Abstract
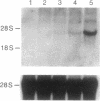
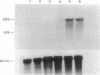
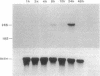
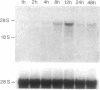
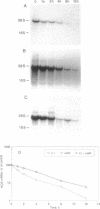
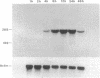
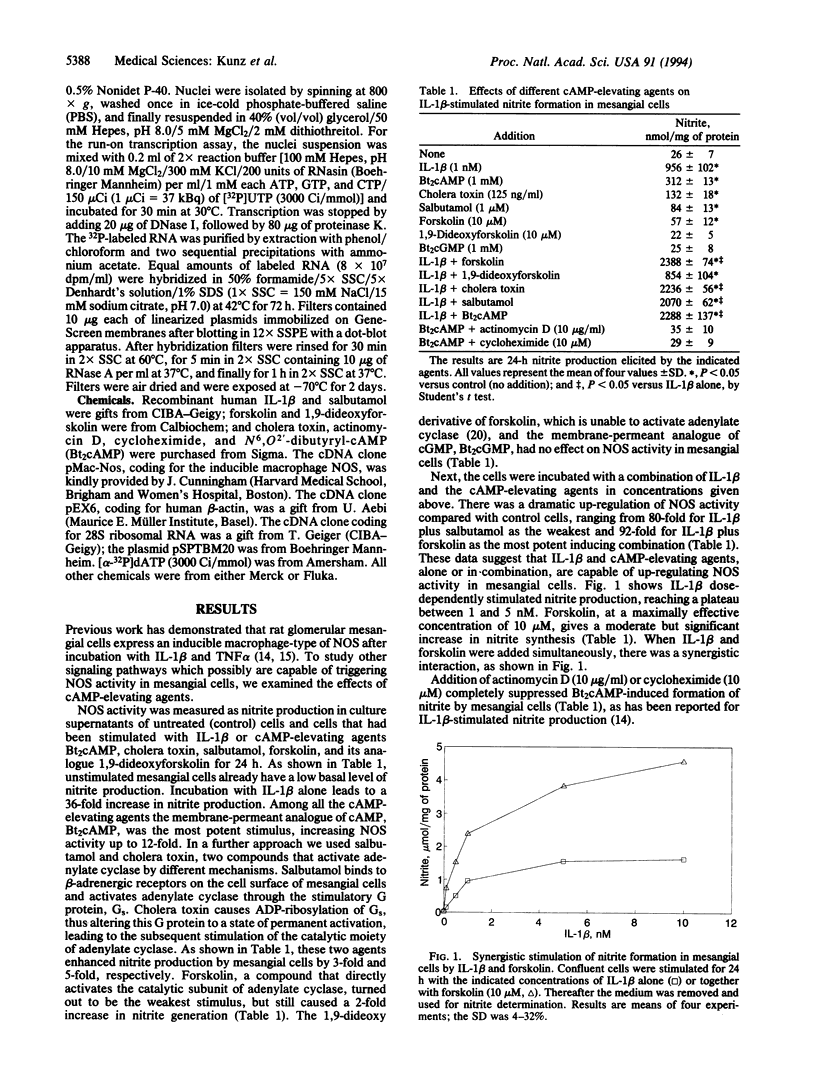
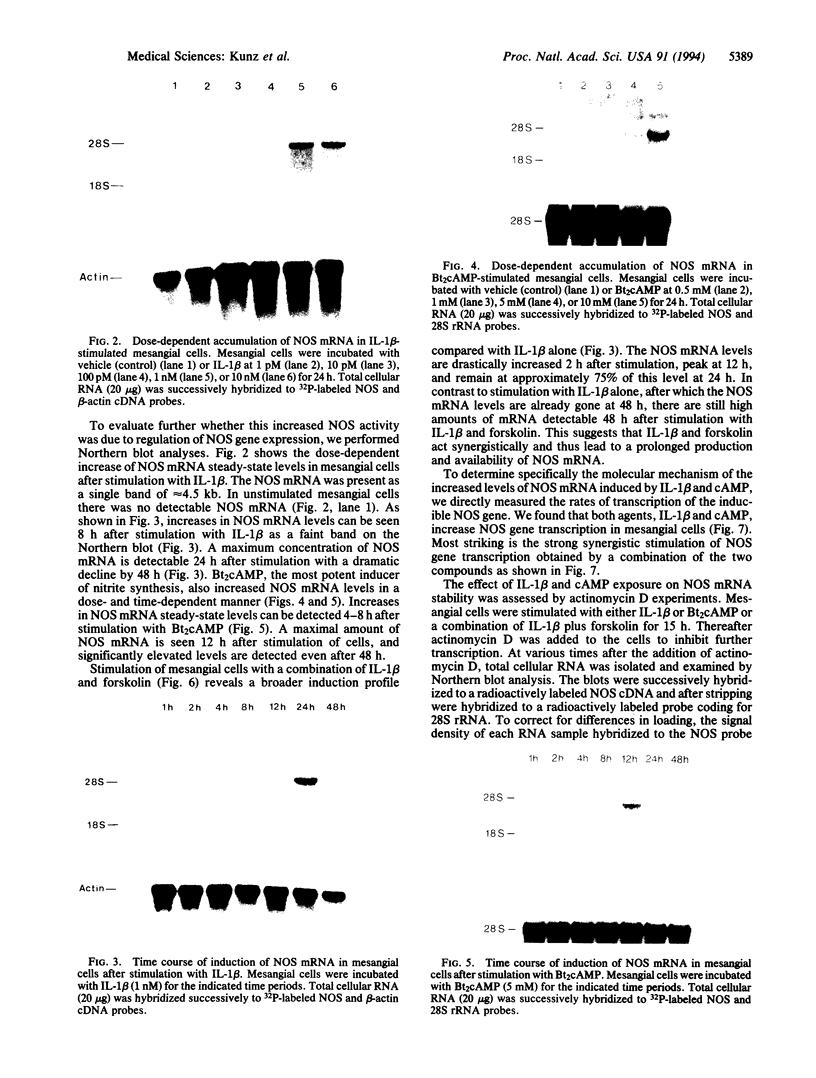
The expression of nitric oxide synthase (NOS; EC 1.14.13.39) is induced in rat glomerular mesangial cells by exposure to the inflammatory cytokine interleukin 1 beta (IL-1 beta) or cAMP-elevating agents. Stimulation with IL-1 beta alone leads to an approximately 40-fold increase in NOS activity and nitrite synthesis, whereas the elevation of cAMP with forskolin, cholera toxin, salbutamol, or dibutyryl-cAMP for 24 h resulted in a 2- to 12-fold increase in NOS activity. Moreover, the combinations of IL-1 beta with each of the cAMP-elevating agents greatly enhanced NOS activity in a synergistic fashion. Northern-blot analysis demonstrated a single band of approximately 4.5 kb for the NOS mRNA in rat mesangial cells. IL-1 beta increased NOS mRNA levels in a dose- and time-dependent fashion with a peak of NOS mRNA at 24 h. Dibutyryl-cAMP also increased NOS mRNA levels in mesangial cells in a dose- and time-dependent manner. Furthermore, combination of IL-1 beta and forskolin revealed a strong synergy with maximal mRNA levels 12 h after stimulation. Nuclear run-on transcription experiments suggest that IL-1 beta and cAMP synergistically interact to increase NOS gene expression at the transcriptional level. Furthermore, message stability studies established that NOS mRNA induced by cAMP has a longer half-life than the IL-1 beta-induced message. Moreover, cAMP exposure markedly prolonged the half-life of NOS mRNA from 1 h to 3 h. These data suggest that the level of NOS mRNA is controlled by at least two different signaling pathways, one involving cAMP and the other being triggered by cytokines such as IL-1 beta. The two pathways act synergistically and thus potently up-regulate the expression of inducible NOS in rat mesangial cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieu-Trautmann O., Fédérici C., Créminon C., Foignant-Chaverot N., Roux F., Claire M., Strosberg A. D., Couraud P. O. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol. 1993 Apr;155(1):104–111. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Cagliero E., Björklund A., Welsh N. Interleukin-1 beta induces the expression of an isoform of nitric oxide synthase in insulin-producing cells, which is similar to that observed in activated macrophages. FEBS Lett. 1992 Aug 24;308(3):249–252. doi: 10.1016/0014-5793(92)81285-t. [DOI] [PubMed] [Google Scholar]
- Gaillard T., Mülsch A., Klein H., Decker K. Regulation by prostaglandin E2 of cytokine-elicited nitric oxide synthesis in rat liver macrophages. Biol Chem Hoppe Seyler. 1992 Sep;373(9):897–902. doi: 10.1515/bchm3.1992.373.2.897. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Geller D. A., Nussler A. K., Di Silvio M., Lowenstein C. J., Shapiro R. A., Wang S. C., Simmons R. L., Billiar T. R. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Kolb-Bachofen V., Berschick B., Burkart V., Kolb H. Activated macrophages kill pancreatic syngeneic islet cells via arginine-dependent nitric oxide generation. Biochem Biophys Res Commun. 1991 Mar 29;175(3):752–758. doi: 10.1016/0006-291x(91)91630-u. [DOI] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Lyons C. R., Orloff G. J., Cunningham J. M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992 Mar 25;267(9):6370–6374. [PubMed] [Google Scholar]
- Marsden P. A., Ballermann B. J. Tumor necrosis factor alpha activates soluble guanylate cyclase in bovine glomerular mesangial cells via an L-arginine-dependent mechanism. J Exp Med. 1990 Dec 1;172(6):1843–1852. doi: 10.1084/jem.172.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden P. A., Brock T. A., Ballermann B. J. Glomerular endothelial cells respond to calcium-mobilizing agonists with release of EDRF. Am J Physiol. 1990 May;258(5 Pt 2):F1295–F1303. doi: 10.1152/ajprenal.1990.258.5.F1295. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Anti-inflammatory steroids inhibit cytokine induction of nitric oxide synthase in rat renal mesangial cells. Eur J Pharmacol. 1991 Mar 19;195(1):179–180. doi: 10.1016/0014-2999(91)90399-b. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Cross-talk between transmembrane signalling systems: a prerequisite for the delicate regulation of glomerular haemodynamics by mesangial cells. Eur J Clin Invest. 1989 Aug;19(4):347–361. doi: 10.1111/j.1365-2362.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Kunz D., Mühl H. Nitric oxide: an inflammatory mediator of glomerular mesangial cells. Nephron. 1993;64(4):518–525. doi: 10.1159/000187394. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Platelet-derived growth factor inhibits cytokine induction of nitric oxide synthase in rat renal mesangial cells. Eur J Pharmacol. 1991 Dec 12;208(4):339–340. doi: 10.1016/0922-4106(91)90081-r. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Rob P., Mülsch A., Fandrey J., Vosbeck K., Busse R. Interleukin 1 beta and tumour necrosis factor alpha induce a macrophage-type of nitric oxide synthase in rat renal mesangial cells. Eur J Biochem. 1992 Jan 15;203(1-2):251–255. doi: 10.1111/j.1432-1033.1992.tb19854.x. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Schwarzenbach H. Interleukin 1 and tumor necrosis factor stimulate cGMP formation in rat renal mesangial cells. FEBS Lett. 1990 Oct 29;273(1-2):185–187. doi: 10.1016/0014-5793(90)81080-8. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Vosbeck K. Transforming growth factor beta 2 inhibits interleukin 1 beta- and tumour necrosis factor alpha-induction of nitric oxide synthase in rat renal mesangial cells. Biochem Biophys Res Commun. 1991 Mar 15;175(2):372–379. doi: 10.1016/0006-291x(91)91574-v. [DOI] [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., Schorer A. E., Raij L. Effects of endothelium-derived relaxing factor and nitric oxide on rat mesangial cells. Am J Physiol. 1990 Jan;258(1 Pt 2):F162–F167. doi: 10.1152/ajprenal.1990.258.1.F162. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993 Jun 1;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]