Abstract
Background
Conventional smallpox vaccines based on replicating vaccinia virus (VV) strains (e.g. Lister Elstree, NYCBOH) are associated with a high incidence of myo-/pericarditis, a severe inflammatory cardiac complication. A new smallpox vaccine candidate based on a non-replicating Modified Vaccinia Ankara (MVA) poxvirus has been assessed for cardiac safety in a large placebo-controlled clinical trial.
Methods
Cardiac safety of one and two doses of MVA compared to placebo was assessed in 745 healthy subjects. Vaccinia-naïve subjects received either one dose of MVA and one dose of placebo, two doses of MVA, or two doses of placebo by subcutaneous injection four weeks apart; vaccinia-experienced subjects received a single dose of MVA. Solicited and unsolicited adverse events (AE) and cardiac safety parameters (recorded as Adverse Events of Special Interest, AESI) were monitored after each injection.
Results
A total of 5 possibly related AESI (3 cases of palpitations, 2 of tachycardia) were reported during the study. No case of myo- or pericarditis occurred. One possibly related serious AE (SAE) was reported during the 6-month follow-up period (sarcoidosis). The most frequently observed AEs were injection site reactions.
Conclusions
Vaccination with MVA was safe and well tolerated and did not increase the risk for development of myo-/pericarditis.
Trial Registration
ClinicalTrials.gov NCT00316524
Introduction
Due to the eradication of smallpox in 1980 and discontinuation of smallpox vaccinations worldwide, an increasing majority of the global population has no existing immunity to Variola virus (VARV). As the release of this highly contagious virus would have devastating effects, the potential use of VARV as an agent in bioterrorism or bio-warfare has raised significant concerns. Thus, many governments recognize the need for a safe and efficacious smallpox vaccine to secure public health interests.
MVA is a highly attenuated strain of VV that fails to replicate in human cells [1] and therefore cannot be transmitted or cause disseminated VV infection, which is an important safety concern of conventional smallpox vaccines[2–4]. Conventional vaccines are also associated with severe adverse events, including myo-/pericarditis or even death [3,5,6]. During the US National Smallpox Pre-Event Vaccination Programs initiated in 2002–2004, an unexpected high incidence of myo-/pericarditis was detected in close temporal relationship to Dryvax administration (> 1:10,000), which was notably higher than the expected rate in non-vaccinated US military personnel (0.21:10,000) [7–13]. In addition to clinical symptoms indicative of a cardiac event, criteria for diagnosis of suspected myo-/pericarditis included elevated serum levels of cardiac enzymes (CK-MB, troponin), usually in the presence of ST-segment elevation on ECG, and wall motion abnormalities on echocardiogram[14]. The vast majority of cases occurred within the first 14 days post-vaccination. Most subjects presented initially with chest pain or prodromal clinical symptoms including fever, chills, myalgia, arthralgia, and headache[15–17].
Moreover, a remarkably high number of suspected and probable myo-/pericarditis cases were reported in the pivotal clinical program of the second generation smallpox vaccine ACAM2000 [18]. In contrast to the national vaccination programs where cases were identified when symptomatic vaccinees presented for medical evaluation, the ACAM2000 trials included active post-vaccination cardiac monitoring, leading to detection of 8 myo-/pericarditis cases in 1,162 primary vaccinees after vaccination with either ACAM2000 or Dryvax (incidence of 1:145). Most cases were symptomatic (i.e. subjects presented with chest pain, reduced tolerance to exercise, dyspnea or palpitations) and diagnosis was confirmed by coexisting ECG changes and/or echocardiogram, findings indicative of myocardial inflammation.
Based on this experience, a causal association between conventional smallpox vaccines and myo-/pericarditis has been postulated, although the pathomechanism for cardiotoxicity remains unclear[13]. This has led to the inclusion of close cardiac monitoring in clinical development programs for poxvirus based smallpox vaccines like MVA.
BN has performed a large placebo-controlled Phase II study to determine immunogenicity and safety of MVA with special focus on cardiac safety. Monitoring of ECG changes, new onset of cardiac symptoms and increase of cardiac enzymes was performed to assess the potential risk of developing myo-/pericarditis after vaccination with MVA.
Material and Methods
The protocol for this study and supporting CONSORT checklist are available as supporting information; see S1 CONSORT Checklist and S1 Protocol.
Vaccine
The MVA strain was derived from the MVA vaccine licensed in Germany by additional passages and serial dilutions in chicken embryo fibroblast (CEF) cells[5,6]. MVA smallpox vaccine was produced by IDT Biologika GmbH (Dessau-Roßlau, Germany) according to GMP and provided by Bavarian Nordic A/S (Kvistgaard, Denmark) in aliquots of 0.5 ml liquid-frozen vaccine containing at least 1 x 108 TCID50 (Tissue Culture Infectious Dose 50) /ml.
Participants and Study Procedures
The study was performed at a Clinical Research Organization in compliance with current standards of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use—Good Clinical Practice (ICH-GCP), the Declaration of Helsinki and local legal and regulatory requirements. A total of 1,322 volunteers between 18 and 55 years of age were screened (Table 1). Healthy subjects with normal baseline ECG, cardiac troponin I within normal limits and no active or history of cardiac disease were eligible for inclusion in the study.
Table 1. Demographics.
| Group 1 (MM) vaccinia-naïve (N = 183) | Group 2 (MP) vaccinia-naïve (N = 181) | Group 3 (PP) vaccinia-naïve (N = 181) | Group 4 (M-) vaccinia-exp. (N = 200) | ||
|---|---|---|---|---|---|
| Age [years] | Mean | 25.3 | 25.4 | 26.0 | 41.5 |
| SD | 5.0 | 4.4 | 5.1 | 7.6 | |
| Median | 24 | 25 | 25 | 42 | |
| Min | 18 | 18 | 18 | 22 | |
| Max | 50 | 44 | 50 | 55 | |
| BMI [kg/m2] | Mean | 23.6 | 23.7 | 23.9 | 24.6 |
| SD | 3.86 | 3.41 | 4.31 | 3.93 | |
| Median | 23.0 | 23.2 | 23.1 | 23.9 | |
| Min | 17.5 | 17.4 | 16.2 | 17.6 | |
| Max | 46.7 | 35.8 | 46.5 | 40.7 | |
| Gender (n/%) | Male | 86 (47.0) | 69 (38.1) | 74 (40.9) | 85 (42.5) |
| Female | 97 (53.0) | 112 (61.9) | 107 (59.1) | 115 (57.5) | |
| Ethnic group (n/%) | Caucasian | 178 (97.3) | 176 (97.2) | 177 (97.8) | 198 (99.0) |
| Asian | 1 (0.5) | 2 (1.1) | 1 (0.6) | 0 (0.0) | |
| Black | 0 (0.0) | 1 (0.6) | 0 (0.0) | 1 (0.5) | |
| Arabic | 2 (1.1) | 1 (0.6) | 1 (0.6) | 0 (0.0) | |
| Other | 2 (1.1) | 1 (0.6) | 2 (1.1) | 1 (0.5) | |
MM = two vaccinations with MVA; MP = first vaccination with MVA, second vaccination with placebo; PP = two vaccinations with placebo; M- = single vaccination with MVA; BMI = body mass index; N = Number of subjects in each group; n = Number of subjects with data available; % = Percentage based on N; SD = Standard deviation
Subjects with coronary heart disease, myocardial infarction, angina, congestive heart failure, cardiomyopathy, stroke or transient ischemic attack, uncontrolled high blood pressure, any other medically treated heart condition, a family history of death due to ischemic heart disease before age 50 and/or a ≥ 10 percent risk for myocardial infarction or coronary death within the next 10 years were excluded. Exclusionary ECG findings included any kind of atrioventricular or intraventricular conditions or blocks such as complete left or right bundle branch block, AV node block, QTc or PR prolongation; premature atrial contractions or other atrial arrhythmia, sustained ventricular arrhythmia, two premature ventricular contractions (PVC) in a row; ST elevation consistent with ischemia.
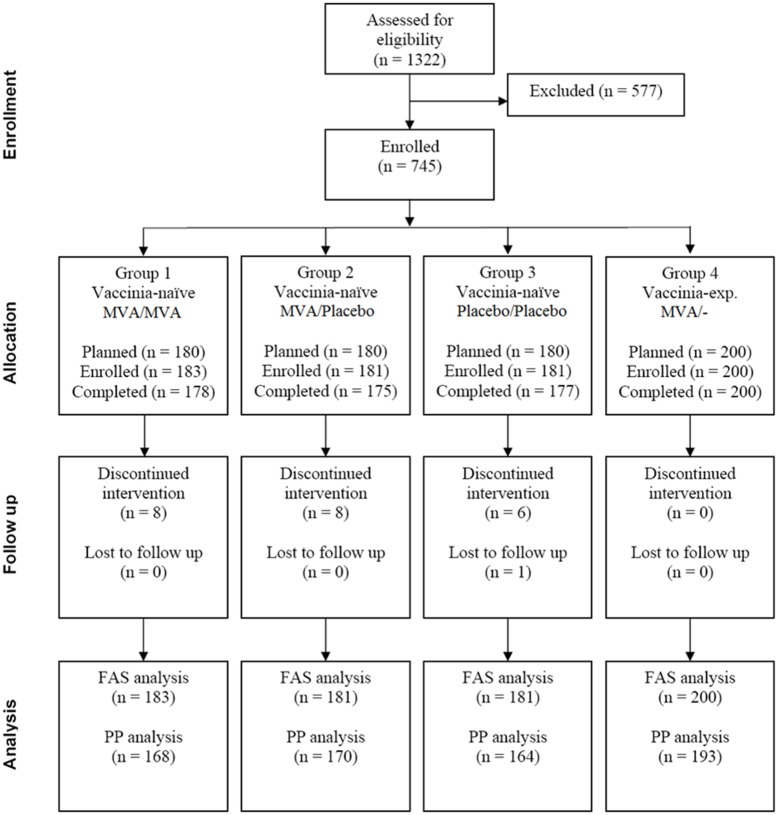
The study was approved by the ethics committee of the Ludwig-Maximilians-Universitaet, Munich, Germany, under the approval number 365–05. Written informed consent was obtained from all subjects. Seven hundred and forty-five (745) subjects were assigned to four groups (Fig 1): Subjects with no history of previous smallpox vaccination (vaccinia-naïve) were randomly assigned to Groups 1 to 3 to receive (under double-blind conditions, including subject handling, trial personnel and data analysis) either two subcutaneous (s.c.) injections of MVA, one dose of MVA and one dose of placebo or two doses of placebo, respectively. Group 4 (open-label) consisted of subjects with a history of previous smallpox vaccination (vaccinia-experienced) that received a single vaccination with MVA. Enrolment started in April 2006, the last follow-up visit was performed in August 2007.
Fig 1. Consolidated Standards of Reporting Trials (CONSORT) subject flow diagram demonstrating the number of patients recruited into the study, number of subjects randomized and vaccinated, and number of subjects analyzed.
MVA: Modified Vaccinia Ankara; FAS: full analysis set: PP: per protocol
Safety Assessments
Safety laboratory tests, including troponin I and ECGs were performed at the screening visit and 10–15 days after each vaccination, and 28 to 35 days after the second (Groups 1–3) or single vaccination (Group 4), as well as at the 6-month follow-up visit, if clinically indicated. All subjects were monitored for ECG changes and cardiac symptoms, such as chest pain, shortness of breath and palpitations, by a physical examination at all trial visits including heart and lung auscultation specifically looking for heart failure, presence of rubs, gallops, murmurs, crackles, and rales.
ECGs were evaluated by the investigator and transmitted electronically to a central database. Subjects with abnormal ECG findings were referred to a cardiologist, which included a thorough examination and performance of an echocardiogram and/or treadmill ECG.
Any cardiac symptom, clinically significant ECG changes or elevated cardiac enzymes (specifically troponin I above upper limit of normal [ULN]) were recorded as an adverse event of special interest (AESI) and followed-up thoroughly to identify or rule-out any cardiac abnormalities. The case definitions as published by the US Center of Disease Control (CDC) [14] were applied to recognize possible cases of acute myocarditis and pericarditis and to distinguish unspecific and isolated ECG changes without or with unclear clinical meaning and ECG changes related to possible or probable cases of acute myocarditis and/or pericarditis.
Unsolicited AE were recorded by the investigator at every study visit by asking the subject for any change in health status since the last visit. Solicited local and general AE (injection site erythema, swelling and induration as well as increased body temperature, headache, myalgia, nausea and fatigue) and any additional symptoms were recorded by the subjects on a diary on the day of vaccination and for the following 7 days, or longer, if lasting beyond the 7-day period. AEs not resolved at the last visit were followed-up and any potentially new SAE and AESI were recorded at the follow-up visit.
Statistical Methods
ECGs were evaluated by a centralized ECG laboratory. Standard ECG results like PQ, QRS, QT and QTc durations and formations as well as heart rate were summarized per visit and group.
Occurrence, relationship and intensity of ECG changes and any other cardiac symptom at any time during the study were assessed and compared between groups by means of the exact test of Fisher. The comparison of the four treatment groups with regard to ECG changes and cardiac symptoms was the primary safety objective of this trial.
Detailed QTc analysis was performed according to FDA/EMA guidance [19]. PR, QT and QRS durations as well as the reported QTc duration based on the Bazett correction, and heart rate were tabulated per group and visit.
AESI were defined as any cardiac symptom, clinically significant ECG changes or cardiac enzymes elevated above the ULN, and were separately listed and tabulated; incidence was compared between groups taking Group 3 (placebo) as the reference group by means of the exact test of Fisher. Here the following commonly used classification of significance was used to display the results: NS = Not Significant (i.e. p≥0.05); * p< 0.05; ** p<0.01; *** p < 0.001).
Unsolicited AE were coded using the MedDRA coding terminology and graded based on intensity. The number of AE and number of subjects with at least one AE for each preferred term were descriptively compared between groups. The occurrence of any Grade 3 or higher AE possibly, probably or definitely related to the study vaccine within 28 days after vaccination was compared between groups.
Occurrence and intensity of solicited general AE within one week after each vaccination was summarized per patient and vaccination. Occurrence and intensity of solicited local AE were documented and graded according to maximum severity. The maximum intensity over the 7-day period after vaccination was used, categorized as Grades 1 to 4 and compared between groups groups using Group 3 as the reference group by means of Fisher’s exact test.
SAE were listed separately and individually described. The number of subjects with at least one SAE was compared between groups using Group 3 as the reference group by means of Fisher’s exact test.
Results
Participants and Demographic Data
Data of 745 randomized subjects who were administered at least one vaccination (564 with MVA and 181 with placebo) were included in the analysis (Fig 1). Of the vaccinia-naïve subjects, 180 subjects (Group 1) received two doses of MVA and three subjects from Group 1 as well as 181 subjects from Group 2 received a single dose of MVA. 174 subjects in Group 2 received placebo with the second injection. 200 vaccinia-experienced subjects received a single dose of MVA (Group 4).
There were slightly more female than male subjects in all groups (Table 1). The vaccinia-naïve population was younger than the vaccinia-experienced population. The median body mass index (BMI) between groups was similar.
Cardiac Monitoring
To ensure that subjects did not have any cardiac abnormalities prior to enrollment, all subjects received an ECG assessment at screening. All ECGs were assessed by the investigator and a central ECG laboratory, and any abnormal or unclear findings were further assessed by a cardiologist. Of the 745 enrolled subjects, 324 (43.5%) had initial ECGs which led to a more thorough cardiac work-up. The ECGs from all enrolled subjects were ultimately assessed to be normal, of these 358 (48.1%) normal ECGs and 386 (51.6%) had ECGs with normal variations such as sinus arrhythmia.
A total of 11 AESI were reported for 10 subjects during the active phase (successful screening until 28 days after last vaccination) of the study. The reported events were palpitations, tachycardia and sinus tachycardia, of which 5 events in 4 subjects were considered as possibly related to the study vaccine (Tables 2 and 3). A further five AESI were reported during follow-up (later than 28 days after last vaccination until end of trial) in five subjects consisting of palpitations, tachycardia and mild pericardial effusion, all assessed as unlikely related.
Table 2. Overview of Unsolicited Adverse Events per Subject.
| Subject based | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| (MM) | (MP) | (PP) | (M/-) | |
| vaccinia-naïve | vaccinia-naïve | vaccinia-naïve | vaccinia-exp. | |
| (N = 183) | (N = 181) | (N = 181) | (N = 200) | |
| SAE | 3 (1.6) NS | 2 (1.1) NS | 5 (2.8) | 1 (0.5) NS |
| Possibly related SAE* | 1 (0.5) NS | 0 (0.0) NS | 0 (0.0) | 0 (0.0) NS |
| AESI | 3 (1.6) NS | 4 (2.2) NS | 1 (0.6) | 8 (4.0) * |
| Possibly related AESI | 0 (0.0) NS | 2 (1.1) NS | 0 (0.0) | 2 (1.0) NS |
| At least one unsolicited AE | 113 (61.7) *** | 107 (59.1) ** | 78 (43.1) | 99 (49.5) NS |
| Related unsolicited AE | 61 (33.3) *** | 61 (33.7) *** | 23 (12.7) | 74 (37.0) *** |
| Related unsolicited AE ≥ Grade 3 | 4 (2.2) NS | 8 (4.4) NS | 4 (2.2) | 1 (0.5) NS |
| AE leading to withdrawal from study | 0 (0.0) NS | 1 (0.6) NS | 1 (0.6) | 0 (0.0) NS |
AE = adverse event (up to 28 days after the first vaccination); SAE = serious adverse event (up to 6 months after the last vaccination); AESI = adverse event of special interest (cardiac event; up to 6 months after the last vaccination); * reported during the 6-month follow-up period. Fishers Exact test of comparison to Group 3; NS = Not Significant (p≥0.05);
* p< 0.05;
** p<0.01;
*** p < 0.001).
Table 3. Cardiac Adverse Events (AESI).
| Subject # | AESI (Diagnosis) | Relationship to vaccine | Onset of symptoms (post last vaccination) | Duration | Other conditions | Cardiac work-up/safety lab | Outcome |
|---|---|---|---|---|---|---|---|
| Group 1 (MM) vaccinia-naïve (N = 183) | |||||||
| 287 | Sinus tachycardia (102 bpm) | Unlikely | 14 days | Minutes | Thyroxin treatment for hypothyreosis | ECG; TSH, T3, T4 (normal) | Recovered |
| 317 | Tachycardia (116 bpm) | Unlikely | 31 days | 4 months | Psychological stress | TSH, T3, T4 (normal) | Recovered |
| 557 | Palpitations | Unlikely | 2 days | 5 seconds | Past history of palpitations during exercise; treatment for hypothyreosis | - | Recovered |
| Group 2 (MP) vaccinia-naïve (N = 181) | |||||||
| 220 | Palpitations | Possibly | 15 hours | 2.5 hours | Treatment for allergic rhinitis | - | Recovered |
| 228 | Sinus tachycardia (111 bpm) | Unlikely | 13 days | < 1 day | Pre-existing HR 98 bpm | Echocardiogram, ECG and TSH/T3/T4 (normal) | Normal Recovered |
| 563 | Tachycardia (105 bpm) | Possibly | 28 days | 2 days | Nervousness, pre-existing HR 81 bpm | - | Recovered |
| 710 | Palpitations (FU) | Unlikely | 193 days | intermittent | Personal distress | - | Ongoing |
| Group 3 (PP) vaccinia-naïve (N = 181) | |||||||
| 689 | Palpitations | Unlikely | 23 days | intermittent | Recurrent pain in shoulder | - | Ongoing |
| Group 4 (M-) vaccinia-exp. (N = 200) | |||||||
| 024 | Palpitations (FU) | Unlikely | >90 days | intermittent | Anxiety, depression | - | Recovered |
| 028 | Palpitations | Possibly | 3 hours | 1 hour | Sweating | - | Recovered |
| Palpitations | Possibly | 3 days | ~ 5 hours | Sweating | ECG, cardiac enzymes (normal) | Recovered | |
| Palpitations (FU) | Unlikely | 92 days | 1,5 hours | Common cold, sweating | Echocardiogram, ECG (normal) | Recovered | |
| 246 | Tachycardia (105 bpm) (FU) | Unlikely | 182 days | 13 days | Nervousness | Echocardiogram, ergometry, TSH, T3, T4 (normal) | Recovered |
| 397 | Tachycardia (bpm 120) | Possibly | ~ 4 hours | 3 days | Nervousness | TSH, T3, T4 (normal) | Recovered |
| 411 | Palpitations | Unlikely | 34 hours | 10 minutes | Hypotension, post-traumatic pain right shoulder | ECG (normal) | |
| Mild pericardial effusion (FU) | Unlikely | 196 | ongoing | Echocardiogram (abnormal); ECG, ergometry (normal) | Abnormal, but clinically not significant Ongoing | ||
Bpm = beats per minute (heart rate); HR = heart rate; Adverse events of special interest (AESI) that were reported during the half-year follow-up phase are marked with “FU”
Subjects showing any ECG abnormalities at any of the post-vaccination visits underwent a thorough cardiac work-up by a cardiologist. Nearly one third of all subjects received echocardiograms and/or treadmill ECGs during the study and all examinations completely ruled out any cardiac abnormalities. Table 4 summarizes all ECG abnormalities observed by group and by visit. Approxinately 20–30% of subjects in each group with normal ECGs at baseline had ECG changes at visit 2 consistent with normal variation and approximately 20–30% of subjects with ECGs demonstrating normal variation at baseline had normal ECGs at visit 2 (Table 4). Similar percentages of subjects with ECG changes were observed at Visit 4, whereby nearly half had reverted back to the original screening assessment.
Table 4. Summary of Electrocardiogram Abnormalities (Safety Analysis Set, N = 745).
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| n (%) N = 183 | n (%) N = 181 | n (%) N = 181 | n (%) N = 200 | |
| Screening | ||||
| Assessment missing | 0 | 0 | 0 | 0 |
| Normal | 87 (47.5%) NS | 78 (43.1%) NS | 76 (42.0%) | 118 (59.0%) ** |
| Abnormal, CS | 0 | 0 | 0 | 0 |
| Abnormal, NCS | 96 (52.5%) | 103 (56.9%) | 105 (58.0%) | 82 (41.0%) |
| AV block (PQ time > 0.20 sec)—first degree | 0 | 0 | 1 (0.6%) | 1 (0.5%) |
| Right bundle branch block—incomplete | 10 (5.5%) | 18 (9.9%) | 4 (2.2%) | 1 (0.5%) |
| ST elevation | 5 (2.7%) | 1 (0.6%) | 4 (2.2%) | 1 (0.5%) |
| ST depression | 0 | 1 (0.6%) | 0 | 1 (0.5%) |
| T inversion—pathological | 42 (23.0%) | 54 (29.8%) | 50 (27.6%) | 48 (24.0%) |
| T inversion—other | 14 (7.7%) | 19 (10.5%) | 22 (12.2%) | 16 (8.0%) |
| Low voltage | 0 | 0 | 0 | 1 (0.5%) |
| Other* | 23 (12.6%) | 23 (12.7%) | 26 (14.4%) | 5 (2.5%) |
| Bradycardia | 30 (16.4%) | 28 (15.5%) | 30 (16.6%) | 6 (3.0%) |
| Arrhythmia | 4 (2.2%) | 3 (1.7%) | 8 (4.4%) | 1 (0.5%) |
| Repolarisation | 3 (1.6%) | 3 (1.7%) | 3 (1.7%) | 0 |
| Q-Abnormalities | 1 (0.5%) | 1 (0.6%) | 0 | 4 (2.0%) |
| Tall T-waves | 0 | 0 | 0 | 0 |
| Two weeks post vaccination 1 | ||||
| Assessment missing | 1 (0.5%) | 2 (1.1%) | 2 (1.1%) | 0 |
| Normal | 91 (49.7%) NS | 81 (44.8%) NS | 84 (46.4%) | 129 (64.5%) *** |
| Abnormal, CS | 0 | 0 | 0 | 0 |
| Abnormal, NCS | 91 (49.7%) | 98 (54.1%) | 95 (52.4%) | 71 (35.5%) |
| Supraventricular arrhythmia | 0 | 1 (0.6%) | 0 | 0 |
| Ventricular arrhythmia | 0 | 1 (0.6%) | 1 (0.6%) | 0 |
| AV block (PQ time > 0.20 sec)—first degree | 0 | 0 | 1 (0.6%) | 1 (0.5%) |
| Right bundle branch block—incomplete | 9 (4.9%) | 17 ((9.4%) | 15 (8.3%) | 15 (7.5%) |
| ST elevation | 3 (1.6%) | 1 (0.6%) | 2 (1.1%) | 0 |
| ST depression | 0 | 1 (0.6%) | 1 (0.6%) | 1 (0.5%) |
| T inversion—pathological | 34 (18.6%) | 46 (25.4%) | 40 (22.1%) | 31 (15.5%) |
| T inversion—other | 11 (6.0%) | 9 (5.0%) | 10 (5.5%) | 7 (3.5%) |
| Other* | 34 (18.6%) | 29 (16.0%) | 34 (18.8%) | 14 (7.0%) |
| Bradycardia | 31 (16.9%) | 25 (13.8%) | 33 (18.2) | 6 (3.0%) |
| Arrhythmia | 4 (2.2%) | 4 (2.2%) | 2 (1.1%) | 5 (2.5%) |
| Repolarisation | 1 (0.5%) | 3 (1.7%) | 2 (1.1%) | 1 (0.5%) |
| Q-Abnormalities | 0 | 3 (1.7%) | 1 (0.6%) | 4 (2.0%) |
| Tall T-waves | 1 (0.5%) | 3 (1.7%) | 0 | 0 |
| Two weeks post vaccination 2 | ||||
| Assessment missing | 7 (3.8%) | 7 (3.9%) | 6 (3.3%) | NA |
| Normal | 89 (48.6%) NS | 82 (45.3%) NS | 82 (45.3%) | |
| Abnormal, CS | 0 | 0 | 0 | |
| Abnormal, NCS | 87 (47.5%) | 92 (50.1%) | 93 (51.4%) | |
| Supraventricular arrhythmia | 1 (0.5%) | 1 (0.6%) | 0 | |
| AV block (PQ time > 0.20 sec)—first degree | 2 (1.1%) | 0 | 1 (0.6%) | |
| Right bundle branch block—incomplete | 10 (5.5%) | 20 (11.0%) | 12 (6.6%) | |
| ST elevation | 1 (0.5%) | 3 (1.7%) | 1 (0.6%) | |
| ST depression | 1 (0.5%) | 0 | 0 | |
| T inversion—pathological | 28 (15.3%) | 37 (20.4%) | 39 (21.5%) | |
| T inversion—other | 3 (1.6%) | 5 (2.8%) | 4 (2.2%) | |
| Other* | 35 (19.1%) | 35 (19.3%) | 33 (18.2%) | |
| Bradycardia | 30 (16.4%) | 23 (12.7%) | 28 (15.5%) | |
| Arrhythmia | 4 (2.2%) | 4 (2.2%) | 2 (1.1%) | |
| Repolarisation | 2 (1.1%) | 3 (1.7%) | 7 (3.9%) | |
| Q-Abnormalities | 1 (0.6%) | 0 | 1 (0.6%) | |
| Tall T-waves | 1 (0.6%) | 1 (0.6%) | 2 (1.1%) | |
N = number of subjects in each group, % = percentage based on N. NA = not applicable; AV = atrioventricular, CS = clinically significant, NCS = not clinically significant, PQ = PQ interval in electrocardiogram, QTc = QT interval corrected for hart rate, ST = ST segment in electrocardiogram,
*Other: non-specific ECG findings not consistent with one of the terms specified in this table
This table includes ECGs obtained in routine study visits only, but no ECGs obtained in potential further workups.
Fishers Exact test of comparison to Group 3; NS = Not Significant (p≥0.05);
* p< 0.05;
** p<0.01;
*** p < 0.001).
Despite the unexpectedly large number of subjects with ECG findings which triggered further work-up, all ECG recordings were ultimately assessed by the investigator and/or the cardiologist to be normal or represent normal variations. No significant QTc prolongations nor changes in PR, QRS and/or RR intervals were considered related to vaccination. Differences in the rates of normal ECGs between the active treatment groups versus placebo were not significant for groups 1 and 2, and showed more normal ECGs than in the placebo group for group 4 (Vaccinia experienced subjects). There were no differences between groups including placebo with regard to ECG changes and cardiac symptoms and no cases of myo- or pericarditis were reported.
Solicited and unsolicited Adverse Events
A total of 718 AEs were reported during the active study phase. The majority of unsolicited AEs were mild or moderate (Grades 1 and 2); of the 18 cases documented as severe (Grade 3), only chills and injection site urticaria were assessed as related to study vaccine. Furthermore, there were no vaccine-related clinically significant laboratory abnormalities.
During the active phase of the study, three SAEs were reported which were assessed as unrelated (ruptured finger tendon) or unlikely related (nervous breakdown, transient motoric hemiparesis) to the study vaccine. The case of transient motoric hemiparesis occurred in a 38-year old female subject 6 days after the first vaccination and was judged by the investigator to be unlikely related to study vaccine but rather related to a previous history of migraine accompagnée,
During the 6-month follow up, nine SAEs were reported, thereof for one (sarcoidosis, diagnosed by bronchoscopy and biopsy), a causal relationship could not be ruled out. Ten weeks following the second vaccination with MVA, a 30 year old, otherwise healthy male subject contracted a urinary tract infection (Chlamydia trachomatis), which was treated with antibiotics. Concomitant arthralgia and fever continued, leading to a chest x-ray, bronchoscopy and finally the diagnosis of sarcoidosis. The responsible Data Safety Monitoring Board members assessed the event to be unlikely related to vaccination and expressed no concerns regarding continuation of the MVA development program. Two further SAEs, colon carcinoma and depression, were judged to be unlikely related to vaccination, while the remaining were considered to be not related.
Solicited general as well as local symptoms were generally moderate in severity and none were considered life-threatening or disabling (Grade 4). Table 5 shows related AEs with a frequency of ≥2% in at least one study group. Most AEs belong to the System Organ Class (SOC) “General disorders and administration site conditions”. As expected, a clear difference between placebo and MVA was observed: 98.9%, 97.8% and 97.0% of subjects in Groups 1, 2 and 4 had at least one vaccine-related solicited or unsolicited AE, respectively, compared to 56.9% receiving placebo. Local injection site reactions such as pain, erythema, induration, swelling, pruritus and warmth presented with a significantly lower frequency in the placebo group and indicate a direct relationship to vaccination with MVA compared to placebo. Nevertheless, pain and erythema did still occur in 20.4% and 21.5%, respectively, of subjects receiving placebo.
Table 5. Related Adverse Events with a frequency of ≥2% in at least one study group; MedDRA Coding by System Organ Class and Preferred Term (Safety dataset).
| N = Number of subjects in the specified group | |||||
|---|---|---|---|---|---|
| SOC | Preferred Term (PT) | Group 1 (N = 183) | Group 2 (N = 181) | Group 3 (N = 181) | Group 4 (N = 200) |
| Number of subjects (%)—Number of events | |||||
| General disorders and administration site conditions | |||||
| Any PT | 181 (98.9) 1392 *** | 177 (97.8) 824 *** | 103 (56.9) 252 | 194 (97.0) 894 *** | |
| Injection site pain | 166 (90.7) 300 | 155 (85.6) 168 | 37 (20.4) 46 | 167 (83.5) 167 | |
| Injection site erythema | 166 (90.7) 304 | 146 (80.7) 160 | 39 (21.5) 51 | 169 (84.5) 169 | |
| Injection site induration | 162 (88.5) 273 | 146 (80.7) 155 | 5 (2.8) 6 | 155 (77.5) 155 | |
| Injection site swelling | 149 (81.4) 247 | 103 (56.9) 112 | 10 (5.5) 12 | 149 (74.5) 149 | |
| Fatigue | 57 (31.1) 75 | 42 (23.2) 52 | 45 (24.9) 53 | 71 (35.5) 71 | |
| Headache | 42 (23.0) 49 | 58 (32.0) 66 | 31 (17.1) 36 | 51 (25.5) 51 | |
| Injection site pruritus | 43 (23.5) 61 | 36 (19.9) 37 | 4 (2.2) 4 | 47 (23.5) 47 | |
| Myalgia | 23 (12.6) 24 | 15 (8.3) 15 | 14 (7.7) 18 | 39 (19.5) 39 | |
| Body temperature increased | 16 (8.7) 18 | 19 (10.5) 19 | 10 (5.5) 10 | 10 (5.0) 10 | |
| Nausea | 12 (6.6) 14 | 17 (9.4) 20 | 8 (4.4) 8 | 16 (8.0) 16 | |
| Injection site hematoma | 6 (3.3) 6 | 9 (5.0) 9 | 4 (2.2) 4 | 4 (2.0) 4 | |
| Injection site warmth | 9 (4.9) 9 | 5 (2.8) 5 | 0 (0.0) 0 | 8 (4.0) 8 | |
| Injection site discoloration | 1 (0.5) 1 | 4 (2.2) 4 | 0 (0.0) 0 | 0 (0.0) 0 | |
| Nervous system disorders | |||||
| Any PT | 1 (0.5) 1 | 6 (3.3) 6 | 3 (1.7) 3 | 6 (3.0) 7 | |
| Dizziness | 1 (0.5) 1 | 2 (1.1) 2 | 2 (1.1) 2 | 4 (2.0) 4 | |
| Infections and infestations | |||||
| Any PT | 6 (3.3) 7 | 1 (0.6) 1 | 5 (2.8) 6 | 2 (1.0) 3 | |
| Nasopharyngitis | 5 (2.7) 5 | 0 (0.0) 0 | 4 (2.2) 4 | 1 (0.5) 1 | |
| Gastrointestinal disorders | |||||
| Any PT | 2 (1.1) 3 | 1 (0.6) 1 | 2 (1.1) 3 | 7 (3.5) 7 | |
| Diarrhoea | 0 (0.0) 0 | 1 (0.6) 1 | 1 (0.6) 1 | 4 (2.0) 4 | |
| Respiratory, thoracic and mediastinal disorders | |||||
| Any PT | 2 (1.1) 2 | 2 (1.1) 2 | 2 (1.1) 2 | 5 (2.5) 5 | |
| Pharyngolaryngeal pain | 2 (1.1) 2 | 1 (0.6) 1 | 1 (0.6) 1 | 4 (2.0) 4 | |
| Blood and lymphatic system disorders | |||||
| Any PT | 2 (1.1) 4 | 5 (2.8) 5 | 0 (0.0) 0 | 2 (1.0) 2 | |
| Lymphadenopathy | 2 (1.1) 3 NS | 4 (2.2) 4 NS | 0 (0.0) 0 | 2 (1.0) 2 NS | |
| Vascular disorders | |||||
| Any PT | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 4 (2.0) 4 | |
| Hot flush | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 4 (2.0) 4 | |
Fishers Exact test of comparison to Group 3; NS = Not Significant (p≥0.05);
* p< 0.05;
** p<0.01;
*** p < 0.001).
When comparing Group 1 with Group 3, fatigue, headache, myalgia and nasopharyngitis occurred slightly less frequently in the placebo group (Table 5). Comparing Group 2 with Group 3, fatigue and myalgia were similarly frequent (23.2% vs. 24.9% and 7.7% vs. 8.3%, respectively). The highest incidence of fatigue and myalgia was observed in subjects in Group 4 (35.5% and 19.5%, respectively).
Overall, there were significantly more AEs in the active treatment groups versus placebo. However, the AE profile was consistent with that of other, approved live-attenuated vaccines.
Discussion
The primary safety objective of this study was to compare the four vaccination groups with regard to ECG changes and cardiac symptoms. Despite rigorous cardiac monitoring of all subjects enrolled in this trial, no cardiac safety signals including cardiac enzyme elevations such as troponin I were detected, reported neither as cardiac symptoms nor as abnormal ECG recordings. Not a single case of myo-/pericarditis was reported following vaccination with MVA, in contrast to the high incidence of myo-/pericarditis observed with Dryvax (10.38 events per thousand vaccinations) and ACAM2000 (5.73 events per thousand immunizations)[20].
Screening revealed an unexpectedly high rate of “abnormal” ECGs in this young healthy population. Of the 543 subjects that failed screening, 139 could not be enrolled due to abnormal ECG findings. Furthermore, routine ECGs performed at visits following the vaccinations also resulted in large numbers of falsely abnormal findings. Extensive cardiac work-up did not reveal any true cardiac condition in all cases. Therefore, it seems that normal variations, rather than cardiac abnormalities were actually being measured. Even in young and healthy individuals, ECG screening frequently reveals abnormalities such as right bundle branch block, fascicular block, first-degree AV blocks or non-specific T-wave abnormalities[21]. When performing routine measurements of ECGs in presumably healthy subjects, one therefore has to consider a potentially high level of background noise [22,23]. This questions whether an ECG represents an appropriate criterion to determine subject eligibility for clinical trials, particularly in healthy young populations. In addition, control ECGs performed throughout ongoing studies without accompanying cardiac symptoms or other clinical signs can lead to the necessity of communicating (falsely) abnormal ECG findings to the volunteers, as was the case in this study. This inevitably leads to anxiety as well as time- and resource-consuming cardiac work up for ruling out any true cardiac condition. Particularly in vaccine studies, which generally by default enroll only healthy volunteers, the often confounding and misleading results of routine ECG monitoring seem to outweigh the benefits. Exceptions are of course vaccines known to cause cardiac complications, such as replication-competent smallpox vaccines [15–17]. For the non-replicating MVA vaccine no confirmed cases of severe cardiac events have been reported in the clinical development program[24–31].
Similar to performing ECGs without a clinical indication, however, the value of routine troponin I measurements in the absence of cardiac symptoms has also been questioned. There are reports that many factors can lead to falsely positive troponin values [32–34]. Furthermore, it is known that isolated elevated troponin I does not necessarily correlate with cardiac damage [35]. Therefore, routine troponin I measurements within the context of a clinical trial have to be regarded as a screening parameter only, as any observed abnormality requires a thorough cardiologist workup to confirm a clinically significant cardiac finding.
Secondary safety objectives of this study were comparisons with regard to overall safety of the four vaccination groups and specifically of Groups 1 and 2 with the placebo group (Group 3) in vaccinia-naive subjects. Commonly observed AEs in this study were local symptoms (pain, erythema, induration and swelling) at the injection site and general symptoms, such as fatigue, headache and myalgia. The significantly lower frequency of local symptoms in the placebo group was expected as these AE are known to be associated with injectable vaccines such as MVA. By far the majority of local symptoms were mild or moderate. Only 3.3% or less of the subjects in any group had Grade 3 local reactions and no Grade 4 events were reported.
None of the SAE historically associated with replicating smallpox vaccines, such as Dryvax or ACAM2000 [7,11–13,36], were reported in this study. Thus, the large amount of safety data, including extensive cardiac monitoring results, collected from the 745 subjects who completed this study confirm the excellent safety and tolerability profile of MVA. The results revealed no safety concerns in this healthy study population, particularly with regard to the development of cardiac events following vaccination with MVA. A Phase III clinical trial evaluating the cardiac safety of MVA in 3,000 subjects compared to placebo is currently ongoing and expected to provide further confirmative data in this regard.
Supporting Information
(DOC)
(PDF)
(ZIP)
Acknowledgments
Design and conduct of the study were managed by Bavarian Nordic A/S. Data collection, management and analysis were performed by Harrison Clinical Research Deutschland GmbH. We wish to gratefully acknowledge the study participants that devoted their time and effort to this research endeavour.
Parts of the data have been presented as posters at the following meetings: XVIII Poxvirus Symposium in Arizona, US in June 2010; CBW Protection Symposium in Stockholm, June 2010; Med Biodefense (Bundeswehr) in Munich, November 2011.
Data Availability
There are no restrictions on sharing of data and/or materials. All relevant documentation, including the trial protocol and the final clinical study report (incl. complete tables, figures and listings) are included in Supporting Information files.
Funding Statement
The study was funded by NIAID contract # N01-Al-40072. The study sponsor was Bavarian Nordic. All authors except for those employed by Bavarian Nordic received research grants from Bavarian Nordic to support the conduct of this trial. AK, JV, NU and GV were employees of Bavarian Nordic at the time of the study conduct. NA and PC are employees of Bavarian Nordic, at the time of study conduct and still currently. The funder provided support in the form of salaries for authors AK, JV, NU, GV, NA and PC, but did not have and additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Suter M, Meisinger-Henschel C, Tzatzaris M, Hulsemann V, Lukassen S, Wulff NH, et al. Modified vaccinia Ankara strains with identical coding sequences actually represent complex mixtures of viruses that determine the biological properties of each strain. Vaccine. [DOI] [PubMed] [Google Scholar]
- 2. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968. N Engl J Med 1969;281: 1201–1208. [DOI] [PubMed] [Google Scholar]
- 3. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis 1970;122: 303–309. [DOI] [PubMed] [Google Scholar]
- 4. Mayr A, Munz E. [Changes in the vaccinia virus through continuing passages in chick embryo fibroblast cultures]. Zentralbl Bakteriol [Orig] 1964;195: 24–35. [PubMed] [Google Scholar]
- 5. Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schafer H, Holzner A. [MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author's transl)]. Dtsch Med Wochenschr 1974;99: 2386–2392. [DOI] [PubMed] [Google Scholar]
- 6. Mayr A, Hochstein-Mintzel V, Stickl H. Passage history, properties, and use of attenuated vaccinia virus strain MVA. Infection 1975;3: 6–14. [Google Scholar]
- 7. Eckart RE, Love SS, Atwood JE, Arness MK, Cassimatis DC, Campbell CL, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol 2004;44: 201–205. [DOI] [PubMed] [Google Scholar]
- 8. Morgan J, Roper MH, Sperling L, Schieber RA, Heffelfinger JD, Casey CG, et al. Myocarditis, pericarditis, and dilated cardiomyopathy after smallpox vaccination among civilians in the United States, January-October 2003. Clin Infect Dis 2008;46 Suppl 3: S242–S250. 10.1086/524747 [DOI] [PubMed] [Google Scholar]
- 9. Mora LF, Khan AH, Sperling LS. Cardiac Complications after Smallpox Vaccination. South Med J 2009;102: 615–619 10.1097/SMJ.0b013e31819fe55b [DOI] [PubMed] [Google Scholar]
- 10. Sharma U, Tak T. A report of 2 cases of myopericarditis after Vaccinia virus (smallpox) immunization. WMJ 2011;110: 291–294. [PubMed] [Google Scholar]
- 11. Grabenstein JD, Winkenwerder W Jr. US military smallpox vaccination program experience. JAMA 2003;289: 3278–3282. [DOI] [PubMed] [Google Scholar]
- 12. Eckart RE, Shry EA, Jones SO, Atwood JE, Grabenstein JD. Comparison of clinical presentation of acute myocarditis following smallpox vaccination to acute coronary syndromes in patients <40 years of age. Am J Cardiol 2005;95: 1252–1255. [DOI] [PubMed] [Google Scholar]
- 13. Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol 2004;43: 1503–1510. [DOI] [PubMed] [Google Scholar]
- 14. MMWR. Update: adverse events following civilian smallpox vaccination—United States, 16.05.2003. MMWR Morb Mortal Wkly Rep 2003;52: 444–446. [PubMed] [Google Scholar]
- 15. Arness MK, Eckart RE, Love SS, Atwood JE, Wells TS, Engler RJ, et al. Myopericarditis following smallpox vaccination. Am J Epidemiol 2004;160: 642–651. [DOI] [PubMed] [Google Scholar]
- 16. Halsell JS, Riddle JR, Atwood JE, Gardner P, Shope R, Poland GA, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 2003;289: 3283–3289. [DOI] [PubMed] [Google Scholar]
- 17. Sniadack MM, Neff LJ, Swerdlow DL, Schieber RA, McCauley MM, Mootrey GT. Follow-up of cardiovascular adverse events after smallpox vaccination among civilians in the United States, 2003. Clin Infect Dis 2008;46 Suppl 3: S251–S257. 10.1086/524741 [DOI] [PubMed] [Google Scholar]
- 18.Acambis (2007) ACAM2000 Smallpox Vaccines: Vaccines and Related Biological Products Advisory Committee (VRBPAC). Acambis Briefing Document, April 2007.
- 19.International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed Regist 2005;70: 61134–61135. [PubMed]
- 20.ACAM2000 Prescribing Information. Package Insert. 2011.
- 21. Kobza R, Cuculi F, Abacherli R, Toggweiler S, Suter Y, Frey F, et al. Twelve-lead electrocardiography in the young: physiologic and pathologic abnormalities. Heart Rhythm 2012;9: 2018–2022. 10.1016/j.hrthm.2012.08.034 [DOI] [PubMed] [Google Scholar]
- 22. Hingorani P, Natekar M, Deshmukh S, Karnad DR, Kothari S, Narula D, et al. Morphological abnormalities in baseline ECGs in healthy normal volunteers participating in phase I studies. Indian J Med Res 2012;135: 322–330. [PMC free article] [PubMed] [Google Scholar]
- 23. Hiss RG, Lamb LE. Electrocardiographic findings in 122,043 individuals. Circulation 1962;25: 947–961. [DOI] [PubMed] [Google Scholar]
- 24. Sano J, Chaitman BR, Swindle J, Frey SE. Electrocardiography screening for cardiotoxicity after modified Vaccinia Ankara vaccination. Am J Med 2009;122: 79–84. 10.1016/j.amjmed.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vollmar J, Arndtz N, Eckl KM, Thomsen T, Petzold B, Mateo L, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine 2006;24: 2065–2070. [DOI] [PubMed] [Google Scholar]
- 26. Frey SE, Newman FK, Kennedy JS, Sobek V, Ennis FA, Hill H, et al. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine 2007;25: 8562–8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Krempelhuber A, Vollmar J, Pokorny R, Rapp P, Wulff N, Petzold B, et al. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine 2010;28: 1209–1216. 10.1016/j.vaccine.2009.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennedy JS, Greenberg RN. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines 2009;8: 13–24. 10.1586/14760584.8.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenberg RN, Overton ET, Haas DW, Frank I, Goldman M, von KA, Virgin G, et al. Safety, Immunogenicity, and Surrogate Markers of Clinical Efficacy for Modified Vaccinia Ankara as a Smallpox Vaccine in HIV-Infected Subjects. J Infect Dis 2013;207: 749–758. 10.1093/infdis/jis753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elizaga ML, Vasan S, Marovich MA, Sato AH, Lawrence DN, Chaitman BR, et al. Prospective surveillance for cardiac adverse events in healthy adults receiving modified vaccinia ankara vaccines: a systematic review. PLoS ONE 2013;8: e54407 10.1371/journal.pone.0054407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frey SE, Winokur PL, Salata RA, El-Kamary SS, Turley CB, Walter EB Jr, et al. Safety and immunogenicity of IMVAMUNE((R)) smallpox vaccine using different strategies for a post event scenario. Vaccine 2013;31: 3025–3033. 10.1016/j.vaccine.2013.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neumayr G, Gaenzer H, Pfister R, Sturm W, Schwarzacher SP, Eibl G, et al. Plasma levels of cardiac troponin I after prolonged strenuous endurance exercise. Am J Cardiol 2001;87: 369–71, A10 [DOI] [PubMed] [Google Scholar]
- 33. Eijsvogels T, George K, Shave R, Gaze D, Levine BD, Hopman MT, et al. Effect of prolonged walking on cardiac troponin levels. Am J Cardiol 2010;105: 267–272. 10.1016/j.amjcard.2009.08.679 [DOI] [PubMed] [Google Scholar]
- 34. Nosanchuk JS, Combs B, Abbott G. False increases of troponin I attributable to incomplete separation of serum. Clin Chem 1999;45: 714 [PubMed] [Google Scholar]
- 35. Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, Valdes R Jr. National Academy of Clinical Biochemistry Standards of Laboratory Practice: recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem 1999;45: 1104–1121. [PubMed] [Google Scholar]
- 36. Neff J, Modlin J, Birkhead GS, Poland G, Robertson RM, Sepkowitz K, et al. Monitoring the safety of a smallpox vaccination program in the United States: report of the joint Smallpox Vaccine Safety Working Group of the advisory committee on immunization practices and the Armed Forces Epidemiological Board. Clin Infect Dis 2008;46 Suppl 3: S258–S270. 10.1086/524749 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(ZIP)
Data Availability Statement
There are no restrictions on sharing of data and/or materials. All relevant documentation, including the trial protocol and the final clinical study report (incl. complete tables, figures and listings) are included in Supporting Information files.