Abstract
Objective
To evaluate whether hormonal contraceptives, used before or in early pregnancy, confer increased risk of preterm birth or reduced fetal growth.
Design
Population-based cohort study conducted by the Norwegian Institute of Public Health (Mother and Child Cohort Study, 1998-2008) with linkage to the Norwegian Prescription Registry and to the Medical Birth Registry of Norway.
Setting
Norway
Population
Of the 48,615 pregnancies meeting study inclusion criteria, 44,734 pregnancies were included in the complete case analysis.
Methods
We characterized hormonal contraception by type (combination oral, progestin only oral, vaginal ring, transdermal, and injectable) and specific progestin component. We used generalized estimating equations to estimate the odds of adverse outcome according to formulation used. Several sensitivity analyses were conducted.
Main outcome measures
Preterm birth, small for gestational age
Results
We observed a positive association between use of a combination oral contraceptive and preterm birth for all exposure periods (e.g., adjusted OR: 1.21, 95% CI: 1.04, 1.41 for last use 12 to >4 months before conception); combination contraceptives containing the progestin norethisterone were consistently related to risk. Other types of hormonal contraception were generally not associated with preterm birth; none were related to small for gestational age. Observed associations were robust to sensitivity analyses.
Conclusion
Hormonally active agents may exert dose-, agent-, and timing-specific effects on growth and development. We found that the particular progestin component is important when assessing the potential for adverse effects among former users of hormonal contraceptives.
Keywords: Mother and child Cohort Study (MoBa), preterm birth, small for gestational age, hormonal contraceptives
Introduction
Exposure to hormonally active agents during pregnancy has been inversely associated with duration of gestation and fetal growth1-3. Much of the literature concerning exposure to hormonally active agents has been centered on exposure to environmental compounds that may have endocrine effects. However pharmacologic agents may also contribute. For example, in women undergoing fresh embryo-based transfer for in vitro fertilization, increased levels of endogenous estrogen at the time of blastocyst implantation, as a result of ovarian hyperstimulation from gonadotropins, may contribute to an increased risk of preterm birth 4, 5. In sows, administering 17β-estradiol throughout pregnancy suppressed offspring growth in early life; in mice 6, administering diethylstilbestrol in early neonatal life resulted in an initial decrease in offspring growth 7.
Possible adverse effects, resulting from use of hormonal contraceptives in pregnancy have been studied, with mixed evidence as to whether use is associated with preterm birth or altered fetal growth. These former studies generally had limited statistical power and most used older statistical methods for data analyses 8-11. Some of the variability in results from these studies may also be attributable to differences in the contraceptives assessed. The effects of hormonal contraceptives vary and are largely driven by the progestin component and the pharmacodynamics of progestin and ethinyl estradiol taken in combination. For example, some progestin formulations, such as levonorgestrel, are androgenic, while others, such as drospirenone, have no affinity for androgen receptor binding.12, 13 The capacity of the progestin to bind to androgen, mineralocorticoid, and glucocorticoid receptors is thought to be a major determinant of the differential actions of progestins in eliciting adverse metabolic effects in women.12, 14-18 These effects are similar to the metabolic changes that occur in women who are overweight or obese.19,20 In the animal models, maternal obesity has led to an increase in the number of apoptotic follicles in the ovaries, smaller and fewer oocytes, and smaller pups at birth.21, 22 To our knowledge, there are no studies of the association between hormonal contraceptive use and birth outcomes whereby exposure to hormonal contraceptives was evaluated according to progestin type. We are also not aware of any studies evaluating birth outcomes for users of the progestin-only oral contraceptive. Given the differences in metabolic effects from hormonal contraceptive formulations with varying progestin components, and the relationship between maternal metabolic factors and offspring birth outcomes, evaluating the association between hormonal contraceptive by progestin type is warranted. These associations may not be limited to exposures incurred during pregnancy. In animal models, developmentally sensitive periods begin even before conception. Specifically, the recruitment of a follicle for selection as a dominant follicle and oocyte maturation can be influenced by exposure to hormonally active agents.23-27.
In the present study we used the Norwegian Mother and Child Cohort Study (MoBa), a population-based cohort study conducted by the Norwegian Institute of Public Health,28, 29 to evaluate the association between hormonal contraceptive use before and in early pregnancy, and birth outcomes. The MoBa data were linked to the Medical Birth Registry of Norway (MBRN) and to the Norwegian Prescription Registry (NorPD). In linking to the prescription registry, the formulation of hormonal contraceptive could be evaluated with finer detail than has been described previously.
Methods
Primary analyses
Study population
MoBa study participants were recruited from 1999 through 2008. Women were identified for eligibility when scheduling the routine prenatal ultrasound offered free of charge to all pregnant women in Norway at 17-20 weeks of gestation. Women were then mailed an invitation to participate before the scheduled ultrasound, with informed consent and enrollment taking place at the ultrasound examination. All hospitals with at least 100 births per year participated in the study recruitment and enrollment. Approximately 42 percent of all pregnant women in Norway were invited to participate in the study. Of these, 39 percent consented to participate. At enrollment, participants were asked to provide a blood sample and to complete an initial, self-administered questionnaire to provide data on demographic characteristics, reproductive health history, disease and medication history, lifestyle factors, and socioeconomic status. Follow-up is ongoing and is conducted through self-administered questionnaires at regular intervals and by linkage to national health registries.
All MBRN birth registry data are collected on a standardized birth notification form completed by the midwife or physician attending the birth. Prescription data from NorPD contains individual-level data on all medications prescribed and dispensed through pharmacies to non-institutionalized individuals in Norway. By Norwegian law, as of January 1, 2004, all pharmacies must provide electronic data for all prescriptions dispensed.
There were 107,308 pregnancies in the MoBa cohort (cohort Version 6). For the present analysis, we included pregnancies resulting in a singleton live birth and excluded pregnancies with documentation of infertility treatment for the index pregnancy, on either the MoBa 17-week questionnaire or the MBRN. We additionally excluded pregnancies to women who had documented pre-pregnancy chronic hypertension (n=527). As the NorPD registry was not initiated until January 1, 2004, we further restricted our study population to pregnancies of women enrolled at least 12 months after the date on which the NorPD registry began collection of data on prescription fills (n=48,615). We excluded pregnancies with missing covariate data (n=4,191). The final study population included 44,734 pregnancies to 42,155 women (Figure 1).
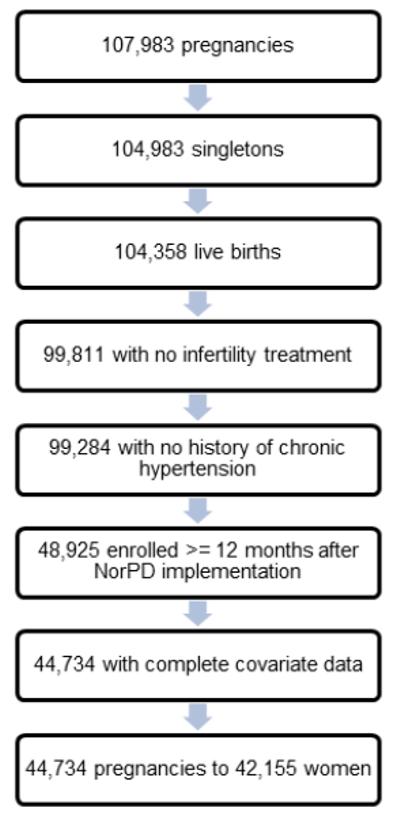
All Norwegian residents are assigned a personal identifier number. Linkage of the MoBa questionnaire, MBRN, and NorPD data files were possible through this identifier. The University of North Carolina at Chapel Hill and the National Institute of Environmental Health Sciences Institutional Review Boards and the Norwegian Southeastern Regional Ethics Committee reviewed and approved this study.
Exposure
Dispensing of hormonal contraceptives prior to conception and in early pregnancy was ascertained via linkage to the Norwegian Prescription Registry Database. These contraceptives were characterized according to the Anatomical Therapeutic Chemical (ATC) Classification System. We characterized exposure by type and route of administration (combination oral contraceptive, progestin-only oral contraceptive, vaginal ring, transdermal, injectable, implant, and hormonal-based intrauterine device), the approach most similar to previous studies evaluating the relationship between hormonal contraceptive use and adverse pregnancy outcomes. Because of the heterogeneity in hormonal contraceptive progestins in their specificity for binding to the progesterone receptor, we also characterized exposure by type, route, and progestin component. All hormonal contraceptives with an estrogen component (combination oral contraceptives, vaginal ring, and the transdermal contraceptive) contained ethinyl estradiol, but there were eight different progestin types used solely or in combination with ethinyl estradiol, including desogestrel, drospirenone, levonorgestrel, norelgestromin, norethisterone, lynestronol, medroxyprogesterone, and etonogestrel. Date of conception was estimated by subtracting 17 days 30 from the total number of days of gestation (to account for the follicular phase prior to conception) and then subtracting this value from the date of birth. We used the last menstrual period (LMP)-based estimated gestational length unless the LMP-based gestational length was missing or ≥2 weeks different from the ultrasound-based estimate of gestational length, in which case we used the ultrasound-based measure 31. We then constructed an exposure window for each hormonal contraceptive prescription filled using the date that the prescription was filled and the number of defined daily doses dispensed (day’s supply). We characterized exposure into discrete categories relative to conception: last use 12 - >4 months before, 4 - >1 months before, and 1 - >0 months before, and 0 -12 weeks after. Women using hormonal contraceptives in early pregnancy were also using hormonal contraceptives before pregnancy. In our analyses, we evaluated outcomes among those within a category of date of last use as compared to those with no hormonal contraceptive use within any of the other exposure periods assessed.
Most oral contraceptives were dispensed in 3-month supply (82%) or 6-month supply (15%). For pregnancies with more than one type of hormonal contraceptive prescribed, we assigned exposure type according to the type of contraceptive used closest to the estimated date of conception. Because many women may choose to stop taking their hormonal contraceptive in order to achieve conception, we characterized women as exposed in early pregnancy only if they reported on the 17-week questionnaire that the pregnancy was unplanned and they had ≥1 day(s) supply of hormonal contraceptive at or after the day of conception as defined above.
Outcome
Preterm birth was defined as delivery before 37 completed weeks of pregnancy 32. Small for gestational age (SGA) was characterized as having been born at <3rd percentile for weight for gestational age 33, 34. Birthweight z-score was calculated by subtracting the observed birthweight from the expected birthweight based on the population standard distribution (at that gestational age) and then dividing this value by the standard deviation for each gestational age.
Covariates
We used a Directed Acyclic Graph (DAG) approach35 to identify a minimally sufficient set of adjustment factors. The selected covariates included in the DAG were factors demonstrated to be antecedents of both hormonal contraceptive use and preterm birth or weight for gestational age, including maternal age (14-19, 20-29, 30-39, 40-49) 36, 37, maternal pre-pregnancy BMI (kg/m2)(<18.5, 18.5-24.9, ≥25.0) 37, 38, parity (0, 1, ≥2) 39, maternal smoking (none, quit, smoker) 37, 40, and maternal education (>4 years of university or technical, 4 year university or technical degree, 3 years of college preparatory high school, 3 years of technical high school, 1-2 years of high school, <9 years of secondary school, other) 41,42. In the analyses we restricted the study population to women without chronic hypertension and adjusted on BMI, age, parity, smoking, and education.
Analysis
Our primary analyses were concerned with assessing the association between hormonal contraceptive use, by type (combination versus progestin-only) and route (oral, vaginal, transdermal, and injectable), and preterm birth or SGA within the discrete categories of period of last use as listed above. For each exposure period, any contraceptive with <10 exposed cases were combined into a single “other” exposure category. We used generalized linear models with a logit link, and generalized estimation equations (GEE) with an independent correlation matrix 43 to estimate associations between exposures and outcomes using robust standard errors, accounting for lack of independence between siblings.
We conducted likelihood ratio tests to assess whether the model fit for preterm birth or SGA was improved by characterizing exposure with increasing detail. We used generalized linear models to obtain the log likelihood for three, nested models; first modeling any hormonal contraceptive use in each of the exposure periods as compared to no use at any of the exposure periods, second, hormonal contraceptive use by type and route as compared to no use, and third, contraceptive use by type, route and progestin formulation as compared to no use. All analyses were conducted using SAS v9.3 (SAS Inc., Cary, North Carolina).
Sensitivity analyses
Examination of the covariate distribution among pregnancies with hormonal contraceptive use in early pregnancy, as compared to non-users, indicated that women with hormonal contraceptive use were generally older and more parous than non-users (Table S1). There were also differences in the characteristics of women using different types of hormonal contraceptives. For example, while 71% of combination oral contraceptive users were nulliparous, just 17.2% of progestin-only oral contraceptive users were nulliparous. These differences suggest that prescribing patterns may differ based on individual factors, thus raising concern about confounding by indication. We explored the robustness of our results through sensitivity analyses designed to mitigate the potential for confounding by indication.
First, because combination oral contraceptive users were much likely to be nulliparous than progestin-only oral contraceptive users, we restricted our analysis to nulliparous pregnancies only. Next, we compared the association comparing combination oral contraceptive use to vaginal ring use (Table S1), because these two groups were socio-demographically similar. Also, to assess whether factors associated with having an unplanned pregnancy (e.g. prenatal care or other behavioral factors) could be confounding the results observed, we restricted our analysis of exposure at 0 -12 weeks after conception to women reporting an unplanned pregnancy, specifically, users of a hormonal contraceptive as compared to unplanned pregnancies not using a hormonal contraceptive. Finally, we conducted a propensity score analysis to reduce residual confounding associated with use of combination oral contraceptives as compared to no use of a hormonal contraceptive.
To construct the propensity scores, we evaluated several models to estimate the predicted probability of obtaining a combination oral contraceptive prescription (propensity for treatment scores). We included in the models those factors believed to be associated with both use of the combination oral contraceptive and preterm birth (parity, maternal pre-pregnancy body mass index, maternal age, maternal education, maternal smoking), but that preceded the use of a combination oral contraceptive. We compared the distribution of propensity scores among those prescribed a combination hormonal contraceptive to those not prescribed any hormonal contraceptive, to evaluate evidence of common support, and trimmed any observations where there was no corresponding propensity score. We then ranked the scores into deciles and assigned each observation a corresponding rank. We used GEE models to assess the relationship of combination oral contraceptive use with preterm birth, with inclusion of an indicator term for rank, and obtained a pooled estimate of the association across strata. In addition to the term for rank, these models included all of the potential confounders from the primary analyses models 44-46. The distribution of study covariates for the exposed and unexposed was similar within propensity score rank (Table S2).
Results
As noted above, 44,734 pregnancies met the inclusionary criteria. Of these, nearly all were to women between the ages of 20-39 (97%), approximately half were first pregnancies (47.1%), and the majority had at least some college education (81.4%). Roughly a third of the pregnancies (30.7%) were in women who were overweight or obese (Table 1).
Table 1.
Study population characteristics by type of hormonal contraceptive used 12 months before conception
| Characteristic | Study population n=44,734 % |
No hormonal contraceptive n=23,727 % |
Combination oral contraceptive n=14,012 % |
Progestin only oral contraceptive n=4,572 % |
Vaginal ring n=1,215 % |
Transdermal n=832 % |
Otherb n=376 % |
|
|---|---|---|---|---|---|---|---|---|
| Maternal age (yrs) | ||||||||
| 14-19 | 0.9 | 0.5 | 1.8 | 0.3 | 0.6 | 1.2 | 0.0 | |
| 20-29 | 42.1 | 33.1 | 56.5 | 37.7 | 54.3 | 59.0 | 47.9 | |
| 30-39 | 54.9 | 63.1 | 41.3 | 60.6 | 44.0 | 39.4 | 49.7 | |
| 40-49 | 2.1 | 3.3 | 0.4 | 1.4 | 1.1 | 0.5 | 2.4 | |
| Maternal BMI (kg/m2) | ||||||||
| <18.5 | 3.2 | 3.3 | 3.1 | 3.3 | 2.8 | 4.1 | 2.9 | |
| 18.5-24.9 | 66.0 | 65.6 | 66.8 | 65.7 | 68.8 | 65.0 | 60.1 | |
| 25.0-29.9 | 21.7 | 21.3 | 21.9 | 22.8 | 22.6 | 21.3 | 28.2 | |
| ≥30.0 | 9.0 | 9.8 | 8.2 | 8.3 | 5.8 | 9.6 | 8.8 | |
| Parity | ||||||||
| 0 | 47.1 | 37.8 | 70.6 | 17.2 | 67.4 | 57.2 | 29.5 | |
| 1 | 35.5 | 39.6 | 20.9 | 63.9 | 21.0 | 27.5 | 41.2 | |
| 2 | 13.7 | 17.6 | 7.0 | 15.0 | 9.3 | 12.7 | 21.8 | |
| 3 | 2.8 | 3.8 | 1.1 | 3.1 | 1.7 | 1.9 | 5.3 | |
| 4 or more | 0.9 | 1.3 | 0.4 | 0.8 | 0.7 | 0.6 | 2.1 | |
| Maternal education | ||||||||
| More than 4 years of university or technical | 27.2 | 29.5 | 23.4 | 30.0 | 25.8 | 16.4 | 17.8 | |
| 4 year university degree, regional technical | 40.8 | 40.0 | 40.5 | 44.7 | 46.0 | 42.0 | 33.8 | |
| 3 years high school, junior college | 13.4 | 12.7 | 15.3 | 10.6 | 13.2 | 17.7 | 17.3 | |
| Technical high school | 11.2 | 10.3 | 12.8 | 9.5 | 10.5 | 14.7 | 16.8 | |
| 1-2 years high school | 3.9 | 3.9 | 4.3 | 2.8 | 2.1 | 4.9 | 7.5 | |
| 9-year secondary | 2.2 | 2.3 | 2.3 | 1.3 | 1.1 | 2.6 | 5.3 | |
| Other | 1.4 | 1.4 | 1.4 | 1.2 | 1.4 | 1.8 | 1.6 | |
| Maternal smoking (at 17 weeks) | ||||||||
| None | 79.3 | 80.0 | 76.8 | 85.0 | 77.8 | 75.0 | 70.5 | |
| Quit | 14.5 | 13.9 | 16.5 | 10.9 | 16.5 | 16.6 | 18.6 | |
| Daily | 1.4 | 1.3 | 1.6 | 1.2 | 1.7 | 2.2 | 2.4 | |
| Sometimes | 4.8 | 4.8 | 5.1 | 3.0 | 4.1 | 6.3 | 8.5 |
represents unique pregnancies conceived without infertility treatments, resulting in a singleton live birth, with a date of birth ≥12 months after NorPD registry began (January 1, 2004)
includes the intrauterine, injectable, emergency, and implant hormonal contraceptives
There were 1,969 (4.4%) births before 37 completed weeks of gestation and 1,167 (2.6%) infants were SGA. Relative to conception, 7,470 pregnancies were last exposed 12 to >4 months before, 5,740 were exposed 4 to >1 months before, 6,465 were exposed 1 to >0 months before, and 1,638 were exposed 0 to 12 weeks after.
In evaluating hormonal contraceptive use by type and route of administration, we observed a positive association between use of a combination oral contraceptive and preterm birth, with the magnitude of the association remaining relatively consistent regardless of the exposure period (Table 2). For the progestin-only oral contraceptive, we observed an inverse association with preterm birth for use 1 to >0 months before conception (aOR: 0.67, 95% CI: 0.46, 0.97) and a positive, but statistically non-significant association for each of the other exposure periods. Other associations with preterm birth were not observed, except use of an injectable contraceptive at 12 - >4 months before conception was positively associated with preterm birth (aOR: 1.83, 95% CI: 1.06, 3.18). Data were too sparse to evaluate the association between the injectable and preterm birth for any of the other exposure periods. The direction of the association between use of a hormonal contraceptive and gestational length, in days, was generally consistent with the estimates obtained from modeling the association with preterm birth.
Table 2.
Hormonal contraceptive use and gestational length at birth by period of last use relative to conception, progestin type, and route of administration
| Preterm birth |
Gestational length (days) |
|||||
|---|---|---|---|---|---|---|
| Exposure | Exposed (n) |
Preterm (n) |
OR (95% CI) | aORa (95% CI) | β (95% CI) unadjusted |
β (95% CI) adjusted* |
| None | 23,421 | 964 | referent | referent | referent | referent |
| 0 - 12 weeks after | ||||||
| Combination OC | 1,062 | 71 | 1.67 (1.30, 2.14) | 1.32 (1.01, 1.73) | −1.19 (−2.08, −0.29) | −0.73 (−1.63, 0.18) |
| Progestin only OC | 359 | 18 | 1.23 (0.76, 1.98) | 1.26 (0.78, 2.04) | −0.52 (−1.75, 0.71) | −0.47 (−1.70, 0.76) |
| Other b | 217 | 5 | 0.55 (0.23, 1.34) | 0.49 (0.20, 1.21) | 1.28 (−0.29, 2.85) | 1.48 (−0.11, 3.06) |
| 1 - >0 months before | ||||||
| Combination OC | 4,660 | 225 | 1.18 (1.02, 1.37) | 1.13 (0.97, 1.31) | 0.11 (−0.30, 0.51) | −0.09 (−0.50, 0.33) |
| Progestin only OC | 1,204 | 30 | 0.60 (0.41, 0.86) | 0.67 (0.46, 0.97) | 0.87 (0.26, 1.48) | 0.66 (0.05, 1.27) |
| Vaginal ring | 356 | 13 | 0.88 (0.51, 1.54) | 0.86 (0.49, 1.51) | 0.18 (−1.06, 1.43) | −0.10 (−1.35, 1.16) |
| Other b | 245 | 7 | 0.69 (0.32, 1.46) | 0.66 (0.31, 1.42) | 0.16 (−1.16, 1.48) | 0.05 (−1.27, 1.36) |
| 4 - >1 month before | ||||||
| Combination OC | 3,833 | 213 | 1.37 (1.18, 1.60) | 1.31 (1.11, 1.53) | −0.49 (−0.93, −0.04) | −0.73 (−1.19, −0.26) |
| Progestin only OC | 1,284 | 53 | 1.00 (0.76, 1.33) | 1.15 (0.87, 1.53) | 0.39 (−0.27, 1.05) | 0.18 (−0.48, 0.85) |
| Vaginal ring | 352 | 12 | 0.82 (0.46, 1.47) | 0.80 (0.45, 1.43) | 0.32 (−0.89, 1.52) | 0.04 (−1.16, 1.25) |
| Other b | 271 | 9 | 0.80 (0.41, 1.56) | 0.76 (0.39, 1.49) | 0.39 (−0.83, 1.61) | 0.30 (−0.93, 1.52) |
| 12 - >4 months before | ||||||
| Combination OC | 4,633 | 241 | 1.28 (1.11, 1.48) | 1.21 (1.04, 1.41) | −0.10 (−0.51, 0.31) | −0.31 (−0.74, 0.11) |
| Progestin only OC | 1,795 | 71 | 0.96 (0.75, 1.23) | 1.10 (0.85, 1.40) | −0.38 (−0.96, 0.19) | −0.56 (−1.14, 0.001) |
| Vaginal ring | 424 | 12 | 0.68 (0.38, 1.21) | 0.68 (0.38, 1.20) | 0.83 (−0.32, 1.98) | 0.54 (−0.63, 1.70) |
| Transdermal | 295 | 10 | 0.82 (0.43, 1.54) | 0.78 (0.41, 1.48) | 1.12 (−0.11, 2.34) | 1.03 (−0.19, 2.25) |
| Injectable | 180 | 14 | 1.96 (1.13, 3.40) | 1.83 (1.06, 3.18) | −1.58 (−3.49, 0.33) | −1.72 (−3.95, 0.50) |
| Other b | 143 | 1 | 0.16 (0.02, 1.17) | 0.18 (0.02, 1.27) | 2.47 (0.99, 3.96) | 2.56 (1.07, 4.05) |
adjusted for parity, maternal education, maternal pre-pregnancy BMI, maternal smoking, and maternal age at birth
hormonal contraceptive types with <10 exposed cases collapsed into a single “other” category
When characterizing exposure by type, route, and progestin component, likelihood ratio tests indicated improved model fit (p<0.05) for exposure 4 to >1 months before, 1 to >0 months before, and 0 to 12 weeks after conception. For example, for exposure 0 - 12 weeks after conception, the magnitude of the association for use of a combination oral contraceptive with norethisterone was much stronger (aOR: 3.33, 95% CI: 1.69, 6.57) than the magnitude of the association for the combination oral contraceptive containing drospirenone (aOR: 1.17, 95% CI: 0.76, 1.80). Similarly, by evaluating the progestin-only oral contraceptive by progestin type, we observed a strong association between use of the norethisterone progestin-only oral contraceptive 0 - 12 weeks after conception and preterm birth (aOR: 2.02, 95% CI: 1.03, 1.79). Data were too sparse to evaluate other forms of the progestin-only oral contraceptive by progestin type for exposure 0 - 12 weeks after conception.
Norethisterone in the combination oral contraceptive was significantly, positively associated with preterm birth across all exposure periods, with the exception of use 1 - >0 months before, which was statistically non-significant. A trend in association magnitude across the four periods was not supported (p=0.41). Norethisterone in the progestin-only oral contraceptive was not associated with preterm birth at any of the other exposure periods (Table 3); however a test of homogeneity of aOR across the four exposure periods suggested no difference (3 d.f., p=0.14).
Table 3.
Hormonal contraceptive use and preterm birth by period of last use relative to conception, progestin type, and route of administration
| Exposure | Exposed(n) | Preterm (n) | OR (95% CI) | aORa (95% CI) |
|---|---|---|---|---|
| None | 23,421 | 964 | referent | referent |
| 0 - 12 weeks after | ||||
| Combination OC | ||||
| drospirenone and EE | 368 | 22 | 1.48 (0.96, 2.29) | 1.17 (0.76, 1.80) |
| levonorgestrel and EE | 545 | 34 | 1.55 (1.09, 2.21) | 1.20 (0.83, 1.74) |
| norethisterone and EE | 75 | 11 | 4.00 (2.10, 7.62) | 3.33 (1.69, 6.57) |
| Progestin only OC | ||||
| norethisterone | 146 | 11 | 1.90 (1.02, 3.52) | 2.02 (1.09, 3.75) |
| Otherb | 504 | 16 | 0.76 (0.46, 1.26) | 0.69 (0.41, 1.15) |
| 1 - >0 months before | ||||
| Combination OC | ||||
| desogestrel and EE | 295 | 25 | 2.16 (1.42, 3.27) | 2.09 (1.38, 3.16) |
| drospirenone and EE | 1,472 | 61 | 1.01 (0.77, 1.31) | 0.95 (0.73, 1.24) |
| levonorgestrel and EE | 2,521 | 120 | 1.16 (0.96, 1.41) | 1.11 (0.91, 1.36) |
| norethisterone and EE | 372 | 19 | 1.25 (0.79, 2.00) | 1.21 (0.76, 1.93) |
| Progestin only OC | ||||
| desogestrel | 690 | 17 | 0.59 (0.36, 0.96) | 0.65 (0.40, 1.06) |
| norethisterone | 483 | 13 | 0.64 (0.37, 1.12) | 0.74 (0.43, 1.29) |
| Vaginal ring | ||||
| etonogestrel and EE | 356 | 13 | 0.88 (0.51, 1.54) | 0.86 (0.49, 1.51) |
| Otherb | 276 | 7 | 0.61 (0.29, 1.29) | 0.60 (0.28, 1.28) |
| 4 - >1 month before | ||||
| Combination OC | ||||
| desogestrel and EE | 197 | 11 | 1.38 (0.75, 2.54) | 1.27 (0.69, 2.35) |
| drospirenone and EE | 1,227 | 56 | 1.11 (0.85, 1.47) | 1.07 (0.80, 1.41) |
| levonorgestrel and EE | 2,107 | 125 | 1.47 (1.21, 1.78) | 1.40 (1.15, 1.70) |
| norethisterone and EE | 302 | 21 | 1.74 (1.11, 2.72) | 1.67 (1.06, 2.62) |
| Progestin only OC | ||||
| desogestrel | 817 | 34 | 1.01 (0.71, 1.43) | 1.15 (0.81, 1.63) |
| norethisterone | 417 | 16 | 0.93 (0.56, 1.54) | 1.09 (0.66, 1.80) |
| Vaginal ring | ||||
| etonogestrel and EE | 352 | 12 | 0.82 (0.46, 1.47) | 0.80 (0.45, 1.43) |
| Otherb | 321 | 12 | 0.90 (0.51, 1.62) | 0.89 (0.50, 1.60) |
| 12 ->4 months before | ||||
| Combination OC | ||||
| desogestrel and EE | 215 | 17 | 2.00 (1.21, 3.30) | 1.86 (1.13, 3.09) |
| drospirenone and EE | 1,475 | 79 | 1.32 (1.04, 1.67) | 1.25 (0.99, 1.60) |
| levonorgestrel and EE | 2,605 | 122 | 1.14 (0.94, 1.39) | 1.09 (0.89, 1.33) |
| norethisterone and EE | 338 | 23 | 1.70 (1.11, 2.61) | 1.61 (1.05, 2.49) |
| Progestin only OC | ||||
| desogestrel | 1,031 | 42 | 0.99 (0.72, 1.36) | 1.13 (0.82, 1.55) |
| norethisterone | 640 | 22 | 0.83 (0.54, 1.28) | 0.95 (0.62, 1.47) |
| Vaginal ring | ||||
| etonogestrel and EE | 424 | 12 | 0.68 (0.38, 1.21) | 0.67 (0.38, 1.20) |
| Transdermal patch | ||||
| norelgestromin | 295 | 10 | 0.82 (0.43, 1.54) | 0.78 (0.41, 1.48) |
| Injectable | ||||
| medroxyprogesterone | 180 | 14 | 1.96 (1.13, 3.40) | 1.83 (1.06, 3.18) |
| Otherb | 267 | 8 | 0.72 (0.36, 1.46) | 0.79 (0.39, 1.60) |
adjusted for parity, maternal education, maternal pre-pregnancy BMI, maternal smoking, and maternal age at birth
hormonal contraceptive types with <10 exposed cases collapsed into a single “other” category
Levonorgestrel was the most commonly prescribed progestin type in combination oral contraceptives in this data. With the exception of use at 4 - >1 month before (aOR: 1.40, 95% CI: 1.15, 1.70), there was little evidence to support an association between levonorgestrel in the combination oral contraceptive and preterm birth (Table 3).
Data were too sparse for evaluating use of desogestrel in early pregnancy, however desogestrel was significantly, positively associated with preterm birth for use 1- >0 months and 12 - >4 months before conception. Use of desogestrel 4 ->1 month before suggested a positive, but non-statistically significant association with preterm birth (Table 3).
With the exception of use 12 - >4 months before conception, the combination oral contraceptive containing drospirenone progestin was generally not associated with preterm birth. Etonogestrel, in the vaginal ring, and norelgestromin, in the transdermal hormonal contraceptive, were also not associated with preterm birth in any of the exposure periods (Table 3).
Medroxyprogesterone was the only progestin used as an injectable among women in this sample and, as described above, was moderately associated with preterm birth for exposure 12 - >4 months before conception (Table 3).
For formulations where sufficient data were available for analyses, we observed no association between use of hormonal contraceptives and increased odds of SGA or decreased birthweight z-score (Tables S3-S4). We observed an inverse relation with use of a progestin-only oral contraceptive and SGA for exposure 4 – >1 month before and 1 – >0 months before conception. Sparse data precluded evaluation of the association between early pregnancy use of a progestin-only oral contraceptive and SGA (Table S3), as well as individual progestin types for the progestin-only oral contraceptive (Table S4).
In sensitivity analyses, estimates observed in our primary analyses were robust to restriction of the study sample to nulliparous pregnancies (Table S5). When restricting the comparator population to vaginal ring users within the same exposure period, the magnitude of the association observed for combination oral contraceptive users and preterm birth strengthened (aOR 12 - >4 months: 1.78, 95% CI: 0.99, 3.21), however the wider confidence intervals reflect the markedly reduced sample size for these analyses (Table S6). The results of the sensitivity analysis evaluating early pregnancy hormonal contraceptive users as compared to unplanned pregnancies without hormonal contraceptive use attenuated the estimates observed in the primary analysis (aOR 1.22, 95% CI: 0.91, 1.63 vs aOR 1.32, 95% CI 1.01, 1.73). The estimate for the combination oral contraceptive containing norethisterone remained significantly, positively associated with preterm birth (aOR: 3.00, 95% CI: 1.53, 5.83) (Table S6). The association between the combination oral contraceptive and preterm birth, at all exposure periods, was similarly robust to approaches employing propensity scores (Table S7).
Discussion
Main findings
In the present study, use of some formulations of hormonal contraceptives, especially oral contraceptives, was associated with preterm birth. The associations observed varied by timing and progestin component, with norethisterone and desogestrel showing the strongest magnitude of association.
For preterm birth, the positive association for the combination oral contraceptive was observed across all exposure periods. The consistency in the magnitude of the association across exposure periods could be evidence of an association with long-term (>12 months) use, in which case proximity of last exposure to conception may be less important.
For inadvertent use of hormonal contraceptives early in pregnancy a few suggestive findings were seen; however, there was no convincing evidence of a causal relationship with preterm birth, and no positive association with SGA was observed.
Strengths and limitations
We used multiple approaches to explore the sensitivity of the estimates to various assumptions made in the analysis, including restriction to nulliparous pregnancies, unplanned pregnancies, and use of a propensity score to ensure covariate balance in the exposed and unexposed groups. As with all observational studies, there remains the potential that residual confounding is contributing to the estimates observed.
The association between the combination oral contraceptive and preterm birth was generally robust to several sensitivity analyses evaluating potential for uncontrolled confounding in our primary analyses. The sensitivity analysis examining use of a hormonal contraceptive in early pregnancy as compared to other women experiencing an unplanned pregnancy resulted in an attenuation of estimates, with the exception of the norethisterone-containing formulation, which remained significantly associated with preterm birth. However, there may be factors we did not include in our models -- factors not in our dataset that lead to differential use of hormonal contraceptives and that were associated with pregnancy outcomes. For example, the specific hormonal contraceptive prescribed is influenced by an individual woman’s estrogen, progesterone, androgen sensitivities 12 and these could confound the association between hormonal contraceptives and preterm birth. Still, the propensity score models provide additional support for the associations observed in our primary analyses, assuming we accurately predicted prescribing of a combination oral contraceptive.
Although use of a pharmacy-based registry offers the benefit of studying specific formulations of contraceptives dispensed at specific times, the registry data are only a proxy for actual use of the contraceptives. Data quality measures are in place for assuring the NorPD is accurate and complete 47. A validation study of hormonal contraceptive use in the NorPD was conducted in adolescents and indicated a sensitivity of 99% and a specificity of 76% for the NorPD as compared to self-reported use 48. In adolescents, hormonal contraceptives may be provided at no cost to the individual, but in adults, hormonal contraceptives are not a reimbursable prescription. This may increase the likelihood that a dispensed prescription will be used by the individual. Classification of exposure in early pregnancy was limited to pregnancies reported to be unplanned to increase the potential that prescribed contraceptives were actually being used, but for exposure in other periods before pregnancy, fewer women may have been taking a hormonal contraceptive than estimated. The consistent attenuation in adjusted associations at the 1 - >0 months before conception exposure interval, as compared to other exposure periods, may reflect the higher potential for misclassification in this exposure period. In some instances, data were too sparse to evaluate all formulations at every exposure period, limiting our capacity to explore differences.
Interpretation
As noted above, previous studies of hormonal contraceptive use in pregnancy and gestational length at birth or size at birth had limited statistical power and were underpowered for evaluating formulation-specific effects10, 11, 49-52. Nonetheless, their results suggest a possible, albeit small, negative association between in utero exposure to hormonal contraceptives and birth weight (Table 4). Contemporary approaches to evaluating birthweight or low birthweight have shifted toward assessment of birthweight z-scores or small for gestational age as these metrics take into account gestational age 53. Applying these contemporary approaches to assessing weight at birth, we found little evidence to support an association with birthweight z-score or small for gestational age. The association with birthweight observed in these previous studies may reflect birth after a shorter gestation. For two of these studies, investigators did not evaluate gestational length at birth. In two other studies, investigators evaluated gestational length; with one small study suggestive of a weak, positive association.
Table 4.
Previous studies of in utero exposure to oral contraceptives and birth weight or gestational length as compared to the present study
| Study (years) | Oral contraceptive |
na | Outcome(s) at birth | Association | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Directio n |
Magnitude | Significance | Test/Estimate | Value | ||||
| Oxford Family Planning Association cohort (1968-1974) (Vessey et al.) |
anyb | 30 | birthweight (kgs) low birth weight (<2,500 g) |
null positive |
-- strong |
-- n.s.f |
noneh crude risk ratio |
noneh 7.0f |
| Upstate New York cohort (1974) (Polednak et al.) |
anyc | 23 | birthweight (kgs) low birth weight (<2,500 g) gestational age at birth (days) |
inverse positive positive |
weak weak weak |
n.s.g n.s.g n.s.g |
noneh noneh noneh |
noneh noneh noneh |
| Chang Mai, Northern Thailand cohort (1984- 1987) (Pardthaisong et al.) |
any | 601 | low birth weight (<2,500 g) | positive | moderate | s.s | adjusted odds ratio | 1.5 (95% CI: 1.2, 2.0) |
| Korean Motherrisk Program cohort (2001- 2006) (Ahn et al.) |
anyd | 120 | birthweight (grams) low birth weight (<2,500 g) gestational age at birth (weeks) preterm birth (<37 weeks of gestation) |
null positive null null |
-- weak -- -- |
-- n.s. -- -- |
Mann-Whitney U-test Chi-square test Mann-Whitney U-test Chi-square test |
p=0.95 p=0.07 p=0.20 p=0.88 |
| Norwegian Mother Child cohort (2004-2008) |
combination only progestin only |
1,062 359 |
weight for gestational age at birth (z-scores) small for gestational age (<3rd percentile) gestational age at birth (days) preterm birth (<37 weeks of gestation) gestational age at birth (days) preterm birth (<37 weeks of gestation) |
null null inverse positive null positive |
-- -- weak moderate -- weak |
-- -- n.s. s.s. -- n.s. |
β coefficient adjusted odds ratio β coefficient adjusted odds ratio β coefficient adjusted odds ratio |
0.02 (95% CI: −0.05, 0.09) 1.10 (95% CI: 0.78, 1.55) −0.73 (95% CI: −1.63, 0.18) 1.32 (95% CI: 1.01, 1.73) −0.47 (95% CI: −1.70, 0.76) 1.26 (95% CI: 0.78, 2.04) |
number exposed
evaluated in parous women only
defined as exposed after the date of last menstrual period
defined as periconceptional use in 4 weeks before or 4 weeks after pregnancy began
n.s. - not significant, s.s. – statistically significant
based on our analysis of their data comparing, among parous women, women with unplanned pregnancies using an oral contraceptive and women with planned pregnancies and no use of an oral contraceptive
our best guess
no statistical test conducted
Several studies have assessed the association between use of hormonal contraceptive before pregnancy and outcomes at birth, including birthweight 10, 11, 49-52 and gestational length 50, 51. In general, these studies suggest a small, negative association between use of an oral contraceptive before pregnancy and birthweight. For preterm birth, findings have been mixed 50, 51. The apparent lack of consistency in results between studies may be attributable to several factors, including differences in formulations, timing of exposure, differences in outcome characterization, such as in using birthweight as opposed to birthweight z-score or weight for gestational age, small sample limitations, differential confounding patterns, or chance.
In the present study, we found that use of a combination oral contraceptive before pregnancy, for all exposure periods examined, was associated with preterm birth. The combination oral contraceptive was not associated with weight for gestational age, while use of a progestin only oral contraceptive was negatively associated with small for gestational age at birth. Compared to former studies10, 11, 49-52, the present project had the benefit of a larger sample size, improved capacity to evaluate formulation-specific effects, better investigation of the potential for confounding by indication, and use of contemporary metrics for weight at birth.
Unusually high concentrations of endogenous estradiol due to ovarian hyperstimulation is associated with impaired fetal growth 54, 55. In the present study, however, exogenous estrogen at the time of conception was unrelated to fetal growth. The estrogenicity of the hormonal contraceptives studied may have been too low to affect growth. The weakly estrogenic environmental contaminant bisphenol A was recently associated with impaired fetal growth 56; that association, however, could be independent of estrogenicity. The hormonally active agent DDE, a degradation product of the insecticide DDT, has been shown to be antiprogestogenic 57 and has been associated with preterm birth 58. The association of norethisterone-containing contraceptives with preterm birth seen here suggests that agents disrupting normal progesterone signaling may increase risk of preterm birth, but other possibilities exist. The variation in effects of hormonal contraceptives is largely driven by the progestin component. The capacity of the progestin to bind to androgen, mineralocorticoid, and glucocorticoid receptors is thought to be a major determinant of the differential actions of progestins in eliciting adverse effects 14.
Conclusion
Pharmacologic sources of exposure to hormonally active agents are prevalent due to the frequent use of hormonal contraceptives among women of childbearing age. The results of this study suggest that certain formulations of hormonal contraceptives may increase risk for preterm birth. We found that the particular progestin component is important when assessing the potential for adverse effects among former users of hormonal contraceptives. Additional resources are needed to evaluate the reproducibility of these findings as these findings potentially have important clinical implications for women and their future pregnancies. Should the results of this study be replicated in additional studies, clinicians prescribing hormonal contraceptives may need to be selective in the formulations they prescribe for women planning to conceive at a later date.
Although this is the largest study to date, examining the association between hormonal contraceptive use and preterm birth or small for gestational age, the relatively small number of exposed cases for some formulations limited study power. The sample size was restricted by the fact that the Norwegian Prescription registry did not begin until 2004, over 5 years after the MoBa cohort enrollment began. The Danish National Birth Cohort (DNBC) would offer the opportunity to study these associations with a larger sample, as the prescription registry predates the initiation of the DNBC.
Supplementary Material
Acknowledgements
We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
Study funding:
This work was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS) (ES102985 to CJW), the University of North Carolina institutional training grant award for reproductive, perinatal, and pediatric epidemiology (grant T32HD052468 to ETJ), and the Carolina Population Center (R24 HD050924 to WRR). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, contract N01-ES-75558 with the NIH/NIEHS, NIH/National Institute of Neurological Disorders and Stroke (grant 1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1), and the Norwegian Research Council/FUGE (grant 151918/S10).
Footnotes
Contribution to authorship:
ETJ, JLD, and MPL contributed to the project conception and all authors contributed to the research design. All analyses were conducted by ETJ and the manuscript was drafted by ETJ. ETJ, JLD, TS, WRR, CJW, KV, PM, and MPL all contributed substantively to interpretation of study results and development of the manuscript.
Conflict of interest disclosures:
TS does not accept personal compensation of any kind from any pharmaceutical company, though he receives salary support from the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, Genentech, Merck, Sanofi) to the Department of Epidemiology, University of North Carolina at Chapel Hill. None of the other authors have any disclosures.
Details of ethics approval:
The University of North Carolina at Chapel Hill (10.3.12) and the National Institute of Environmental Health Sciences Institutional Review Boards (4.22.02) and the Norwegian Southeastern Regional Ethics Committee (1.28.13) reviewed and approved this study.
References
- 1.Cantonwine DE, Hauser R, Meeker JD. Bisphenol A and Human Reproductive Health. Expert Rev Obstet Gynecol. 2013 Jul 1;8(4) doi: 10.1586/17474108.2013.811939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson KK, O’Neill MS, Meeker JD. Environmental contaminant exposures and preterm birth: a comprehensive review. J Toxicol Environ Health B Crit Rev. 2013;16(2):69–113. doi: 10.1080/10937404.2013.775048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjeldsen LS, Bonefeld-Jorgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int. 2013 Nov;20(11):8031–44. doi: 10.1007/s11356-013-1753-3. [DOI] [PubMed] [Google Scholar]
- 4.Marino JL, Moore VM, Willson KJ, Rumbold A, Whitrow MJ, Giles LC, et al. Perinatal outcomes by mode of assisted conception and sub-fertility in an Australian data linkage cohort. PLoS One. 2014;9(1):e80398. doi: 10.1371/journal.pone.0080398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari AM, Hyden-Granskog C, et al. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995-2006. Hum Reprod. 2010 Apr;25(4):914–23. doi: 10.1093/humrep/dep477. [DOI] [PubMed] [Google Scholar]
- 6.Werner Fürst R, Pistek VL, Kliem H, Skurk T, Hauner H, Meyer HHD, et al. Maternal low-dose estradiol-17β exposure during pregnancy impairs postnatal progeny weight development and body composition. Toxicology and Applied Pharmacology. 2012;263(3):338–44. doi: 10.1016/j.taap.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009 May 25;304(1-2):84–9. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn HK, Choi JS, Han JY, Kim MH, Chung JH, Ryu HM, et al. Pregnancy outcome after exposure to oral contraceptives during the periconceptional period. Hum Exp Toxicol. 2008 Apr;27(4):307–13. doi: 10.1177/0960327108092290. [DOI] [PubMed] [Google Scholar]
- 9.Pardthaisong T, Gray RH. In utero exposure to steroid contraceptives and outcome of pregnancy. American Journal of Epidemiology. 1991;134(8):795–803. doi: 10.1093/oxfordjournals.aje.a116152. [DOI] [PubMed] [Google Scholar]
- 10.Polednak AP, Janerich DT, Glebatis DM. Maternal exposure to exogenous sex hormones in relation to birth weight of offspring. Teratology. 1983 Apr;27(2):223–9. doi: 10.1002/tera.1420270210. [DOI] [PubMed] [Google Scholar]
- 11.Vessey M, Meisler L, Flavel R, Yeates D. Outcome of pregnancy in women using different methods of contraception. Br J Obstet Gynaecol. 1979 Jul;86(7):548–56. doi: 10.1111/j.1471-0528.1979.tb10808.x. [DOI] [PubMed] [Google Scholar]
- 12.Dickey RP. Managing contraceptive pill patients/drug patients. 14th ed EMIS, Inc.; 2010. [Google Scholar]
- 13.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. Classification and pharmacology of progestins. Maturitas. 2003 Dec 10;46(Suppl 1):S7–S16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Hapgood JP, Koubovec D, Louw A, Africander D. Not all progestins are the same: Implications for usage. Trends Pharmacol Sci. 2004 Nov;25(11):554–7. doi: 10.1016/j.tips.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Frempong BA, Ricks M, Sen S, Sumner AE. Effect of low-dose oral contraceptives on metabolic risk factors in African-American women. J Clin Endocrinol Metab. 2008 Jun;93(6):2097–103. doi: 10.1210/jc.2007-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen KR. Pharmacodynamic effects of oral contraceptive steroids on biochemical markers for arterial thrombosis. Studies in non-diabetic women and in women with insulin-dependent diabetes mellitus. Dan Med Bull. 2002 Feb;49(1):43–60. [PubMed] [Google Scholar]
- 17.Winkler UH, Sudik R. The effects of two monophasic oral contraceptives containing 30 mcg of ethinyl estradiol and either 2 mg of chlormadinone acetate or 0.15 mg of desogestrel on lipid, hormone and metabolic parameters. Contraception. 2009 Jan;79(1):15–23. doi: 10.1016/j.contraception.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Jensen ET, Daniels JL, Sturmer T, Robinson WR, Williams CJ, Moster D, et al. Maternal hormonal contraceptive use and offspring overweight or obesity. Int J Obes. 2014 doi: 10.1038/ijo.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannel WB, Wilson PW, Nam BH, D’Agostino RB. Risk stratification of obesity as a coronary risk factor. Am J Cardiol. 2002 Oct 1;90(7):697–701. doi: 10.1016/s0002-9149(02)02592-4. [DOI] [PubMed] [Google Scholar]
- 20.Pietilainen KH, Sysi-Aho M, Rissanen A, Seppanen-Laakso T, Yki-Jarvinen H, Kaprio J, et al. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects--a monozygotic twin study. PLoS One. 2007;2(2):e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell M, Schulz SL, Armstrong DT, Lane M. Metabolic and mitochondrial dysfunction in early mouse embryos following maternal dietary protein intervention. Biology of Reproduction. 2009 Apr;80(4):622–30. doi: 10.1095/biolreprod.108.072595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010 Aug;151(8):4039–46. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicology and Applied Pharmacology. 2008;233(2):286–96. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pocar P, Brevini T, Fischer B, Gandolfi F. The impact of endocrine disruptors on oocyte competence. Reproduction. 2003 Mar 1;125(3):313–25. doi: 10.1530/rep.0.1250313. 2003. [DOI] [PubMed] [Google Scholar]
- 25.Pocar P, Nestler D, Risch M, Fischer B. Apoptosis in bovine cumulus–oocyte complexes after exposure to polychlorinated biphenyl mixtures during in vitro maturation. Reproduction. 2005 Dec 1;130(6):857–68. doi: 10.1530/rep.1.00761. 2005. [DOI] [PubMed] [Google Scholar]
- 26.Campagna C, Sirard M-A, Ayotte P, Bailey JL. Impaired Maturation, Fertilization, and Embryonic Development of Porcine Oocytes Following Exposure to an Environmentally Relevant Organochlorine Mixture. Biology of Reproduction. 2001 2001 Aug 1;65(2):554–60. doi: 10.1095/biolreprod65.2.554. [DOI] [PubMed] [Google Scholar]
- 27.Gandolfi F, Pocar P, Brevini TAL, Fischer B. Impact of endocrine disrupters on ovarian function and embryonic development. Domestic Animal Endocrinology. 2002;23(1-2):189–201. doi: 10.1016/s0739-7240(02)00156-x. [DOI] [PubMed] [Google Scholar]
- 28.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) International journal of epidemiology. 2006 Oct;35(5):1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 29.Nilsen RM, Vollset SE, Gjessing HK, Skjærven R, Melve KK, Schreuder P, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatric and perinatal epidemiology. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 30.Jukic AM, Weinberg CR, Baird DD, Wilcox AJ. Lifestyle and reproductive factors associated with follicular phase length. J Womens Health (Larchmt) 2007 Nov;16(9):1340–7. doi: 10.1089/jwh.2007.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatric and perinatal epidemiology. 2007;21(Suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. Journal Article. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council . Preterm Birth: Causes, Consequences, and Prevention. National Academies Press; Washington, DC: 2007. [PubMed] [Google Scholar]
- 33.Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gulmezoglu AM, et al. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011 May 28;377(9780):1855–61. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 34.Goldenberg RL, Cliver SP. Small for gestational age and intrauterine growth restriction: definitions and standards. Clin Obstet Gynecol. 1997 Dec;40(4):704–14. doi: 10.1097/00003081-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 36.Axmon A, Rylander L, Albin M, Hagmar L. Factors affecting time to pregnancy. Hum Reprod. 2006 May;21(5):1279–84. doi: 10.1093/humrep/dei469. [DOI] [PubMed] [Google Scholar]
- 37.Mutsaerts MA, Groen H, Huiting HG, Kuchenbecker WK, Sauer PJ, Land JA, et al. The influence of maternal and paternal factors on time to pregnancy--a Dutch population-based birth-cohort study: the GECKO Drenthe study. Hum Reprod. 2012 Feb;27(2):583–93. doi: 10.1093/humrep/der429. [DOI] [PubMed] [Google Scholar]
- 38.Edelman AB, Carlson NE, Cherala G, Munar MY, Stouffer RL, Cameron JL, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009 Aug;80(2):119–27. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pittman ME, Secura GM, Allsworth JE, Homco JB, Madden T, Peipert JF. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011 Apr;83(4):340–5. doi: 10.1016/j.contraception.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal A, Aponte-Mellado A, Premkumar B, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reproductive Biology and Endocrinology. 2012;10(1):49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception. 1995 May;51(5):283–8. doi: 10.1016/0010-7824(95)00074-k. [DOI] [PubMed] [Google Scholar]
- 42.Dempsey AR, Johnson SS, Westhoff CL. Predicting oral contraceptive continuation using the transtheoretical model of health behavior change. Perspect Sex Reprod Health. 2011 Mar;43(1):23–9. doi: 10.1363/4302311. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan Pepe M, Anderson GL. A cautionary note on inference for marginal regression models with longitudinal data and general correlated response data. Communications in Statistics - Simulation and Computation. 1994 Jan 01;23(4):939–51. 1994. [Google Scholar]
- 44.Rosenbaum PR. Quantiles in Nonrandom Samples and Observational Studies. Journal of the American Statistical Association. 1995;90(432):1424–31. [Google Scholar]
- 45.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010 Sep;15(3):234–49. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. American Journal of Epidemiology. 2006 Jun 15;163(12):1149–56. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furu K, Wettermark Br, Andersen M, Martikainen J, Almarsdottir A, SÃrensen H. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86–94. doi: 10.1111/j.1742-7843.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 48.Skurtveit S, Selmer R, Tverdal A, Furu K. The validity of self-reported prescription medication use among adolescents varied by therapeutic class. J Clin Epidemiol. 2008 Jul;61(7):714–7. doi: 10.1016/j.jclinepi.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Alberman E, Pharoah P, Chamberlain G, Roman E, Evans S. Outcome of pregnancies following the use of oral contraceptives. International journal of epidemiology. 1980 Sep;9(3):207–13. doi: 10.1093/ije/9.3.207. [DOI] [PubMed] [Google Scholar]
- 50.Chen XK, Wen SW, Sun LM, Yang Q, Walker MC, Krewski D. Recent oral contraceptive use and adverse birth outcomes. European journal of obstetrics, gynecology, and reproductive biology. 2009;144(1):40–3. doi: 10.1016/j.ejogrb.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Mucci LA, Lagiou P, Hsieh CC, Tamimi R, Hellerstein S, Vatten L, et al. A prospective study of pregravid oral contraceptive use in relation to fetal growth. Bjog. 2004 Sep;111(9):989–95. doi: 10.1111/j.1471-0528.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 52.Rothman KJ. Fetal loss, twinning and birth weight after oral-contraceptive use. N Engl J Med. 1977 Sep 1;297(9):468–71. doi: 10.1056/NEJM197709012970903. [DOI] [PubMed] [Google Scholar]
- 53.Wilcox AJ. On the importance--and the unimportance--of birthweight. International journal of epidemiology. 2001 Dec;30(6):1233–41. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 54.Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012 Jun;97(6):1374–9. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 55.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol. 2011 Oct;118(4):863–71. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snijder CA, Heederik D, Pierik FH, Hofman A, Jaddoe VW, Koch HM, et al. Fetal growth and prenatal exposure to bisphenol A: the generation R study. Environ Health Perspect. 2013 Mar;121(3):393–8. doi: 10.1289/ehp.1205296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scippo ML, Argiris C, Van De Weerdt C, Muller M, Willemsen P, Martial J, et al. Recombinant human estrogen, androgen and progesterone receptors for detection of potential endocrine disruptors. Anal Bioanal Chem. 2004 Feb;378(3):664–9. doi: 10.1007/s00216-003-2251-0. [DOI] [PubMed] [Google Scholar]
- 58.Longnecker MP, Klebanoff MA, Zhou H, Brock JW. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet. 2001 Jul 14;358(9276):110–4. doi: 10.1016/S0140-6736(01)05329-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


