Abstract
The development of inexpensive and effective approaches to transiently decrease gene expression in vivo would be useful for the study of physiological processes in living animals. DNAzymes are a novel class of DNA oligonucleotides that can catalytically cleave target mRNAs and thereby reduce protein production. However, current methods for their delivery in vivo are limited and inefficient. In this study, we show that electroporation can be used to deliver DNAzymes to the intact mesenteric vasculature of rats. With the use of PKC-ε as a target, a set of wild-type and mutant control DNAzymes was designed and shown to reduce both PKC-ε mRNA and protein levels in cultured smooth muscle cells in a specific manner. The wild-type DNAzyme reduced PKC-ε protein levels by 70% at 24 h in two different cell lines without decreasing the levels of the five other PKC isoforms tested. When delivered to the intact vasculature using electroporation, the DNAzyme reduced PKC-ε protein levels by >60% without affecting these other PKC isoforms. Electro-poration was required for oligonucleotide transfer and was able to deliver the DNAzymes to multiple cell layers in the vessel wall. Protein levels were reduced maximally by 24 h postelectroporation and returned to normal by 48 h. These results suggest that electroporation can be used to deliver DNAzymes and other DNA oligonucleotides to the vasculature in vivo and can decrease gene expression for a window of time that can be used for experimental studies.
Keywords: DNAzyme, protein kinase C-ε, transient knockout, gene delivery
At present, there are relatively few rapid and easy ways to experimentally ablate or reduce enzyme and protein activity in vivo (38). While the use of pharmacological approaches to inhibit or stimulate specific pathways remains the standard for physiologists, the use of such approaches is often complicated by unwanted side effects related to the specificity of the compound. Alternatively, gene transfer can be used to alter pathways at the molecular level by modulating the activity of a gene product at the genetic level with much more specificity and control than drugs. The most common way to do this is through the use of “knockout” transgenic mice. The major benefit to this approach is that the gene product is ablated from the animal (or specific tissues, depending on the strategy used) for the lifetime of the animal. However, such knockouts are generally restricted to mice, are very costly to produce, and can take up to several years to produce, depending on the desired target. In addition, compensatory regulatory mechanisms can often mask the physiological effect of the knocked out gene. As an alternative to this approach, several methods for creating “transient transgenics” have been developed that use molecular approaches to inhibit gene expression or reduce protein levels in desired tissues for limited amounts of time. The benefit to these approaches is that they are very well suited for the physiologist to study various physiological pathways and responses in vivo. Such approaches include the use of antisense technology, dominant negative genes, small interfering RNA, and DNAzymes.
DNAzymes are single-stranded DNA molecules that selectively bind to an RNA substrate by Watson-Crick base pairing and cleave phosphodiester bonds (6, 11, 22, 24, 25, 28). The DNAzyme is composed of two 7- or 8-mer binding arms flanking a catalytic domain of 15 nucleotides called the “10–23” sequence (24, 25). The DNAzyme binds the target mRNA molecule, cleaves it, releases the cleavage products, and is then able to hybridize with another molecule of the mRNA target. This sequence of events repeats itself many times until the DNAzyme itself is degraded by nucleases. Through careful design of the binding arms, a DNAzyme can be engineered to bind to and specifically cleave any target mRNA that contains a purine-pyrimidine junction (11–13, 24, 25, 34). Because they have a high catalytic activity, relatively low concentrations can be used to degrade even abundant mRNAs in the cell, leading to decreased translation and ultimately reduced protein levels. While they have been used extensively in cell culture using liposome-mediated delivery agents, their use in vivo has been limited by a lack of efficient delivery methods (10, 12, 14, 15, 23, 39).
DNAzymes have been shown to efficiently target and cleave specific mRNA sequences to prevent protein translation both in vitro (7, 24, 25, 36) and in vivo (10, 12, 14, 15, 20, 23, 39). Limited therapeutic efficacy has been demonstrated in vivo in studies targeting early growth response factor-1 in the vasculature (14, 15, 23) and kidney (20), TNF-α in the coronary artery (10), and VEGF receptor 2 in the vasculature (39). The majority of these studies has relied on liposomal delivery of the DNAzyme to the intact vasculature. Liposomes deliver DNA by binding to the cell membrane, becoming endocytosed and releasing DNA into the cytoplasm. Liposomal delivery of oligonucleotides and DNA results in diffuse delivery only to the surface layer of cells in the target tissue; if the liposome-DNA complexes are delivered via the lumen of a vessel, DNA transfer is only obtained in endothelial cells. Thus, although this delivery method lacks the ability to penetrate into the tissue, the results using DNAzymes in the vasculature and in other tissues are promising. Clearly, the development of additional delivery methods with increased tissue penetration and localized delivery would be of great benefit.
An alternative delivery method, electroporation, shows promise as a nonviral delivery vehicle for oligo-nucleotides. The technique works by applying a pulsed electric field that causes transient pores to form in the cell membrane. The electroporative uptake of DNA is preceded by field-induced structural changes in the membrane, resulting in transient permeation sites, through which DNA can diffuse into the cell (26, 29). When the electric field is removed, the pores close and the DNA is trapped in the cytoplasm. In vivo electro-poration was first demonstrated in skeletal muscle and dramatically increased gene expression of plasmid DNA (1, 17, 19). Electroporation has been used in a number of other tissues and results in reproducible, high-level gene expression in defined areas (2, 4, 5, 8, 21, 31, 35). Furthermore, because plasmids are used, the technique does not generate the immune or inflammatory responses that continue to limit the clinical use of viral vectors. Electroporation also has been shown to deliver plasmids efficiently to the intact vasculature, resulting in high-level expression in all cell layers (i.e., adventitia, media and intima, and endothelium) (16, 18, 37). Electroporated vessels are indistinguishable from naïve vessels by histological analysis, physiological responses, and DNA microarray analysis (16, 37).
In the present study, we designed a DNAzyme to target PKC-ε and tested the ability of electroporation to deliver this oligonucleotide to the intact vasculature of rats. The choice of PKC-ε as the target was made due to its suggested role in Ca2+-independent smooth muscle cell contraction and because it can be quantified on mRNA and protein levels (3, 9, 27, 32). Thus we can measure the amount of PKC-ε in cells and tissues to determine the efficacy of DNAzyme delivery and activity. More importantly, because PKC exists in multiple isoforms, the use of the highly selective DNAzymes allows us to specifically knock down the level of one isoform without altering the levels of the others. We show that the DNAzymes efficiently and specifically decrease PKC-ε mRNA and protein levels in both cultured cells and the intact vasculature. Together, these findings have important implications in the study of vascular biology and transient transgenics.
METHODS
Preparation of DNA enzymes
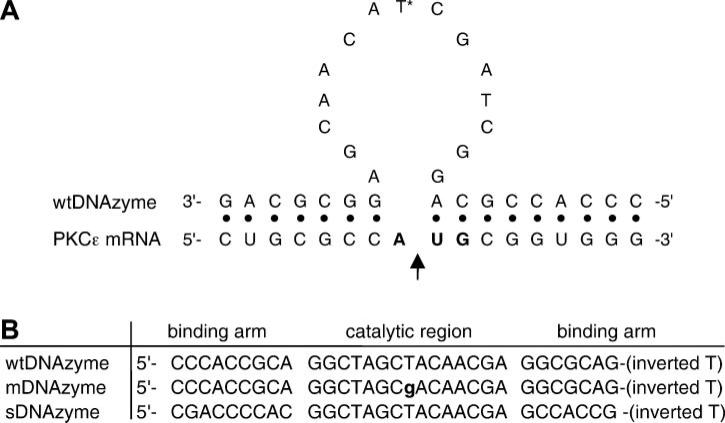
The 31-mer single-stranded DNA sequences used in this study are shown in Fig. 1. All three oligonucleotides [wild-type (wt)DNAzyme, mutant (m)DNAzyme, and scrambled (s)DNAzyme] have an inverted T at the 3′ end to increase stability. A FITC-labeled single-stranded DNA oligonucleotide of the same sequence as the wtDNAzyme was synthesized without a 3′-inverted T but with a fluorescein molecule attached to the 5′ end. All oligo-nucleotides were synthesized by Oligo's (Wilsonville, OR) and purified by HPLC. Upon receipt of the lyophilized oligonucleotides, they were diluted in sterile water to a concentration of 1 mM and stored at −80°C.
Fig. 1.
PKC-ε DNAzymes. A: model of a DNAzyme-PKC-ε mRNA hybrid. The wild-type (wt)DNAzyme binds to the PKC-ε mRNA strand through two recognition arms; the arrow indicates the location of target mRNA cleavage. *Location of the single base mutation of the mDNAzyme. AUG codon is shown in bold. B: sequences of the wtDNAzyme, mutant (m)DNAzyme, and scrambled (s)DNAzyme. The binding arms and catalytic region are indicated. The point mutation in the catalytic region of the mDNAzyme is lowercase and bold.
Cell culture and transfection
Human pulmonary artery smooth muscle cells (hPASMC; a gift of Dr. Paul Babal, Department of Pharmacology, University of South Alabama) and A7r5 cells (ATCC No. CRL-1444) were maintained in 100-mm plates containing DMEM with 10% FBS, 1× Kanamycin, and 1× antimycotic solution (Life Technologies; Gaithersberg, MD) at 37°C and 5% CO2. Transfections were done when the cultured cells achieved 70% confluency within the 100-mm plate. Each plate was treated with 20 μl lipofectin (20 μg, Life Technologies) complexed without (“lipofectin only”) or with one of the three DNAzymes (wtDNAzyme, mDNAzyme, and sDNAzyme) at a final oligonucleotide concentration of 1.5 μM in 2 ml of DMEM without serum. After 4 h of serum starvation, DMEM with serum was added to each plate. Twenty-four hours after transfection, the cells were harvested for Northern or Western blots.
Application of the electroporation technique to the intact vasculature
Rats were anesthetized using isoflurane, and a midline incision was made for the exteriorization of the small intestine and access to the mesenteric arterial tree. Vessels were prepared and electroporated as described previously (16). Electroporation conditions for DNAzymes were determined from previous experience with plasmid DNA and optimized for the DNAzymes (200 V/cm, 8 pulses of 10-ms duration). Oligonucleotide solutions were made at varying concentrations (10, 50, and 100 μM) in 10 mM Tris (pH 8.0) containing 1 mM EDTA and 140 mM NaCl. After the electroporation of 12 vessels/animal (3 vessels per condition: wtDNAzyme, sDNAzyme, mDNAzyme, and buffer only), the abdominal cavity was closed in two layers with sutures and staples. All animals recovered without incident. At the indicated times of postdelivery (12–48 h), animals were anesthetized, and the treated vessels were identified and removed before euthanasia by thoracotomy and ventricular dissection. All experiments were conducted in accordance with institutional guidelines in compliance with the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Northern blot analyses
RNA was harvested from hPASMC and A7r5 cells 24 h posttransfection using a Qiagen RNeasy kit according to the recommendations of the manufacturer (Qiagen; Chatsworth, CA). Twenty micrograms of total RNA of each sample were mixed with 25 μl of RNA loading dye (48% deionized formamide, 20 mM MOPS, 0.06% formalde-hyde, 50% glycerol, and 0.05% saturated bromophenol blue in diethyl pyrocarbonate-treated water). The samples were denatured at 65°C for 15 min, loaded onto an agarose gel (0.8% agarose, 20 mM MOPS, 2% formaldehyde, and 300 ng/ml ethidium bromide), and separated via electrophoresis at a field strength of 4.2 V/cm for 90 min at room temperature in 20 mM MOPS running buffer. The gel was denatured for 30 min with 0.05 N NaOH and 0.15 M NaCl, renatured for 30 min with 0.1 M Tris (pH 7.5) and 0.15 M NaCl, rinsed briefly in 10× SSC (1.5 M NaCl and 0.15 M sodium citrate; pH 7.0), and transferred to a nylon membrane. RNA was UV cross-linked to the nylon membrane and prehybridized at 68°C for 30 min in ExpressHyb solution (Clontech; Palo Alto, CA).
DNA fragments to be labeled were PCR amplified from a human quick clone cDNA library (Clontech) and gel purified. Fragments were 32P labeled using the Stratagene PrimeIt II labeling kit, boiled for 5 min, added to the prehybridization solution, and incubated at 68°C for 1 h. The blots were washed three times for 10 min each at room temperature in 2× SSC containing 0.1% SDS and twice for 10 min each at 50°C in 0.1× SSC containing 0.1% SDS before exposure on X-ray film (Kodak X-OMAT AR film) at −80°C. Autoradio-graphs were digitized, and the bands were quantified by densitometry using NIH Image.
Western blot analyses
Cultured cells (100-mm dishes) were washed twice with PBS and lysed with 1 ml of 50 mM Tris (pH 7.5) containing 1% Triton X-100, 150 mM NaCl, 5 mM EDTA, and complete protease inhibitor (Roche). Cells were scraped from the plate and vortexed, and debris was removed by centrifugation before the determination of protein concentration. For in vivo analyses, mesenteric neurovascular bundles were excised from rats and frozen in liquid nitrogen before mechanical disruption using a drill press as previously described (16). The ground tissue was suspended in Promega lysis buffer containing 1 mM dithiothreitol and subjected to three freeze-thaw cycles. Debris was removed by centrifugation, and the protein concentration of each sample was determined.
Forty micrograms of protein from cell cultures or 100 μg of protein from vessel lysates were separated in 12.5% polyacrylamide gels by electrophoresis and transferred to nitro-cellulose membranes (Osmonics). Membranes were blocked with 5% milk in PBS containing 0.05% NaN3 for 1 h at room temperature and then hybridized with monoclonal 1:500 anti-PKC-ε antibody (BD Transduction Laboratories) in 5% milk for 2 h at room temperature. The blots were washed and incubated with 1:10,000 horseradish peroxidase-labeled anti-mouse secondary antibodies (Jackson ImmunoResearch Laboratories) in 5% milk for 2 h at room temperature and washed again before chemiluminescent detection using SuperSignal West Dura Extended Duration Substrate (Pierce). The blots were then stripped and reprobed with 1:4,000 anti-β-tubulin antibodies (Sigma) in the case of cell culture samples or 1:1,000 anti-β-actin antibodies (Sigma), followed by washes, secondary antibodies, and detection as for PKC-ε. Films were digitized, and the bands for PKC-ε, β-actin, and β-tubulin were quantified by densitometry using NIH Image. PKC band intensities were normalilzed to those of β-actin and β-tubulin, and the results are expressed as means ± SE.
Levels of other PKC isoforms were determined by separating 750 μg of total protein from either cell cultures or vessel lysates by preparative polyacrylamide gel electrophoresis, transferring to nitrocellulose, and reacting strips of the blot with antisera to the various isoforms. All antibodies were obtained from BD Biosciences and were used at the following dilutions: Rack-1 (1:3,000), PKC-α (1:200), PKC-β (1:200), PKC-δ (1:200), PKC-ι (1:100), and PKC-γ (1:100). Horseradish peroxidase-labeled secondary antibodies were added at 1:10,000 dilution, and the blots were detected as above and normalized to β-tubulin (cultured cells) or β-actin (vessels).
Immunohistochemistry
Treated vessels were harvested and fixed in 4% buffered formalin for 24 h before paraffin embedding by the Northwestern Pathology Core Laboratory. Sections (6 μm thick) were cut for immunohistochemistry. Sections were deparaffinized, hydrated, and probed following the Vectastain mouse IgG ABC protocol (Vector Laboratories) using a 1:500 mouse anti-fluorescein antibody (Chemicon; Temecula, CA). The slides were developed according to the Vector Blue Alkaline Phosphatase Substrate Kit III (Vector Laboratories) and imaged using a Leica DMRX upright microscope and SPOT digital camera interfaced to a G4 Macintosh computer.
RESULTS
Design of DNAzymes
To determine whether electro-poration could be used to efficiently deliver oligonucleotides and DNAzymes to the intact vasculature, we chose to target the ε-isoform of PKC. This enzyme can be quantified in both cell culture and vessels at the mRNA, protein, and activity levels, making it an attractive choice to test our methods. The mRNA target sequence for PKC-ε is shown in Fig. 1A. This target sequence is at the translation start site for PKC-ε (AUG is shown in bold). This sequence is conserved in mouse, rat, and human genes, allowing the same oligonucleotides to be used for each species. Above the mRNA sequence is that of the wtDNAzyme. The wtDNAzyme contains a 7-nucleotide 5′ binding arm and a 9-nucleotide 3′ binding arm for target site recognition. Between these two arms is the 10–23 catalytic domain, which cleaves the target between the unpaired A and U of the start codon (arrow) (24, 25). Figure 1B shows the sequence of the three oligonucleotides used in this study, with their binding arms and catalytic domains indicated. Two control oligonucleo-tides were used in this study, including a catalytically inactive mutant that can bind to the target but has an inactive 10–23 domain (mDNAzyme) and a scrambled oligonucleotide (sDNAzyme) that is catalytically active but contains binding arms that have been scrambled so that they cannot bind to the target mRNA. The mDNAzyme acts as a control for any antisense effects (i.e., RNaseH-dependent degradation) that the oligonucleo-tides may have, whereas the sDNAzymes acts as a control for any nonspecific oligonucleotide effects.
wtDNAzymes decrease PKC-ε expression in cultured cells
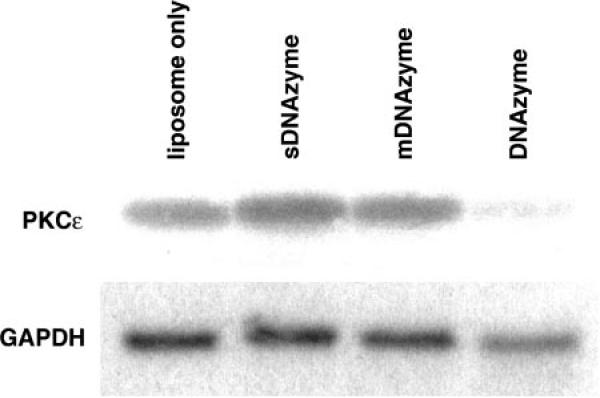
hPASMCs and A7r5 cells were transfected with 1.5 μM wtDNAzyme, sDNAzyme, mDNAzyme, or liposomes alone, and PKC-ε mRNA levels were measured by Northern blot analysis 24 h posttransfection (Fig. 2). When normalized to GAPDH mRNA as a loading control, a 96% decrease in PKC-ε mRNA levels was observed in wtDNAzyme-treated cells compared with liposome only-treated cells. A similar decrease in mRNA levels was seen in both cell types. In contrast, neither the sDNAzyme nor mDNAzyme decreased PKC-ε mRNA levels compared with liposome only-treated cells in either cell type. In fact, in multiple experiments, the levels of PKC-ε mRNA appeared to increase slightly (<50%) when cells were transfected with these oligonucleotides.
Fig. 2.
Effect of DNAzyme treatment on steady-state PKC-ε mRNA levels in cultured cells. Representative Northern blots for PKC-ε (top) or GAPDH mRNA (bottom) from human pulmonary artery smooth muscle cells (hPASMCs) treated with liposome alone, wtDNAzyme, mDNAzyme, or sDNAzyme are shown.
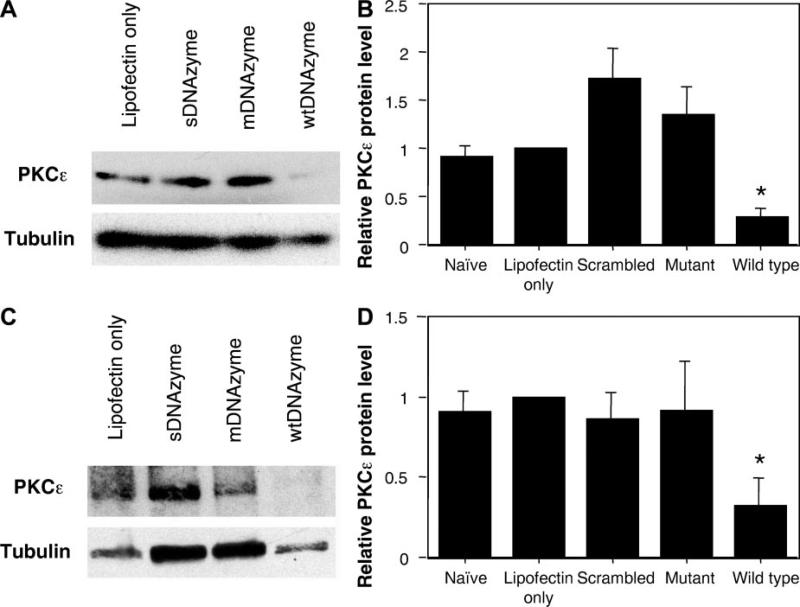
PKC-ε protein levels were also quantified in hPASMCs that had been transfected with the various oligonucleotides. Twenty-four hours posttransfection, PKC-ε protein levels were measured by Western blot and normalized to β-tubulin (Fig. 3, A and B). A 71 ± 8% decrease in the PKC-ε protein level was reproducibly measured in the wtDNAzyme-treated hPASMCs compared with liposome only-transfected cells. Similar to the results seen with mRNA levels, both sDNAzyme and mDNAzyme increased PKC-ε protein levels compared with liposome only-treated cells. Data from five separate experiments showed that sDNAzyme and mDNAzyme increased PKC-ε by 85 ± 33% and 47 ± 30%, respectively. Mann-Whitney U-test indicated that the wtDNAzyme-induced PKC-ε decrease was statistically significant compared with the other conditions (P = 0.016) but that the increases seen with mDNAzyme and sDNAzyme were not statistically different from the naïve or liposome only-transfected cells.
Fig. 3.
Effects of DNAzymes on PKC-ε protein levels in cultured cells. A: Western blots of hPASMCs. Representative Western blots for PKC-ε (top) or β-tubulin (bottom) from hPASMCs treated with liposome alone, wtDNAzyme, mDNAzyme, or sDNAzyme are shown. B: quantification of protein levels in hPASMCs. Western blots from 5 separate transfection experiments using hPASMCs were digitized, analyzed by densitometry using NIH Image, and normalized to liposome only treatment. Values are expressed as normalized averages ± SE. A Mann-Whitney U-test gave a P value of 0.016 for the wtDNAzyme-induced PKC-ε decrease compared with the other conditions. C: Western blots of A7r5 cells. Representative Western blots for PKC-ε (top) or β-tubulin (bottom) from rat A7r5 cells treated with liposome alone, wtDNAzyme, mDNAzyme, or sDNAzyme are shown. D: quantification of protein levels in A7r5 cells. Western blots from 3 separate transfection experiments using A7r5 cells were digitized, analyzed by densitometry using NIH Image, and normalized to liposome only treatment, as described in B. *P = 0.003 for PKC-ε levels in wtDNAzyme-treated cells vs. all other conditions by Mann-Whitney U-test.
When PKC-ε protein levels were measured in transfected A7r5 cells, an almost identical decrease in PKC-ε compared with liposome only-treated or naïve cells (68 ± 17%) was seen as in the primary cells (Fig. 3, C and D). However, there was no increase or decrease in PKC-ε levels with the two control oligonucleotides, as was seen in hPASMCs.
For this approach to be of use in the study of PKC-ε-specific pathways, it is necessary that the PKC-ε wtDNAzyme shows specificity for the ε-isoform. To determine whether this was the case, protein samples from transfected hPASMCs and A7r5 cells were also analyzed for changes in the levels of several other PKC isoforms by Western blot. None of the experimental treatments (wtDNAzyme, mDNAzyme, or sDNAzyme) caused any changes in the levels of PKC-α, PKC-β, PKC-δ, PKC-ι, PKC-γ, or Rack-1 (data not shown). Taken together, these results demonstrate that the appropriately designed PKC-ε wtDNAzyme can specifically reduce both mRNA and protein levels of PKC-ε.
Electroporation can deliver oligonucleotides to multiple cell layers of the intact mesenteric vasculature
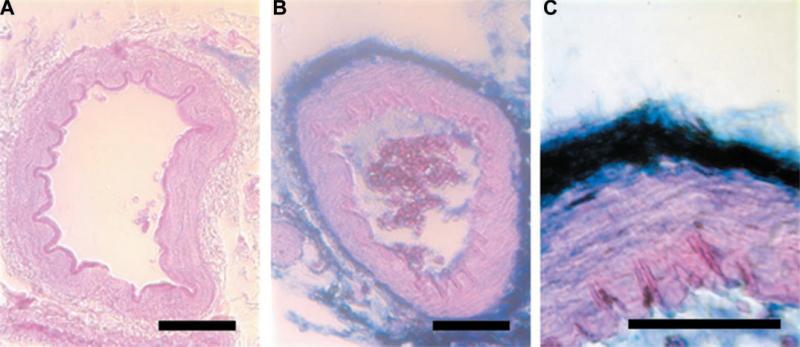
Before determining the activities of the DNAzymes in the rat mesenteric vasculature, we wanted to ensure that electroporation could be used to deliver the oligo-nucleotides to the intact vasculature. Rat mesenteric neurovascular bundles were either electroporated (8 pulses at 10-ms duration each, 200 V/cm) with a 100 μM FITC-labeled wtDNAzyme solution or bathed in the labeled DNAzyme solution in the absence of an electric field. At various times after electroporation (between 1 min and 24 h), vessels were excised, rinsed, fixed in formalin, paraffin embedded, and sectioned. Immunohistochemistry using antibodies against fluorescein was performed to visualize the location of the wtDNAzyme within the neurovascular bundle (Fig. 4). In the absence of an electric field, no oligonucleotide was detected within the vessel wall, although some staining could be seen in the surrounding adipose tissue of the bathed vessels (results not shown) at times up to 5 min after electroporation. In contrast, within 1 min of electroporation, the presence of the oligonucleotide can be seen in the surrounding adipose tissue, adventitial cells, and smooth muscle cells within the artery wall. Similar vessel wall localization of the oligonucleotide could be detected up to 10 min after electroporation, but by 30 min, the signal decreased to undetectable levels. Thus electroporation can be used to deliver oligonucleotides to intact vessels.
Fig. 4.
Electroporation-mediated delivery of oligonucleotides to the intact vasculature. FITC-labeled wtDNAzyme [100 μM in 10 mM Tris (pH 8), 1 mM EDTA, and 140 mM NaCl] was transferred to rat mesenteric arteries using electroporation (8 pulses at 10 ms each, 200 V/cm). One minute to 24 h postelectroporation, vessels were excised, rinsed extensively with PBS, fixed in formalin, paraffin embedded, and sectioned to 10 μm. Immunohistochemistry was performed with antibodies against fluorescein and visualized using Vector Blue (Vector Labs). Control vessel receiving no oligonucleotide (A) or vessels electroporated with oligonucleotide and removed at 1 min postelectroporation (B and C) are shown. The oligonucleotide is stained in blue, and the tissue was counterstained with eosin. Bars = 50 μm.
wtDNAzymes decrease PKC-ε expression in vivo
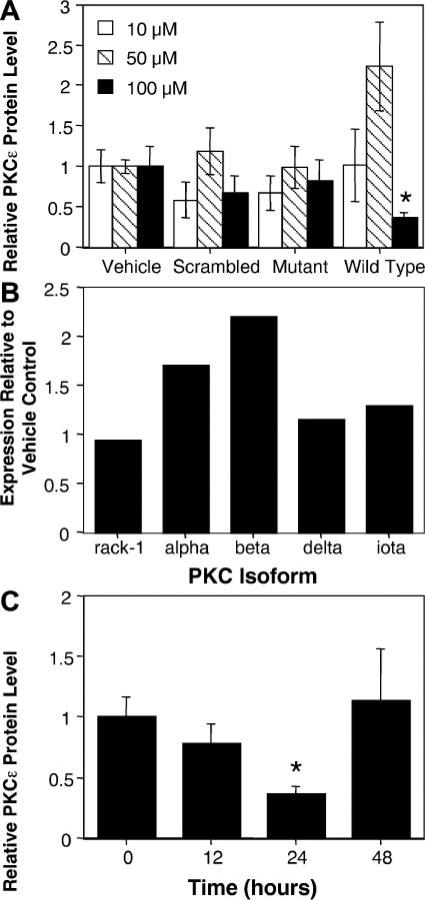
On the basis of previous studies (10, 14, 15, 23) using liposomes to deliver DNAzymes to the vasculature, we chose a concentration range of between 10 and 100 μM oligonucleotide for the in vivo studies. Vessels were bathed in a solution of DNAzyme in 10 mM Tris (pH 8), 1 mM EDTA, and 140 mM NaCl and electroporated using a series of eight square wave pulses (10-ms each) at 200 V/cm. One minute after the last pulse, the vessels were removed from the electrode, and the next vessel was treated. These electroporation parameters were chosen based on our extensive experience using electroporation to transfer plasmids to the intact vasculature. To directly compare the effects of the DNAzymes on PKC-ε levels, three vessels in each animal were treated with each DNAzyme, for a total of 12 electroporated vessels/animal, and the experiments were repeated in at least 7 animals/experimental condition. Twenty-four hours after electroporation, vessels were removed and used to prepare a cell lysate for Western blot analysis. All three vessels receiving the same DNAzyme in a given animal were combined for extract preparation. When PKC-ε protein levels were normalized to β-actin and compared with PKC-ε levels in vessels electroporated in the absence of oligonucleotides, neither 10 nor 50 μM wtDNAzyme decreased PKC-ε levels in vivo (Fig. 5A). In fact, at 50 μM wtDNAzyme, PKC-ε levels actually appeared to increase by approximately twofold, but this was not considered significant by a Mann-Whitney U-test (P = 0.122). However, at 100 μM wtDNAzyme, PKC-ε expression was inhibited by 64% compared with controls (P = 0.006). Neither the mDNAzyme nor sDNAzyme had any significant effects at any of the tested concentrations. We also tested the effects of the DNAzymes on the expression of other isoforms of PKC, and, similar to the findings in cell culture, no significant decreases in the expression of PKC-α, PKC-β, PKC-δ, PKC-ι, or Rack-1 were detected in vivo with any of the DNAzymes (Fig. 5B).
Fig. 5.
Effects of DNAzymes on PKC-ε protein levels in the intact rat mesenteric vasculature A: dose response of wtDNAzyme and mDNAzyme. Rat mesenteric arteries were electroporated with DNAzymes (10, 50, or 100 μM) or buffer alone (vehicle) as described in Fig. 4. Twenty-four hours postelectroporation, vessels were removed, and protein levels were determined by Western blot and densitometry as described in Fig. 3. Mean values are given normalized to vehicle-treated vessels ± SE; n = 7 vessels. The decrease in PKC-ε levels caused by 100 μM wtDNAzyme compared with vehicle-treated vessels was statistically significant by Mann-Whitney U-test (*P = 0.006). B: effects of wtDNAzyme on expression of other PKC isoforms. Western blots were performed on protein lysates from untreated vessels or vessels electroporated with 100 μM wtDNAzyme 24 h postelectroporation using antisera directed against the indicated isoforms. The individual isoform levels were quantified from digitized blots and normalized to the levels seen in untreated vessels (vehicle control). C: time course of wtDNAzyme effects on PKC-ε protein levels. Vessels were electroporated with 100 μM wtDNAzyme and removed from animals at 12, 24, or 48 h postelectroporation. PKC-ε protein levels were quantified from digitized Western blots, as in A. Values represent the average normalized PKC-ε protein levels ± SE; n = 7 vessels. *P < 0.005 compared with control (vehicle alone) by Mann-Whitney U-test.
To determine how long it would take to downregu-late PKC-ε and how long the downregulation would last, a time-course study was performed using 100 μM DNAzymes (Fig. 5C). As found at 24 h (Fig. 5A), neither the mDNAzyme nor the sDNAzyme had any effect on PKC-ε levels in the vessels at 12 or 48 h postelectroporation (data not shown). In contrast, the wtDNAzyme slightly inhibited PKC-ε expression as early as 12 h compared with vessels electroporated without any oligonucleotide, but by 24 h, PKC-ε protein levels dropped by 64% (P = 0.006). By 48 h postelectroporation, PKC-ε levels returned to normal. These results demonstrate that DNAzymes can be delivered to the intact vasculature using electroporation and elicit their effects for a limited window of time.
DISCUSSION
We (16, 37) have previously demonstrated that electroporation can be used to transfer plasmids to the intact vasculature of living rats, resulting in high-level gene transfer and expression in all layers of the vessel wall. Using this approach, we have been able to transfer and increase the expression of exogenous genes in the vasculature. Using a similar delivery method, we show here that DNAzymes can be transferred to the intact vasculature as well and can effectively reduce the endogenous gene expression of a target gene. DNAzymes were directed against the PKC-ε isoform and were shown to reduce mRNA levels by over 90% and protein production by almost 70% in cultured smooth muscle cells. This inhibition of protein production was due to the catalytic activity of the DNAzymes, based on the lack of PKC-ε reduction seen with a catalytically dead DNAzyme (mDNAzyme), and was specific for the ε-isoform, because the levels of several other PKC isoforms were unaffected by DNAzyme treatment. When transferred to the mesenteric vasculature of rats by electroporation, a 60% reduction in PKC-ε protein levels was detected at 24 h with 100 μM wtDNAzyme. Inhibition of protein production began as early as 12 h postelectroporation and reached its maximum at 24 h. PKC-ε levels returned to normal by 48 h. Thus electroporation can be used to deliver DNAzymes to the intact vasculature, and the use of these oligonucleotides can greatly diminish the level of a desired protein over a 24-h period to create a transient transgenic model for physiological studies.
Several previous studies (14, 23) employing DNAzymes in the vasculature have used liposome complexes to deliver the oligonucleotides to the lumen of the carotid artery via a balloon catheter. Because liposomes can only deliver their contents to the cell layer with which they interact, delivery was limited to the lumen and there was no penetration of the DNAzyme into the intimal or advential layers of the vascular wall (10, 14, 15, 23, 39). In contrast, we and others (16, 18, 37) have previously shown that electroporation can be used to deliver reporter or therapeutic plasmids to all layers of the intact vasculature, using either luminal or adventitial delivery. By using a labeled oligonucleo-tide, we were able to show that electroporation causes transfer of the DNA to both adventitial cells as well as several layers of smooth muscle cells within the vessel wall, a result similar to that seen with entire plasmids. Although it appeared that the labeled DNAzyme could diffuse into the adipose tissue surrounding the artery within the neurovascular bundle, it was not able to penetrate into the arterial wall by simple diffusion; electro-poration was needed for vessel delivery. Recently, it was demonstrated that electroporation can also be used to deliver DNAzymes to the kidney to decrease interstitial fibrosis in a rat ureteral obstruction model (20). Our results support the contention that electroporation can be used to successfully deliver DNAzymes in vivo.
We had expected the amount of DNAzyme to decrease over time, but we had not anticipated the quickness with which the detectable signal diminished. The signal, although intense at the initial time points, quickly faded to undetectable levels after 10 min. As a single-stranded DNA molecule, the DNAzyme is subject to nucleases. The FITC-labeled oligonucleotide used in the immunohistochemistry study did not have an inverted T at the 3′ end, making it more vulnerable to nuclease degradation than the DNAzyme used in the experiments in which protein levels were measured. Sun et al. (30) showed that an unmodified DNAzyme had a half-life of <2 h in vitro. Because stability in vivo is usually greatly decreased compared with the in vitro setting, degradation likely occurs even faster in vivo and would explain the quick deterioration of the signal. However, because we see functional activity of the DNAzyme at 24 h postdelivery in vivo, at least some of the inverted T-containing oligonucleotides must be present at these late times.
This is the first study to demonstrate electroporation-mediated DNAzyme delivery to the intact vasculature. These combined results indicate the potential use of DNAzymes to selectively knockout a gene for the development of transient transgenics. As seen in Fig. 5C, there is a window of protein modulation that could be valuable to a researcher looking at the effect of a specific gene at a given point in time. In other studies (6, 7, 24, 25, 30, 33, 36), decreases in target RNA levels have been seen as early as 6 h and have lasted 24–48 h in cultured cells and in vivo. Whereas with the PKC-ε DNAzyme designed for these experiments we obtained a 60–70% reduction in protein levels in vivo, other DNAzymes for different target mRNAs may have greater effects. Depending on the protein being targeted, this may or may not be enough to elicit a biological effect. Thus, while removal of 50% of a vital structural protein may be enough to have significant effects on the cell, even a 90% decrease in the levels of a regulatory protein may not be sufficient to alter a biological response. Thus, as in any experiment, the properties of the target are important. This approach could be useful because one could measure a physiological process, transiently knockout a gene, and measure the physiological process at various intervals until normal physiological function is restored, all in the same animal. This could be a useful platform for studying complex biological pathways, disease pathogenesis, or elucidating the function of novel genes in the vasculature.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL-59956 (to D. A. Dean) and DK-51430 (to J. N. Benoit).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
DISCLOSURES
We thank Jennifer Young and Lori Crosson for advice and helpful discussions.
REFERENCES
- 1.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 2.Blair-Parks K, Weston BC, Dean DA. Gene delivery to the cornea by plasmid injection and electroporation. J Gene Med. 2002;4:92–100. doi: 10.1002/jgm.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins EM, Walsh MP, Morgan KG. Contraction of single vascular smooth muscle cells by phenylephrine at constant [Ca2+]i. Am J Physiol Heart Circ Physiol. 1992;262:H754–H762. doi: 10.1152/ajpheart.1992.262.3.H754. [DOI] [PubMed] [Google Scholar]
- 4.Dezawa M, Takano M, Negishi H, Mo X, Oshitari T, Sawada H. Gene transfer into retinal ganglion cells by in vivo electroporation: a new approach. Micron. 2002;33:1–6. doi: 10.1016/s0968-4328(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 5.Dujardin N, Staes E, Kalia Y, Clarys P, Guy R, Preat V. In vivo assessment of skin electroporation using square wave pulses. J Control Release. 2002;79:219–227. doi: 10.1016/s0168-3659(01)00548-x. [DOI] [PubMed] [Google Scholar]
- 6.Emilsson GM, Breaker RR. Deoxyribozymes: new activities and new applications. Cell Mol Life Sci. 2002;59:596–607. doi: 10.1007/s00018-002-8452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goila R, Banerjea AC. Sequence specific cleavage of the HIV-1 coreceptor CCR5 gene by a hammer-head ribozyme and a DNA-enzyme: inhibition of the coreceptor function by DNA-enzyme. FEBS Lett. 1998;436:233–238. doi: 10.1016/s0014-5793(98)01137-5. [DOI] [PubMed] [Google Scholar]
- 8.Harrison RL, Byrne BJ, Tung L. Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett. 1998;435:1–5. doi: 10.1016/s0014-5793(98)00987-9. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz A, Clement-Chomienne O, Walsh MP, Morgan KG. ε-Isoenzyme of protein kinase C induces a Ca2+-independent contraction in vascular smooth muscle. Am J Physiol Cell Physiol. 1996;271:C589–C594. doi: 10.1152/ajpcell.1996.271.2.C589. [DOI] [PubMed] [Google Scholar]
- 10.Iversen PO, Nicolaysen G, Sioud M. DNA enzyme targeting TNF-α mRNA improves hemodynamic performance in rats with postinfarction heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H2211–H2217. doi: 10.1152/ajpheart.2001.281.5.H2211. [DOI] [PubMed] [Google Scholar]
- 11.Joyce GF. Nucleic acid enzymes: playing with a fuller deck. Proc Natl Acad Sci USA. 1998;95:5845–5847. doi: 10.1073/pnas.95.11.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khachigian LM. Catalytic DNAs as potential therapeutic agents and sequence-specific molecular tools to dissect biological function. J Clin Invest. 2000;106:1189–1195. doi: 10.1172/JCI11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Sen D. Toward an efficient DNAzyme. Biochemistry. 1997;36:5589–5599. doi: 10.1021/bi962694n. [DOI] [PubMed] [Google Scholar]
- 14.Lowe HC, Chesterman CN, Khachigian LM. Catalytic antisense DNA molecules targeting Egr-1 inhibit neointima formation following permanent ligation of rat common carotid arteries. Thromb Haemost. 2002;87:134–140. [PubMed] [Google Scholar]
- 15.Lowe HC, Fahmy RG, Kavurma MM, Baker A, Chesterman CN, Khachigian LM. Catalytic oligodeoxynucleotides define a key regulatory role for early growth response factor-1 in the porcine model of coronary in-stent restenosis. Circ Res. 2001;89:670–677. doi: 10.1161/hh2001.097867. [DOI] [PubMed] [Google Scholar]
- 16.Martin JB, Young JL, Benoit JN, Dean DA. Gene transfer to intact mesenteric arteries by electroporation. J Vasc Res. 2000;37:372–380. doi: 10.1159/000025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 1999;6:508–514. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto T, Komori K, Shoji T, Kuma S, Kume M, Yamaoka T, Mori E, Furuyama T, Yonemitsu Y, Sugimachi K. Successful and optimized in vivo gene transfer to rabbit carotid artery mediated by electronic pulse. Gene Ther. 2001;8:1174–1179. doi: 10.1038/sj.gt.3301502. [DOI] [PubMed] [Google Scholar]
- 19.Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura H, Isaka Y, Tsujie M, Rupprecht HD, Akagi Y, Ueda N, Imai E, Hori M. Introduction of DNA enzyme for Egr-1 into tubulointerstitial fibroblasts by electroporation reduced interstitial alpha-smooth muscle actin expression and fibrosis in unilateral ureteral obstruction (UUO) rats. Gene Ther. 2002;9:495–502. doi: 10.1038/sj.gt.3301681. [DOI] [PubMed] [Google Scholar]
- 21.Oshima Y, Sakamoto T, Yamanaka I, Nishi T, Ishibashi T, Inomata H. Targeted gene transfer to corneal endothelium in vivo by electric pulse. Gene Ther. 1998;5:1347–1354. doi: 10.1038/sj.gt.3300725. [DOI] [PubMed] [Google Scholar]
- 22.Pyle AM, Chu VT, Jankowsky E, Boudvillain M. Using DNAzymes to cut, process, and map RNA molecules for structural studies or modification. Methods Enzymol. 2000;317:140–146. doi: 10.1016/s0076-6879(00)17012-0. [DOI] [PubMed] [Google Scholar]
- 23.Santiago FS, Lowe HC, Kavurma MM, Chesterman CN, Baker A, Atkins DG, Khachigian LM. New DNA enzyme targeting Egr-1 mRNA inhibits vascular smooth muscle proliferation and regrowth after injury. Nat Med. 1999;5:1438. doi: 10.1038/71020. [DOI] [PubMed] [Google Scholar]
- 24.Santoro SW, Joyce GF. A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci USA. 1997;94:4262–4266. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santoro SW, Joyce GF. Mechanism and utility of an RNA-cleaving DNA enzyme. Biochemistry. 1998;37:13330–13342. doi: 10.1021/bi9812221. [DOI] [PubMed] [Google Scholar]
- 26.Satkauskas S, Bureau M, Puc M, Mahfoudi A, Scherman D, Miklavcic D, Mir L. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol Ther. 2002;5:133–140. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- 27.Shirasawa Y, Young JL, Dean DA, Benoit JN. Involvement of protein kinase C-ε (PKCε) in mesenteric arterial vasoconstriction (Abstract). FASEB J. 2000;15:A489. [Google Scholar]
- 28.Sioud M, Leirdal M. Therapeutic RNA and DNA enzymes. Biochem Pharmacol. 2000;60:1023–1026. doi: 10.1016/s0006-2952(00)00395-6. [DOI] [PubMed] [Google Scholar]
- 29.Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, Malone RW. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000;2:178–187. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 30.Sun LQ, Cairns MJ, Gerlach WL, Witherington C, Wang L, King A. Suppression of smooth muscle cell proliferation by a c-myc RNA-cleaving deoxyribozyme. J Biol Chem. 1999;274:17236–17241. doi: 10.1074/jbc.274.24.17236. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki T, Shin BC, Fujikura K, Matsuzaki T, Takata K. Direct gene transfer into rat liver cells by in vivo electropo-ration. FEBS Lett. 1998;425:436–440. doi: 10.1016/s0014-5793(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 32.Taylor MS, McMahon AM, Gardner JD, Benoit JN. Cyclic nucleotides and vasoconstrictor function: physiological and pathophysiological considerations. Pathophysiology. 1999;5:233–245. [Google Scholar]
- 33.Toyoda T, Imamura Y, Takaku H, Kashiwagi T, Hara K, Iwahashi J, Ohtsu Y, Tsumura N, Kato H, Hamada N. Inhibition of influenza virus replication in cultured cells by RNA-cleaving DNA enzyme. FEBS Lett. 2000;481:113–116. doi: 10.1016/s0014-5793(00)01974-8. [DOI] [PubMed] [Google Scholar]
- 34.Tsang J, Joyce GF. Specialization of the DNA-cleaving activity of a group I ribozyme through in vitro evolution. J Mol Biol. 1996;262:31–42. doi: 10.1006/jmbi.1996.0496. [DOI] [PubMed] [Google Scholar]
- 35.Tsujie M, Isaka Y, Nakamura H, Imai E, Hori M. Electroporation-mediated gene transfer that targets glomeruli. J Am Soc Nephrol. 2001;12:949–954. doi: 10.1681/ASN.V125949. [DOI] [PubMed] [Google Scholar]
- 36.Unwalla H, Banerjea Akhil. Inhibition of HIV-1 gene expression by novel macrophage-tropic DNA enzymes targeted to cleave HIV-1 TAT/Rev RNA. Biochem J. 2001;357:147–155. doi: 10.1042/0264-6021:3570147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young JL, Benoit JN, Dean DA. Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature. Gene Ther. 2003;10:1465–1470. doi: 10.1038/sj.gt.3302021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young JL, Dean DA. Non-viral gene transfer strategies for the vasculature. Microcirc Res. 2002;9:35–50. doi: 10.1038/sj/mn/7800120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Gasper WJ, Stass SA, Ioffe OB, Davis MA, Mixson AJ. Angiogenic inhibition mediated by a DNAzyme that targets vascular endothelial growth factor receptor 2. Cancer Res. 2002;62:5463–5469. [PubMed] [Google Scholar]