Abstract
Objective(s)
To determine how adults in the United States (US) view non-invasive prenatal testing using cell-free fetal DNA (cffDNA testing) in order to help estimate uptake.
Study Design
A national sample of 1,861 US-based adults was surveyed using a validated online survey instrument. The survey was administered by a commercial survey research company. Respondents were randomized to receive a survey about prenatal testing for trisomy 13 and 18 or trisomy 21. Participants were asked to select among testing modalities, including cffDNA testing, and rank the features of testing that they considered most important to decision making.
Results
There was substantive interest in the use of cffDNA testing rather than traditional screening mechanisms with a minority of respondents reporting that they would support the use of both methods in combination. The lower rates of false negative and false positive test results and the ability to use the test earlier in the pregnancy were the most highly rated benefits of cffDNA testing. Participants expressed strong support for diagnostic confirmation via invasive testing after a positive result from either screening or cffDNA testing. However, almost one-third of participants reported that they would not endorse the use of either invasive or non-invasive prenatal testing.
Conclusion(s)
There appears to be support for uptake of non-invasive prenatal tests. Clinical guidelines should therefor go forward in providing guidance on how to integrate non-invasive methods into current standard of care. However, our findings indicate that even when accuracy, which is rated by patients as the most important aspect of prenatal testing, is significantly improved over existing screening methods and testing is offered non-invasively, the number of individuals who reported that they would decline any testing remained the same. Attention should therefor be directed at ensuring that the right of informed refusal of prenatal testing is not impacted by new, non-invasive methods.
Keywords: Prenatal Testing, Prenatal Diagnosis, Ethics
Introduction
The emergence of non-invasive forms of prenatal genetic testing has the potential to expand the availability of highly sensitive and specific prenatal testing. In particular, new tests that target and analyze cell-free fetal DNA (cffDNA) in the maternal bloodstream as early as nine weeks into pregnancy (1) have generated considerable public interest (2). The growing interest in these technologies stems from cffDNA testing’s distinguishing features: the absence of procedure-related risks of miscarriage and the potential to obtain results earlier in gestation than currently available prenatal diagnostic tests (3,4). Over the past decade, several biotechnology companies - including Sequenom, Verinata Health, Natera and Ariosa Diagnostics - and academic laboratories have validated cffDNA techniques for the detection of fetal RhD blood type, sex, and most recently, trisomy 13, 18, and 21 (Down syndrome), monosomy X, and other sex chromosome aneuploidies (1,5,6). Tests using these techniques are now entering an expanding $1.3 billion prenatal testing market in the United States (US) (7,8). Industry expects non-invasive prenatal testing methods using cffDNA (cffDNA testing) to supplement, and potentially replace, existing first-trimester combined screening and significantly reduce the number of invasive procedures performed (9) because cffDNA testing offers increased sensitivity and specificity (between 98.0 and 99.0% sensitivity and 99.5% and 99.8% specificity) over existing screening measures together with early usage and without the procedure-related miscarriage associated with diagnostic testing (1). Professional societies have begun to release preliminary guidelines supporting the use of cffDNA testing as second-tier screening in high-risk pregnancies (10,11).
This study explores the interest of individuals of reproductive age in cffDNA testing in relation to other prenatal testing mechanisms. Past studies on American attitudes towards prenatal testing were largely conducted before non-invasive testing methods became clinically available (12). One of few studies addressing non-invasive testing, by Tischler et al., found that 71.9% of a small sample of pregnant women were interested in cffDNA testing as a replacement for amniocentesis because of the decreased risk to the fetus (13). Zamerowski et al. similarly found that women undergoing invasive diagnostic testing in four United States metropolitan clinics responded positively to the potential of non-invasive prenatal testing using whole fetal cells (14). In the United Kingdom, Lewis et al. found positive attitudes towards non-invasive testing for fetal sex among women at risk for transmitting sex-linked genetic disorders (15).
Although professional stakeholders, such as healthcare providers, have been assessed regarding the potential usage of cffDNA testing (16), studies conducted on views of non-invasive testing among end users in the US have been limited to small, specific populations of pregnant women (13). There has yet to be a comprehensive national study on the target users of this technology – individuals of reproductive age - and their view of cffDNA testing as a possible supplement to or replacement for other methods of testing, including invasive measures such as amniocentesis.
Methods
Survey Design
We designed a 25 question online survey for distribution to a representative selection of the general public in the US regarding their choices among cffDNA testing, conventional screening, and diagnostic procedures such as amniocentesis. Here, we present data on the responses of individuals of reproductive age, as defined by age self-reported between 18 and 44 years. Participants were randomized between one of two versions of the survey - focusing on testing for either trisomy 13 and 18 or trisomy 21 - in order to asses whether the severity of the condition being tested affected uptake rates. Participants were randomized in order to avoid any cognitive bias associated with the order in which conditions were presented (17). We were interested in testing whether allowing individuals to consider higher morbidity versus lower morbidity conditions independently would impact testing decisions.
At the beginning of the survey, short descriptions of either trisomy 13 and 18 or trisomy 21 were provided to the viewer (see Appendix A) in conjunction with a table highlighting the features of different testing options and their costs. The tests presented were designed to mirror the current abilities of first-trimester combined screening and cffDNA testing. cffDNA testing was described as more expensive than screening in accordance with the current state of the US prenatal testing market (7). Participants were asked to state a preference as to which test should be used in the event of a pregnancy in themselves or a loved one. Survey questions were derived from previous empirical data on prenatal testing as well as pertinent concepts in the prenatal medicine literature (18,19). A broader team of bioethics scholars reviewed the survey’s text for readability and content. The survey consisted of 10 questions on the topic of prenatal testing choices, some of which used a five-point Likert scale to determine the importance the participant assigned to the features of different tests. Participants were then asked to consider invasive follow-up and whether they would consider termination of an affected pregnancy. Fifteen demographic questions were included.
Data collection and analysis
The research team contracted a web-based survey provider to electronically distribute both versions of the study to individuals aged 18 to 44 years throughout the United States. The online survey company provides a quality-assured sample of survey respondents by validating prospective panelists to ensure that they are providing accurate data and de-duplicating survey responses by using digital fingerprinting.
Participants were compensated for their time completing the survey via the commercial provider. Responses were downloaded to a spreadsheet via a secure webpage by the research team, and statistical analysis was performed using the SPSS Statistics Standard program version 20.0 in consultation with the Stanford University biostatistics consultation service. This study was approved by the Stanford University Institutional Review Board.
Results
A pilot version of the study was sent to approximately 200 users (100 individuals for each version of the survey) to ensure the participants could understand and complete the survey. Minor revisions were made to enhance the clarity of the questions, and the final survey was sent to members of the company’s panel, representative of the primary demographic features of the United States general public. Including the pilot sample, 1861 individuals of reproductive age completed the survey. Because the commercial survey provider measures participation in terms of completed surveys rather than invitations sent, the total response rate is unknown.
Population Characteristics
Both income and education were evenly distributed and most (90%+) participants had a high school degree or higher. Age and gender in the sample were well distributed, with slightly more women than men responding (52.6% female to 47.3% male for trisomy 13 and 18 and 55.8% female to 44.1% male for trisomy 21). A majority, over 80% of the sample, identified as white; over 80% also identified as non-Hispanic or Latino. A majority of the sample identified as somewhat or very religious, with strong Catholic representation (21.6% for trisomy 13 and 18, 24.5% for trisomy 21). Approximately a quarter of the sample marked themselves as unaffiliated with the religions listed. Demographic features of the sample are included in Table 1.
Table 1.
Demographics of respondent population
| N = 3133 | trisomy 13 & 18 n = 1642 |
trisomy 21 n = 1491 |
National |
|---|---|---|---|
| 18 – 24 | 14% | 15% | 13% |
| 25 – 34 | 23% | 21% | 18% |
| 35 – 44 | 22% | 22% | 18% |
| 45 – 54 | 16% | 16% | 19% |
| 55 – 64 | 11% | 10% | 16% |
| 65 or older | 14% | 16% | 17% |
|
| |||
| Male | 46% | 44% | 49% |
| Female | 54% | 56% | 51% |
|
| |||
| Hispanic or Latino | |||
| Yes | 8% | 7% | 16% |
| No | 92% | 93% | 84% |
|
| |||
| American Indian or Alaska Native | 2% | 2% | 2% |
| Asian | 6% | 6% | 6% |
| Black or African American | 8% | 8% | 14% |
| Native Hawaiian or Other Pacific Islander | 1% | 1% | <1% |
| White | 84% | 83% | 75% |
| Other | 2% | 3% | N/A |
|
| |||
| Catholic | 23% | 24% | 23% |
| Evangelical Protestant | 9% | 10% | 27% |
| Other Protestant | 18% | 16% | 27% |
| Mormon | 2% | 2% | 2% |
| Jewish | 4% | 4% | 2% |
| Buddhist | 1% | 1% | 1% |
| Muslim | 1% | 1% | <1% |
| Unaffiliated | 24% | 20% | 14% |
| Other | 20% | 24% | N/A |
|
| |||
| Very Religious | 21% | 23% | Not Measured |
| Somewhat Religious | 41% | 42% | Not Measured |
| Not Very Religious | 22% | 20% | Not Measured |
| Not Religious | 17% | 16% | Not Measured |
|
| |||
| Under $25,000 | 20% | 20% | 25% |
| $25,000 – $49,999 | 29% | 27% | 25% |
| $50,000 – $74,999 | 23% | 23% | 18% |
| $75,000 – $99,999 | 14% | 15% | 12% |
| Over $100,000 | 15% | 15% | 20% |
|
| |||
| Some High School | 2% | 2% | 9% |
| High School Diploma | 18% | 20% | 30% |
| Some College | 31% | 30% | 23% |
| College Diploma | 28% | 28% | |
| Some Graduate School | 7% | 5% | 27% |
| Graduate School Diploma | 14% | 15% | 11% |
|
| |||
| Are you a parent? | |||
| Yes | 58% | 61% | Not Measured |
| No | 42% | 39% | Not Measured |
In order to establish representativeness, we compared demographic measures to the 2010 US Census data (for gender, age, ethnicity, race, educational attainment, household income, and health insurance status) using chi-square goodness-of-fit tests with α=0.05. The survey sample under-represented males (χ2(1, N=1861)=14.8, p<0.001), individuals who identify as Hispanic or Latino (χ2(1, N=1738)=90.7, p<0.001) and Black or African-American (χ2(1, N=1861)=7.7, p<0.001). Additionally, the distributions of respondent age (χ2(5, N=1861)=8.3, p<0.001) and educational attainment (χ2(4, N=1861)=490.4, p<0.001) were statistically different from those of the national population. Age matched national statistics were not available for participant religion or educational achievement. No statistically significant differences were found in the demographics between the T13/18 and T21 samples.
Choosing between screening and cffDNA testing
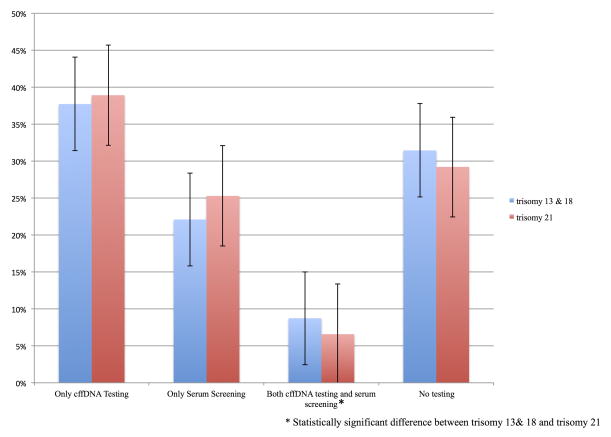
As Figure 1 (CI = 95%) demonstrates, when asked to choose between testing modalities, respondents reported a substantive projected uptake of cffDNA testing (trisomy 13/18 37.7%, trisomy 21 38.9%) over traditional screening mechanisms (trisomy 13/18 22.1%, trisomy 21 25.2%) with a small minority (trisomy 13/18 8.7%, trisomy 21 6.5%) expressing an interest in using both tests. There was no statistically significant difference between the two cohorts in those who chose cffDNA over screening. However there was a statistically significant difference (p=0.001) in those that wished to undergo both tests, with a higher percentage electing to undergo both tests for trisomy 13 and 18. A significant segment of the respondents (trisomy 13/18 31.4%, trisomy 21 29.2%) declined either form of testing.
Figure 1.
Preferences between non-invasive testing methods.
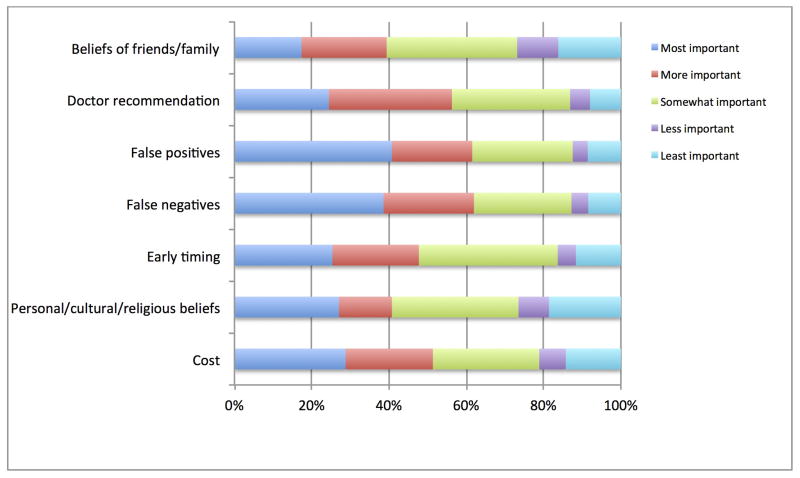
Participants were asked to rank the features of testing that they considered most important to decision making (see Figure 2). The lowered rate of false negatives and false positives and the ability to use the test earlier in the pregnancy were the most highly rated benefits of cffDNA testing. Two other strongly rated factors were the cost of cffDNA testing and the recommendation of a health care provider. Finally, the beliefs of family and friends were consistently ranked lowest among the factors provided.
Figure 2.
Factors influencing support for testing choices among non-invasive methodologies. No statistical difference between priorities for trisomy 13 and 18 and trisomy 21 were observed.
Choosing whether or not to undergo invasive testing
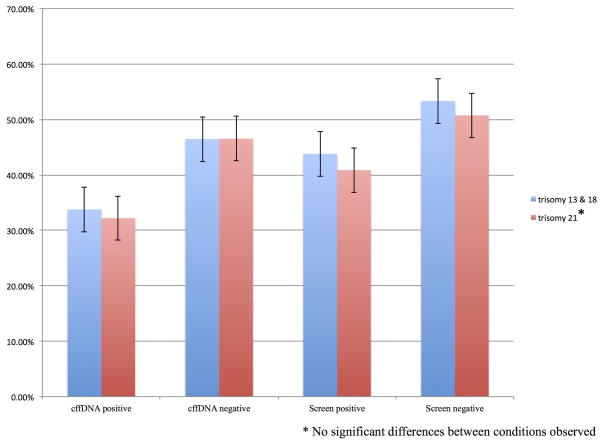
Participants were asked whether they would elect for invasive confirmatory testing after receiving a prenatal diagnosis of trisomy 13 or 18 or trisomy 21. Results are included in Figure 2 (CI = 95%). Desire for invasive confirmation was higher after receiving a positive result from both screening and cffDNA testing with approximately half of participants electing to receive confirmatory testing after a positive screening result (trisomy 13/18 53.3%, trisomy 21 50.7%) or after a positive cffDNA testing result (trisomy 13/18 46.4%, trisomy 21 46.5%). Uptake rates were lower following a negative testing result from screening (trisomy 13/18 43.8%, trisomy 21 40.8%) and cffDNA testing (trisomy 13/18 33.7%, trisomy 21 32.1%). There were no significant differences between rates for trisomy 13 and 18 and rates for trisomy 21.
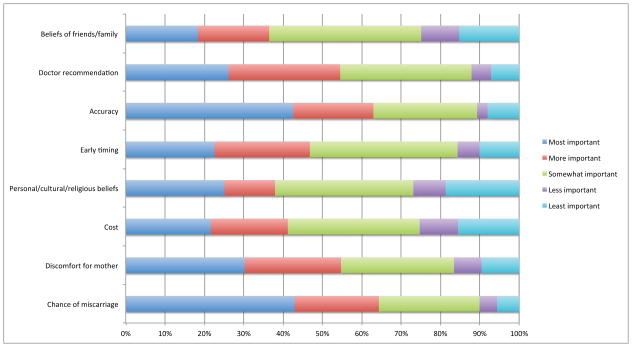
The accuracy of invasive testing was the highest rated factor when respondents were asked which factors were most influential in deciding whether or not to undergo invasive testing. However, this was closely followed by the risk of miscarriage. The discomfort of invasive procedures and the recommendation of a medical provider were also ranked as considerations for both conditions. Timing was also ranked fairly low, and cost was ranked considerably lower as a decision-making factor when considering invasive versus non-invasive testing. Finally, the belief of family and friends was once again ranked lowest in considering testing. Rankings are displayed in Figure 4.
Figure 4.
Factors influencing support for undergoing invasive confirmatory testing. No statistical difference between priorities for trisomy 13 and 18 and trisomy 21 were observed.
Finally, participants were asked whether they would consider termination following a diagnosis of trisomy. (When trisomy 13 or 18 is detected in a fetus, most parents choose not to continue the pregnancy. Do you think this is a decision you would consider?) As shown in Figure 5, a statistically significant (p=0.001) percentage (trisomy 13/18 49.8%, trisomy 21 41.3%) were more likely to consider termination for trisomy 13/18 than for trisomy 21. Approximately half (trisomy 13/18 50.1%, trisomy 21 58.6%), however, reported that they would not consider termination after a diagnosis of trisomy 13 and 18 or 21.
Figure 5.

Expressed willingness to consider termination after an invasive diagnosis. Statistically significant difference between conditions. P <0.001, CI = 95%.
Discussion
Reported support for cffDNA testing
Overall, these data show support among US adults of reproductive age for the uptake of non-invasive prenatal testing for aneuploidy over current first-trimester combined screening methods. Recent studies have suggested that cffDNA testing is more cost effective as a follow-up to a positive first-trimester screen (20). However, our results suggest that patients would not necessarily support such an approach, as only 8.7%, for trisomy 13/18, or 6.5%, for trisomy 21, indicated that they would opt for both screening and cffDNA testing. Furthermore, unlike previous testing estimates that the availability of cffDNA testing would decrease the uptake of invasive testing for 66% (9), 46% of our participant population indicated that they would request confirmatory testing, even after a negative result from cffDNA. This result suggests that, in the hypothetical, participants devalue the risk of invasive procedures in favor of a desire for more conclusive information. Given the decline in uptake of invasive procedures in general (21), it is probable that this number is significantly higher than actual clinical uptake of invasive procedures. It does, however, emphasize the importance potential parents place on certainty in prenatal results.
Respondents clearly indicated the value of clinical recommendation in selecting among testing options. However, a pilot study of providers also indicated that many felt unprepared to have a knowledgeable conversation about cffDNA testing, suggesting that broader clinician education about the capabilities and limitations of non-invasive testing will be necessary (16). Furthermore, genetic counselors have expressed concerns that other medical professionals do not offer adequate pre-test counseling to ensure informed consent for cffDNA testing (3,22–24), while physicians have reported a preference for professional genetic counseling services to be administered after a positive test result, indicating a discomfort with physicians attempting to offer effective genetic counseling. Plans to expand the offer of cffDNA testing into wider clinical use for average risk pregnancies will exacerbate the lack of adequate counseling services available to women considering prenatal testing options. On a broader level, the input of pertinent regulatory agencies may become increasingly important as non-invasive prenatal testing expands. Given that the accuracy of testing is the most highly valued feature in prenatal testing, additional measures will be necessary to ensure that novel tests are rigorously validated and monitored.
Approximately a third of participants indicated that they would continue to decline all testing, including cffDNA testing, despite the potential to receive more accurate results non-invasively. These results are supported by Tischler et al (2011) and are consistent with limited clinical reports from academic medical centers (25). However, other studies involving individuals who decline invasive prenatal testing have emphasized that risk to the fetus is a significant motivation for declining testing (26). One explanation for this may be that the risk to the fetus may serve as a form of proxy anxiety for cultural and religious beliefs surrounding the permissibility of termination; because patients may be uncomfortable considering elective termination as a valid option, risk is used as an alternative explanation for why testing is unacceptable. Previous studies have supported the idea that personal and ethical beliefs can significantly mediate patient choices regarding prenatal testing (27). Despite the modest importance assigned to personal beliefs in the current sample, it is probable that those who do express strong cultural or religious values contribute significantly to the proportion of individuals in the sample who said they would decline testing.
The number of individuals who continue to express a desire to avoid all prenatal testing is potentially worrisome because of the potential for cffDNA testing to compress the informed consent process leading up to near-diagnostic results. cffDNA testing can be carried out at a time in the pregnancy when women generally undergo blood draws for a variety of reasons and observers have frequently expressed concern that pregnant women will have difficulty distinguishing aneuploidy testing from other routine maternal tests (28–30). Indeed, recent data show that even women who remember formulating a plan, with regard to whether or not to receive quad screen testing, end up inadvertently acting against their intentions due to a lack of awareness of what kind of testing to which they are agreeing (31). Furthermore, due to the lack of physical risk in cffDNA testing, there is concern that medical professionals may be inclined to view non-invasive testing as information with ‘no downside’ (32,33), an attitude that may erode the informed consent process (34). For instance, van den Heuvel et al. found that service providers recommended less stringent informed consent procedures when hypothetically referring for non-invasive versus invasive testing (35). We echo the concerns of other observers who note that it is important to maintain the integrity of the informed consent process, even when testing is conducted early and non-invasively (35–38).
Finally, it is clear that cost remains a factor in prenatal decision-making, highlighting the significant challenges of integrating cffDNA testing into the US health care system. While a few states have subsidized prenatal screening programs using first-trimester combined screening, it is unclear how quickly, if at all, cffDNA testing will be taken up by public payors, including both Medicaid and state screening programs. For third-party payors who might otherwise cover these costs, cffDNA testing will have to prove that it significantly improves detection rates of targeted conditions or substantively reduces the number of invasive procedures before it will be widely reimbursable. Reimbursement for non-invasive tests using cffDNA among private insurers has thus far been inconsistent among US providers (3, 22); to date, California is the only state that includes NIPT in its universally available prenatal screening regime (39). Furthermore, if patients are unwilling to rely on cffDNA testing in making decisions about a pregnancy and decide to undergo amniocentesis regardless of their results, then the timing advantages of cffDNA testing may be reduced (3).
Differences between trisomy 13/18 and 21
Reported uptake of testing of any kind did not significantly differ across trisomies, despite the fact that trisomies 13/18 were presented as most often fatal whereas trisomy 21 was presented as a condition with which affected individuals may lead long, relatively healthy lives (see Appendix A). The only significant difference came in the small proportion of people who indicated that they would choose to undergo both screening and cffDNA testing suggesting that the additional reassurance was considered important when considering a fatal condition. On the one hand, as we report elsewhere, the inherent value of information is frequently cited as a motivation for undergoing testing, even when participants report that they intend to carry the pregnancy to term regardless of testing results. This finding may suggest that respondents who said they would choose testing value prenatal information in and of itself, regardless of the condition being detected. Further, participants were informed that current standard of care is the integrated screen and that doctors are thus likely to suggest this option to patients as a first tier test. Indeed, the recommendation of a health care provider was listed higher than personal or family beliefs in testing decision-making factors. The recommendation of a health care provider may therefore be a significant contributor to the decision to undergo integrated screening rather than cffDNA testing. Similarly, those who said they would refuse testing may regard the information as unnecessary, regardless of the condition.
Significant difference was shown in the consideration of termination between trisomy 13/18 and trisomy 21. A higher percentage of respondents indicated that they would be more likely to consider terminating a pregnancy affected by trisomy 13/18 than a pregnancy affected by trisomy 21, which most likely indicates consideration of the increased severity of trisomy 13/18. However, the reported willingness to consider termination seen here are considerably lower than actual statistics for termination post trisomy 21 diagnosis, which range from 67 to 85% in the US (40). The high reported willingness to consider termination for trisomy 21 is also at odds with previous studies of individuals and families with trisomy 21, which report high levels of satisfaction and quality of life, although there is some selection bias in these findings (41,42). Patient advocates have long suggested that this dichotomy reflects a lack of education and nuanced understanding of the reality of living with genetic conditions which can be overcome with better educational approaches to prenatal counseling (43).
Limitations
Finally, as noted in the results section, there are statistically significant differences between the study sample and the national census on several demographic factors. In particular, as reported elsewhere, educational achievement and income may affect the reported willingness to undergo testing. By the same token the underrepresentation of certain minority populations may give the impression of greater enthusiasm for testing among the population as a whole; past studies have shown that minority populations have lower uptake rates of prenatal testing than Caucasian populations (44). On the other hand, these demographics may be a more accurate portrayal of those populations that are most likely to be offered non-invasive prenatal testing methods. Given high price points and uncertain insurance coverage, it is likely that this testing will be accessed, at least initially, by more socio-economically advantaged populations.
Another limitation concerns the fact that participants received relatively little information on the complex nature of risk calculations and phenotypic expression of trisomies. This is counter to the recommendation of many disability advocates, who frequently campaign for more nuanced information in the genetic counseling process (45,46). However, it may not be dissimilar to actual clinical testing presentations, especially in resource-limited settings (47). A third limitation is suggested by the disparity between anticipated invasive testing and termination rates and the experience of clinical practitioners. This disparity suggests that individuals cannot always accurately predict their future actions, which may restrict the generalizability of the data gathered here. There is a well-known expectation bias among participants in social science research, particularly that which deals with morally controversial subjects. Especially among populations with strong cultural or religious stigma surrounding abortion, individuals are less likely to publically confess a willingness to undergo pregnancy termination. The way in which these attitudes change when confronted with the reality of a positive diagnosis may account in part for the discrepancy in reported versus actual terminations.
Conclusion
Overall, these results suggest moderate support for the use of cffDNA testing in high-risk pregnancies for the detection of trisomy. Despite differences in mortality and morbidity between trisomy 13 and 18 and trisomy 21, there were no statistical differences between uptake of testing for the two sets of conditions, with the exception of a slight increase in those who indicated that they would prefer to have both screening and cffDNA testing for trisomy 13 & 18. There was also greater support for consideration of termination when considering a positive result for trisomy 13 and 18 as opposed to trisomy 21. While these numbers seem to suggest a measured consideration of the probable quality of life impact of these respective conditions, reported willingness to consider termination remained significantly lower than the rates of actual termination reported clinically.
While the potential to test for aneuploidy earlier in the pregnancy does offer benefits in terms of the possibility of earlier reassurance or decisions surrounding termination when medical, rather than surgical, abortions are still possible, it also condenses the informed consent process and may discourage healthcare providers and patients from having comprehensive conversations about the realities of living with a genetic condition (32,37). It is of some concern to note that respondents consistently valued the recommendation of a physician when making prenatal testing decisions despite the fact that most obstetric clinicians do not have training in genetics and may be unprepared to have knowledgeable conversations about the implications of expanded prenatal genetic testing.
In addition, a small but significant portion of the sample indicated that they would continue to support the refusal of any form of prenatal testing, even when offered a non-invasive testing option and despite differences in the severity of the condition being tested. This is contrary to previous prediction, which suggested that the limiting factor for many women who decline testing is the risk of miscarriage (26). We suggest that stakeholders should direct proactive attention to reforming and strengthening the informed consent process in order to effect the most ethical implementation of cffDNA testing.
Supplementary Material
Figure 3.
Preferences for invasive follow-up testing following a negative or positive test result.
Acknowledgments
This work is funded by NIH grant P50 HG003389 (Center for Integrating Ethics and Genetic Research). Dr. Cho is additionally supported by NIH grant 1 U54 RR024374-01A1 (Stanford Center for Clinical and Translational Education and Research).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Contributor Information
Megan Allyse, Email: megan.allyse@duke.edu, Science & Society, Duke University, 101 Science Drive, Box 3882, Durham, North Carolina 27708, Telephone: 919.684.4151, Mobile: (415) 860 -7135, Fax number: 919.668.0795.
Lauren C. Sayres, Duke University School of Medicine.
Taylor A. Goodspeed, Stanford University.
Mildred K. Cho, Stanford Center for Biomedical Ethics, Stanford University, Department of Pediatrics, Division of Genetics, Stanford University.
Bibliography
- 1.Zimmermann B, Hill M, Gemelos G, Demko Z, Banjevic M, Baner J, et al. Noninvasive prenatal aneuploidy testing of chromosomes 13, 18, 21, X, and Y, using targeted sequencing of polymorphic loci. Prenat Diagn. 2012;32(13):1233–1241. doi: 10.1002/pd.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heger M. As rivals vie for share of noninvasive trisomy testing market, physicians see value for screening. GenomeWeb. 2012 Jul 3; [Google Scholar]
- 3.Allyse M, Sayres C, King S, Norton E, Cho K. Cell-free fetal DNA testing for fetal aneuploidy and beyond: Clinical integration challenges in the US context. Human Reproduction. 2012;27(11):3123–3131. doi: 10.1093/humrep/des286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sayres LC, Cho MK. Cell-Free fetal nucleic acid testing: A review of the technology and its applications. Obstetrical & Gynecological Survey. 2011;66(7):431. doi: 10.1097/OGX.0b013e31822dfbe2. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP. Genome-Wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119(5) doi: 10.1097/AOG.0b013e31824fb482. [DOI] [PubMed] [Google Scholar]
- 6.Norton ME, Brar H, Weiss J, Karimi A, Laurent LC, Caughey AB, et al. Non-Invasive chromosomal evaluation (NICE) study: Results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207(2):137.e1–8. doi: 10.1016/j.ajog.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal A, Sayres LC, Cho MK, Cook-Deegan R, Chandrasekharan S. Commercial landscape of noninvasive prenatal testing in the united states. Prenat Diagn. 2013;33(6):521–31. doi: 10.1002/pd.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan W. Prenatal markets remain strong and fertile. Genetic and Engineering News. 2011;31(18) [Google Scholar]
- 9.Garfield SS, Armstrong SO. Clinical and cost consequences of incorporating a novel non-invasive prenatal test into the diagnostic pathway for fetal trisomies. Journal of Managed Care Medicine. 2012;15(2):34–41. [Google Scholar]
- 10.The American College of Obstetricians and Gynecologists Committee on Genetics, The Society for Maternal-Fetal Medicine Publications Committee. [last accessed January 2014];Committee Opinion Number. 2012 545 Available at http://www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Genetics/Noninvasive_Prenatal_Testing_for_Fetal_Aneuploidy. [Google Scholar]
- 11.Devers P, Cronister S, Ormond K, Facio F, Brasington C, Flodman Noninvasive prenatal testing/noninvasive prenatal diagnosis: The position of the national society of genetic counselors. J Genet Couns. 2013;22(13):291–295. doi: 10.1007/s10897-012-9564-0. [DOI] [PubMed] [Google Scholar]
- 12.Kalfoglou AL, Doksum T, Bernhardt B, Geller G, LeRoy L, Mathews DJ, et al. Opinions about new reproductive genetic technologies: Hopes and fears for our genetic future. Fertil Steril. 2005;83(6):1612–21. doi: 10.1016/j.fertnstert.2005.01.090. [DOI] [PubMed] [Google Scholar]
- 13.Tischler R, Hudgins L, Blumenfeld YJ, Greely HT, Ormond KE. Noninvasive prenatal diagnosis: Pregnant women’s interest and expected uptake. Prenat Diagn. 2011;31(13):1292–9. doi: 10.1002/pd.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamerowski S, Lumley M, Arreola R, Dukes K, Sullivan L. Favorable attitudes toward testing for chromosomal abnormalities via analysis of fetal cells in maternal blood. Genetics in Medicine. 2001;3(4):301. doi: 10.1097/00125817-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Lewis C, Hill M, Skirton H, Chitty LS. Non-invasive prenatal diagnosis for fetal sex determination: Benefits and disadvantages from the service users’ perspective. Eur J Hum Genet. 2012;20(11):1127–33. doi: 10.1038/ejhg.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayres LC, Allyse M, Norton ME, Cho MK. Cell-free fetal DNA testing: A pilot study of obstetric healthcare provider attitudes toward clinical implementation. Prenat Diagn. 2011;31(11):1070–6. doi: 10.1002/pd.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ubel PA, Smith DM, Zikmund-Fisher BJ, Derry HA, McClure J, Stark A, et al. Testing whether decision aids introduce cognitive biases: Results of a randomized trial. Patient Educ Couns. 2010;80(2):158–63. doi: 10.1016/j.pec.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrimond R, Kelly E. Public viewpoints on new non-invasive prenatal genetic tests. Public Understanding of Science. 2013;22(6):730–44. doi: 10.1177/0963662511424359. [DOI] [PubMed] [Google Scholar]
- 19.Kelly SE, Farrimond HR. Non-invasive prenatal genetic testing: A study of public attitudes. Public Health Genomics. 2012;15(2):73–81. doi: 10.1159/000331254. [DOI] [PubMed] [Google Scholar]
- 20.Wald NJ, Bestwick JP. Incorporating DNA sequencing into current prenatal screening practice for Down’s syndrome. PLoS One. 2013;8(3):e58732. doi: 10.1371/journal.pone.0058732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose NC, Lagrave D, Hafen B, Jackson M. The impact of utilization of early aneuploidy screening on amniocenteses available for training in obstetrics and fetal medicine. Prenat Diagn. 2013;33(3):242–4. doi: 10.1002/pd.4052. [DOI] [PubMed] [Google Scholar]
- 22.Horsting JM, Dlouhy SR, Hanson K, Quaid K, Bai S, Hines KA. Genetic counselors’ experience with cell-free fetal DNA testing as a prenatal screening option for aneuploidy. J Genet Couns. 2013 Dec 19; doi: 10.1007/s10897-013-9673-4. [DOI] [PubMed] [Google Scholar]
- 23.Musci TJ, Fairbrother G, Batey A, Bruursema J, Struble C, Song K. Non-invasive prenatal testing with cell-free DNA: US physician attitudes toward implementation in clinical practice. Prenat Diagn. 2013;33(5):424–428. doi: 10.1002/pd.4091. [DOI] [PubMed] [Google Scholar]
- 24.Norton ME, Rose NC, Benn P. Noninvasive prenatal testing for fetal aneuploidy: Clinical assessment and a plea for restraint. Obstetrics & Gynecology. 2013;121(4):847–50. doi: 10.1097/AOG.0b013e31828642c6. [DOI] [PubMed] [Google Scholar]
- 25.Chetty S, Garabedian MJ, Norton ME. Uptake of noninvasive prenatal testing (NIPT) in women following positive aneuploidy screening. Prenat Diagn. 2013;33(6):542–6. doi: 10.1002/pd.4125. [DOI] [PubMed] [Google Scholar]
- 26.Markens S, Browner C, Press N. ‘Because of the risks’: How US pregnant women account for refusing prenatal screening. Social Science & Medicine. 1999;49:359–69. doi: 10.1016/s0277-9536(99)00097-0. [DOI] [PubMed] [Google Scholar]
- 27.García E, Timmermans DR, van Leeuwen E. The impact of ethical beliefs on decisions about prenatal screening tests: Searching for justification. Soc Sci Med. 2008;66(3):753–64. doi: 10.1016/j.socscimed.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Dahl K, Hvidman L, Jørgensen FS, Kesmodel US. Knowledge of prenatal screening and psychological management of test decisions. Ultrasound Obstet Gynecol. 2011;38(2):152–7. doi: 10.1002/uog.8856. [DOI] [PubMed] [Google Scholar]
- 29.Favre R, Moutel G, Duchange N, Vayssière C, Kohler M, Bouffet N, et al. What about informed consent in first-trimester ultrasound screening for Down syndrome? Fetal Diagn Ther. 2008;23(3):173–84. doi: 10.1159/000116738. [DOI] [PubMed] [Google Scholar]
- 30.Ormond KE, Banuvar S, Daly A, Iris M, Minogue J, Elias S. Information preferences of high literacy pregnant women regarding informed consent models for genetic carrier screening. Patient Educ Couns. 2009;75(2):244–50. doi: 10.1016/j.pec.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Constantine M, Allyse M, Rockwood T, Wall M, De Vries R. Imperfect informed consent for prenatal screening: Lessons from the quad screen. Clinical Ethics (In press) [Google Scholar]
- 32.Schmitz, Netzer, Henn An offer you can’t refuse? Ethical implications of non-invasive prenatal diagnosis. Nature Reviews Genetics. 2009;10:515. doi: 10.1038/nrg2631. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz D, Henn W, Netzer C. Clinical review: Commentary: No risk, no objections? Ethical pitfalls of cell-free fetal DNA and RNA testing. BMJ. 2009;339:b2690. doi: 10.1136/bmj.b2690. [DOI] [PubMed] [Google Scholar]
- 34.Deans Z, Newson AJ. Should non-invasiveness change informed consent procedures for prenatal diagnosis? Health Care Anal. 2011;19(2):122–32. doi: 10.1007/s10728-010-0146-8. [DOI] [PubMed] [Google Scholar]
- 35.van den Heuvel A, Chitty L, Dormandy E, Newson A, Deans Z, Attwood S, et al. Will the introduction of non-invasive prenatal diagnostic testing erode informed choices? An experimental study of health care professionals. Patient Educ Couns. 2010;78(1):24–8. doi: 10.1016/j.pec.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Benn PA, Chapman AR. Ethical challenges in providing noninvasive prenatal diagnosis. Curr Opin Obstet Gynecol. 2010;22(2):128–34. doi: 10.1097/GCO.0b013e3283372352. [DOI] [PubMed] [Google Scholar]
- 37.de Jong A, Dondorp WJ, Frints SG, de Die-Smulders CE, de Wert GM. Advances in prenatal screening: The ethical dimension. Nat Rev Genet. 2011;12(9):657–63. doi: 10.1038/nrg3036. [DOI] [PubMed] [Google Scholar]
- 38.Allyse MA, Sayres LC, Havard M, King JS, Greely HT, Hudgins L, et al. Best ethical practices for clinicians and laboratories in the provision of non-invasive prenatal testing. Prenat Diagn. 2013;33(7):656–61. doi: 10.1002/pd.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flessel M, Goldman S. California prenatal screening program to include noninvasive testing. Acog.org. 2013 http://www.acog.org/About_ACOG/ACOG_Departments/District_Newsletters/District_IX/September_2013/California_Prenatal_Screening_Program.
- 40.Natoli JL, Ackerman DL, McDermott S, Edwards JG. Prenatal diagnosis of down syndrome: A systematic review of termination rates (1995–2011) Prenat Diagn. 2012;32(2):142–53. doi: 10.1002/pd.2910. [DOI] [PubMed] [Google Scholar]
- 41.Skotko BG, Levine SP, Goldstein R. Having a son or daughter with Down syndrome: Perspectives from mothers and fathers. Am J Med Genet A. 2011;155A(10):2335–47. doi: 10.1002/ajmg.a.34293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skotko BG, Levine SP, Goldstein R. Self-perceptions from people with Down syndrome. Am J Med Genet A. 2011;155A(10):2360–9. doi: 10.1002/ajmg.a.34235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheets KB, Crissman BG, Feist CD, Sell SL, Johnson LR, Donahue KC, et al. Practice guidelines for communicating a prenatal or postnatal diagnosis of down syndrome: Recommendations of the national society of genetic counselors. J Genet Couns. 2011;20(5):432–41. doi: 10.1007/s10897-011-9375-8. [DOI] [PubMed] [Google Scholar]
- 44.Kuppermann M, Learman LA, Gates E, Gregorich SE, Nease RF, Jr, Lewis J, Washington AE. Beyond race or ethnicity and socioeconomic status: Predictors of prenatal testing for Down syndrome. Obstetrics & Gynecology. 2006;107(5):1087. doi: 10.1097/01.AOG.0000214953.90248.db. [DOI] [PubMed] [Google Scholar]
- 45.Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, Bittles AH. The changing survival profile of people with Down’s syndrome: Implications for genetic counselling. Clinical Genetics. 2002;62(5):390–3. doi: 10.1034/j.1399-0004.2002.620506.x. [DOI] [PubMed] [Google Scholar]
- 46.Skotko BG, Kishnani PS, Capone GT Down Syndrome Diagnosis Study Group. Prenatal diagnosis of Down syndrome: How best to deliver the news. Am J Med Genet A. 2009;149A(11):2361–7. doi: 10.1002/ajmg.a.33082. [DOI] [PubMed] [Google Scholar]
- 47.Madeo AC, Biesecker BB, Brasington C, Erby LH, Peters KF. The relationship between the genetic counseling profession and the disability community: A commentary. Am J Med Genet A. 2011;155A(8):1777–85. doi: 10.1002/ajmg.a.34054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.