Abstract
Introduction/Purpose
Fracture risk is increased in patients with type 2 diabetes mellitus (DM2) despite normal areal bone mineral density (aBMD). DM2 is more common in African-Americans than in Caucasians. It is not known whether African-American women with DM2 have deficits in bone microstructure.
Methods
We measured aBMD at the spine and hip by DXA, and volumetric BMD (vBMD) and microarchitecture at the distal radius and tibia by HR-pQCT in 22 DM2 and 78 non-diabetic African-American women participating in the Study of Women Across the Nation (SWAN). We also measured fasting glucose and HOMA-IR.
Results
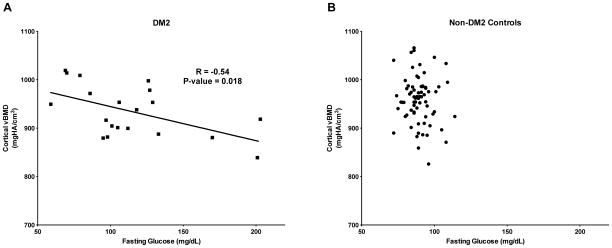
Age, weight, and aBMD at all sites were similar in both groups. At the radius, cortical porosity was 26% greater, while cortical vBMD and tissue mineral density were lower in women with DM2 than in controls. There were no differences in radius total vBMD or trabecular vBMD between groups. Despite inferior cortical bone properties at the radius, FEA-estimated failure load was similar between groups. Tibia vBMD and microarchitecture were also similar between groups. There were no significant associations between cortical parameters and duration of DM2 or HOMA-IR. However, among women with DM2, higher fasting glucose levels were associated with lower cortical vBMD (r=−0.54, p=0.018).
Conclusions
DM2 and higher fasting glucose are associated with unfavorable cortical bone microarchitecture at the distal radius in African-American women. These structural deficits may contribute to the increased fracture risk among women with DM2. Further our results suggest that hyperglycemia may be involved in mechanisms of skeletal fragility associated with DM2.
Keywords: diabetes mellitus type 2, African-American, HR-pQCT, bone microarchitecture, microfinite element analysis
Introduction
African-American women have a higher risk of developing type 2 diabetes mellitus (DM2) than other ethnic groups [1]. Fracture risk is increased in patients with DM2 even though they have normal or higher bone mineral density (BMD) than adults without diabetes [2, 3]. While it is possible that microvascular complications associated with DM2 may increase risk of falls [4, 5], fracture risk is elevated in diabetics even after accounting for their increased fall incidence [6, 7]. Moreover, it has recently become apparent that the effects of hyperglycemia may also have direct negative effects upon bone strength that may not be reflected in DXA measurements of aBMD [8].
High-resolution peripheral quantitative computed tomography (HR-pQCT) allows in vivo assessment of trabecular and cortical bone microarchitecture that may contribute to bone strength independently of vBMD. Using this technique, two recent publications have reported abnormalities in cortical bone microarchitecture in adults with DM2 [9, 10]. These studies are limited by small sample sizes and having racially-mixed populations in the DM2 and control groups. We previously demonstrated that, in comparison to Caucasians, African-American women have higher trabecular vBMD at the radius, higher cortical vBMD and lower cortical porosity at the tibia, and larger cortical area and thickness at both the radius and tibia [11].
Given these differences in bone microarchitecture by race and the high prevalence of DM2 in African Americans, we sought to determine whether the adverse effects of DM2 on cortical bone seen in Caucasians are also seen in African-American women. We hypothesized that, despite overall improvements in cortical microstructure in African-American women as a whole, African-American women with DM2 would have cortical bone abnormalities as compared to non-diabetic women. Furthermore, we examined whether microarchitectural deficits associated with DM2 impact bone strength, as estimated by microfinite element analysis (μFEA). Lastly, to explore possible mechanisms that may contribute to altered bone microarchitecture, we determined the association between hyperglycemia, insulin resistance and bone microarchitecture.
Materials and Methods
Study cohort
We studied a subset of African-American women (n=100) who were participating in The Study of Women’s Health Across the Nation (SWAN). Details of this subset of SWAN participants have been described in detail previously [11]. Briefly, SWAN is a multisite, multiethnic longitudinal study designed to characterize the biological and psychosocial changes that occur during the menopausal transition in a community-based cohort of 3302 women. All sites enrolled Caucasians, and each site also enrolled women belonging to one prespecified minority ethnic group. The Boston site specifically recruited African-American women. DXA and HR-pQCT measurements were performed at the Boston site in African-American women at study visit 11 or 12 (September 2008 – April 2011). The SWAN parent study and HR-pQCT substudy protocols were approved by the Institutional Review Board at MGH, and all women provided written informed consent.
Clinical data
Height and weight were measured using a fixed stadiometer and a digital scale with the participants wearing light clothing and no shoes. BMI was calculated as weight (in kilograms) divided by the square of height (in meters). Standardized interviews and self-administered questionnaires were used to obtain information on current clinical factors, including age (years), cigarette smoking (yes/no), alcohol intake (yes/no), medical diagnoses (including diabetes type 2), medication use, menopause stage, and physical activity (modified Baeke interview) [12]. History of fractures occurring after age 20 years was obtained by self–report. At the Boston SWAN site, all fractures in the preceding 15 years were confirmed by X–ray or physician reports. For the present analysis, fractures of the hand, foot, and face were excluded. Medications were self-reported, including any use of diabetes medications, steroids (defined by self report of glucocorticoid use >3 months at the baseline visit or report of use at ≥3 subsequent follow up visits), and/or osteoporosis medications (including all oral and intravenous bisphosphonates, selective estrogen receptor modulators, teriparatide, and calcitonin).
Diagnosis of DM2
Subjects were asked whether they had diabetes or used any medications for diabetes at every annual SWAN visit, from the baseline visit (1996–1997) through the current visits 11 (2008–2009) and 12 (2010–2011). Subjects were considered to have DM2 if they self-reported a history of diabetes or use of diabetic medications, or if they had a fasting blood glucose ≥126 mg/dL at any SWAN visit. Incident cases of DM2 were noted prospectively at the annual visits and allowed ascertainment of DM2 duration. Those subjects reporting history of diabetes at the baseline visit (n=7) were presumed to have a duration of diabetes of ≥12 years.
Glycemic indices
Serum insulin and glucose was measured from blood drawn after an overnight 12-h fast. Serum insulin was measured using a RIA procedure (Coat-a-Count; Diagnostic Products Corp., Los Angeles, CA). The quality control program for serum insulin in SWAN has been previously described [13]. Serum glucose was measured using a hexokinase-coupled reaction (Roche Molecular Biochemicals Diagnostics, Indianapolis, IN). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting serum glucose (in mg/dL) times fasting serum insulin (in uIU/mL) divided by the constant 405 [14].
Areal bone mineral density
Areal BMD (aBMD) of the posterior-anterior (PA) and lateral lumbar spine, total hip, femoral neck, and total body were measured by dual-energy X-ray absorptiometry, DXA (QDR4500A, Hologic Inc, Bedford, MA). The head was excluded from total body DXA measurements to avoid artifacts from metal jewelry and dental fillings. A standard quality control program was employed that included daily measurement of a Hologic DXA anthropomorphic spine phantom and visual review of every scan image by a local site investigator experienced in bone densitometry.
Volumetric bone density and bone microarchitecture measurements
On the same day as the DXA measurement, volumetric bone density and microarchitecture of the distal radius and tibia were assessed using high-resolution peripheral quantitative computed tomography (HR-pQCT, XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland) as previously described [11]. Quality control was maintained with daily scanning of the manufacturer’s phantom, as well as visual inspection of each HR-pQCT scan by an investigator experienced in this technology. The standard analysis program (Scanco software version V6.0) was used to calculate trabecular geometry, density, and microarchitecture. To characterize cortical microarchitecture in greater detail, HR-pQCT images were processed by a semi-automated cortical bone segmentation technique as previously described [15]. After image segmentation, measures were obtained for cortical geometry, density, and porosity. This more precise segmentation was also used to calculate trabecular area and total area. Linear microfinite element analysis (μFEA) was used to estimate stiffness and failure load following uniaxial compression as previously described [16].
Statistical Analysis
African-American women with DM2 were compared with a control group of African-American women without DM2. Clinical characteristics of women with and without DM2 were compared using independent samples two-sided t-tests and/or chi-square tests. Unadjusted differences in means of HR-pQCT parameters between women with and without DM2 were examined using independent samples two-sided t-tests. In addition, the group comparisons were repeated using a multivariate linear regression model (PROC REG) while adjusting for covariates that were significantly different between groups and/or might have a strong independent effect on skeletal outcomes (e.g. use of osteoporosis medications, thiazolinediones, or glucocorticoids). Three women (1 with DM2, 2 without) were not fasting at the time of the blood draw and were therefore excluded from fasting glucose and HOMA-IR analyses. Pearson’s correlations were used to test associations of HR-pQCT parameters with fasting glucose and HOMA-IR in the women with and without DM2. Women were also analyzed by categories of prediabetic/diabetic (fasting glucose ≥ 100 mg/dL) or normoglycemic (fasting glucose < 100 mg/dL). Statistical analysis was performed using SAS 9.3 software (SAS Institute Inc., Cary, NC). Data are reported as mean ± standard deviation (SD), unless otherwise noted.
Results
Cohort Characteristics
One hundred African-American women underwent HR-pQCT scanning of distal radius and tibia, of whom 22 had DM2 (Table 1). Women with and without DM2 were of similar age, weight/BMI, and had similar time since menopause. On average, women were 59.6 ± 2.6 years old and 94% of the cohort was postmenopausal at the time of the study visit. Women with DM2 were more likely to be active smokers (p=0.02). There were no differences in use of osteoporosis medications or glucocorticoids. As expected, women with DM2 had higher mean fasting glucose (p=0.02). DXA-based aBMD measurements at the spine, hip, and total body were similar in women with and without DM2. All of the 22 women with DM2 were taking diabetic medications, including 2 who were taking thiazolinediones. Median duration of DM2 was 11 years (range 1 to ≥12 years). Duration since diagnosis of DM2 was ≥10 years in 59% of women.
Table 1.
Cohort characteristics, mean ± SD
| DM2 n=22 |
Non-DM2 n=78 |
p-value (t-test or chi-square) | |
|---|---|---|---|
| Age, yr | 60.1 ± 2.8 | 59.4 ± 2.5 | 0.26 |
| Weight, kg | 87 ± 20 | 84 ± 19 | 0.58 |
| BMI, kg/m2 | 32 ± 7 | 31 ± 7 | 0.58 |
| Time since menopause, yr | 8.7 ± 2.9 | 7.7 ± 3.7 | 0.28 |
| Previous fracture, N (%) | 4 (18%) | 5 (6%) | 0.10 |
| Current smoking, N (%) | 8 (36%) | 10 (13%) | 0.02 |
| Current alcohol ≥1 drink/day, N (%) | 14 (64%) | 50 (64%) | 0.20 |
| Physical activity score* | 7.2 ± 2.1 | 7.8 ± 1.8 | 0.18 |
| Osteoporosis medication, N (%) | 8 (36%) | 29 (37%) | 1.00 |
| Glucocorticoid medication, N (%) | 4 (18%) | 9 (12%) | 0.47 |
| Fasting glucose, mg/dL | 115 ± 41 | 91 ± 12 | 0.02 |
| Fasting insulin, uIU/mL | 23 ± 29 | 14 ± 17 | 0.11 |
| HOMA-IR | 6.3 ± 7.4 | 3.5 ± 6.6 | 0.11 |
| Spine BMD, g/cm2 | 1.079 ± 0.220 | 1.061 ± 0.155 | 0.67 |
| Total hip BMD, g/cm2 | 1.019 ± 0.172 | 1.002 ± 0.144 | 0.64 |
| Femoral neck BMD, g/cm2 | 0.896 ± 0.130 | 0.873 ± 0.144 | 0.51 |
| Total body BMD, g/cm2 | 1.170 ± 0.120 | 1.154 ± 0.126 | 0.60 |
Scores range 3–9 with higher scores indicating increased physical activity(12)
Volumetric BMD, microarchitecture, and estimated strength
Although there were no differences in total or trabecular bone density, women with DM2 had worse cortical bone microarchitecture at the radius compared to controls (Figure 1, Table 2). Specifically, radius cortical vBMD was 3% lower (p=0.040), while cortical porosity was 26% higher (p = 0.025) in women with DM2 than in controls. Furthermore, radius cortical TMD was also 2% lower in diabetics (p=0.045), which reflects a lower bone tissue density independent of macroscopic cortical pores. Trabecular microarchitecture at the radius did not differ between groups. Bone density and microarchitecture at the tibia were similar in women with DM2 and controls. Estimates of failure load and stiffness at the radius and tibia were also similar between women with DM2 and controls (Table 2).
Figure 1.
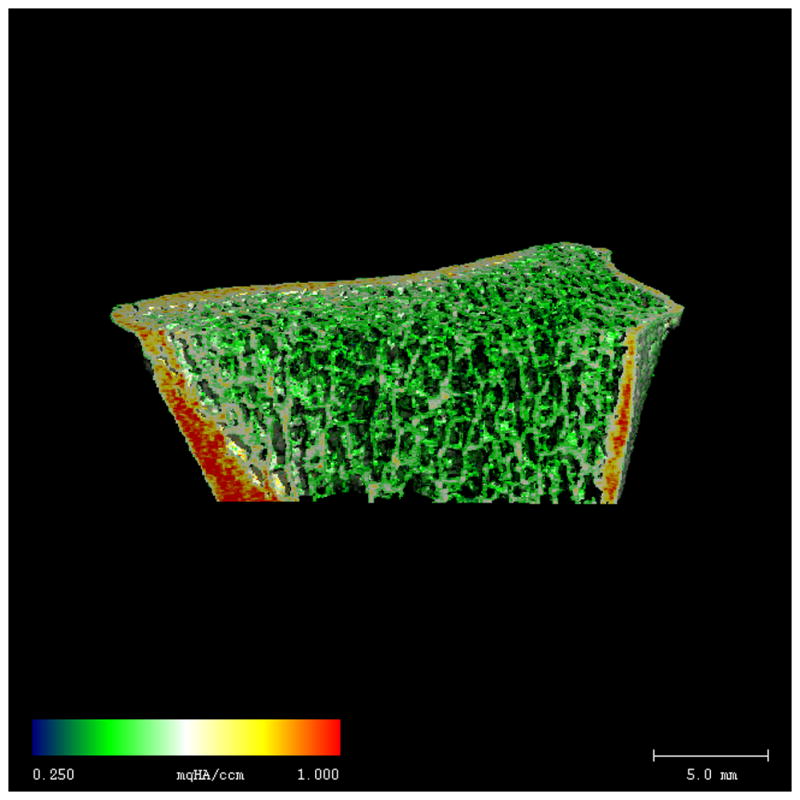
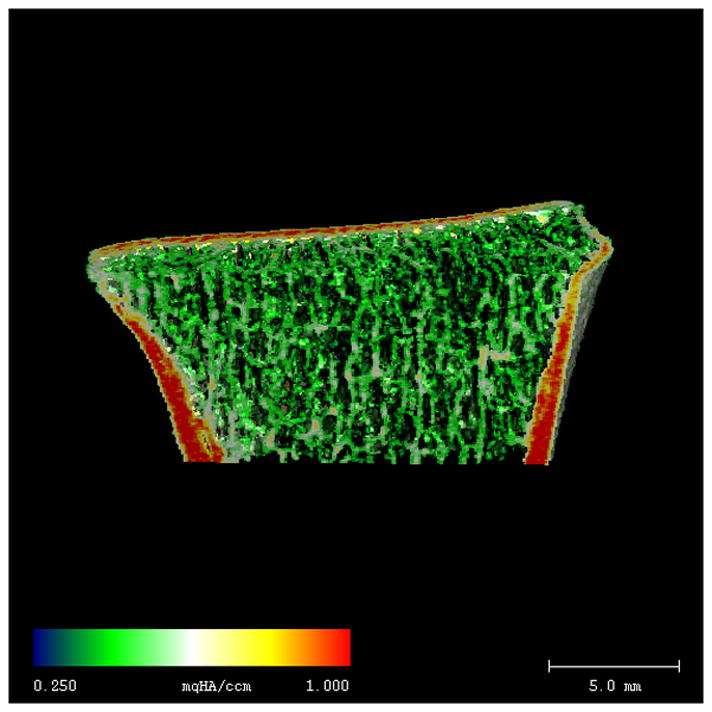
Radius HR-pQCT images from representative African-American women with DM2 (A) and non-DM2 (B). Color shading indicates ranges of BMD (lowest BMD = blue, highest BMD = red). Despite similar cortical size and thickness, cortical BMD is lower in DM2, as indicated by a more heterogeneous pattern of red/yellow shading within the cortex. Trabecular density and microstructure are similar in the DM2 and non-DM2 subjects.
Table 2.
Bone density, microarchitecture, and biomechanical parameters at the distal radius and tibia in African-American women with and without DM2 (mean ± SD)
| Radius | Tibia | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| DM2 (n=22) | Controls (n=78) | p-value | DM2 (n=22) | Controls (n=78) | p-value | |
| Total BMD (mgHA/cm3) | 308.6 ± 60.9 | 325.5 ± 73.3 | 0.325 | 287.3 ± 68 | 289.4 ± 53.8 | 0.878 |
| Geometry | ||||||
| Total Area (mm2) | 284.6 ± 42.4 | 269.4 ± 52.7 | 0.216 | 717.8 ± 107.0 | 712.4 ± 120.5 | 0.849 |
| Trabecular Area (mm2) | 227.4 ± 44.4 | 213.0 ± 53.6 | 0.254 | 599.9 ± 113.5 | 597.0 ± 124.0 | 0.921 |
| Cortical Area (mm2) | 60.4 ± 7.0 | 59.2 ± 10.8 | 0.547 | 123.1 ± 21.4 | 120.3 ± 23.6 | 0.622 |
| Cortical Area / Total area (%) | 21.7 ± 4.2 | 22.8 ± 6.1 | 0.433 | 17.6 ± 4.5 | 17.4 ± 4.6 | 0.830 |
| Cortical density and structure | ||||||
| Cortical BMD (mgHA/cm3) | 933.3 ± 52.7 | 957.7 ± 47.0 | 0.040 | 878.6 ± 65.1 | 894.9 ± 64.9 | 0.304 |
| Cortical Tissue Mineral Density (mgHA/cm3) | 975.8 ± 40.3 | 993.9 ± 35.4 | 0.045 | 975.3 ± 31.7 | 982.2 ± 35.3 | 0.450 |
| Cortical Thickness (mm) | 0.91 ± 0.14 | 0.93 ± 0.20 | 0.729 | 1.26 ± 0.26 | 1.23 ± 0.27 | 0.607 |
| Cortical Porosity (%) | 2.9 ± 1.5 | 2.3 ± 1.1 | 0.025 | 7.2 ± 2.8 | 6.3 ± 2.6 | 0.161 |
| Cortical Pore Volume (mm3) | 14.8 ± 7.5 | 11.5 ± 6.2 | 0.037 | 74.0 ± 25.0 | 63.4 ± 26.8 | 0.102 |
| Cortical Pore Diameter (mm) | 0.17 ± 0.02 | 0.16 ± 0.02 | 0.319 | 0.19 ± 0.03 | 0.18 ± 0.03 | 0.169 |
| Trabecular density and structure | ||||||
| Trabecular BMD (mgHA/cm3) | 156.7 ± 44.9 | 160.4 ± 36.0 | 0.690 | 163.6 ± 41.7 | 165.9 ± 33.7 | 0.791 |
| Trabecular Number (1/mm) | 1.83 ± 0.4 | 1.89 ± 0.32 | 0.483 | 1.79 ± 0.43 | 1.87 ± 0.34 | 0.341 |
| Trabecular Thickness (mm) | 0.071 ± 0.014 | 0.071 ± 0.014 | 0.934 | 0.077 ± 0.012 | 0.075 ± 0.014 | 0.570 |
| Trabecular Separation (mm) | 0.519 ± 0.235 | 0.475 ± 0.101 | 0.208 | 0.514 ± 0.145 | 0.477 ± 0.097 | 0.274 |
| Heterogeneity of Network (mm) | 0.234 ± 0.146 | 0.211 ± 0.087 | 0.486 | 0.261 ± 0.137 | 0.22 ± 0.069 | 0.060 |
| Biomechanics | ||||||
| Strength (kN/mm) | 75.5 ± 15.6 | 74.6 ± 14.6 | 0.792 | 201.4 ± 34.1 | 200.1 ± 43.8 | 0.882 |
| Failure Load (N) | 3774 ± 765 | 3744 ± 719 | 0.867 | 10193 ± 1678 | 10073 ± 2200 | 0.784 |
Differences in cortical bone microstructure and density between women with DM2 and controls persisted after adjustment for smoking with minimal change in the point estimates (Supplemental Table 1), although some parameters were no longer statistically significant. Additional adjustments for osteoporosis medication use, glucocorticoid use, and thiazolinedione use did not substantially alter the results. Within the diabetic group, there were no differences in cortical microarchitecture by prevalent fracture status or by disease duration.
Correlations with fasting glucose and insulin resistance
Among the women with DM2, fasting glucose was negatively associated with cortical vBMD (r=−0.54, p=0.018) and cortical TMD (r=−0.58, p=0.009) at the radius (Figure 2A). Significant associations were not observed between fasting glucose and cortical vBMD or TMD among the controls (Figure 2B). Overall, women with fasting glucose ≥100 mg/dL had significantly greater cortical porosity (p=0.043) and cortical pore volume (p=0.009) than those with fasting glucose <100 mg/dL. There were no significant associations between fasting glucose and microarchitectural parameters at the tibia. Lastly, there were no significant associations between HOMA-IR and microarchitectural parameters at the radius or tibia.
Figure 2.
Scatterplot of cortical vBMD at the radius versus fasting glucose among African-American women with DM2 (A) and non-DM2 controls (B). Among women with DM2, cortical vBMD is negatively associated with fasting glucose (r = −0.54, p=0.018).
Discussion
In this cross-sectional study of African-American women, most of whom were postmenopausal, we found that women with DM2 had lower cortical density and worse cortical microarchitecture at the distal radius. These cortical defects occurred despite being of similar age and weight, and having similar DXA-measured aBMD at the spine, hip and total body as controls. Furthermore, the differences in cortical porosity persisted after adjustment for smoking, use of osteoporosis medications, and glucocorticoids. These results demonstrate that HR-pQCT is providing information about skeletal fragility above and beyond what is possible with standard bone densitometry. Furthermore, the association of high fasting glucose with worse cortical parameters suggests that hyperglycemia may mediate these negative skeletal effects.
Our findings are consistent with two studies that identified high cortical porosity at the distal radius in mostly Caucasian women with DM2 [9], and in women with DM2 and prevalent fracture [10]. In contrast, other studies did not identify any differences in peripheral bone structure in subjects with DM2 as assessed by HR-pQCT [17, 18]. We did not find any significant differences in volumetric density or microarchitecture at the tibia, suggesting that the negative skeletal effects of DM2 were mitigated at this weight-bearing site. Furthermore, we did not find any evidence of disordered trabecular microarchitecture, which is consistent with most previous HR-pQCT studies [9, 10, 17, 18]. A study using magnetic resonance imaging (MRI) suggested that DM2 was associated with larger trabecular bone holes at the distal radius, but MRI is limited by lower resolution than is afforded by HR-pQCT [19]. In addition, although two studies using Trabecular Bone Score (TBS) suggested that DM2 is associated with defects in trabecular structure at the spine [20, 21], it should be noted that because it is derived from the projected measurement by DXA, TBS might also reflect defects in vertebral cortical architecture.
Importantly, many of the previously published studies may have been confounded by differences in racial composition in the comparison groups with and without DM2 [9, 10, 19]. We and others have previously reported that both cortical and trabecular microarchitectural differences exist between Caucasians, African-Americans [11], and Chinese-Americans [22, 23]. Our demonstration of microarchitectural differences between women with DM2 and controls within a homogeneous population of African-American women suggests that DM2, and not racial heterogeneity, explains the observed differences.
The mechanisms by which DM2 causes deficits in cortical bone density and microstructure are unknown. Some diabetes medications, such as thiazolinediones, may lower BMD [24], but adjustment for thiazolinedione use did not affect the significance of our findings. We did find that higher fasting glucose was associated with worsened cortical parameters, but the link between hyperglycemia and osteoclast or osteoblast activity is incompletely understood [25]. In addition, while a significant correlation was noted on a cohort level, there was a wide variability such that it would be difficult to predict an individual’s cortical bone density based on any given fasting glucose assessment. This is perhaps not surprising as fasting glucose measurements provide only a snapshot of glycemic control and are highly variable in individuals over time. Unfortunately we did not have a more integrated measure of long-term glycemic control (such as HbA1c) available for analysis.
Although we detected a statistically significant difference in radius cortical porosity between women with DM2 and controls, it is unclear what the impact of a <1% absolute difference in cortical porosity has on overall mechanical strength and fracture risk. In combination with a decreased cortical tissue mineral density at the radius, these changes led to an overall lower cortical vBMD. Nevertheless, as in other studies [9, 10], estimated stiffness and failure load of the whole bone did not differ between the women with DM2 and controls. It may be that other factors that are unrelated to the density and structure of the bone may further contribute to increased skeletal fragility in women with DM2 [25]. For example, a recent study found that women with longstanding DM2 had decreased cortical “bone material strength (BMS)”, as assessed by reference point indentation of the tibia diaphysis [18]. Furthermore, BMS was negatively associated with long-term glycemic control (assessed by HbA1c), again suggesting that hyperglycemia itself may contribute to impaired bone quality. Defects in BMS would not be captured in HR-pQCT-derived estimations of bone strength.
Our study has several limitations. First, our sample size was relatively small. Further, assessment of intracortical pores was limited by the resolution of the HR-pQCT machine, which is only able to detect macroscopic pores of > ~100 microns [26]. Therefore, assessment of differences in cortical porosity may be incomplete and measurement of cortical TMD may partially reflect differences in microscopic pores. Nevertheless, the contribution of microscopic pores that are below the limit of detection to bone strength remains controversial. As mentioned earlier, our finite element models were constrained by assumptions of homogenous material properties, and therefore could not account for differences in bone material properties that may exist between study groups. Lastly, although we found associations of cortical microarchitecture and fasting glucose levels, we were unable to detect an association with duration of DM2, and we did not have information on glycohemoglobin or other proxies of long-term glycemic control.
In conclusion, we found that DM2 was associated with increased cortical porosity and decreased cortical density at the distal radius in a cohort of African-American women. These cortical deficits may contribute in part to the higher fracture risk observed among adults with DM2. Further studies are required to determine whether hyperglycemia is directly involved in mechanisms of skeletal fragility associated with DM2.
Supplementary Material
Acknowledgments
NIH Grants: 1S10RR023405; U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495.
We would like to thank the Massachusetts General Hospital (MGH) Bone Density Center and the MGH HR-pQCT Core Facility, which is supported by NIH grant 1S10RR023405. The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495).
Footnotes
Disclosures: Elaine Yu, Melissa Putman, Nicolas Derrico, Gabriela Abrishamanian-Garcia, Joel Finkelstein, and Mary Bouxsein declare that they have no conflicts of interest.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
References
- 1.Behavioral Risk Factor Surveillance System; Centers for Disease Control and Prevention. Department of Health and Human Services . [Accessed April 16, 2014];2010 http://phstwlp2.partners.org:2692/brfss/
- 2.Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–1200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- 3.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study. J Bone Miner Res. 2004;19:1084–1091. doi: 10.1359/JBMR.040311. [DOI] [PubMed] [Google Scholar]
- 4.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ Blue Mountains Eye S. Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care. 2001;24:1198–1203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]
- 5.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 6.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91:3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR Study of Osteoporotic Features Research G. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 8.Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep. 2007;5:62–66. doi: 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- 9.Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 95:5045–5055. doi: 10.1210/jc.2010-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28:313–324. doi: 10.1002/jbmr.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putman MS, Yu EW, Lee H, Neer RM, Schindler E, Taylor AP, Cheston E, Bouxsein ML, Finkelstein JS. Differences in Skeletal Microarchitecture and Strength in African-American and Caucasian Women. J Bone Miner Res. 2013;28:2177–2185. doi: 10.1002/jbmr.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 13.Santoro N, Torrens J, Crawford S, et al. Correlates of circulating androgens in mid-life women: the study of women’s health across the nation. J Clin Endocrinol Metab. 2005;90:4836–4845. doi: 10.1210/jc.2004-2063. [DOI] [PubMed] [Google Scholar]
- 14.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 15.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47:519–528. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein M, Delmas P. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23:392–399. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 17.Shu A, Yin MT, Stein E, Cremers S, Dworakowski E, Ives R, Rubin MR. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int. 2012;23:635–641. doi: 10.1007/s00198-011-1595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farr JN, Drake MT, Amin S, Melton LJ, 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29:787–795. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchard JM, Giangregorio LM, Atkinson SA, Beattie KA, Inglis D, Ioannidis G, Punthakee Z, Adachi JD, Papaioannou A. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis care & research. 2012;64:83–91. doi: 10.1002/acr.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leslie WD, Aubry-Rozier B, Lamy O, Hans D Manitoba Bone Density P. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 21.Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014 doi: 10.1007/s00198-014-2704-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Wang X-F, Wang Q, Ghasem-Zadeh A, Evans A, Mcleod C, Iuliano-Burns S, Seeman E. Differences in macro- and microarchitecture of the appendicular skeleton in young Chinese and white women. J Bone Miner Res. 2009;24:1946–1952. doi: 10.1359/jbmr.090529. [DOI] [PubMed] [Google Scholar]
- 23.Walker MD, Mcmahon DJ, Udesky J, Liu G, Bilezikian JP. Application of high-resolution skeletal imaging to measurements of volumetric BMD and skeletal microarchitecture in Chinese-American and white women: explanation of a paradox. J Bone Miner Res. 2009;24:1953–1959. doi: 10.1359/JBMR.090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz AV. Diabetes, TZDs, and Bone: A Review of the Clinical Evidence. PPAR research. 2006;2006:24502. doi: 10.1155/PPAR/2006/24502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27:2231–2237. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 26.Zebaze R, Ghasem-Zadeh A, Mbala A, Seeman E. A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantification of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone. 2013;54:8–20. doi: 10.1016/j.bone.2013.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.