Abstract
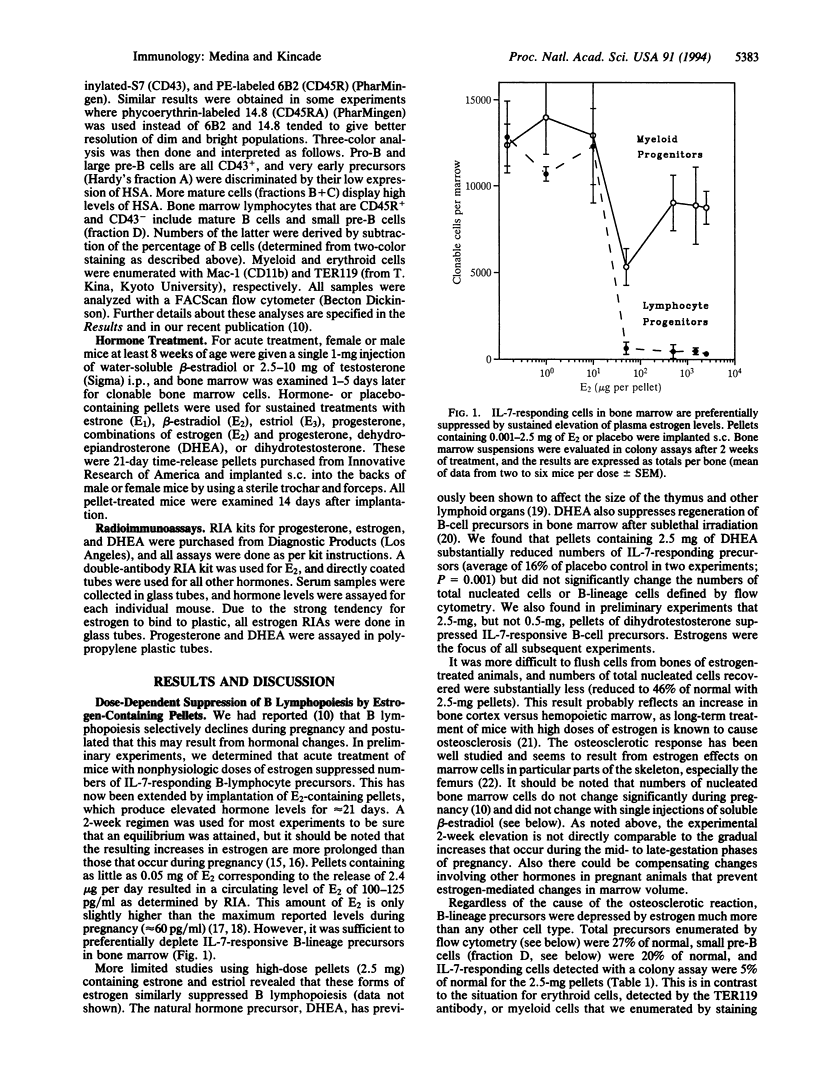
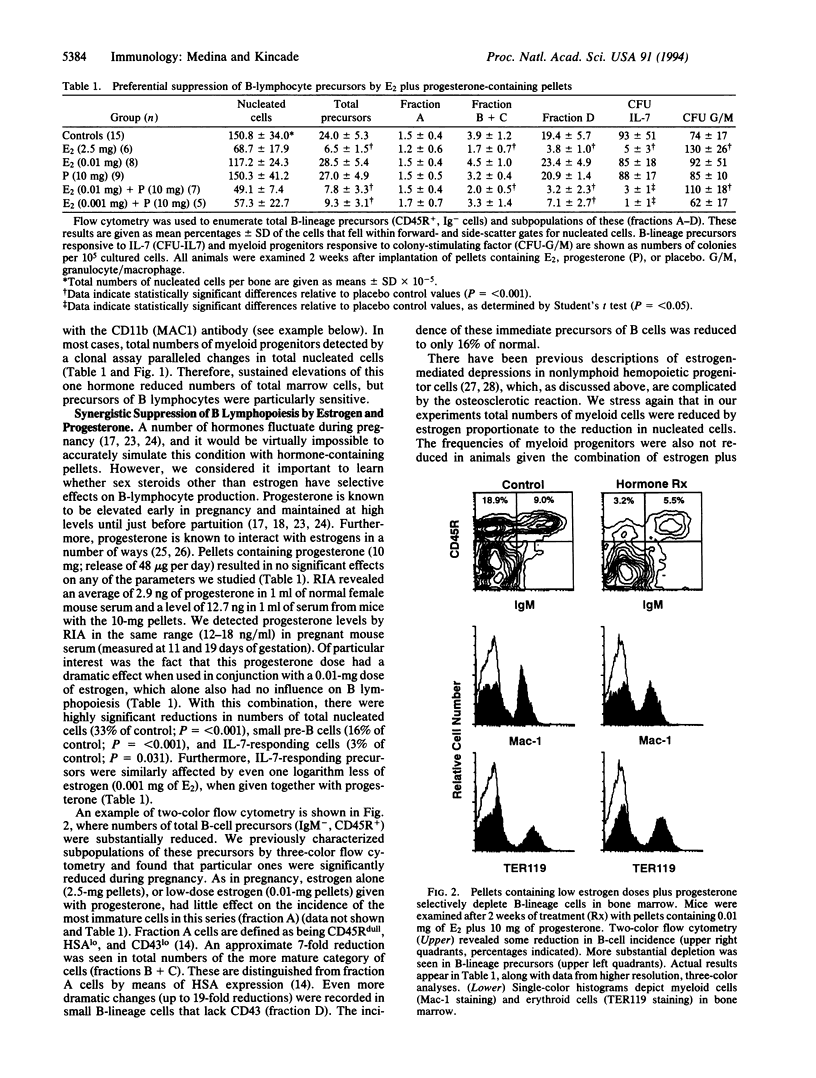
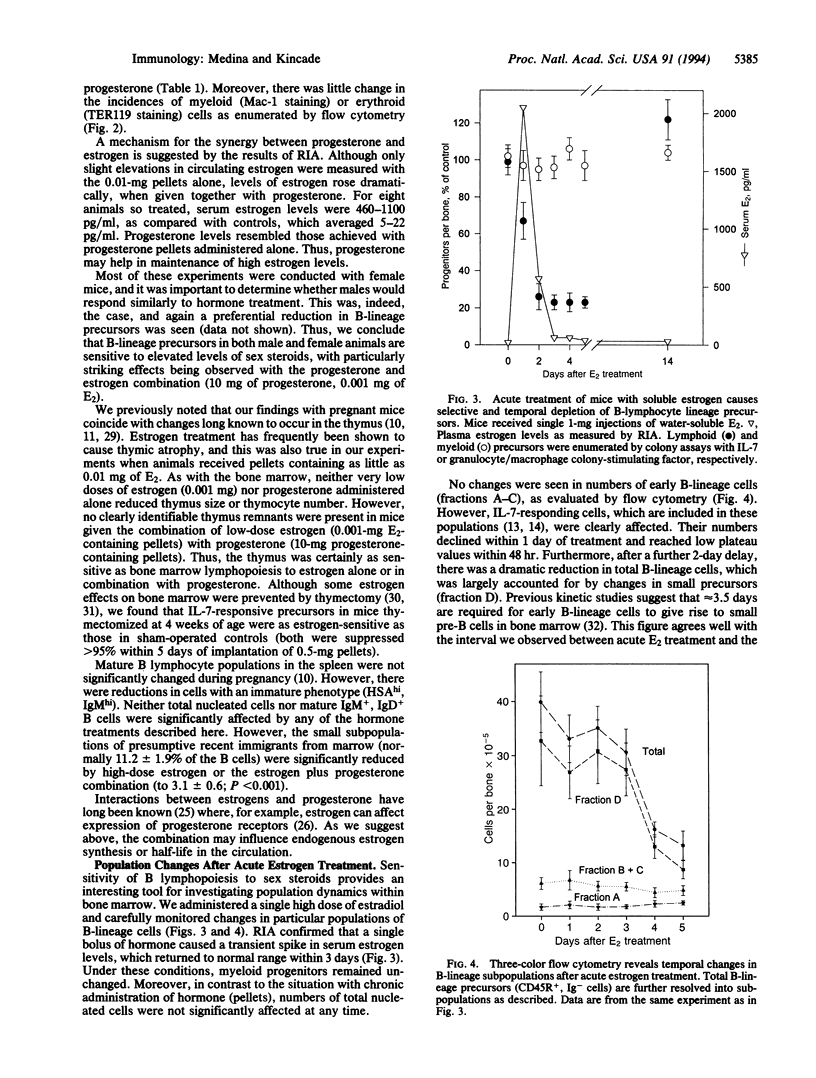
B lymphopoiesis is selectively suppressed in normal pregnant mice, suggesting that fluctuations in systemic hormone levels might influence local events within bone marrow. This has now been tested by sustained experimental elevation of sex steroids by hormone-containing pellet implants. We found that while numbers of total nucleated cells declined after treatment with estrone, beta-estradiol, or estriol, there was preferential suppression of B-lymphocyte lineage precursors. Progesterone pellets had no effect when used alone, but mice exposed to progesterone were sensitive to several-logarithm lower concentrations of estrogen. Changes in subpopulations of B-lymphocyte lineage cells with hormone pellets were similar to those previously recorded in pregnancy. B-lymphocyte lineage precursors in male and female mice were sensitive to these sex hormones. Acute treatment with single injections of water-soluble beta-estradiol allowed temporal effects on B-lineage cells to be documented. With this protocol, total numbers of nucleated cells and myeloid progenitor cells remained unchanged. Interleukin 7-responsive precursors dramatically declined within 1 day of injection, suggesting that estrogen influences that stage in the B-lymphocyte lineage. There was a subsequent sharp drop in small pre-B cells 4 days after this transient elevation in estrogen. These experiments demonstrate that B lymphopoiesis is sensitive to negative regulation by sex steroids. They extend findings made with pregnant animals and parallel previous studies of the thymus. Sex steroids might contribute to control of steady-state lymphopoiesis, and fluctuations in their levels could have implications for human disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkley M. S., Lasley B. L., Thompson M. A., Shackleton C. H. Equol: a contributor to enigmatic immunoassay measurements of estrogen. Steroids. 1985 Jul;46(1):587–608. doi: 10.1016/0039-128x(85)90024-8. [DOI] [PubMed] [Google Scholar]
- Bellido T., Girasole G., Passeri G., Yu X. P., Mocharla H., Jilka R. L., Notides A., Manolagas S. C. Demonstration of estrogen and vitamin D receptors in bone marrow-derived stromal cells: up-regulation of the estrogen receptor by 1,25-dihydroxyvitamin-D3. Endocrinology. 1993 Aug;133(2):553–562. doi: 10.1210/endo.133.2.8393768. [DOI] [PubMed] [Google Scholar]
- Billips L. G., Petitte D., Landreth K. S. Bone marrow stromal cell regulation of B lymphopoiesis: interleukin-1 (IL-1) and IL-4 regulate stromal cell support of pre-B cell production in vitro. Blood. 1990 Feb 1;75(3):611–619. [PubMed] [Google Scholar]
- Boorman G. A., Luster M. I., Dean J. H., Wilson R. E. The effect of adult exposure to diethylstilbestrol in the mouse on macrophage function and numbers. J Reticuloendothel Soc. 1980 Dec;28(6):547–560. [PubMed] [Google Scholar]
- De M., Sanford T. R., Wood G. W. Interleukin-1, interleukin-6, and tumor necrosis factor alpha are produced in the mouse uterus during the estrous cycle and are induced by estrogen and progesterone. Dev Biol. 1992 May;151(1):297–305. doi: 10.1016/0012-1606(92)90234-8. [DOI] [PubMed] [Google Scholar]
- Dorshkind K. IL-1 inhibits B cell differentiation in long term bone marrow cultures. J Immunol. 1988 Jul 15;141(2):531–538. [PubMed] [Google Scholar]
- Dorshkind K. In vivo administration of recombinant granulocyte-macrophage colony-stimulating factor results in a reversible inhibition of primary B lymphopoiesis. J Immunol. 1991 Jun 15;146(12):4204–4208. [PubMed] [Google Scholar]
- Gaunt S. D., Pierce K. R. Myelopoiesis and marrow adherent cells in estradiol-treated mice. Vet Pathol. 1985 Jul;22(4):403–408. doi: 10.1177/030098588502200416. [DOI] [PubMed] [Google Scholar]
- Gimble J. M., Medina K., Hudson J., Robinson M., Kincade P. W. Modulation of lymphohematopoiesis in long-term cultures by gamma interferon: direct and indirect action on lymphoid and stromal cells. Exp Hematol. 1993 Feb;21(2):224–230. [PubMed] [Google Scholar]
- Grabstein K. H., Waldschmidt T. J., Finkelman F. D., Hess B. W., Alpert A. R., Boiani N. E., Namen A. E., Morrissey P. J. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993 Jul 1;178(1):257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawunder U., Melchers F., Rolink A. Interferon-gamma arrests proliferation and causes apoptosis in stromal cell/interleukin-7-dependent normal murine pre-B cell lines and clones in vitro, but does not induce differentiation to surface immunoglobulin-positive B cells. Eur J Immunol. 1993 Feb;23(2):544–551. doi: 10.1002/eji.1830230237. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Gimble J. M., Henley A., Ellingsworth L. R., Kincade P. W. Differential effects of TGF-beta 1 on lymphohemopoiesis in long-term bone marrow cultures. Blood. 1989 Oct;74(5):1711–1717. [PubMed] [Google Scholar]
- Hirayama F., Shih J. P., Awgulewitsch A., Warr G. W., Clark S. C., Ogawa M. Clonal proliferation of murine lymphohemopoietic progenitors in culture. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5907–5911. doi: 10.1073/pnas.89.13.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO T., HOSHINO T. Studies of the influences of pregnancy and lactation on the thymus in the mouse. Z Zellforsch Mikrosk Anat. 1962;57:667–678. doi: 10.1007/BF00410229. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Labhsetwar A. P., Watson D. J. Temporal relationship between secretory patterns of gonadotropins, estrogens, and prostaglandin-F in periparturient rats. Biol Reprod. 1974 Feb;10(1):103–110. doi: 10.1095/biolreprod10.1.103. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Michel G., Baulieu E. E. Studies of testosterone-induced involution of the bursa of Fabricius. Dev Biol. 1980 Mar 15;75(2):288–302. doi: 10.1016/0012-1606(80)90164-5. [DOI] [PubMed] [Google Scholar]
- Lee G., Namen A. E., Gillis S., Ellingsworth L. R., Kincade P. W. Normal B cell precursors responsive to recombinant murine IL-7 and inhibition of IL-7 activity by transforming growth factor-beta. J Immunol. 1989 Jun 1;142(11):3875–3883. [PubMed] [Google Scholar]
- Luster M. I., Boorman G. A., Korach K. S., Dieter M. P., Hong L. Mechanisms of estrogen-induced myelotoxicity: evidence of thymic regulation. Int J Immunopharmacol. 1984;6(4):287–297. doi: 10.1016/0192-0561(84)90045-6. [DOI] [PubMed] [Google Scholar]
- McCormack J. T., Greenwald G. S. Progesterone and oestradiol-17beta concentrations in the peripheral plasma during pregnancy in the mouse. J Endocrinol. 1974 Jul;62(1):101–107. doi: 10.1677/joe.0.0620101. [DOI] [PubMed] [Google Scholar]
- Medina K. L., Smithson G., Kincade P. W. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993 Nov 1;178(5):1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael S. D., Geschwind I. I., Bradford G. E., Stabenfeldt G. H. Pregnancy in mice selected for small litter size: reproductive hormone levels and effect of exogenous hormones. Biol Reprod. 1975 Apr;12(3):400–407. doi: 10.1095/biolreprod12.3.400. [DOI] [PubMed] [Google Scholar]
- Mountz J. Animal models of systemic lupus erythematosus and Sjögren's syndrome. Curr Opin Rheumatol. 1990 Oct;2(5):740–748. doi: 10.1097/00002281-199002050-00010. [DOI] [PubMed] [Google Scholar]
- Murr S. M., Stabenfeldt G. H., Bradford G. E., Geschwind I. I. Plasma progesterone during pregnancy in the mouse. Endocrinology. 1974 Apr;94(4):1209–1211. doi: 10.1210/endo-94-4-1209. [DOI] [PubMed] [Google Scholar]
- Osmond D. G. B cell development in the bone marrow. Semin Immunol. 1990 May;2(3):173–180. [PubMed] [Google Scholar]
- Rennick D., Yang G., Muller-Sieburg C., Smith C., Arai N., Takabe Y., Gemmell L. Interleukin 4 (B-cell stimulatory factor 1) can enhance or antagonize the factor-dependent growth of hemopoietic progenitor cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6889–6893. doi: 10.1073/pnas.84.19.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risdon G., Cope J., Bennett M. Mechanisms of chemoprevention by dietary dehydroisoandrosterone. Inhibition of lymphopoiesis. Am J Pathol. 1990 Apr;136(4):759–769. [PMC free article] [PubMed] [Google Scholar]
- Risdon G., Kumar V., Bennett M. Differential effects of dehydroepiandrosterone (DHEA) on murine lymphopoiesis and myelopoiesis. Exp Hematol. 1991 Feb;19(2):128–131. [PubMed] [Google Scholar]
- Screpanti I., Morrone S., Meco D., Santoni A., Gulino A., Paolini R., Crisanti A., Mathieson B. J., Frati L. Steroid sensitivity of thymocyte subpopulations during intrathymic differentiation. Effects of 17 beta-estradiol and dexamethasone on subsets expressing T cell antigen receptor or IL-2 receptor. J Immunol. 1989 May 15;142(10):3378–3383. [PubMed] [Google Scholar]
- Seaman W. E., Gindhart T. D., Greenspan J. S., Blackman M. A., Talal N. Natural killer cells, bone, and the bone marrow: studies in estrogen-treated mice and in congenitally osteopetrotic (mi/mi) mice. J Immunol. 1979 Jun;122(6):2541–2547. [PubMed] [Google Scholar]
- Slavin S., Strober S. Spontaneous murine B-cell leukaemia. Nature. 1978 Apr 13;272(5654):624–626. doi: 10.1038/272624a0. [DOI] [PubMed] [Google Scholar]
- Stimson W. H., Hunter I. C. Oestrogen-induced immunoregulation mediated through the thymus. J Clin Lab Immunol. 1980 Jul;4(1):27–33. [PubMed] [Google Scholar]
- Thompson E. A., Jr The effects of estradiol upon the thymus of the sexually immature female mouse. J Steroid Biochem. 1981 Feb;14(2):167–174. doi: 10.1016/0022-4731(81)90170-9. [DOI] [PubMed] [Google Scholar]
- URIST M. R., BUDY A. M., McLEAN F. C. Endosteal-bone formation in estrogen-treated mice. J Bone Joint Surg Am. 1950 Jan;32A(1):143-62, illust. [PubMed] [Google Scholar]
- Vitetta E. S., Yuan D., Krolick K., Isakson P., Knapp M., Slavin S., Strober S. Characterization of the spontaneous murine B cell leukemia (BCL1). III. Evidence for monoclonality by using an anti-idiotype antibody. J Immunol. 1979 May;122(5):1649–1654. [PubMed] [Google Scholar]


