Abstract
A complex integration of molecular and electrical signals is needed to transform a quiescent uterus into a contractile organ at the end of pregnancy. Despite the discovery of key regulators of uterine contractility, this process is still not fully understood. Transgenic mice provide an ideal model in which to study parturition. Previously, the only method to study uterine contractility in the mouse was ex vivo isometric tension recordings, which are suboptimal for several reasons. The uterus must be removed from its physiological environment, a limited time course of investigation is possible, and the mice must be sacrificed. The recent development of radiometric telemetry has allowed for longitudinal, real-time measurements of in vivo intrauterine pressure in mice. Here, the implantation of an intrauterine telemeter to measure pressure changes in the mouse uterus from mid-pregnancy until delivery is described. By comparing differences in pressures between wild type and transgenic mice, the physiological impact of a gene of interest can be elucidated. This technique should expedite the development of therapeutics used to treat myometrial disorders during pregnancy, including preterm labor.
Keywords: Medicine, Issue 98, Intrauterine pressure, telemetry, pregnancy, mouse, uterus, in vivo recording, preterm labor, reproductive
Introduction
Preterm birth is the leading cause of perinatal morbidity and mortality in developed countries; it is responsible for 50% of perinatal morbidity and 75% of perinatal mortality1,2. Preterm labor is multifaceted and can be idiopathic. Although much research has emerged on the molecular and electrical pathways transforming the myometrium from a quiescent tissue into a contractile one, the exact pathophysiology of preterm labor remains elusive. Endocrine, inflammatory, and gene regulation have all been linked to preterm labor3,4. However, ethical concerns limit the ability to conduct research on the mechanisms of preterm labor in humans.
Given these limitations, many researchers have turned to mice as a model system with which to study the physiology of parturition. Mice have short gestations lasting approximately three weeks and can be easily genetically manipulated. Additionally, multiple genetic mouse models have been developed to determine the signaling pathways that are essential for labor5. Despite key differences between mouse and human parturition, mice and humans share many of the same mechanisms that are essential for labor, including intrauterine inflammation and infection6. Thus, mice serve as an invaluable tool for examining uterine activity. To date, the gold standard to measure uterine contractility in mice has been ex vivo isometric tension recordings; however, this is limited to one gestational time point per experiment and requires removal of the uterus from its physiological environment. Another important factor is the large number of animals required for these investigations. Lastly, this methodology does not allow for longitudinal studies examining induction of labor and preterm pathophysiology.
Recent advancements made in radiometric telemetry devices used to study arterial pressure changes7 in mice and intrauterine pressure in rats8 begged the question to whether the same technology could be used to study changes in intrauterine pressure in mice during pregnancy. After initial troubleshooting, a method was successfully developed to measure labor induction and progression in a mouse. This in vivo approach can measure the transition from the low-pressure state of the quiescent uterus to the high-pressure state indicative of forceful labor contractions. This method has also been able to detect significant pressure differences between transgenic mice with compromised parturition and wild type mice, demonstrating the physiological impact of gene expression9. This protocol provides a real-time, in vivo method to study mouse uterine pressure during pregnancy.
Protocol
NOTE: All animal procedures complied with guidelines for the care and use of animals set forth by the National Institutes of Health. All protocols were approved by the Animal Studies Committee at Washington University in St. Louis. NOTE: Specific materials and equipment for this protocol are listed Materials and Equipment table.
1. Timed Breeding of Female Mice
Breed adult females between two and six months of age in a 2 hr time window with males. Specific breeding hr are not needed as long as they are kept consistent for the entirety of the experiment.
Confirm pregnancy by the presence of a copulatory plug. Designate the day and hr on which the plug is first seen as day post-coitus 0 (dpc0). Remember the specific hour when starting pressure recording.
Perform surgeries on dpc8–13. The exact dpc will depend on the mouse strain.
To determine if a mouse is pregnant before surgery, perform ultrasound imaging of the abdomen with a 40 MHz linear array probe coupled to a imaging system before administering anesthesia. This will allow visualization of gestational sacs. If an ultrasound system is not available, use the weight of the mouse. NOTE: A weight gain of at least 2 g between dpc0 and dpc8 and distension of the abdomen is a good indicator of pregnancy.
2. Telemetry Surgery Prep
Use an autoclave to sterilize instruments, gauze, cotton swabs, surgical drapes, and pipette tips within 24 hr before surgery.
Clean surgery surface with 70% ethanol.
Turn on heating pad (specialized for rodents) and bead sterilizer.
3. Telemetry Surgery
For anesthesia, use 4-5% isoflurane with an oxygen flow rate of 1-2 L/min.
Wait until the mouse shows signs of anesthetization and then inject subcutaneously with buprenorphine (analgesic: 0.1 ml per 20 g).
Shave the abdomen of the mouse from the bottom of the ribs down to the bladder.
Bring the mouse to the surgery table on pre-warmed heating pad, and cover the mouse’s eyes with ophthalmic ointment to prevent drying.
Begin sterile technique. Put on sterile gloves and keep the wrapper as a sterile surface on which to place the iodine and ethanol scrubs.
- Remove the telemeter from the package or saline and use sterile forceps to place it on sterile gauze.
- Use the syringe (provided by the telemeter supplier) to fill the catheter tip with gel. Gently squeeze the catheter creating enough negative pressure to draw the gel into the catheter.
Place a sterile tip on the pipette. Pipette 2 µl of surgical glue and set the pipette on a sterile surface.
To clean the surgical site, swab the area with iodine and then with 70% ethanol.
Cut a hole in the surgical drape just large enough for the area needed for the incision and place over the mouse.
Perform a foot pinch (pedal response) to ensure the mouse is completely anesthetized before making an incision.
Make a small vertical midline incision. Next, make a similar incision through the underlying body wall muscle.
- Gently pull the uterus out of the body cavity and locate the uterine horn containing the most pups that are viable.
- Optional - For addition of miRNA, inject viral vector into muscle layer of uterus before insertion of telemeter.
Make a small incision at the tip of the uterine horn with 3 mm cutting edge spring scissors.
Thread the catheter, without squeezing, between the uterine wall and fetal sacs. Insert the catheter past several sacs to reduce its accidental removal. Make sure the fetal sacs are not disturbed by the insertion.
Pipette the surgical glue to the site of telemeter insertion to adhere the catheter to the uterine horn and prevent the catheter from sliding out of the uterine horn. Wait a few seconds for the glue to become rigid, and then carefully place the uterus back inside the body cavity.
Place the telemeter in the body cavity on the opposite side of the catheter insertion site.
Suture the body cavity closed with 6.0 absorbable sutures. First, do a purse stitch and then three to four individual stitches.
Suture the skin by using the same technique, but with 6-0 non-absorbable suture.
Swab dibucaine (topical analgesic) ointment over the incision site.
Inject 0.3-0.4 ml of sterile saline subcutaneously to help rehydrate the animal.
Monitor the animal after surgery until it is fully awake. Do not place the mouse back in its cage until it is able to freely hold its head above the table as certain types of bedding can suffocate a heavily anesthetized mouse. For a mouse that has been anesthetized for 30-45 min, one should expect the mouse to be fully awake by 1 hr and 15 min.
If performing additional surgeries, wipe instruments with 70% ethanol and bead sterilize before reuse.
4. Post-surgery Recovery
- Monitor daily post-surgery progress in a log. Catalog weight gain and loss and watch for lethargy, bleeding, or incomplete closure of the surgical incision.
- Feed the mouse softened mouse chow (~the consistency of applesauce) or specialized recovery diet food.
- Examine the sutures and make sure they are not pulled out, but try not to handle the mouse excessively.
5. Telemetry Recording
On the day of interest (at least a two to three days after the mouse has recovered from surgery), place a magnet near the mouse to turn on the telemeter. Put the mouse cage on the receiver. The light on the receiver should turn on if the telemeter is activated.
Calibrate brand new telemeters with manufacturer specific calibrations provided. Reused telemeter calibration specifications will be kept in software.
Within the software, start recording by right clicking on “animal” and click “start sampling, continuous,” which allows sampling at rates of up to 500 Hz. Make sure the software is set to save AND trace. NOTE: When the sampling mouse icon turns green, pressures are being recorded. Disturbing the mouse during delivery can interrupt or alter labor, so observe cautiously.
After delivery or time period of interest, stop recording by right clicking on “animal” and selecting “stop recordings, all”. Use a magnet to turn off telemeter to save battery life, as the telemeter battery will typically only last for 1.5 months of continuous recording.
Euthanize the mouse by CO2 overdose and recover the telemeter. Pups are euthanized by decapitation.
6. Telemeter Sterilization
Place the telemeter in a solution of 1% enzyme detergent in deionized water overnight at room temperature.
Rinse the telemeter in deionized water and remove all tissue and glue, being careful not to squeeze the catheter.
Incubate the telemeter in 2% glutaraldehyde for 24 hr at room temperature to sterilize.
Rinse the telemeter with sterile saline and store it in sterile saline until next implantation or to be sent back to manufacturer to be refurbished. Telemeters should be refurbished if tissue is located inside the catheter after sterilization. Typically telemeters can be used 4-5 times before needing to be refurbished.
Representative Results
Real-time in vivo intrauterine pressures can be recorded by using a telemetric acquisition system. Pressure sampling paired with simultaneous video recording of the mouse was used to capture the exact time of each pup’s delivery and correlate delivery time to the intrauterine pressure of the mother. All the data points can then be plotted throughout the recording to generate a pressure-versus-time plot (Figure 1A). This plot shows the points during gestation when the intrauterine pressure changes as well as the difference in pressure between the dark and light cycles. Additionally, a moving average of the intrauterine pressure can be plotted (Figure 1B). This is useful to more easily visualize sustained increases in intrauterine pressure characteristic of the contractions associated with the labor process (recordings obtained during delivery have been published previously9).
Data can be further analyzed by exporting data points to analysis software and averaging data over the time period of interest. When comparing different experimental conditions, such as wild type versus transgenic mice, daily or hourly pressures from different mice can be averaged to examine contractile patterns dependent on the gene of interest. Additionally, the statistical significance of any differences can be determined. Intrauterine pressures can be examined during delivery, or a longitudinal study can be conducted to examine the entire gestational period under different conditions.
Intrauterine telemetry can be used to test in vivo responses to pharmacological agents during pregnancy. Specifically, this method allows for early drug testing prior to clinical trials. Differences in response to specific agents can be tested in transgenic mice, as well as in non-pregnant or pregnant mice. One specific pharmacological agent known to increase uterine contraction, oxytocin, can be delivered subcutaneously as previously described in a rat model10. The telemeter can be implanted at the same time as a micro-osmotic pump to deliver a steady rate of oxytocin. Differences in recordings between non-pregnant (Figure 2A) and pregnant (Figure 2B) mice with administration of oxytocin can also be examined.
Another potential application of intrauterine telemetry is looking at uterine contractions after miRNA/shRNA silencing of proteins. Mouse models are not available for all genes that are essential for parturition. Thus, gene silencing in combination with intrauterine pressure measurements can be used to investigate proteins of interest. By injecting miRNA-expressing lentiviral vectors into the myometrium during the implantation of the telemeter, uterine-specific knockdown of a gene of interest can be examined, as seen in Figure 3A. A recent study shows a specific example demonstrating that the Kir7.1 channel has an important role in uterine quiescence after using both miRNA and telemetry to examine changes to intrauterine pressure throughout gestation11. Representative tracings of scrambled miRNA (Figure 3A) or Kir7.1 miRNA (Figure 3B) show that knocking down expression of the potassium channel Kir7.1 caused intrauterine pressure to increase. These representative tracings are similar to what is seen in transgenic mouse models as previously described9.
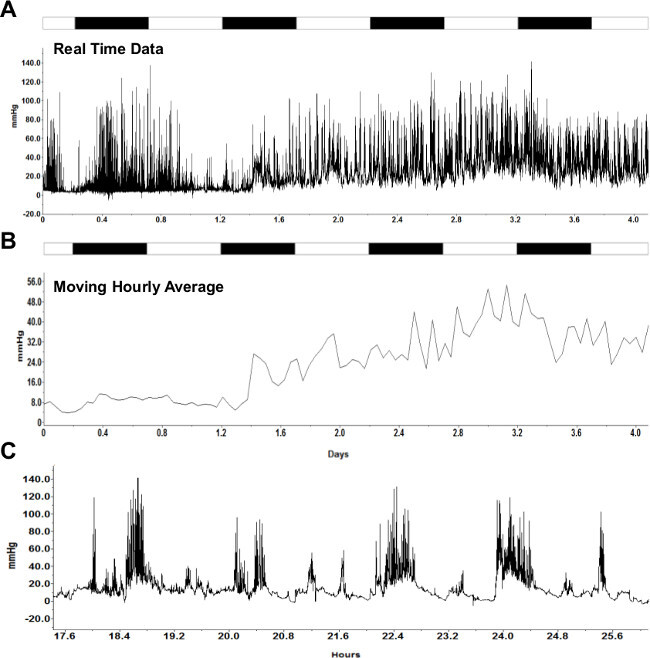 Figure 1: Representative continuous intrauterine pressure recording. (A) Real-time intrauterine pressure data recorded in vivo continuously for four days (15-19 dpc). (B) Moving hourly average of data in (A). White and black bars on top of recordings indicate 12 hr light and dark cycles, respectively. (C) A zoomed in trace of mouse in vivo individual contractions. Please click here to view a larger version of this figure.
Figure 1: Representative continuous intrauterine pressure recording. (A) Real-time intrauterine pressure data recorded in vivo continuously for four days (15-19 dpc). (B) Moving hourly average of data in (A). White and black bars on top of recordings indicate 12 hr light and dark cycles, respectively. (C) A zoomed in trace of mouse in vivo individual contractions. Please click here to view a larger version of this figure.
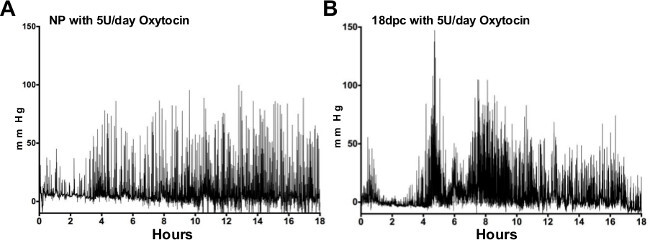 Figure 2:Intrauterine pressures in response to oxytocin. Representative tracings of intrauterine pressure from non-pregnant (NP) myometrium (A) and late pregnant (18 dpc) myometrium (B) exposed to 5 units/day of oxytocin for 24 hr show increases in pressure in the late pregnant versus NP mice. Please click here to view a larger version of this figure.
Figure 2:Intrauterine pressures in response to oxytocin. Representative tracings of intrauterine pressure from non-pregnant (NP) myometrium (A) and late pregnant (18 dpc) myometrium (B) exposed to 5 units/day of oxytocin for 24 hr show increases in pressure in the late pregnant versus NP mice. Please click here to view a larger version of this figure.
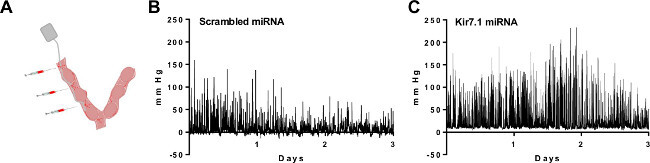 Figure 3:Measurement of intrauterine pressure after silencing of a potassium channel by miRNA/shRNA. (A) Schematic of the placement of the telemeter and sites of injection of miRNA-expressing lentiviruses. Recent studies have shown the representative tracings of intrauterine pressure from myometrium transduced with (B) scrambled miRNA or (C) Kir7.1 miRNA. Please click here to view a larger version of this figure.
Figure 3:Measurement of intrauterine pressure after silencing of a potassium channel by miRNA/shRNA. (A) Schematic of the placement of the telemeter and sites of injection of miRNA-expressing lentiviruses. Recent studies have shown the representative tracings of intrauterine pressure from myometrium transduced with (B) scrambled miRNA or (C) Kir7.1 miRNA. Please click here to view a larger version of this figure.
Discussion
Investigations of myometrial contractility have relied on ex vivo measurements of muscle tension. This methodology can be useful for early-stage testing of newly developed uterotonic drugs that cannot be administered to live animals. However, in vivo approaches are necessary to longitudinally understand the progression of labor. The in vivo measurements serve several purposes. First, they enable capture of a complete picture of changes in uterine contractile pattern over the duration of pregnancy. Second, they eliminate the bias of ex vivo recordings that require uterine tissue to be mounted either vertically or horizontally, limiting our understanding of the complete role of the circular and longitudinal muscle layer of the uterus. Third, they allow recording of uterine pressure in the endogenous hormonal environment, thus making it possible to more accurately study parturition in mouse models. Finally, they allow for more translational investigations between mouse models and humans as uterine telemetry more closely resembles measurements taken in pregnant women with intrauterine pressure catheters.
The methods for mouse uterine telemetry are easy to learn, but difficult to master. Investigators with previous mouse surgery experience may achieve success faster, but this shouldn’t deter labs from using this valuable technique. The original studies using mouse uterine telemetry were done by a novice with great success after a few tries, so labs should be prepared for the first couple of surgeries to cause the dam to lose all fetuses. Here are a few pointers to keep in mind. As with any surgery, the shortest duration is the best for the animal. A quick surgery will limit dehydration and the need for additional doses of anesthesia. If the researcher is having a difficult time finishing the procedure before the mouse wakes up, isoflurane can be used to keep the mouse anesthetized longer. We have also found that the use of isoflurane increases the number of viable pups and is preferred over ketamine. The smallest incision possible, just slightly bigger than the body of the telemeter, will reduce the chance of the wound gaping later, decrease time needed to suture, and improve mouse recovery. With a small abdominal incision, pulling the uterus outside of the body cavity will allow for better visibility during catheter insertion. When reusing a telemeter, factor in the previous curve of the catheter when threading it down the uterine horn. Allowing the surgical glue to harden completely before placing the uterine horn back inside the body cavity will help keep the catheter in place and prevent the glue from sticking to the intestines. The telemeter body can damage the liver if placed too high, and the mouse’s abdomen is capable of expanding extensively, so do not hesitate to place the telemeter lower in the body. These methods will have to be customized to fit the needs of different labs and different mouse models, but the advantages of uterine telemetry far outweigh the obstacles.
There are some limitations that should be considered when implementing this methodology. One of the main challenges that have to be overcome is the degree of fetal loss following implantation of the transmitter. In the intrauterine telemetry initial report9, all transgenic SK3T/T and wild type mice had some degree of fetal loss in one or both uterine horns regardless if that horn was chosen for telemeter placement. Optimization of the choice of day during pregnancy when the telemeter was surgically inserted improved our rate of successful term pregnancies, but a full complement of pups was never obtained. Notably, the optimal day of surgery for SK3T/T mice differed from that in wild type, with better reproductive outcomes noted at dpc12-13 for SK3T/T mice versus dpc8-9 for WT mice, suggesting that the surgery protocol may need to be modified for each mouse model of interest. Our data indicated that the number of pups birthed did not correlate with overall intrauterine pressure. Fetal outcomes did improve with improved surgical skills by the experimenter, and others who have implemented this methodology in their laboratory have noted that fetal outcomes improved with the use of isoflurane instead of ketamine. It is expected that as more laboratories use this methodology, the transmitter technology and intrauterine analysis software will become more refined. This will undoubtedly advance the usefulness of this technology in studies of the mechanisms that contribute to labor.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by funding from the March of Dimes FY12-133 (S.K.E.) and the National Institutes of Health R01HD037831 (S.K.E.). We would like to thank Dr. Deborah Frank for critical review of the manuscript.
References
- Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keelan JA. Pharmacological inhibition of inflammatory pathways for the prevention of preterm birth. Journal Of Reproductive Immunology. 2011;88:176–184. doi: 10.1016/j.jri.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Voltolini C, et al. Understanding spontaneous preterm birth: from underlying mechanisms to predictive and preventive interventions. Reproductive Sciences. 2013;20:1274–1292. doi: 10.1177/1933719113477496. [DOI] [PubMed] [Google Scholar]
- Ratajczak CK, Muglia LJ. Insights into parturition biology from genetically altered mice. Pediatric research. 2008;64:581–589. doi: 10.1203/PDR.0b013e31818718d2. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, et al. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. American Journal Of Physiology. Regulatory, Integrative And Comparative Physiology. 2007;293 doi: 10.1152/ajpregu.00097.2007. [DOI] [PubMed] [Google Scholar]
- Whitesall SE, Hoff JB, Vollmer AP, D'Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. American journal of physiology. Heart And Circulatory Physiology. 2004;286:H2408–H2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- Mackay LB, Shi LB, Maul H., Maner WL, Garfield RE. The effect of bilateral pelvic neurectomy on cervical ripening in pregnant rats. Journal Of Perinatal Medicine. 2009;37:263–269. doi: 10.1515/JPM.2009.043. [DOI] [PubMed] [Google Scholar]
- Pierce SL, Kutschke W, Cabeza R, England SK. In vivo measurement of intrauterine pressure by telemetry: a new approach for studying parturition in mouse models. Physiol Genomics. 2010;42:310–316. doi: 10.1152/physiolgenomics.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Luedke CE, Vogt SK, Muglia LJ. Oxytocin modulates the onset of murine parturition by competing ovarian and uterine effects. American Journal Of Physiology. Regulatory, Integrative And Comparative Physiology. 2000;279:R1061–R1067. doi: 10.1152/ajpregu.2000.279.3.R1061. [DOI] [PubMed] [Google Scholar]
- McCloskey C, et al. The inwardly rectifying K+ channel KIR7.1 controls uterine excitability throughout pregnancy. EMBO Mol Med. 2014;6(9):1161–1174. doi: 10.15252/emmm.201403944. [DOI] [PMC free article] [PubMed] [Google Scholar]


