Abstract
The induction of systemic responses in plants is associated with the connectivity between damaged and undamaged leaves, as determined by vascular architecture. Despite the widespread appreciation for studying variation in induced plant defense, few studies have characterized spatial variability of induction in the model species, Arabidopsis thaliana. Here we show that plant architecture generates fine scale spatial variation in the systemic induction of invertase and phenolic compounds. We examined whether the arrangement of leaves along the stem (phyllotaxy) produces predictable spatial patterns of cell-wall bound and soluble invertase activities, and downstream phenolic accumulation following feeding by the dietary specialist herbivore, Pieris rapae and the generalist, Spodoptera exigua. Responses were measured in leaves within and outside of the damaged orthostichy (leaves sharing direct vascular connections), and compared to those from plants where source-sink transport was disrupted by source leaf removal and by an insertional mutation in a sucrose transporter gene (suc2-1). Following herbivore damage to a single, middle-aged leaf, induction of cell-wall and soluble invertase was most pronounced in young and old leaves within the damaged orthostichy. The pattern of accumulation of phenolics was also predicted by these vascular connections and was, in part, dependent on the presence of source leaves and intact sucrose transporter function. Induction also occurred in leaves outside of the damaged orthostichy, suggesting that mechanisms may exist to overcome vascular constraints in this system. Our results demonstrate that systemic responses vary widely according to orthostichy, are often herbivore-specific, and partially rely on transport between source and sink leaves. We also provide evidence that patterns of induction are more integrated in A. thaliana than previously described. This work highlights the importance of plant vascular architecture in determining patterns of systemic induction, which is likely to be ecologically important to insect herbivores and plant pathogens.
Introduction
Attack by insect herbivores elicits chemical defenses as a critical step in the development of resistance in plants. This induction can occur locally, within a damaged leaf, or in undamaged, system leaves. Many studies have found that systemic defense responses are often highly variable in space and time. Such variation has been attributed to developmental plasticity and plant modularity, to variation in transport of systemic signals, to within-plant genetic variation, and to constraints arising from vascular architecture [1, 2]. Because it can impact herbivore feeding and movement [3] and might slow the evolution of insect adaptation [4] attack-induced variation has even been described as an adaptation [5].
If vascular constraint determines the pattern of plant defense heterogeneity, plant responses to their insect herbivores should often be related to the arrangement of leaves along the stem (phyllotaxy), and the vascular connectivity between these leaves (orthostichy), which are dynamically linked to plant ontogeny [6]. The importance of orthostichy is well known in long-distance nutrient transport, where young, sink leaves are not equally supplied by the same source leaves. Instead, sources preferentially serve sinks that share direct vascular connections along an orthostichy [7]. If resistance-inducing signals travel via the vascular system, we should be able to predict spatial and temporal pattern of defense as it spreads from the site of attack.
Several studies have demonstrated that the spread of induced defense may be related to the degree of connectivity between damaged and undamaged leaves [8–12]. In tomato, the intensity of systemic proteinase inhibitor accumulation correlates well with the degree of vascular linkage between and within leaves, which may be driven by the release of the chemical elicitor, systemin, into the phloem [9, 13–15]. Similar observations have been made in Nicotiana spp., where salicylic acid transport [16], the induction of defense-related gene expression [10], and the accumulation of trypsin protease inhibitors [8] all appear related to source-sink relationships. In Populus hybrids, local production of phenolics elicited by jasmonates or insect attack depends on movement of photosynthate from sources to directly connected sink leaves [17, 18].
Surprisingly few studies of vascular constraints on systemic responses have employed the model plant Arabidopsis thaliana, despite its widespread use in studies of plant responses to insects and pathogens. The relationship between orthostichy and phyllotaxy is well worked out in this plant making it comparatively easy to predict the spatial distribution of systemically induced defenses. A. thaliana has a 3 + 5 spiral leaf phyllotaxy with a divergence angle between successive leaves of 137.5° [19]. Leaves within an orthostichy are arranged in an approximately vertical line on the stem above each other in the phyllotaxy [20, 21]. Vascular architecture appears to constrain defense responses to pathogen infection to leaves within the orthostichy of an inoculated leaf [20, 21]. We recently used the glucose surrogate, 2-[18F]fluoro-2-deoxy-D-glucose, and 11C-photosynthate administered to plants as 11CO2, to link the movement of potential defense-related substrates to plant architecture in response to wounding and herbivore-related elicitation [22, 23]. Here we showed that radioactivity was most concentrated in the petiole of the radiolabelled, middle-aged leaf and in the younger and older leaves most directly above and below it in the phyllotaxy.
With this information, and our current knowledge of vascular connections within the rosette of A. thaliana, we used source-sink manipulations and genetic approaches to determine whether plant phyllotaxy produces predictable spatial patterns in two functions associated with systemic defense induction: cell-wall bound and soluble invertases activities, and phenolic accumulation, following herbivore damage to a single leaf. We hypothesized that herbivore-induced changes in invertase activity and phenolic compounds in source and sink leaves are directly attributable to leaf age and location relative to site of damage. If transported carbohydrates serve as building blocks for the production of phenolics in developing sink leaves, we also predicted that intact transport mechanisms would be required as a prerequisite to induction in young leaves.
Materials and Methods
Study System
Arabidopsis thaliana (Col-0) plants were grown in individual 4cm round pots at 22°C and 62% relative humidity on an 8L:16D photoperiod (180μM PAR) in Metro-Mix 200TM soil (Sun Gro Horticulture, Bellvue, WA) supplemented with 1.8 kg of OsmocoteTM slow-release fertilizer (The Scotts Company, Marysville, OH) per cubic meter of soil. Plants were bottom-watered when necessary (approximately every 4 days). Plants were used in the experiments when they were 5 weeks post-germination (contained approximately 15 leaves) and remained in the vegetative state during the experiments.
Insects
Pieris rapae were reared at 24°C on A. thaliana plants and are the progeny of biological stock originally obtained from Carolina Biological and the Jander laboratory (Cornell University, Ithaca, NY). Spodoptera exigua eggs were obtained from Benzon Research (Carlisle, PA) and reared on Beet Armyworm Diet (Southland Products Inc., Lake Village, AR) at 27°C. Post-ecdysial 3rd instar caterpillars were used for all experiments. Insects raised on artificial diet were placed on a pre-feeding diet of A. thaliana 24h prior to experiment and removed from these plants for a maximum of 3h before the feeding assay.
Insect Induction Experiments
Does plant vascular architecture constrain systemically induced responses elicited by P. rapae and S. exigua
Carbon allocation in A. thaliana is integrated along a gradient from young leaves at the top, which are entirely sinks, to older leaves at the bottom of the rosette that are weak sinks and/or sources; thus, younger leaves import carbon for growth and metabolism from older sources. Young leaves are particularly responsive to attack, and studies show that they draw carbon from older leaves, stems, and roots [17, 24–26]. That said, we decided to focus on the role of leaves in the middle-aged, transitional leaves located in the center of this gradient, because they act as both sinks and sources and are shared targets of both P. rapae and S. exigua feeding on A. thaliana [27].
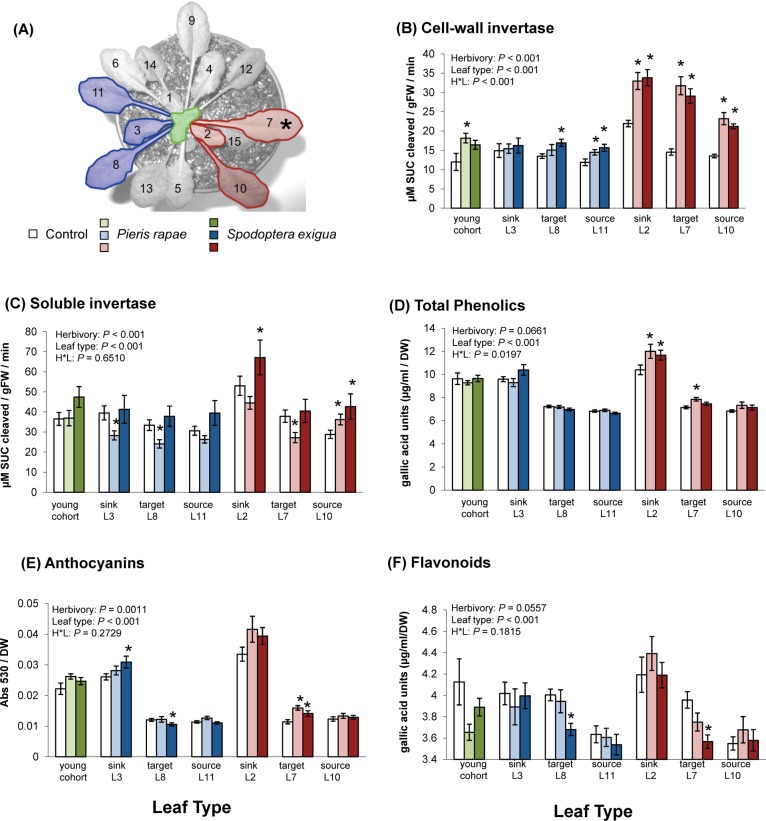
During insect treatments, individual P. rapae and S. exigua larvae were confined via clip cage (diameter of 3 cm with nylon mesh screening on top and bottom) to the seventh leaf (L7) from the apex (counting down from the first fully expanded leaf >1mm). Insects were allowed to feed until approximately 30% of the leaf was removed. Clip cages without insects were affixed to L7 of control plants. Toothpicks were used to denote undamaged control leaves after clip cages were removed. Leaves were harvested into liquid N2 48h after insect treatment according to their location within (red, L2, 10) and outside (blue, L3, 8, 11) of the orthostichy of insect-damaged L7 (Fig 1A). Young, undeveloped leaves located at the apex of each rosette (green, Fig 1A) were also pooled and harvested.
Fig 1. Herbivore-induced changes in foliar invertase activity and phenolic accumulation are determined by orthostichy.
(A) A. thaliana rosette depicting leaves within (red) and outside (blue) of the damaged orthostichy. Asterisk denotes the middle-aged leaf targeted for herbivore damage. Cell-wall (B) and soluble (C) invertase activities were measured 48h following damage of P. rapae or S. exigua larva to leaf 7. Foliar accumulation of total phenolics (D), anthocyanins (E), flavonoids (F) were also quantified within and outside of the damaged orthostichy. All bars represent means ±SE. n = 20/treatment group. Results of two-way analysis of variance are shown. Asterisks indicate significant differences between clip-caged control and herbivore elicited plants for a given leaf type (P < 0.05 Tukey’s post hoc comparisons).
Is source-to-sink flow of carbon required for defense induction in young, developing leaves
The importance of carbon import for induced phenolic production was examined further in two experiments. The first involved physically removing all source leaves located below young, damaged leaves. The second experiment utilized sucrose transporter mutants.
Source Leaf Removal Study
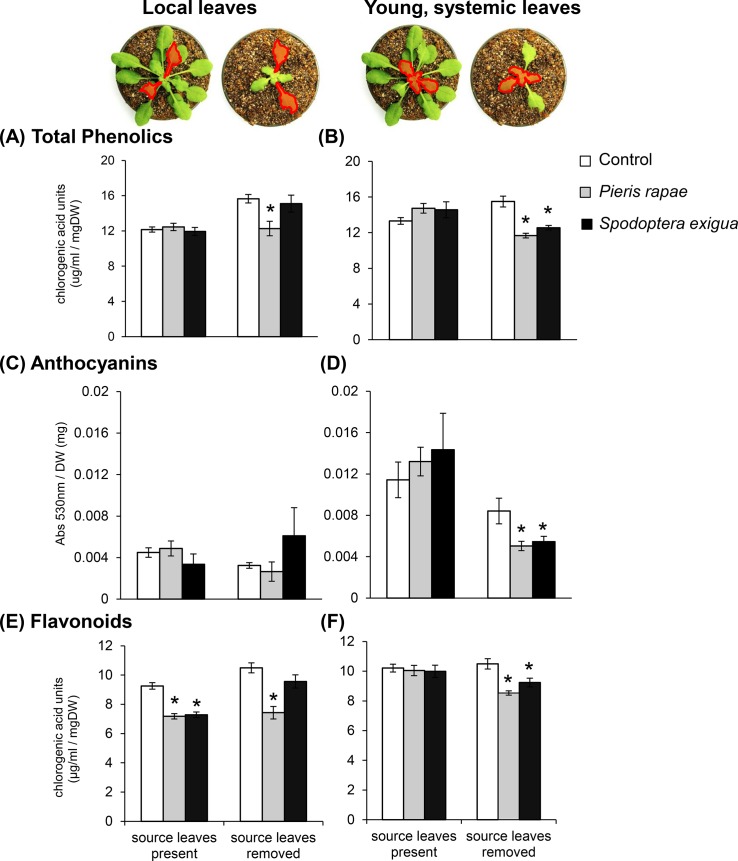
The effects of source leaf removal on response to insect treatments were assessed by randomly assigning plants (n = 16) to one of the following treatments: (1) control, with source leaves, (2) P. rapae, with source leaves, (3) S. exigua, with source leaves, (4) control, source leaves removed, (5) P. rapae, source leaves removed (6) S. exigua, source leaves removed. Source leaves (leaf 4 and greater, see Fig 2) were removed from the appropriate plants at the base of the petiole immediately prior to insect treatments. Larvae were clip-caged to sink leaves 2 and 4 and allowed to feed until they removed approximately 30% of the leaf area. Sink leaves and the young cohort of undeveloped leaves at the top of the rosette were harvested in liquid N2 48h later for phenolic measurements.
Fig 2. Herbivore-induced phenolic accumulation is dependent on the presence of intact source leaves.
The accumulation of total phenolics (A,B), anthocyanins (C,D) and flavonoids (E,F) were measured locally, within herbivore-damaged leaves (A,C,E), and in young, systemic leaves (B,D,F). Bars represent means ±SE. n = 16/treatment group. Results of analysis of variance are summarized in Tables A-C in S1 File. Asterisks indicate significant differences between clip-caged control and herbivore-damaged plants for a given leaf type (P < 0.05 Tukey’s post hoc comparisons).
suc2-1 Study
In source leaves of A. thaliana, photosynthate and presumably other phloem-transported metabolites are loaded apoplastically by the companion cell/sieve-element complex [28]—a process driven by an energy-dependent transport system involving sucrose transporters [29]. Source leaves of the A. thaliana T-DNA insertion line in AtSUC2 (suc2-1, At1G22710), a phloem-specific sucrose transporter gene [29], contain excess starch, and fail to transport radiolabeled sugar efficiently to roots and inflorescences [30]. Work using this genotype not only provides strong evidence in support of apoplastic loading as the primary method for initiating long distance transport in A. thaliana, but also offers a unique opportunity to test whether phloem loading at the source and subsequent transport to sink tissues are required for induced systemic responses.
AtSUC2-silenced plants were grown side by side with their wild-type (WT) background (Ws-2) for controlled comparisons. Seeds (TAIR) were surface sterilized and germinated on agar plants containing Murashige and Skoog media [31]. Homozygote suc2-1 seedlings were screened for experiments by their characteristic phenotype following germination on media without sucrose—mutants are visibly smaller than wild-type, with very short primary roots, and translucent cotyledons [30]. For controls, wild-type Ws-2 plants were grown without selection under the same conditions as AtSUC2-silenced plants. Approximately 2 weeks post-germination, homozygous suc2-1 plants and Ws-2 controls were rescued on fresh media containing 1% supplemental sucrose. Plants that were successfully rescued were transplanted to pots and maintained under the same growth conditions as described above. Homozygous and wild-type plants were approximately 6-weeks post-germination at the time of the experiment. Homozygous plants were generally slower to development than their wild type siblings, and so were slightly smaller at the time of the experiment.
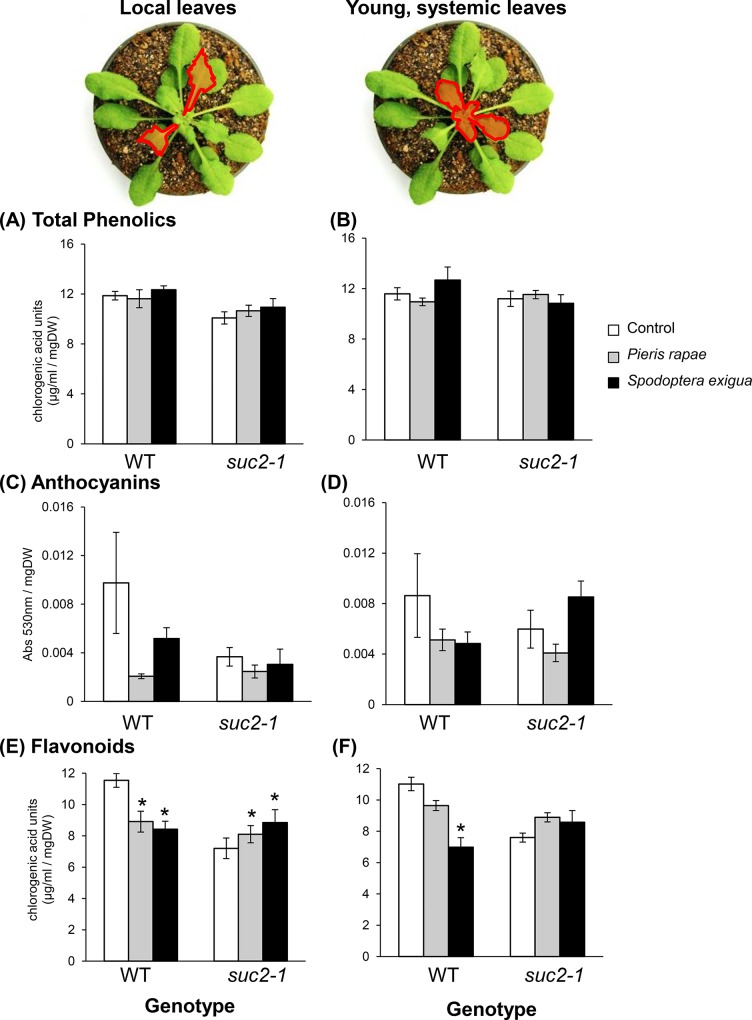
We used a factorial design comprised of 8 plants allotted to one of the following treatments: (1) control, wild-type, (2) P. rapae, wild-type (3) S. exigua, wild-type (4) control, suc2-1 (5) P. rapae, suc2-1 (6) S. exigua, suc2-1. As in the source leaf removal experiment, larvae were clip-caged to sink leaves 2 and 4 where they were allowed to feed until 30% of each leaf was eaten. Damaged sink leaves and leaves from the apex of the rosette were harvested in liquid N2 48h later for phenolic measurements.
Invertase Activity Assays
The activity of cell-wall and soluble invertases was measured in leaves according to previously described methods [22]. Proteins were extracted from fresh frozen and ground tissue in 6 volumes (vol/gFW) of MES buffer (pH 7.0) containing 5mM EDTA, 5% w/v PVPP, serine proteinase inhibitors dithiothreitol (20mM), and benzamidine (2.5mM). Cell-wall bound invertase was assayed in the washed pellets and supernatants were pooled for soluble invertase measurements. Sucrose cleaved by cell-wall and soluble invertase fractions was assayed separately by the generation of glucose monomers during a 15 minute incubation period (pH 4.5, 37°C). Invertase activity was quantified colorimetrically by adding 100 μl of Sumner’s reagent to an equal volume of assay fraction in a 96-well plate (Greiner Bio-One) and incubating at 105°C for 10 min; 33 μl of 40% Rochelle salt was then added and absorbance was recorded immediately at 562 nm. Invertase activity was quantified (μM sucrose cleaved/gFW/min) by standard curve method using glucose as a standard.
Phenolics Extraction and Quantification
Individual leaves and roots were freeze-dried (4–6mg DW) and ground in a Talboys High Throughput Homogenizer (Troemner, NJ, USA). Phenolics were extracted overnight in 200 μl of 1% (v/v) HCl in methanol at 4°C. An additional extraction with 250 μl distilled water and 500 μl chloroform was used to remove chlorophyll from all samples [32]. Samples were vortexed and centrifuged for 3 min at 3000 × g. Relative anthocyanin levels in the aqueous phase were determined spectrophotometrically by measuring absorbance at 530nm. Total flavonoid compounds were also estimated in the same extracts at absorbance 320nm [33, 34]. The concentration of total reactive phenolics present in leaf extracts was determined using the Folin-Denis assay. Standard curves were developed using chlorogenic acid as a standard [35].
Statistical Analysis
The effect of P. rapae and S. exigua feeding on local and systemic induction was assessed with two-way analysis of variance (PROC ANOVA) or general linear models (unequal sample sizes, PROC GLM). These procedures were followed by Tukey’s HSD post hoc comparisons to determine individual insect effects relative to controls, as well as differences between the insect species. Normality and equality of variance were verified using Kolmogorov-Smirnov and Levene’s tests, respectively. All statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Insect Induction
Measurements of invertase activity and phenolic accumulation demonstrate that herbivore-induced response patterns in A. thaliana are controlled, in part, by the vascular architecture of the plant (Fig 1). The activity of cell-wall invertase, the form of this enzyme associated with tissue sink strength, was altered by both P. rapae and S. exigua feeding, and the magnitude of such changes was dependent on the age and location of a given leaf relative to the site of herbivore damage. Both herbivores increased cell-wall invertase locally (target L7), as well as within orthostichous source and sink leaves (Fig 1B; P. rapae L2 P = 0.0002, L10 P < 0.0001; S. exigua L2 P < 0.0001, L10 P < 0.0001). P. rapae feeding also increased cell-wall invertase in the youngest cohort of leaves harvested from the apex of the rosette (P = 0.0311), whereas S. exigua did not (P = 0.1714). Cell-wall invertase activity of undamaged source leaves was frequently increased following herbivore damage (Fig 1B; P. rapae L11 P = 0.0400; S. exigua L8 P = 0.0072, L11 P = 0.0090). S. exigua feeding increased soluble invertase within orthostichous, source leaves (Fig 1C; L10 P = 0.059) while damage by P. rapae suppressed soluble invertase locally (L7 P = 0.0175), and within undamaged, systemic leaves (L3 P = 0.0179; L8 P = 0.0148).
P. rapae feeding increased the accumulation of total phenolic compounds locally (Fig 1D; L7 P = 0.0013), as well as in the young leaf directly above the damaged leaf (Fig 1D; L2 P = 0.0410). Similar patterns of induction were observed following feeding by S. exigua; however, only induction in young, orthostichous sinks could be supported statistically (Fig 1D; L7 P = 0.1200; L10 P = 0.2402; L2 P = 0.0441). For both insect species, the accumulation of phenolics in orthostichous sinks co-occurred with visible reddening of leaf petioles during the course of the experiment (Figure A in S1 File). Reddening corresponded with an increase in local anthocyanin production (Fig 1E; L7 P. rapae P = 0.0260; S. exigua P = 0.0001). Anthocyanin accumulation was also observed for sink leaves outside of the attacked orthostichy (Fig 1E; S. exigua L3 P = 0.0361) and the youngest cohort of leaves (Fig 1E; P. rapae P = 0.0600). Feeding by S. exigua suppressed flavonoid production locally (Fig 1F; L7 P = 0.0005) and in middle-aged leaves outside of the damaged orthostichy (Fig 1F; L8 P = 0.0003), while P. rapae feeding marginally reduced flavonoids within young, undamaged systemic leaves (Fig 1F; P = 0.0516).
Source Constraint on Induction
Removing source leaves alone not only increased total phenolics in A. thaliana plants, but was found to significantly influence the magnitude and direction of leaf responses following herbivory (Fig 2A and 2B and Table A in S1 File, significant effect of source leaf removal P = 0.0373; interaction with herbivory P < 0.0001). In undamaged control plants, removing source leaves reduced anthocyanin accumulation in middle-aged (Table B in S1 File, P = 0.0020; Fig 2C) and young, systemic leaves (Fig 2D), but did not incur significant changes to flavonoid levels (Fig 2E and 2F and Table C in S1 File, P = 0.1436). In plants with intact source leaves, we found that P. rapae and S. exigua suppressed flavonoids locally (Fig 2E; P < 0.05), but elicited no changes to undamaged, systemic leaves (Fig 2F). We also observed a tendency for young, systemic leaves to accumulate phenolics upon damage by P. rapae and S. exigua to intact plants (Fig 2B). In contrast, the concentrations of all phenolic compounds were significantly reduced in young leaves of plants where source leaves were removed following damage by either P. rapae or S. exigua (Fig 2B, 2D and 2F).
In general, middle-aged leaves of suc2-1 plants contained lower levels of total phenolic compounds (Fig 3A and Table D in S1 File, significant effect of genotype P = 0.0083), anthocyanins (Fig 3C and Table E in S1 File) and flavonoids (Fig 3E and Table F in S1 File) relative to their WT counterparts. While P. rapae and S. exigua did not elicit local or systemic changes to total phenolics (Fig 3A and 3B) or anthocyanins (Fig 3C and 3D), flavonoid levels were significantly suppressed in locally damaged leaves of WT plants (Fig 3E). S. exigua also suppressed flavonoids in young, undamaged systemic leaves (Fig 3F). In contrast to WT responses, both herbivores elicited an increase in local flavonoid levels within suc2-1 plants relative to control (Fig 3E).
Fig 3. Herbivore-induced changes in foliar phenolic accumulation is independent of intact AtSUC2 function.
Herbivore-damaged leaves (A,C,E), and young, systemic leaves (B,D,F) are shown in red. Total phenolics (A,B), anthocyanins (C,D) and flavonoids (E,F) were measured 48h following damage by P. rapae or S. exigua larvae to wild-type (WT) and AtSUC2-silenced plants (suc2-1). Bars represent means ±SE. n = 8/treatment group. Results of analysis of variance are summarized in Tables D-F in S1 File. Asterisks indicate significant differences between clip-caged control and herbivore-damaged plants for a given leaf type (P < 0.05 Tukey’s post hoc comparisons).
Discussion
Here we show that plant architecture generates fine scale spatial variation in the systemic induction of invertase and phenolic compounds. These patterns of induction could be predicted by orthostichous sectors and known vascular connections between source and sink leaves previously identified for A. thaliana [20–23]. These findings align with those in Populus [36], tomato [9], and tobacco [10] demonstrating strong vascular control over patterns of systemic induction. The observed increase in cell-wall invertase in young leaves following herbivore damage is consistent with our previous work demonstrating that the ability of developing leaves to respond defensively to jasmonates, wounding, or herbivory relies on their capacity to induce sink strength for carbon-based resources by increasing cell-wall invertase and importing carbon from orthostichous source leaves [17, 18]. Young sink leaves typically have little in the way of endogenous reserves [37], and often have lower photosynthetic rates compared to mature source leaves, yet are often the most responsive to herbivores [38]. As a result, an increase in invertase activity within sink leaves can act to facilitate greater transport of carbohydrates between source and sink tissues to sustain growth and provide potential building blocks to support the production of carbon-based metabolites, as we have confirmed with labelling studies [17, 18, 22, 23]. Interestingly, we also found that mature source leaves located beneath the damaged leaf also exhibited higher invertase activities following herbivore damage. Given this result, it is also possible that young leaves exhibit induced sink strength by proxy, in which their phenolic accumulation occurs as a byproduct of increased invertase activities in their orthostichous source leaves, creating a need to dispose of excess sugars [39].
The increase in phenolics observed within young, sink leaves in our studies are in agreement with previous findings that an up-regulation of gene transcripts and enzyme activities associated with phenylpropanoid biosynthesis, and the accumulation of phenolic metabolites in leaves following herbivore damage or treatment with jasmonates [17, 40, 41]. Optimal defense theory predicts that the induction of phenolics in young leaves in particular may be favored as these leaves have greater current and future photosynthetic potential for the plant, and are therefore of greater value to potential fitness. In fact, this hypothesis is in agreement with a previous study in A. thaliana showing that the loss of young leaves incurs more negative effects on reproductive fitness compared to other tissues [42]. In tobacco, young leaves at the apex of the plant are also found to respond most strongly to herbivory with the accumulation of proteinase inhibitor and threonine deaminase transcripts when leaves at a lower nodal position are damaged [10]. This species is also capable of targeting nicotine defense to leaves of higher fitness value, which are not always the wounded tissues [43].
Here we show that within plants, systemic regions orthostichous to sites of herbivore damage accumulate phenolics to a greater extent than non-orthostichous regions. Such constraints in the pattern of induction may be ecologically relevant if they occur at the expense of resistance in non-orthostichous sectors of the plant. Indeed, this has been supported by work in Solanum dulcamara where induced resistance in orthostichous leaves coincided with an increase in susceptibility of non-orthostichous leaves to potato beetle, Lema trilinea [44]. Since all plants are comprised of modular units defined by plant vasculature, the distribution of signals and resources may also influence where and when herbivores feed. Within-plant and within-leaf resource heterogeneity may also affect the performance and behavior of insect herbivores that exhibit preferences among leaves on a plant. Spatial and temporal variation in leaf quality may force herbivores to make compromises between diet quality and the costs of foraging [3], which may subsequently reinforce defensive traits in plants that are associated with heterogeneity of induced responses [45].
In our study, responses in systemic leaves were not only common, but also showed unique patterns for P. rapae and S. exigua. This is consistent with herbivore-specific responses observed in other systems [46, 47] and may be attributed to herbivore-specific salivary components and their ability to elicit different hormonal and metabolic effects in plants [48]. Despite these overall differences, both P. rapae and S. exigua appeared to suppress flavonoid accumulation within the leaf they fed on. This is consistent with our previous work observing transcriptional changes in A. thaliana after herbivory which found that plant responses to both P. rapae and S. exigua differed greatly and were frequently weaker or absent in response to the specialist, P. rapae [49, 50]. Indeed, feeding by P. rapae and S. exigua both down-regulated the expression of known transcriptional regulators of flavonoid biosynthesis, including members of the ERF/AP2, MYB, Homeobox, C2H2, BHLH and WRKY gene families within damaged leaves in A. thaliana [49]. The suppression of secondary metabolites by herbivores is often thought to be mediated by hormone release in plants, including salicylic acid, ethylene and jasmonic acid, which may interact to fine-tune the plant response towards specific attackers [51]. Future studies may explore how such mechanisms may regulate flavonoid levels in this system following herbivore attack.
We found that plants with source leaves removed contained constitutively higher total phenolic levels compared to intact plants. This suggests that the removal of subtending source leaves increased accumulation of carbohydrates into the strongest sinks of the rosette (the young leaves remaining on the plant). As we expected, phenolic induction did not occur in leaves that could not import resources from source tissues below. In fact, in systemic leaves the concentrations of total Folin-reactive phenolics, anthocyanins, and flavonoids were all suppressed following insect damage on plants without intact source leaves. Similar findings have been observed in Populus when carbohydrate transport between source and sink leaves was disrupted via steam girdling and sink leaves of girdled plants failed to accumulate phenolics and tannins to the same extent following exposure to jasmonic acid [17, 18].
Systemic induction is likely determined by the impact of phyllotaxy on signaling and material movement through the plant. The type of signal (phloem- or xylem-based, or both) elicited by herbivore feeding determines the spatial and temporal pattern of systemic responses in addition to the impact of vascular architecture itself, and there is evidence for both phloem and xylem transport of signal molecules associated with chemical induction [52]. In A. thaliana, we found that invertases were commonly induced in source leaves located below the damaged leaf; however this induction did not correspond to changes in phenolic chemistry in any of the classes measured within these mature leaves. This finding sheds light on the directionality of induction and is in agreement with the existence of phloem-based signaling during systemic induction. Yet, the fact that we observed induction of flavonoids in suc2-1 plants argues against the hypothesis that all responses depend on functional phloem loading. This result is interesting as it suggests that xylem-based signaling or greater mass flow driven by high sieve element and plasmodesmatal densities in developing leaves [53, 54] may facilitate signaling between damaged and un-damaged tissues in suc2-1 plants.
While vascular architecture was the most consistent predictor of systemic patterns of induction, we found that both a specialist and generalist herbivore frequently induced changes in invertase and phenolic compounds in undamaged leaves located outside of the attacked orthostichy. While plant phyllotaxy links source and sink tissues by a common transport stream, minor connections among other leaves on the stem still exist that may facilitate such responses [28]. Indeed, our recent work demonstrates that the glucose surrogate, 2-[18F]fluoro-2-deoxy-D-glucose, is capable of traveling to leaves that are non-orthostichous to the tracer administration site, despite that plant’s highly sectorial vascular architecture, which consists of the leaf petiole that received the radiolabel, and the vertical column of younger and older leaves above and below it [23]. Induction outside of a damaged orthostichy may result from developmentally decreased sensitivity to elicitation (young leaves are more inducible than older leaves) or reflect greater connectivity among orthostichies in young leaves whose vascular connections are not fully matured [54]. Given the compact nature of A. thaliana rosette leaves, it is also likely that within-plant volatile signaling may act as a mechanism to bypass vascular constraints in systemic induction [55]. While this phenomenon has been well-documented in other species, including Populus [56] and lima bean [57], further studies are required to determine whether A. thaliana uses such mechanisms to facilitate signaling across orthostichies.
In summary, we found that systemic responses in A. thaliana rosettes were best predicted by orthostichy, were often herbivore-specific, and relied, in part, on intact vasculature between source and sink leaves. While induction was most apparent within leaves of the herbivore-damaged orthostichy, our results also provide evidence that induction is sometimes less constrained and more integrated in A. thaliana than previously described. Nevertheless, future studies which aim to describe herbivore-induced plant responses will benefit from taking into account the potential constraints imposed by plant vascular architecture.
Supporting Information
White arrows point to petioles of young leaves where color differences were evident. Table A, Source leaf removal increases total phenolics in A. thaliana plants. Summary of 3-way ANOVA comparing total phenolics within two herbivore-damaged leaves and young, systemic leaves of intact A. thaliana rosettes or those with source leaves removed. Table B, Source leaf removal reduces anthocyanin accumulation in A. thaliana plants. Summary of 3-way ANOVA comparing anthocyanin accumulation within two herbivore-damaged leaves and young, systemic leaves of intact A. thaliana rosettes or those with source leaves removed. Table C, Source leaf removal does not influence flavonoid accumulation in A. thaliana plants. Summary of 3-way ANOVA comparing flavonoid accumulation within two herbivore-damaged leaves and young, systemic leaves of intact A. thaliana rosettes or those with source leaves removed. Table D, Middle-aged leaves of suc2-1 plants contain lower levels of total phenolic compounds relative to WT plants. Summary of 3-way ANOVA comparing total phenolics within two herbivore-damaged leaves and young, systemic leaves of wild-type (Ws-2) and mutant (suc2-1) lines. Table E, Middle-aged leaves of suc2-1 plants contain lower levels of anthocyanin relative to WT plants. Summary of 3-way ANOVA comparing anthocyanin accumulation within two herbivore-damaged leaves and young, systemic leaves of wild-type (Ws-2) and mutant (suc2-1) lines. Table F, Middle-aged leaves of suc2-1 plants contain lower levels of flavonoids relative to WT plants. Summary of 3-way ANOVA comparing flavonoid accumulation within two herbivore-damaged leaves and young, systemic leaves of wild-type (Ws-2) and mutant (suc2-1) lines.
(PDF)
Acknowledgments
We would like to thank members of the Schultz-Appel lab, especially Dean Bergstrom and Clayton Coffman, for assistance with experiments, and three reviewers who provided helpful comments on an earlier version of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Research was supported by National Science Foundation grant IOS-0805272 to JCS and HMA, and the University of Missouri Life Sciences Fellowship Program, which supported APF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schultz JC, Appel HM, Ferrieri AP, Arnold TM. Flexible resource allocation during plant defense responses. Front Plant Sci 2013;4:324 Epub 2013/08/30. 10.3389/fpls.2013.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whitham T, Slobodchikoff C. Evolution by individuals, plant-herbivore interactions, and mosaics of genetic variability: the adaptive significance of somatic mutations in plants. Oecologia. 1981;49(3):287–92. [DOI] [PubMed] [Google Scholar]
- 3. Schultz J. Habitat selection and foraging tactics of caterpillars in heterogeneous trees In: Denno R, McClure M, editors. Variable Plants and Herbivores in Nautral and Managed Systems. New York: Academic Press; 1983. pp. 61–86. [Google Scholar]
- 4. Whitham T. Host manipulation of parasites: within-plant variation as a defense against rapidly evolving pests In: Denno R, McClure M, editors. Variable Plants and Herbivores in Nautral and Managed Systems. New York: Academic Press; 1983. pp. 15–41. [Google Scholar]
- 5. Schultz J, Baldwin I. Oak leaf quality declines in response to defoliation by Gypsy moth larvae. Science. 1982;217:149–151. [DOI] [PubMed] [Google Scholar]
- 6. Barton K, Koricheva J. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Amer Nat. 2010;175(4):481–493. 10.1086/650722 [DOI] [PubMed] [Google Scholar]
- 7. Larson P, Dickson R. Distribution of imported 14C in developing leaves of eastern cottonwood according to phyllotaxy. Planta. 1973;111(2):95–112. 10.1007/BF00386270 [DOI] [PubMed] [Google Scholar]
- 8. Van Dam NM, Horn M, Mareš M, Baldwin IT. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata . J Chem Ecol. 2001;27(3):547–568. [DOI] [PubMed] [Google Scholar]
- 9. Orians C, Pomerleau J, Ricco R. Vascular architecture generates fine scale variation in systemic induction of proteinase inhibitors in tomato. J Chem Ecol. 2000;26(2):471–485. [Google Scholar]
- 10. Schittko U, Baldwin I. Constraints to herbivore-induced systemic responses: bidirectional signaling along orthostichies in Nicotiana attenuata . J Chem Ecol. 2003;29(3):763–770. [DOI] [PubMed] [Google Scholar]
- 11. Orians C. Herbivores, vascular pathways, and systemic induction: facts and artifacts. J Chem Ecol. 2005;31(10):2231–2242. [DOI] [PubMed] [Google Scholar]
- 12. Thorpe M, Ferrieri A, Herth M, Ferrieri R. 11C-imaging: methyl jasmonate moves in both phloem and xylem, promotes transport of jasmonate, and of photoassimilate even after proton transport is decoupled. Planta. 2007;226(2):541–551. [DOI] [PubMed] [Google Scholar]
- 13. Rhodes J, Thain J, Wildon D. Evidence for physically distinct systemic signalling pathways in the wounded tomato plant. Ann Bot. 1999;84(1):109–16. [Google Scholar]
- 14. Ryan CA. The systemin signaling pathway: differential activation of plant defensive genes. Biochim Biophys Acta. 2000;1477(1–2):112–121. [DOI] [PubMed] [Google Scholar]
- 15. Bowles D. Signal transduction in the wound response of tomato plants. Philos Trans R Soc London (Biol). 1998;353(1374):1495–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shulaev V, León J, Raskin I. Is salicylic acid a translocated signal of systemic acquired resistance in tobacco? Plant Cell. 1995;7(10):1691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnold T, Appel H, Patel V, Stocum E, Kavalier A, Schultz J. Carbohydrate translocation determines the phenolic content of Populus foliage: a test of the sink-source model of plant defense. New Phytol. 2004;164(1):157–164. [DOI] [PubMed] [Google Scholar]
- 18. Arnold T, Schultz J. Induced sink strength as a prerequisite for induced tannin biosynthesis in developing leaves of Populus . Oecologia. 2002;130(4):585–593. [DOI] [PubMed] [Google Scholar]
- 19. Callos J, Medford J. Organ positions and pattern formation in the shoot apex. Plant J. 1994;6(1):1–7. [Google Scholar]
- 20. Kiefer I, Slusarenko A. The pattern of systemic acquired resistance induction within the Arabidopsis rosette in relation to the pattern of translocation. Plant Physiol. 2003;132(2):840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberts K, Love A, Laval V, Laird J, Tomos A, Hooks M, et al. Long distance movement of Cauliflower mosaic virus and host defence responses in Arabidopsis follow a predictable pattern that is determined by the leaf orthostichy. New Phytol. 2007;175(4):707–717. [DOI] [PubMed] [Google Scholar]
- 22. Ferrieri AP, Agtuca B, Appel HM, Ferrieri RA, Schultz JC. Temporal changes in allocation and partitioning of new carbon as 11C elicited by simulated herbivory suggest that roots shape aboveground responses in Arabidopsis . Plant Physiol. 2013;161(2):692–704. 10.1104/pp.112.208868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrieri AP, Appel H, Ferrieri RA, Schultz JC. Novel application of 2-[18F]fluoro-2-deoxy-d-glucose to study plant defenses. Nuc Med and Bio. 2012;39(8):1152–60. 10.1016/j.nucmedbio.2012.06.005. 10.1016/j.nucmedbio.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 24. Kearsley M, Whitham T. The developmental stream of cottonwoods affects ramet growth and resistance to galling aphids. Ecology. 1998;79(1):178–191. [Google Scholar]
- 25. Cipollini D, Bergelson J. Environmental and developmental regulation of trypsin inhibitor activity in Brassica napus . J Chem Ecol. 2000;26(6):1411–1422. [Google Scholar]
- 26. Agrawal A, Gorski P, Tallamy D. Polymorphism in plant defense against herbivory: constitutive and induced resistance in Cucumis sativus . J Chem Ecol. 1999;25(10):2285–2304. [Google Scholar]
- 27. McCartney N. Analysis of Pieris rapae and Spodoptera exigua feeding site preferences on Arabidopsis thaliana “Columbia” ecotype in relation to within-plant chemical and structural heterogeneity University Park, PA: MS, The Pennsylvania State University; 2007. [Google Scholar]
- 28. Haritatos E, Medville R, Turgeon R. Minor vein structure and sugar transport in Arabidopsis thaliana . Planta. 2000;211(1):105–111. [DOI] [PubMed] [Google Scholar]
- 29. Stadler R, Sauer N. The Arabidopsis thaliana AtSUC2 gene is specifically expressed in companion cells. Bot Act. 1996;109(4):299–306. [Google Scholar]
- 30. Gottwald J, Krysan P, Young J, Evert R, Sussman M. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA. 2000;97(25):13979–13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15(3):473–97. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- 32. Choi S, Kwon Y, Hossain M. A mutation in ELA1, an age-dependent negative regulator of PAP1/MYB75, causes UV-and cold stress-tolerance in Arabidopsis thaliana seedlings. Plant Sci. 2009;176(5):678–686. [Google Scholar]
- 33. Fukumoto L, Mazza G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J Agric Food Chem. 2000;48(8):3597–3604. [DOI] [PubMed] [Google Scholar]
- 34. Shao L, Shu Z, Peng C, Lin Z, Yang C, Gu Q. Enhanced sensitivity of Arabidopsis anthocyanin mutants to photooxidation: a study with fluorescence imaging. Func Plant Bio. 2008;35(8):714–724. 10.1097/OLQ.0b013e3181723d93 [DOI] [PubMed] [Google Scholar]
- 35. Appel HM, Govenor HL, D'Ascenzo M, Siska E, Schultz JC. Limitations of Folin assays of foliar phenolics in ecological studies. J Chem Ecol. 2001;27(4):761–78. Epub 2001/07/12. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 36. Davis J, Gordon M, Smit B. Assimilate movement dictates remote sites of wound-induced gene expression in poplar leaves. Proc Natl Acad Sci USA. 1991;88(6):2393–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kozlowski T, Pallardy S. Growth control in woody plants: Academic Press, Inc; San Diego, CA, USA; 1997. [Google Scholar]
- 38. Harper J. The value of a leaf. Oecologia. 1989;80(1):53–58. 10.1007/BF00789931 [DOI] [PubMed] [Google Scholar]
- 39. Arnold TM, Appel HM, Schultz JC. Is polyphenol induction simply a result of altered carbon and nitrogen accumulation? Plant Signal Behav. 2012;7(11):1498–1500. Epub 2012/09/11. 10.4161/psb.21900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hanik N, Gómez S, Best M, Schueller M, Orians C, Ferrieri R. Partitioning of new carbon as 11C in Nicotiana tabacum reveals insight into methyl jasmonate induced changes in metabolism. J Chem Ecol. 2010;36(10): 1058–1067. 10.1007/s10886-010-9835-x [DOI] [PubMed] [Google Scholar]
- 41. Richard S, Lapointe G, Rutledge R, Seguin A. Induction of chalcone synthase expression in white spruce by wounding and jasmonate. Plant Cell Phys. 2000;41(8):982–987. [DOI] [PubMed] [Google Scholar]
- 42. Barto EK, Cipollini D. Testing the optimal defense theory and the growth-differentiation balance hypothesis in Arabidopsis thaliana . Oecologia. 2005;146(2):169–178. [DOI] [PubMed] [Google Scholar]
- 43. Ohnmeiss TE, Baldwin IT. Optimal defense theory predicts the ontogeny of an induced nicotine defense. Ecology. 2000;81(7):1765–1783. [Google Scholar]
- 44. Viswanathan D, Thaler J. Plant vascular architecture and within-plant spatial patterns in resource quality following herbivory. J Chem Ecol. 2004;30(3):531–543. [DOI] [PubMed] [Google Scholar]
- 45. Orians C, Jones C. Plants as resource mosaics: a functional model for predicting patterns of within-plant resource heterogeneity to consumers based on vascular architecture and local environmental variability. Oikos. 2001;94(3):493–504. [Google Scholar]
- 46. Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001;125(2):711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steinbrenner AD, Gómez S, Osorio S, Fernie AR, Orians CM. Herbivore-induced changes in tomato (Solanum lycopersicum) primary metabolism: a whole plant perspective. J Chem Ecol. 2011;37(12):1294–1303. 10.1007/s10886-011-0042-1 [DOI] [PubMed] [Google Scholar]
- 48. Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009;150(3):1576–1586. 10.1104/pp.109.139550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rehrig EM, Appel HM, Jones AD, Schultz JC. Roles for jasmonate- and ethylene- induced transcription factors in the ability of Arabidopsis to respond differentially to damage caused by two insect herbivores. Front Plant Sci. 2014;5 10.3389/fpls.2014.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Appel HM, Fescemyer H, Ehlting J, Weston D, Rehrig EM, Joshi T, et al. Transcriptional responses of Arabidopsis thaliana to chewing and sucking insect herbivores. Front Plant Sci. 2014;in press. [DOI] [PMC free article] [PubMed]
- 51. Reymond P, Farmer E. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1(5):404–411. [DOI] [PubMed] [Google Scholar]
- 52. Stratmann J. Long distance run in the wound response-jasmonic acid is pulling ahead. Trends Plant Sci. 2003;8(6):247–250. [DOI] [PubMed] [Google Scholar]
- 53. Thorpe M, Minchin P, Gould N, McQueen J, Zwieniecki M, Holbrook N. The stem apoplast: a potential communication channel in plant growth regulation In: Holbrook NM, Zwieniecki M, editors. Vascular Transport in Plants. Oxford: Elsevier/AP Co-imprint; 2005. pp. 201–220. [Google Scholar]
- 54. Turgeon R. Phloem loading: how leaves gain their independence. BioSci. 2006;6:15–24. [Google Scholar]
- 55. Heil M, Ton J. Long-distance signalling in plant defence. Trends Plant Sci. 2008;13(6):264–272. 10.1016/j.tplants.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 56. Frost C, Appel H, Carlson J, De Moraes C, Mescher M, Schultz J. Within plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett. 2007;10(6):490–498. [DOI] [PubMed] [Google Scholar]
- 57. Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA. 2007;104(13):5467–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
White arrows point to petioles of young leaves where color differences were evident. Table A, Source leaf removal increases total phenolics in A. thaliana plants. Summary of 3-way ANOVA comparing total phenolics within two herbivore-damaged leaves and young, systemic leaves of intact A. thaliana rosettes or those with source leaves removed. Table B, Source leaf removal reduces anthocyanin accumulation in A. thaliana plants. Summary of 3-way ANOVA comparing anthocyanin accumulation within two herbivore-damaged leaves and young, systemic leaves of intact A. thaliana rosettes or those with source leaves removed. Table C, Source leaf removal does not influence flavonoid accumulation in A. thaliana plants. Summary of 3-way ANOVA comparing flavonoid accumulation within two herbivore-damaged leaves and young, systemic leaves of intact A. thaliana rosettes or those with source leaves removed. Table D, Middle-aged leaves of suc2-1 plants contain lower levels of total phenolic compounds relative to WT plants. Summary of 3-way ANOVA comparing total phenolics within two herbivore-damaged leaves and young, systemic leaves of wild-type (Ws-2) and mutant (suc2-1) lines. Table E, Middle-aged leaves of suc2-1 plants contain lower levels of anthocyanin relative to WT plants. Summary of 3-way ANOVA comparing anthocyanin accumulation within two herbivore-damaged leaves and young, systemic leaves of wild-type (Ws-2) and mutant (suc2-1) lines. Table F, Middle-aged leaves of suc2-1 plants contain lower levels of flavonoids relative to WT plants. Summary of 3-way ANOVA comparing flavonoid accumulation within two herbivore-damaged leaves and young, systemic leaves of wild-type (Ws-2) and mutant (suc2-1) lines.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.