cognitive assessment tool is recommended in any elderly patient with a cognitive complaint. Many tools are suitable for use in a primary care setting, and no single test is clearly superior. Considerations in selecting a test include time, education level, language barriers, severity of deficits, and cognitive domains of interest (Table 2). Performance on cognitive assessments may sometimes provide clues to the underlying etiology. For example, patients with depression may exhibit inattention and poor motivation. Patients with AD may have marked loss of short-term memory and executive function, whereas impairments primarily in language may point toward frontotemporal dementia.
Table 2.
Common brief cognitive screening tools
| Test | Time (min) |
Advantages | Limitations |
|---|---|---|---|
| Mini-Mental Status Examination118 (MMSE) | 7–10 | Most widely used and studied worldwide Often used as a reference for comparative evaluations of other assessments Required for some drug insurance reimbursements |
Education/age/language/culture bias Ceiling effect (highly educated impaired subjects pass) Proprietary: unless used from memory, test needs to be purchased Best performance for at least moderate cognitive impairment |
| Montreal Cognitive Assessment119 (MoCA) | 10–15 | Designed to test for mild cognitive impairment Multiple languages accessible Tests many separate domains (7) |
Lacks studies in general practice settings Education bias (≤12 y) Limited use and evidence: published data are relatively new (2005) Administration time ≥10 min |
| St Louis University Mental Status Examination120 (SLUMS) | 7 | No education bias Tests many separate domains (7) |
Limited use and evidence: published data are relatively new (2006) Studied in Veterans Affairs geriatric clinic (predominantly white males) |
| Mini-Cog121 | 2–4 | Developed for and validated in primary care and multiple languages/cultures Little or no education/language/race bias Short administration time |
Use of different word lists may affect failure rates Some study results based on longer tests with the Mini-Cog elements reviewed independently |
Adapted from Cordell CB, Borson S, Boustani M, et al. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement 2013;9(2):147. http://dx.doi.org/10.1016/j.jalz.2012.09.011; with permission.
Neuropsychiatric Symptoms
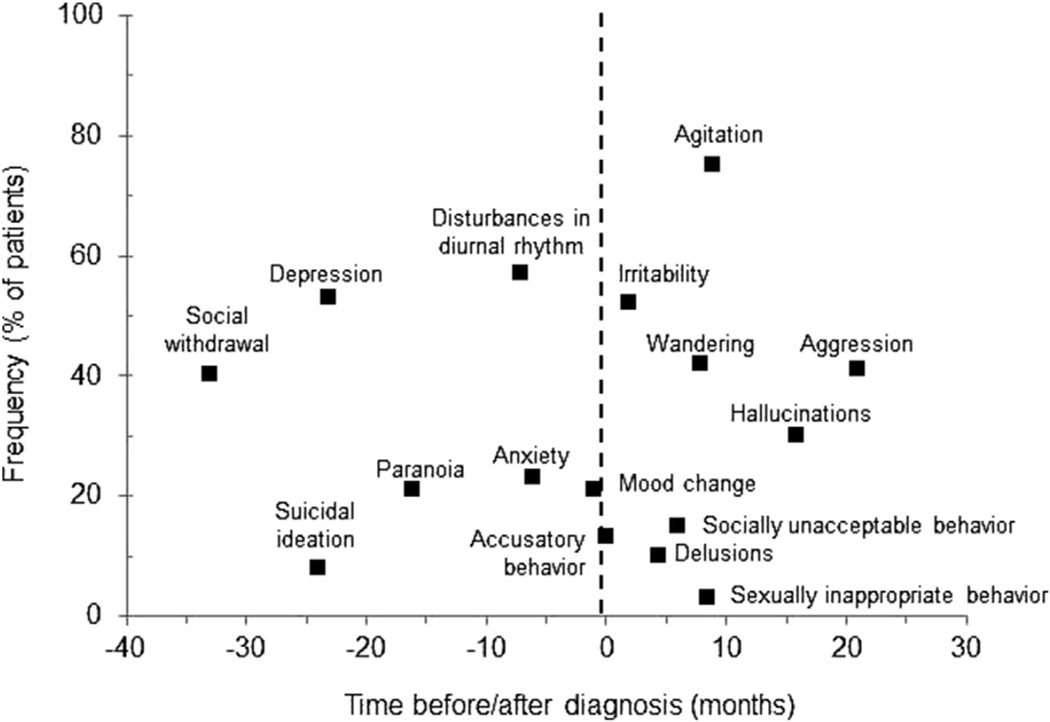
Neuropsychiatric symptoms such as depression, anxiety, irritability, and agitation are common, developing in 90% of patients with dementia within 5 years of diagnosis (Fig. 1).15 Frequently these symptoms precede the diagnosis of dementia, and can be helpful in pointing toward a cause in some cases. Prominent neuropsychiatric findings or behavioral problems on initial evaluation may suggest cognitive impairment resulting from non-AD dementias. Disinhibition and personality changes with relatively preserved memory and executive function are characteristic of frontotemporal dementias. Visual hallucinations and delusions paired with motor abnormalities are often seen in Lewy body dementia (LBD).
Fig. 1.
Frequency and timing of behavioral disturbances in Alzheimer disease. (From Ballard CG, Gauthier S, Cummings JL, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol 2009;5(5):246; with permission.)
Imaging and Additional Testing
At present there are no laboratory tests or imaging studies that definitively diagnose dementia in the clinical setting. Although significant progress has been made in identifying biomarkers for AD, particularly amyloid imaging and cerebrospinal fluid (CSF) markers, these tests are not sufficiently standardized or widely available outside of research settings. Basic laboratory testing is recommended to evaluate for metabolic and other disorders that may cause or contribute to cognitive impairment, including complete blood count, comprehensive metabolic panel, thyroid function testing, and vitamin B12. Testing for human immunodeficiency virus and neurosyphilis can be considered in individuals at risk, but are not recommended as part of the routine evaluation.
The American Academy of Neurology recommends obtaining a noncontrast head computed tomography (CT) or MRI scan as part of the initial evaluation of cognitive impairment21; however, in primary care practice this decision is often guided instead by the presenting signs and symptoms, and the likelihood that imaging will provide useful information. For example, in patients with focal neurologic deficits, recent head trauma, or neurologic complaints such as headache, imaging may help identify or eliminate other possible causes of the cognitive complaints. Patients in whom the cognitive complaints are acute, rapidly progressive, or atypical should also receive imaging with the choice of modality depending on availability and suspicion for causes such as hemorrhage or mass lesions for which CT would be sufficient. Conversely, in patients with moderate to severe dementia and a typical clinical presentation and progression, imaging is unlikely to be useful. In patients with dementia, neuroimaging may be normal, or show global or focal atrophy. Findings of cortical or subcortical infarcts or a high burden of chronic small-vessel disease may suggest a vascular cause, and the location may correlate with the clinical presentation. Functional MRI and PET imaging are additional imaging modalities being studied for use in the diagnosis of dementia. These techniques may better elucidate the pathophysiology of cognitive impairment and dementia, but, as with amyloid imaging, they are not currently recommended for the evaluation of cognitive complaints.21
Lumbar puncture and CSF analysis is not recommended in the standard workup of cognitive impairment, but may be helpful in rapidly progressive or atypical dementias. Genetic testing is likewise not routinely performed. To date, the only gene identified as increasing susceptibility to late-onset, sporadic AD, which accounts for greater than 95% of cases, is the apolipoprotein (APOE)-ε4 allele. Although heterozygosity and homozygosity for APOE-ε4 increase the risk of AD by approximately 2-fold and 10-fold, respectively, it is neither necessary nor sufficient for the development of AD. In rare cases of strongly familial early-onset disease, referral for genetic testing for identified mutations in the processing of amyloid precursor protein may be considered.22
Diagnostic Dilemmas
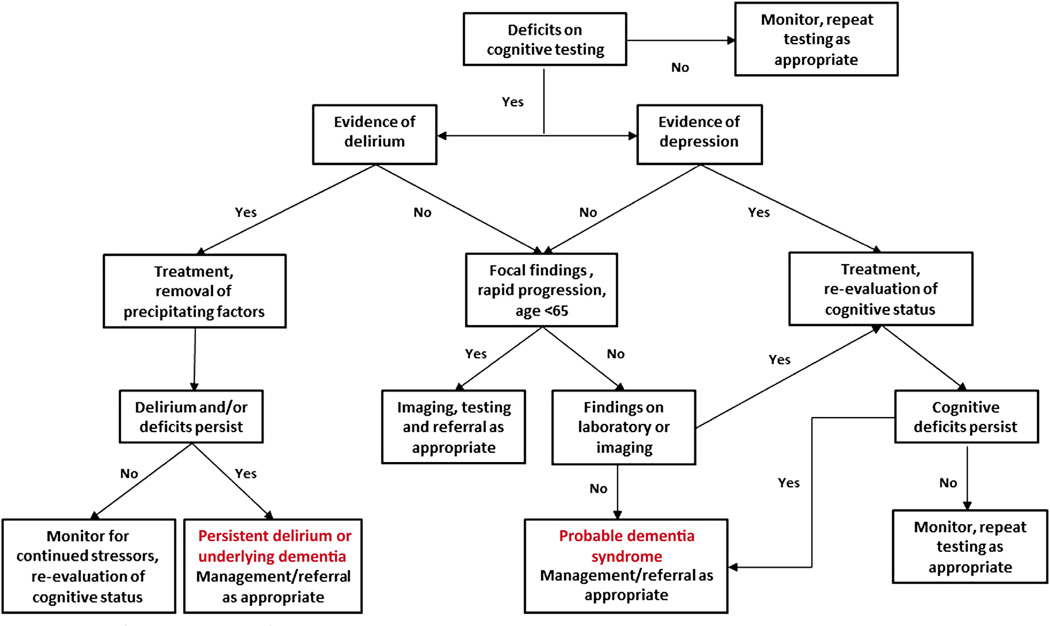
A general algorithm for the evaluation of cognitive complaints in older adults is presented in Fig. 2. Uncertainty about the diagnosis, or atypical presentations such as early onset (<65 years), rapid progression (≤6 months), or isolated cognitive deficits should prompt referral for neurologic and/or psychological evaluation. Less common types of dementia (eg, prion disease, autoimmune, infectious, neoplastic) may require specialized diagnostic and management skills. Formal neuropsychological testing can be informative in cases where psychiatric disease is suggested, or for suspected impairment in highly educated individuals who may score within the normal range on standard assessments despite significant deficits. These sensitive and detailed assessments can also provide anticipatory guidance and compensatory strategies for patients and families, and facilitate reevaluation, tracking of disease progression, and prognostic guidance in some cases.
Fig. 2.
Algorithm for the evaluation of cognitive complaints in elderly adults.
PATIENT MANAGEMENT
Management Goals
For all patients with cognitive complaints, primary goals include maintaining function and independence, preventing further cognitive decline, and ensuring quality of life. Depending on the presumed cause and the severity of impairment, specific goals and strategies will vary significantly. The management of cognitive impairment is unique in that the caregiver is a critical part of the alliance between patient and provider, and must be highly involved and supported for successful attainment of goals.
Subjective cognitive impairment
For patients with only subjective cognitive complaints, the goals are reassurance, optimizing management of any comorbid conditions, and promoting a healthy lifestyle. However, these patients should be monitored carefully for any signs of progression, as studies have suggested that subjective cognitive impairment is predictive of future MCI.3,4
Mild cognitive impairment
An important goal for patients with MCI is acceptance of the uncertainty surrounding this diagnosis given the possibility of progression, stability, or even improvement. Collaboration with the health care provider and utilization of community resources such as the Alzheimer’s Association for support and education can help patients and caregivers manage the inevitable frustration and fear associated with this diagnosis.
Careful attention to the management of comorbid conditions that may worsen cognitive impairment (eg, depression, heart failure, sleep apnea, hypertension, diabetes) should be part of the management plan, particularly with respect to the management of vascular risk factors. Clinicians should conduct a rigorous and ongoing review of all prescription and nonprescription medications that may affect cognition, and consider alternatives when possible. Impairments in memory and executive function may affect adherence to medications and treatment plans; regimens should be simplified and other methods, such as automated medication dispensers, pill boxes, and caregiver oversight should be considered to help prevent errors. Patients should be encouraged to remain physically, socially, and intellectually active while realizing that their usual activities may need to be modified to compensate for cognitive deficits. The caregiver’s physical and mental health need to be monitored and supported, so assessment of the caregiver’s level of ability and understanding of the disease are essential.
Although many patients with MCI remain stable over time, the risk of progression to dementia, usually AD, is significantly higher than in cognitively normal older adults.6–8 Thus, establishing a safety net and advance care planning are important. Ideally, patients and caregivers will discuss potential issues such as driving, eventual need for a higher level of care (eg, home care, assisted living), financial planning, and end-of-life goals and preferences while the patient is able to participate in a meaningful way.
Dementia
In patients with dementia, maintaining function, facilitating independence, and ensuring quality of life remain key management goals, although they may appear very different than in patients with MCI. Dementia encompasses a wide spectrum of disease from mild to advanced; furthermore, the progressive nature of dementia requires ongoing reevaluation and adjustment of goals. Caregiver support becomes increasingly important as disease progresses and dependence increases. Continued vigilance and early intervention for problems such as neuropsychiatric symptoms, sleep disturbance, and incontinence can help maintain the quality of life for both patients and caregivers.
Although early attention to these issues may delay institutionalization, nursing home admission is expected for 75% of those with dementia by age 80 years, compared with just 4% of the general population.23 Advance care planning and addressing end-of-life wishes increasingly fall to the caregiver as the patient loses capacity to make these decisions, emphasizing the importance of confronting these issues as early as possible in patients with any degree of cognitive impairment. Helping patients and caregivers accept the diagnosis of a terminal illness, and introducing the role of palliative care and hospice are also important management goals for patients with dementia (see later discussion). A summary of management goals at various stages is provided in Table 3.
Table 3.
Management goals for elderly patients with cognitive complaints
| Management Goal | Subjective Cognitive Complaint |
MCI | Dementia |
|---|---|---|---|
| Maintain independence and quality of life | |||
| Optimize management of comorbidities | |||
| Treat vascular risk factors | |||
| Eliminate/minimize medications affecting cognitive function | |||
| Promote physical and mental health | |||
| Advance care planning | |||
| Ongoing monitoring for progression | |||
| Acceptance and adjusting expectations | |||
| Referral to support organization | |||
| Partnership with patient and caregiver | |||
| Caregiver support | |||
| Meeting goals/preferences for end-of-life care | |||
Abbreviation: MCI, mild cognitive impairment.
Nonpharmacologic Strategies
Subjective cognitive impairment and mild cognitive impairment
To date, no nonpharmacologic interventions have been shown to prevent further decline in patients with either subjective cognitive impairment or MCI. Subjective cognitive impairment is a relatively new construct, and few strategies to delay progression have been studied. Investigations into nonpharmacologic strategies for MCI have been complicated by the heterogeneity and instability of this diagnosis. Two promising strategies that have been studied in MCI are exercise24–27 and cognitive training,26–33 although the quality and quantity of evidence is limited, and more rigorous controlled trials in well-defined populations are needed. Because both exercise and cognitive training are associated with few, if any, risks and may have beneficial effects on cognition, patients with subjective cognitive impairment or MCI should be advised to adopt both of these activities as part of an overall healthy lifestyle. Both the US Centers for Disease Control and Prevention34 and the National Institute on Aging34 have guidelines for physical activity in older adults and materials that can be used to help patients initiate and maintain a regular exercise program. Patients should be encouraged to engage in enjoyable, cognitively stimulating activities. There are no compelling data to support any specific program or activity; patients should pursue activities that match their interests and abilities.
Dementia
Numerous nonpharmacologic interventions targeting patients with dementia, their caregivers, or the patient-caregiver dyad have been investigated (Box 1). To date, high-quality evidence for any strategy is lacking, as most studies tend to be small, heterogeneous with respect to populations, interventions, and outcomes, and/or of poor quality.
Box 1. Nonpharmacologic interventions investigated in dementia.
One of the more promising nonpharmacologic interventions is exercise. Possible mechanisms by which exercise may improve or maintain cognitive function include improving central adiposity and insulin resistance,35–37 decreasing oxidative stress and low-grade inflammation,38 improving vascular function,39 and increasing cerebral blood flow.40 Combined with the established benefits of exercise on multiple chronic illnesses and lack of side effects when properly performed, exercise presents an extremely attractive potential therapy for dementia. Epidemiologic evidence strongly supports a beneficial effect of higher levels of physical activity on cognitive function and the risk of dementia,41 but results from prospective randomized controlled trials (RCTs) have been mixed. A recent review of 6 good-quality RCTs found that all reported significant positive results on functional outcomes.42 The most recent Cochrane review concluded that exercise interventions may have positive effects on cognitive function and activities of daily living, but emphasized that significant heterogeneity among studies requires cautious interpretation of results.43 In addition, research to determine the optimal type, duration, and intensity of exercise for patients with dementia is needed. As with MCI, regular physical activity should be recommended for all patients with dementia.
Cognitive stimulation uses enjoyable activities to engage memory and concentration in a social setting. Two of the larger studies using this approach reported improvements in cognitive function44,45 and quality of life,44 but not in functional status, mood, or behavioral symptoms. Cognitive training or rehabilitation has also received considerable attention, resulting in a burgeoning commercial industry promoting “brain training” programs to enhance or maintain cognitive function. To date, studies of these interventions in patients with mild to moderate dementia have not provided strong evidence of benefit on cognition, function, or mood,46 and patients and caregivers should be cautioned against expensive programs that promise to prevent or reverse dementia. Most studies are of low to moderate quality, and have been small and of short duration. Outcomes such as trajectory of decline or time to institutionalization have not been studied. However, given the risk/benefit ratio and the lack of effective treatments for dementia, patients should be encouraged to participate in enjoyable, cognitively stimulating activities appropriate for their interests and level of impairment.
Neuropsychiatric symptoms
Managing neuropsychiatric symptoms in patients with dementia can be extremely challenging and distressing for caregivers, and often serve as the trigger for institutionalization. These symptoms may reflect unmet needs (eg, pain, constipation, urinary tract infection), unintentional reinforcement of behaviors (eg, repetitive questioning or yelling to receive attention), or lack of fit between the environment and the patient’s abilities (eg, excess stimulation or distraction).47 Nonpharmacologic approaches to managing these symptoms are strongly preferred because of the limited benefits and substantial risks of pharmacologic therapies (discussed in the next section). General strategies for managing behavioral symptoms are described in Table 4.48–50 Specific interventions should match the patient’s needs and abilities, which will change as the disease progresses. Successful implementation of these strategies is more likely with a team approach involving the patient, caregiver(s), and multiple health professionals. This approach can be time consuming and labor intensive, and is not well supported by current reimbursement systems, presenting significant barriers to more widespread use.
Table 4.
Nonpharmacologic strategies for neuropsychiatric symptoms in dementia
| Symptom | Strategies |
|---|---|
| Depression/apathy | Introduce/encourage enjoyable activities Modify activities patient has enjoyed in the past to fit current level of function to avoid frustration Consider repetitive activities (eg, folding laundry, sorting papers) Introduce/encourage social activities, outings Address caregiver depression |
| Agitation | Identify and avoid triggers Maintain structured daily routines Stay calm; avoid arguing, reasoning Redirect and distract |
| Wandering | Provide labels or visual cues (eg, arrows to bathroom, bedroom, stop signs) Disguise exits Provide supervision (eg, family, paid caregivers, adult day programs) Introduce/encourage enjoyable activities Develop a safety plan (eg, “Safe Return” program) |
| Hallucinations/delusions | If not disturbing or frightening, allow patient their experience of the truth; avoid reasoning or attempting to correct patient’s perceptions If disturbing or frightening provide calm reassurance, distract and redirect, consider medication |
| Disorientation | Simplify environment, reduce clutter, noise Provide visual cues and reminders Provide verbal prompts Identify self and others |
| Sleep disturbance | Maintain consistent sleep times and routines Avoid daytime napping Reduce/eliminate alcohol and caffeine Reduce/eliminate noise, distractions Consider bright light exposure |
Pharmacologic Strategies
Options for pharmacologic treatment of cognitive impairment are few and limited to dementia. There are no approved pharmacologic therapies for MCI, and no studies have yet been conducted in patients with subjective cognitive impairment. Despite 30 years of drug development efforts, there are only 4 medications in 2 drug classes approved by the Food and Drug Administration that are available for the treatment of dementia (Table 5). These drugs are approved only for AD with the exception of rivastigmine, which is also approved for Parkinson disease dementia (PDD).
Table 5.
Approved pharmacologic treatments for dementia
| Drug | Year Approved |
FDA-Labeled Indications | Dosing | Route | Frequency | |
|---|---|---|---|---|---|---|
| Initial | Maintenance | |||||
| Cholinesterase Inhibitors | ||||||
| Donepezil | 1996 | AD, all stages | 5 mg | 10 or 23 mg | Oral | Daily |
| Galantamine-IR | 2000 | AD, mild to moderate | 4 mg | 8–12 mg | Oral | BID |
| Galantamine-ER | 2004 | AD, mild to moderate | 8 mg | 16–24 mg | Oral | Daily |
| Rivastigmine | 2001 | AD, mild to moderate PDD, mild to moderate |
1.5 mg | 6 mg | Oral | BID |
| Rivastigmine patch | 2007 | AD, all stagesa PDD, mild to moderate |
4.6 mg/24 h | 9.5–13.3 mg/24 h | Transdermal | Daily |
| NDMA Receptor Antagonists | ||||||
| Memantine | 2003 | AD, moderate to severe | 5 mg | 10 mg | Oral | BID |
| Memantine-ER | 2010 | AD, moderate to severe | 7 mg | 28 mg | Oral | Daily |
Abbreviations: AD, Alzheimer disease; BID, twice daily; ER, extended release; FDA, Food and Drug Administration; IR, immediate release; NMDA, N-methyl-d-aspartate; PDD, Parkinson disease dementia.
High-dose patch (13.3 mg/24 h) approved for severe dementia in 2013.
Cholinesterase inhibitors
The cholinesterase inhibitors (ChEIs) prevent the enzymatic breakdown of the neurotransmitter acetylcholine in the synaptic cleft. Cholinergic transmission is thought to be crucial for attention and memory, in addition to neuronal plasticity, and as early as 1976 severe cortical cholinergic loss was noted in AD patients.51,52 Although enthusiasm for ChEIs waned as attempts at disease-modifying amyloid-based therapies began to dominate research efforts, these drugs remain the mainstay of pharmacologic therapy for dementia.
The effectiveness of the ChEIs has been summarized in several systematic reviews and meta-analyses.53–55 The data support modest benefits in stabilizing or slowing cognitive decline, behavior, and function, but the clinical significance of the changes is uncertain. Comparisons between ChEIs suggest that all 3 drugs are similarly effective.
ChEIs have not been as well studied in dementias other than AD. Small improvements in cognition, behavior, and function in PDD have been reported, but the clinical benefit in LBD is not clear.56 Donepezil has been studied in patients with probable mild to moderate vascular dementia, with small improvements in cognition and function compared with placebo57; evidence for benefit with rivastigmine58 and galantamine59 are less certain. ChEIs should not be used in the treatment of frontotemporal dementia, in which no cholinergic deficit has been demonstrated.60 Although one small, open-label study showed improvement in some behavioral symptoms with rivastigmine,61 other studies of ChEIs in frontotemporal dementia showed no benefit62 and potential worsening63 of symptoms.
Side effects of ChEIs are related to increases in cholinergic activity and most commonly include nausea, vomiting, and diarrhea, which can often be minimized with dose titration over 4 to 6 weeks. In addition, augmentation of parasympathetic activity has been associated with an increased risk of syncope,64,65 hospitalization for bradycardia,66 pacemaker insertion,64 and hip fracture.64 In addition to awareness of potential side effects, it is important for patients and caregivers to understand that although ChEIs may provide some symptomatic benefits, they do not change the underlying course of the disease. There is no clear consensus on the appropriate duration of treatment with ChEIs. Recent guidelines from the American Geriatrics Society as part of the “Choosing Wisely” Campaign recommended a trial of 12 weeks if the medication is tolerated, as any benefit is likely to be observed by then.67 Because “improvement” may manifest as stability or a slower rate of decline, and bedside cognitive assessments are unlikely to show significant change, the decision to continue the medication often depends on the patient and caregivers’ perceptions of benefit. If a clinically significant decline is observed after stopping a ChEI, therapy can be restarted. Such treatment gaps do not seem to increase the risk of institutionalization or mortality.68
Memantine
The second class of drugs approved for the treatment of dementia is the N-methyl-d-aspartate (NMDA) receptor antagonist memantine. Excessive stimulation of the NMDA receptor by the excitatory neurotransmitter glutamate may result in neuronal excitotoxicity. Memantine is purported to prevent pathologic activation of the NMDA receptor via low to moderate affinity binding, thereby providing neuroprotection. Memantine is approved only for moderate to severe AD, with small benefits on cognition, behavior, and function demonstrated with 6 months of treatment in several trials.69 Memantine does not seem to be effective in mild AD, although its use in this population is common.70 Memantine is typically well tolerated.
Data supporting the use of memantine in dementia other than AD are mixed. There is some evidence of benefit in patients with vascular dementia,71,72 but studies in PDD and LBD are inconsistent.73,74 The few studies conducted to date have not supported a role for memantine in frontotemporal dementia.75,76
Combination therapy with ChEI and memantine is appealing given the different mechanisms of action of these drugs, but studies to date have not clearly supported this approach.77–79 Hopes that ChEIs or memantine would prevent further cognitive or functional decline in MCI have not been realized. One study reported a decreased rate of conversion from MCI to AD with donepezil compared with placebo at 12 months, but by 36 months the conversion rates were not different.80 Despite the lack of evidence, ChEIs and memantine are frequently used in patients with MCI81; availability of generic forms of most of these drugs has also likely tilted the cost-benefit calculation toward more widespread use.
In summary, a trial of a ChEI or, in patients with moderate to severe dementia, memantine, is recommended in patients without contraindications to the medications after discussion of reasonable expectations of symptomatic benefit and potential side effects. If there is no perceived benefit after at least 12 weeks of therapy, these medications should be discontinued.
Supplements and medical foods
There is no evidence to date that any dietary supplement is effective in delaying or preventing cognitive decline.82 Commonly used supplements for cognitive complaints include gingko biloba, ginseng, B vitamins, vitamin E, omega-3 fatty acids, and phospholipids. Many of these supplements have potential adverse effects and drug interactions,83 yet patients often do not inform their health care providers about their use of these supplements unless specifically queried.
Among supplements, the antioxidant vitamin E has been studied most extensively in clinical trials for potential cognitive benefits. Although a positive association between vitamin E and cognitive function has been reported,84 controlled trials have not convincingly demonstrated benefit. Two trials have reported that vitamin E treatment slowed progression of dementia compared with placebo (measured as decline in functional status, institutionalization, or development of severe dementia or death),85,86 but no benefit on secondary cognitive outcomes. In addition, both studies had methodological and/or statistical limitations that limit interpretation of results.
As preventive therapy, vitamin E did not delay progression to dementia over 3 years in patients with MCI.80 Furthermore, concerns about the adverse effects87,88 and possible increased mortality with vitamin E supplementation89 have dampened enthusiasm for its use.
Medical foods are intended for “the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.”90 AC-1202 (Axona) is the only medical food currently available for AD. AC-1202 is a medium-chain triglyceride that is metabolized into ketone bodies, which can be used as an alternative to glucose as an energy source for neurons. In a 90-day RCT of 152 participants with mild to moderate AD, AC-1202 did not improve cognition compared with placebo, although a significant benefit was found in APOE-ε4–negative participants.91
Pharmacologic treatment of neuropsychiatric symptoms in dementia
The off-label use of antipsychotics for neuropsychiatric symptoms in dementia is common, with an estimated 20% to 30% of patients receiving these medications.92 Data for the efficacy of antipsychotics are limited, and suggest that the clinical benefits are modest at best.93–95 Furthermore, these small benefits may be offset by adverse effects and harms. Both typical and atypical antipsychotics are associated with extrapyramidal symptoms, sedation, confusion, and falls. Because patients with LBD are particularly sensitive to these agents, consultation with a specialist (eg, neurologist, geriatric psychiatrist) is recommended.
In addition to adverse effects, both typical and atypical antipsychotic medications are associated with increased mortality.96–98 Given their limited, uncertain benefits and potential for serious harm, antipsychotics should be reserved for those cases whereby nonpharmacologic strategies have failed and the patient poses a serious threat to himself/herself or others.99 If started, a risk-benefit discussion with the caregiver should be performed and documented, and frequent review and attempts to withdraw the medications are recommended. Limited data suggest that these agents may be safely withdrawn in patients with dementia without risk of relapse.100
Comorbid depression should be considered as a contributor to behavioral disturbance in patients with dementia. Depression may be difficult to diagnose in this population, and geriatric or geriatric psychiatry consultation should be considered if available. There is no strong evidence for the benefit of selective serotonin reuptake inhibitors (SSRIs) in the treatment of depression in patients with dementia,93,101 and potential adverse effects including hyponatremia, QT prolongation, and falls must be considered. However, SSRIs may be beneficial in individual patients and are recommended by some experts.
Pharmacologic treatment of sleep disturbances is generally avoided in patients with dementia, as this population is even more vulnerable to the negative side effects of sedative-hypnotics than older adults in general. One exception is melatonin, which seems to be well tolerated, although studies in patients with dementia and sleep disturbance have had mixed results.102–104 Additional data suggest that melatonin may also have a positive effect on nonsleep behavioral disturbances in dementia.105 Trazodone or mirtazapine are sometimes used, but have not been well studied in patients with dementia. Owing to the risk of adverse events, benzodiazepine receptor agonists, benzodiazepines, and sedating antipsychotics should only be used when all other options have been exhausted and with a risk-benefit discussion with caregivers, as these agents can convey a significant risk of delirium, oversedation, and falls.
Evaluation, Adjustment, and Recurrence
Ongoing evaluation of cognitive status and review of management goals should be incorporated into regular medical visits for patients with any cognitive complaint or impairment. Although some patients may be followed by a neurologist, day-to-day management in the context of the patient’s other medical conditions will generally fall to the primary care provider. The primary care provider has a critical role in coordinating care among multiple disciplines such as physical, occupational, and speech therapy, nutrition, social work, and pharmacy. The primary care provider should also keep apprised of care the patient is receiving from any specialists, especially with respect to medications prescribed that may affect cognition, and ensure that the plan of care is consistent with the patient’s and family members’ goals.
As the disease inevitably advances, the question of discontinuing medications frequently arises. These decisions should be guided by the patient’s goals of care, but it is often appropriate to reduce or discontinue medications that are not providing symptomatic benefits, and de-escalate treatment of conditions such as hypertension and diabetes. It may be appropriate to taper and discontinue ChEIs when patients reach an advanced stage of dementia, as benefits are unlikely to be realized. Although memantine is approved for use in severe dementia, the benefits in very advanced stages are uncertain. As noted earlier, the medications can be restarted if a significant decline is noted in relation to stopping.
End-of-life care
Providing quality end-of-life care in patients with cognitive impairment poses special challenges that increase with the degree of impairment, thus the importance of early advance care planning cannot be overstated. Palliative care is appropriate for all patients with a diagnosis of dementia, given the terminal nature of this disease and its associated physical and psychological symptoms. Difficulty with swallowing and walking, weakness, incontinence, and sleep disruption often occur as disease progresses. Symptoms related to comorbid conditions such as pain and dyspnea may also be present. One of the major challenges in providing palliative care to patients with cognitive impairment is communication, as patients may be unable to describe their symptoms or whether interventions are effective.
Hospice care has the potential to provide significant benefits for patients with dementia, including better quality of life106,107 and increased caregiver and family satisfaction.108 Compared with nonhospice dementia patients, those enrolled in hospice are more likely to die in their location of choice108 and to have a better overall dying experience.106,107 Use of hospice for dementia patients has been steadily increasing; an analysis of Medicare data reported that the proportion of patients with dementia enrolled in hospice at the time of death increased from 19.5% in 2000 to 48.5% in 2009.109 Countering this encouraging trend, however, were increases in intensive care unit utilization and mechanical ventilation within the last 30 days of life, an increase in the proportion of patients with dementia who transitioned between sites of care in the last 3 days of life, and in late hospice referrals (enrollment within 3 days of death). Alarmingly, a recent study of greater than 4700 discharges from hospice care found that patients with dementia were significantly more likely than patients with other diagnoses to have received tube feeding,110 even though ample evidence suggests that tube feeding provides no morbidity or mortality benefit in this population.111 These data suggest that despite increased hospice utilization in patients with dementia, significant barriers to providing high-quality end-of-life care for this population remain.
Several characteristics of dementia may act as potential barriers to improving end-of-life care. Despite heightened awareness of dementia, both the public and professional communities have difficulty recognizing dementia as a terminal disease. Indeed, one of the most common questions about dementia is what patients with dementia actually die from; the terminal event (eg, pneumonia, urinary tract infection), rather than the dementia, is often considered the proximate cause of death.112 Acceptance of dementia as a terminal disease is further complicated by the trajectory of death and the considerable prognostic uncertainty, which has been cited as the primary barrier to hospice enrollment.113 Current Medicare hospice eligibility requirements are based on assessment of functional status and the occurrence of specific medical conditions.114 These somewhat rigid criteria have been shown to be poor predictors of life expectancy in dementia,115–117 which may explain in part the high hospice disenrollment rates in patients with this diagnosis.110 The lack of fit between the current hospice model and the nature of dementia has led some experts to suggest a shift in focus from prognosis to early palliative care guided by a preference for comfort.110 Choosing to forgo life-prolonging interventions in dementia can be extremely challenging for both caregivers and health care providers, especially when the treatment seems relatively nonburdensome. For example, a decision not to provide intravenous fluids and antibiotic treatment for a urinary tract infection to a patient with advanced dementia may be fraught with guilt and uncertainty, given that withholding this simple intervention may be perceived as hastening the patient’s death.
The primary care provider has an essential role in end-of-life care for patients with dementia. Establishing and frequently revisiting goals of care and providing information on risks and benefits of specific treatments can help prevent burdensome interventions and transitions at the end of life. Ensuring the timely introduction of palliative care and hospice services can provide a better quality of life and better dying experience for both patients and their caregivers, although challenges to delivering these effectively and efficiently within the current system remain.
FUTURE CONSIDERATIONS AND SUMMARY
As the population of older adults continues to increase, health care providers in virtually all specialties will be caring for growing numbers of patients with cognitive impairment and dementia, most commonly AD. At present, our ability to diagnose and treat conditions along the spectrum of cognitive impairment is disappointingly limited. Approved medications provide modest symptomatic benefits in some patients, but do not affect the underlying course of the disease. Nonpharmacologic approaches form the cornerstone of management, with a focus on maintaining function and independence, and providing caregiver support.
To date, efforts to develop effective preventive and treatment strategies for dementia, particularly AD, have been hampered by the long time lag between neurologic damage and the onset of clinical disease. Although significant progress has been made in the development of biomarkers to diagnose AD both earlier and with more certainty, the heterogeneity of AD will likely require a multifaceted approach to prevention and treatment that addresses the varying risk factors that may contribute to the development and progression of cognitive impairment in different populations.
KEY POINTS.
Cognitive complaints in elderly patients are common and may range from normal aging to dementia; complaints should always be evaluated rather than be attributed to aging.
Cognitive impairment in elderly patients is often multifactorial, and potential roles of medications, depression, delirium, alcohol use, and other comorbid conditions should be considered.
Dementia is a clinical diagnosis, with laboratory and imaging studies used to eliminate other explanations for the impairments.
Management goals for patients with cognitive complaints center on preserving function and quality of life, advance care planning, and caregiver support; goals will change with progression of disease.
Although no pharmacologic or nonpharmacologic therapies have been shown to alter the progression of Alzheimer dementia, they may modestly improve symptoms in some patients.
Footnotes
The authors have no disclosures to report.
REFERENCES
- 1.Lin JS, O’Connor E, Rossom RC, et al. Screening for cognitive impairment in older adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(9):601–612. doi: 10.7326/0003-4819-159-9-201311050-00730. [DOI] [PubMed] [Google Scholar]
- 2.Cordell CB, Borson S, Boustani M, et al. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare annual wellness visit in a primary care setting. Alzheimers Dement. 2013;9(2):141–150. doi: 10.1016/j.jalz.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr. 2008;20(1):1–16. doi: 10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- 4.Reisberg B, Shulman MB, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyle PA, Wilson RS, Aggarwal NT, et al. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67(3):441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 8.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56(1):37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 10.Ganguli M, Dodge HH, Shen C, et al. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63(1):115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 11.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- 15.Ballard CG, Gauthier S, Cummings JL, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. 2009;5(5):245–255. doi: 10.1038/nrneurol.2009.39. [DOI] [PubMed] [Google Scholar]
- 16.Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 17.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 18.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 19.Dickson DW. Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol. 2009;3(1):1–23. [PMC free article] [PubMed] [Google Scholar]
- 20.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369(24):2275–2277. doi: 10.1056/NEJMp1311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 22.Duthie EH, Katz PR, Malone ML. Practice of geriatrics. 4th edition. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 23.Arrighi HM, Neumann PJ, Lieberburg IM, et al. Lethality of Alzheimer disease and its impact on nursing home placement. Alzheimer Dis Assoc Disord. 2010;24(1):90–95. doi: 10.1097/WAD.0b013e31819fe7d1. [DOI] [PubMed] [Google Scholar]
- 24.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira CV, Gobbi LT, Corazza DI, et al. Non-pharmacological interventions on cognitive functions in older people with mild cognitive impairment (MCI) Arch Gerontol Geriatr. 2012;54(1):175–180. doi: 10.1016/j.archger.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Yu JT, Wang HF, et al. Non-pharmacological interventions for patients with mild cognitive impairment: a meta-analysis of randomized controlled trials of cognition-based and exercise interventions. J Alzheimers Dis. 2014;42:663–678. doi: 10.3233/JAD-140660. [DOI] [PubMed] [Google Scholar]
- 28.Martin M, Clare L, Altgassen AM, et al. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 2011;(1):CD006220. doi: 10.1002/14651858.CD006220.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Olchik MR, Farina J, Steibel N, et al. Memory training (MT) in mild cognitive impairment (MCI) generates change in cognitive performance. Arch Gerontol Geriatr. 2013;56(3):442–447. doi: 10.1016/j.archger.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Gagnon LG, Belleville S. Training of attentional control in mild cognitive impairment with executive deficits: results from a double-blind randomised controlled study. Neuropsychol Rehabil. 2012;22(6):809–835. doi: 10.1080/09602011.2012.691044. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Li J, Li N, et al. Cognitive intervention for persons with mild cognitive impairment: a meta-analysis. Ageing Res Rev. 2011;10(2):285–296. doi: 10.1016/j.arr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Belleville S, Gilbert B, Fontaine F, et al. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: evidence from a cognitive intervention program. Dement Geriatr Cogn Disord. 2006;22(5–6):486–499. doi: 10.1159/000096316. [DOI] [PubMed] [Google Scholar]
- 33.Jean L, Bergeron ME, Thivierge S, et al. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry. 2010;18(4):281–296. doi: 10.1097/JGP.0b013e3181c37ce9. [DOI] [PubMed] [Google Scholar]
- 34. [Accessed September 10, 2014]; Available at: http://go4life.nia.nih.gov/.
- 35.Baker LD, Frank LL, Foster-Schubert K, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30(5):327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 37.Goodpaster BH, Kelley DE, Wing RR, et al. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48(4):839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira-Lemos E, Nunes S, Teixeira F, et al. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. 2011;10:12. doi: 10.1186/1475-2840-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita S, Rasmussen BB, Cadenas JG, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56(6):1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolduc V, Thorin-Trescases N, Thorin E. Endothelium-dependent control of cerebrovascular functions through age: exercise for healthy cerebrovascular aging. Am J Physiol Heart Circ Physiol. 2013;305(5):H620–H633. doi: 10.1152/ajpheart.00624.2012. [DOI] [PubMed] [Google Scholar]
- 41.Rolland Y, Abellan van Kan G, Vellas B. Healthy brain aging: role of exercise and physical activity. Clin Geriatr Med. 2010;26(1):75–87. doi: 10.1016/j.cger.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 42.McLaren AN, Lamantia MA, Callahan CM. Systematic review of non-pharmacologic interventions to delay functional decline in community-dwelling patients with dementia. Aging Ment Health. 2013;17(6):655–666. doi: 10.1080/13607863.2013.781121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forbes D, Thiessen EJ, Blake CM, et al. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2013;(12):CD006489. doi: 10.1002/14651858.CD006489.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Spector A, Thorgrimsen L, Woods B, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry. 2003;183:248–254. doi: 10.1192/bjp.183.3.248. [DOI] [PubMed] [Google Scholar]
- 45.Onder G, Zanetti O, Giacobini E, et al. Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer’s disease: randomised controlled trial. Br J Psychiatry. 2005;187:450–455. doi: 10.1192/bjp.187.5.450. [DOI] [PubMed] [Google Scholar]
- 46.Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2013;(6):CD003260. doi: 10.1002/14651858.CD003260.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. Am J Geriatr Psychiatry. 2001;9(4):361–381. [PubMed] [Google Scholar]
- 48.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25(3):350–366. doi: 10.3122/jabfm.2012.03.100183. [DOI] [PubMed] [Google Scholar]
- 50.Teri L, Logsdon RG, McCurry SM. Nonpharmacologic treatment of behavioral disturbance in dementia. Med Clin North Am. 2002;86(3):641–656. viii. doi: 10.1016/s0025-7125(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 51.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2(8000):1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 52.Bowen DM, Smith CB, White P, et al. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99(3):459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- 53.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen RA, Gartlehner G, Webb AP, et al. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3(2):211–225. [PMC free article] [PubMed] [Google Scholar]
- 55.Tan CC, Yu JT, Wang HF, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41(2):615–631. doi: 10.3233/JAD-132690. [DOI] [PubMed] [Google Scholar]
- 56.Rolinski M, Fox C, Maidment I, et al. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev. 2012;(3):CD006504. doi: 10.1002/14651858.CD006504.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malouf R, Birks J. Donepezil for vascular cognitive impairment. Cochrane Database Syst Rev. 2004;(1):CD004395. doi: 10.1002/14651858.CD004395.pub2. [DOI] [PubMed] [Google Scholar]
- 58.Birks J, McGuinness B, Craig D. Rivastigmine for vascular cognitive impairment. Cochrane Database Syst Rev. 2013;(5):CD004744. doi: 10.1002/14651858.CD004744.pub3. [DOI] [PubMed] [Google Scholar]
- 59.Birks J, Craig D. Galantamine for vascular cognitive impairment. Cochrane Database Syst Rev. 2013;(4):CD004746. doi: 10.1002/14651858.CD004746.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Francis PT, Holmes C, Webster MT, et al. Preliminary neurochemical findings in non-Alzheimer dementia due to lobar atrophy. Dementia. 1993;4(3–4):172–177. doi: 10.1159/000107319. [DOI] [PubMed] [Google Scholar]
- 61.Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931–937. doi: 10.2165/00002512-200421140-00003. [DOI] [PubMed] [Google Scholar]
- 62.Kertesz A, Morlog D, Light M, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord. 2008;25(2):178–185. doi: 10.1159/000113034. [DOI] [PubMed] [Google Scholar]
- 63.Mendez MF, Shapira JS, McMurtray A, et al. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15(1):84–87. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 64.Gill SS, Anderson GM, Fischer HD, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169(9):867–873. doi: 10.1001/archinternmed.2009.43. [DOI] [PubMed] [Google Scholar]
- 65.Kim DH, Brown RT, Ding EL, et al. Dementia medications and risk of falls, syncope, and related adverse events: meta-analysis of randomized controlled trials. J Am Geriatr Soc. 2011;59(6):1019–1031. doi: 10.1111/j.1532-5415.2011.03450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park-Wyllie LY, Mamdani MM, Li P, et al. Cholinesterase inhibitors and hospitalization for bradycardia: a population-based study. PLoS Med. 2009;6(9):e1000157. doi: 10.1371/journal.pmed.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950–960. doi: 10.1111/jgs.12770. [DOI] [PubMed] [Google Scholar]
- 68.Pariente A, Fourrier-Reglat A, Bazin F, et al. Effect of treatment gaps in elderly patients with dementia treated with cholinesterase inhibitors. Neurology. 2012;78(13):957–963. doi: 10.1212/WNL.0b013e31824d5773. [DOI] [PubMed] [Google Scholar]
- 69.McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154. doi: 10.1002/14651858.CD003154.pub5. [DOI] [PubMed] [Google Scholar]
- 70.Schneider LS, Dagerman KS, Higgins JP, et al. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011;68(8):991–998. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- 71.Orgogozo JM, Rigaud AS, Stoffler A, et al. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300) Stroke. 2002;33(7):1834–1839. doi: 10.1161/01.str.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- 72.Wilcock G, Mobius HJ, Stoffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500) Int Clin Psychopharmacol. 2002;17(6):297–305. doi: 10.1097/00004850-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613–618. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- 74.Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969–977. doi: 10.1016/S1474-4422(10)70194-0. [DOI] [PubMed] [Google Scholar]
- 75.Boxer AL, Lipton AM, Womack K, et al. An open-label study of memantine treatment in 3 subtypes of frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2009;23(3):211–217. doi: 10.1097/WAD.0b013e318197852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diehl-Schmid J, Forstl H, Perneczky R, et al. 6-month, open-label study of memantine in patients with frontotemporal dementia. Int J Geriatr Psychiatry. 2008;23(7):754–759. doi: 10.1002/gps.1973. [DOI] [PubMed] [Google Scholar]
- 77.Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 78.Porsteinsson AP, Grossberg GT, Mintzer J, et al. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5(1):83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 79.Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366(10):893–903. doi: 10.1056/NEJMoa1106668. [DOI] [PubMed] [Google Scholar]
- 80.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 81.Cooper C, Li R, Lyketsos C, et al. Treatment for mild cognitive impairment: systematic review. Br J Psychiatry. 2013;203(3):255–264. doi: 10.1192/bjp.bp.113.127811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer disease and cognitive decline. Ann Intern Med. 2010;153(3):176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 83.Ernst E. The risk-benefit profile of commonly used herbal therapies: ginkgo, St. John’s wort, ginseng, echinacea, saw palmetto, and kava. Ann Intern Med. 2002;136(1):42–53. doi: 10.7326/0003-4819-136-1-200201010-00010. [DOI] [PubMed] [Google Scholar]
- 84.Mangialasche F, Xu W, Kivipelto M, et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol Aging. 2012;33(10):2282–2290. doi: 10.1016/j.neurobiolaging.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 85.Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311(1):33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alphatocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336(17):1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 87.Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 88.Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 90.U.S. Food and Drug Administration. Medical foods guidance documents & regulatory information. [Accessed August 4, 2014]; Available at: http://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/medicalfoods/default.htm.
- 91.Henderson ST, Vogel JL, Barr LJ, et al. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab. 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schulze J, Glaeske G, van den Bussche H, et al. Prescribing of antipsychotic drugs in patients with dementia: a comparison with age-matched and sex-matched non-demented controls. Pharmacoepidemiol Drug Saf. 2013;22(12):1308–1316. doi: 10.1002/pds.3527. [DOI] [PubMed] [Google Scholar]
- 93.Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- 94.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 95.Maher AR, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306(12):1359–1369. doi: 10.1001/jama.2011.1360. [DOI] [PubMed] [Google Scholar]
- 96.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 97.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 98.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627–632. doi: 10.1503/cmaj.061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.AGS Choosing Wisely Workgroup. American Geriatrics Society identifies five things that healthcare providers and patients should question. J Am Geriatr Soc. 2013;61(4):622–631. doi: 10.1111/jgs.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;(3):CD007726. doi: 10.1002/14651858.CD007726.pub2. [DOI] [PubMed] [Google Scholar]
- 101.Sepehry AA, Lee PE, Hsiung GY, et al. Effect of selective serotonin reuptake inhibitors in Alzheimer’s disease with comorbid depression: a meta-analysis of depression and cognitive outcomes. Drugs Aging. 2012;29(10):793–806. doi: 10.1007/s40266-012-0012-5. [DOI] [PubMed] [Google Scholar]
- 102.Serfaty M, Kennell-Webb S, Warner J, et al. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int J Geriatr Psychiatry. 2002;17(12):1120–1127. doi: 10.1002/gps.760. [DOI] [PubMed] [Google Scholar]
- 103.Gehrman PR, Connor DJ, Martin JL, et al. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry. 2009;17(2):166–169. doi: 10.1097/JGP.0b013e318187de18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26(7):893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jansen SL, Forbes DA, Duncan V, et al. Melatonin for cognitive impairment. Cochrane Database Syst Rev. 2006;(1):CD003802. doi: 10.1002/14651858.CD003802.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiely DK, Givens JL, Shaffer ML, et al. Hospice use and outcomes in nursing home residents with advanced dementia. J Am Geriatr Soc. 2010;58(12):2284–2291. doi: 10.1111/j.1532-5415.2010.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teno JM, Gozalo PL, Lee IC, et al. Does hospice improve quality of care for persons dying from dementia? J Am Geriatr Soc. 2011;59(8):1531–1536. doi: 10.1111/j.1532-5415.2011.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shega JW, Hougham GW, Stocking CB, et al. Patients dying with dementia: experience at the end of life and impact of hospice care. J Pain Symptom Manage. 2008;35(5):499–507. doi: 10.1016/j.jpainsymman.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 109.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Albrecht JS, Gruber-Baldini AL, Fromme EK, et al. Quality of hospice care for individuals with dementia. J Am Geriatr Soc. 2013;61(7):1060–1065. doi: 10.1111/jgs.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sampson EL, Candy B, Jones L. Enteral tube feeding for older people with advanced dementia. Cochrane Database Syst Rev. 2009;(2):CD007209. doi: 10.1002/14651858.CD007209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Michel JP, Pautex S, Zekry D, et al. End-of-life care of persons with dementia. J Gerontol A Biol Sci Med Sci. 2002;57(10):M640–M644. doi: 10.1093/gerona/57.10.m640. [DOI] [PubMed] [Google Scholar]
- 113.Hanrahan P, Luchins DJ. Access to hospice programs in end-stage dementia: a national survey of hospice programs. J Am Geriatr Soc. 1995;43(1):56–59. doi: 10.1111/j.1532-5415.1995.tb06243.x. [DOI] [PubMed] [Google Scholar]
- 114.Medical guidelines for determining prognosis in selected non-cancer diseases. The National Hospice Organization. Hosp J. 1996;11(2):47–63. doi: 10.1080/0742-969x.1996.11882820. [DOI] [PubMed] [Google Scholar]
- 115.Hanrahan P, Raymond M, McGowan E, et al. Criteria for enrolling dementia patients in hospice: a replication. Am J Hosp Palliat Care. 1999;16(1):395–400. doi: 10.1177/104990919901600110. [DOI] [PubMed] [Google Scholar]
- 116.Schonwetter RS, Han B, Small BJ, et al. Predictors of six-month survival among patients with dementia: an evaluation of hospice Medicare guidelines. Am J Hosp Palliat Care. 2003;20(2):105–113. doi: 10.1177/104990910302000208. [DOI] [PubMed] [Google Scholar]
- 117.Mitchell SL, Miller SC, Teno JM, et al. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304(17):1929–1935. doi: 10.1001/jama.2010.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 119.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 120.Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900–910. doi: 10.1097/01.JGP.0000221510.33817.86. [DOI] [PubMed] [Google Scholar]
- 121.Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive ’vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 122.Vink AC, Birks JS, Bruinsma MS, et al. Music therapy for people with dementia. Cochrane Database Syst Rev. 2004;(3):CD003477. doi: 10.1002/14651858.CD003477.pub2. [DOI] [PubMed] [Google Scholar]
- 123.Ueda T, Suzukamo Y, Sato M, et al. Effects of music therapy on behavioral and psychological symptoms of dementia: a systematic review and meta-analysis. Ageing Res Rev. 2013;12(2):628–641. doi: 10.1016/j.arr.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 124.Aguirre E, Woods RT, Spector A, et al. Cognitive stimulation for dementia: a systematic review of the evidence of effectiveness from randomised controlled trials. Ageing Res Rev. 2013;12(1):253–262. doi: 10.1016/j.arr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 125.Thorgrimsen L, Spector A, Wiles A, et al. Aroma therapy for dementia. Cochrane Database Syst Rev. 2003;(3):CD003150. doi: 10.1002/14651858.CD003150. [DOI] [PubMed] [Google Scholar]
- 126.Fung JK, Tsang HW, Chung RC. A systematic review of the use of aromatherapy in treatment of behavioral problems in dementia. Geriatr Gerontol Int. 2012;12(3):372–382. doi: 10.1111/j.1447-0594.2012.00849.x. [DOI] [PubMed] [Google Scholar]
- 127.Cameron M, Lonergan E, Lee H. Transcutaneous electrical nerve stimulation (TENS) for dementia. Cochrane Database Syst Rev. 2003;(3):CD004032. doi: 10.1002/14651858.CD004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cotelli M, Manenti R, Zanetti O. Reminiscence therapy in dementia: a review. Maturitas. 2012;72(3):203–205. doi: 10.1016/j.maturitas.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 129.Woods B, Spector A, Jones C, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;(2):CD001120. doi: 10.1002/14651858.CD001120.pub2. [DOI] [PubMed] [Google Scholar]
- 130.Maayan N, Soares-Weiser K, Lee H. Respite care for people with dementia and their carers. Cochrane Database Syst Rev. 2014;(1):CD004396. doi: 10.1002/14651858.CD004396.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Forbes D, Culum I, Lischka AR, et al. Light therapy for managing cognitive, sleep, functional, behavioural, or psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2009;(4):CD003946. doi: 10.1002/14651858.CD003946.pub3. [DOI] [PubMed] [Google Scholar]
- 132.Vernooij-Dassen M, Draskovic I, McCleery J, et al. Cognitive reframing for carers of people with dementia. Cochrane Database Syst Rev. 2011;(11):CD005318. doi: 10.1002/14651858.CD005318.pub2. [DOI] [PubMed] [Google Scholar]
- 133.Viggo Hansen N, Jorgensen T, Ortenblad L. Massage and touch for dementia. Cochrane Database Syst Rev. 2006;(4):CD004989. doi: 10.1002/14651858.CD004989.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]