Abstract
Objectives
High frequency ultrasound scanning may be used for prevention, detection and monitoring of pressure ulcers in patients at risk and is amenable for portable, bedside use by a variety of clinicians. Limited data are available about the criteria to determine an ideal image or measures of tissue changes representative of tissue injury. We developed and evaluated criteria for overall image quality and measures of tissue integrity.
Methods
In 40 mechanically ventilated adults in 3 ICUs, 241 HFUS sacral images were evaluated for agreement using criteria for overall image quality and tissue changes (dermal, hypodermal layer thickness and layer density).
Results
HFUS sacral images (N= 241) were evaluated in three analyses and showed poor agreement in all three analyses using the specific criteria for global quality, however when criteria were collapsed agreement was good to substantial. Evaluator agreement for layer thickness and layer density was also good.
Conclusions
A global rating is adequate for identifying good images. Agreement for measurements of layer thickness and density were also good and may be useful to identify early changes in tissue integrity leading to tissue injury. Additional data are needed concerning the association of changes in layer thickness and layer density to eventual tissue injury.
Ultrasonography has been widely used in clinical practice as an inexpensive and portable diagnostic tool. Recently high frequency ultrasound (HFUS) using a 20MHz probe has been used to provide images for both dermatologic practice and research to evaluate lymph nodes,1 chronic ulcers,2;3 skin lesions and tumors.1;4–6 However, more limited data are available about the use of HFUS to evaluate the development of pressure ulcers (PUs).
Pressure ulcers are common and costly,7–11,12;13 but methods to quantitatively evaluate patients at risk or identify early stages of pressure ulcer development, especially the more elusive, deep tissue injuries are limited.14 Certain deep tissue injuries are currently thought to develop through a “deep-to-superficial” pattern resulting in delayed clinical recognition of significant and often extensive tissue changes.15;16 Since deep tissue ulcers are difficult to identify, identification of quantitative measures to determine risk and early stage injury would be clinically useful.
HFUS scanning identifies the existence of a hypo-echogenic subepidermal layer at the location of pressure ulcers17 and may demonstrate soft-tissue damage and edema before clinical signs are visible.2;4;18 The ability to identify early changes in deep tissues may enhance tissue injury prevention strategies. Changes in dermal echogenicity reflect alternations of dermal water content associated with inflammation. HFUS dermal echogenicity and skin water content using nuclear magnetic resonance were evaluated and HFUS was shown to be a sensitive method to assess dermal hydration and skin pathology associated with edema formation.19
Visual skin assessments by clinical providers may not always correspond to changes in HFUS images. Quintavalle et al.18 compared HFUS from 119 long-term-care (LTC) facility residents at risk for PUs with 15 healthy volunteers. 55.3% of the images from LTC residents showed areas within the skin layers that were not visible, indicative of fluid or edema, while those from healthy volunteer had homogeneous patterns of reflection. Most images (79.7%) with abnormal ultrasounds did not have documentation of skin erythema in the clinical record. More recently Porter-Armstrong et al.20 evaluated whether HFUS supported clinical skin assessment in an inpatient population by comparing HFUS to clinical assessments of heel and sacral skin. They found that qualitative classification of ultrasound images did not match outcomes yielded through the clinical skin assessment.
HFUS has been shown to identify deep tissue changes. To compare rates of visualized heel PUs to hidden injury using HFUS in geriatric medical patients, Helvig et al.21 evaluated 100 hospital patients and found that HFUS detected occult injury more often than visual assessment did. Yabunaka et al.,22 in comparing HFUS images in patients with PUs to normal skin areas, found subcutaneous fat edema in the ulcerated area and no edema in normal skin areas; fat edema was identified in all PUs, regardless of wound depth. Further, follow-up images showed that as the wounds improved, fat edema was reduced. These authors suggest that the inflammation caused by pressure ulcers may have a greater effect on subcutaneous fat than any other tissue and that resulting edema occurs early in the development of PUs, which can then be detected by HFUS.22 In addition, HFUS has also been used to monitor healing of PUs in experimentally induced wounds in guinea pigs.23
HFUS is increasingly marketed and used for the prevention, detection and monitoring of PUs in patients at risk. These systems purport to provide users with images of high resolution and clarity with a user friendly interface amenable for portable, bedside use by a variety of clinicians such as physicians, nurses, technicians and other care providers. This technology may also be able to detect deep tissue injury upon hospital or long term care admission, although few data are available to support this ability. Since a variety of clinicians may obtain and evaluate these images, it is important that quality images are obtained and processes to evaluate them are standardized. However, limited data are available about the criteria to determine an ideal image or processes used to determine image changes representative of tissue injury.
This paper will summarize the process developed in our project to determine the effect of backrest elevation on skin integrity (R01 NR010381, Grap PI) using HFUS as an adjunctive measure of sacral skin integrity. The goals of this sub-project were to 1) develop and test the reliability of criteria for overall image quality; 2) develop and test the reliability of criteria to evaluate change in tissue integrity (dermal and hypodermal layer thickness, dermal and hypodermal layer density).
METHODS
Sample and Setting
The parent study, from which this analysis is derived, is a descriptive, longitudinal study of skin integrity of 150 intubated and mechanically ventilated adult patients from a medical respiratory ICU (MRICU), surgical trauma ICU (STICU) or neuroscience ICU (NSICU) in an academic medical center. Since this evaluation was completely “new ground” there was no effect size to consider, nor expectations with respect to variation to specifically determine sample size. The quality criteria described below were being developed during data collection and we used results of the first set of scans to help refine and develop those criteria. The study was approved by the university IRB and informed consent was obtained from the subject’s legally authorized representative (LAR). Because the parent study included use of a pressure mapping system that may enhance skin moisture, exclusion criteria included patients who had significant skin moisture risk as determined by the Braden scale of “constantly moist”.24 Subjects were enrolled in the study within 24 hours of intubation and sacral HFUSs were obtained daily for up to seven days. Data were collected from February 2010 through May 2012.
Image Quality Components
Sacral tissue was evaluated using a high (20MHz) frequency ultrasound dermal scanning system (EPISCAN, Longport, Inc, Glen Mills, PA). The EPISCAN settings on all images were as per manufacturer’s recommendation for sacral scans and were consistent to insure uniformity of measurement between images: size – 512×1024; TGC srt = 0%; pos = 16.0; depth = 10.2mm; TGC = 10%; gain = 20%. However, in this critically ill population, it was not possible to obtain scans in the prone position as recommended by the manufacturer, rather HFUS sacral images were obtained in patient side-lying positions as close to a side-lying, 90 degree lateral rotation as possible, based on the clinical condition of the patient. All sacral scans were therefore obtained in the lateral position with the subject turned from 60 to 90 degrees.
Overall HFUS Image Quality
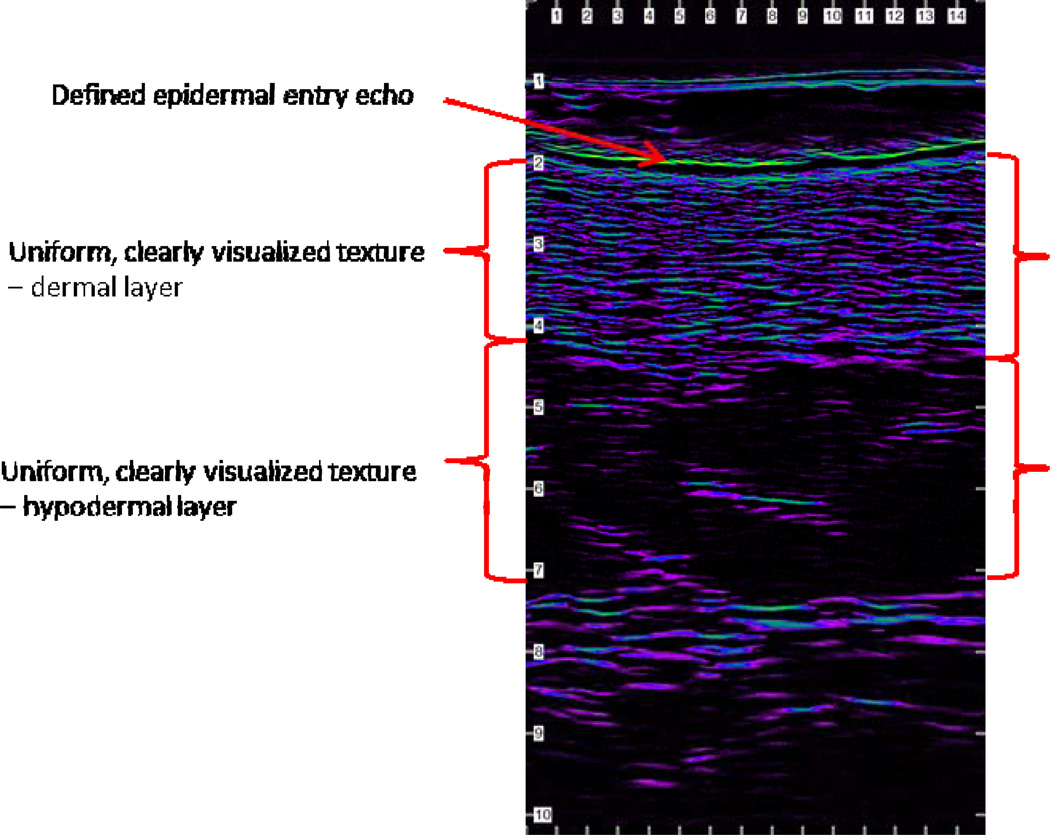
Highest quality HFUS images have specific components: a defined epidermal entry echo and uniform, clearly visualized texture (collagen bundles and ground substance) differences between the dermal and hypodermal skin layers (Figure 1). Defects and artifacts in the image resulting from bubbles in the gel, improper probe placement, and improper probe technique may distort or occlude image components preventing optimal evaluation and should be considered in any rating scale. Therefore we developed a rating scale (Table 1) and tested it in a pilot study25 reflecting the presence or absence of the specific components described above which resulted in an acceptable image having a rating of 3.0 to 4.0. To further delineate image quality between the rating of 3 (acceptable image) to a rating of 4 (essentially a perfect image), a further gradation was developed to differentiate specific features of images between 3.0 and 4.0 (Table 1).
Figure 1. High quality HFUS image with specific components.
Table 1.
HFUS Overall Image Quality Rating Scale
| Rating | Definition |
|---|---|
| 1.0 | dermis and hypodermis not evaluable due to poor image |
| 2.0 | at least 1 layer evaluable but other is not or at least 50% of both layers are evaluable* |
| 3.0 | at least 50% of both layers are evaluable; no well-defined epidermal entry echo, no defined layer margin between all 3 layers |
| 3.1 | approximated epidermal entry echo, no clearly defined layer margin between dermis and hypodermis |
| 3.2 | defined epidermal entry echo, no clearly defined layer margin between dermis and hypodermis |
| 3.3 | well-defined epidermal entry echo, no clearly defined layer margin between dermis and hypodermis |
| 3.4 | approximated epidermal entry echo, layer margin between dermis and hypodermis can be approximated |
| 3.5 | defined epidermal entry echo, layer margin between dermis and hypodermis can be approximated |
| 3.6 | defined epidermal entry echo, layer margin between dermis and hypodermis can be determined |
| 3.7 | defined epidermal entry echo, well-defined layer margin between dermis and hypodermis |
| 3.8 | well-defined epidermal entry echo, well-defined layer margin between dermis and hypodermis |
| 3.9 | very high quality image, some minor image defects on lateral areas |
| 4.0 | all of both layers are evaluable, no interfering image artifacts present, no visible image defects - essentially flawless image |
at least 50% of the image widths of both layers are evaluable with the evaluable portions positioned over each other
Evaluation of Tissue Changes
Early changes in tissue integrity may begin with tissue inflammation either in the dermal or hypodermal layer and may occur well before visual changes in the skin. Recent use of HFUS to evaluate tissue injury has indicated that patterns of fluid accumulation or layer edema within dermal and hypodermal tissue may be early indicators of tissue injury.17;18;21 The ability to identify tissue changes prior to visual skin changes would be beneficial to prevent further damage to skin integrity. Changes in dermal and hypodermal layer thickness (in millimeters) and dermal and hypodermal layer density (median signal intensity distribution) may reflect tissue inflammation. EPISCAN HFUS software-generated measurements include measures for layer thickness and layer density. Layer thickness is a measure of the width of each layer (dermal and hypodermal), that is, as inflammation and edema occurs, layers become thicker.
Layer density measured in this study is the median signal intensity distribution of the image. Colors represent the intensity of the signal reflected from the tissue and therefore the structure of the tissue. Statistical analysis of the color distribution may detect subtle changes that cannot be reliably detected by the visual assessment of the images. The median intensity (or color) is the intensity/color at the center of the distribution profile.26 Therefore layer density may decrease as fluid accumulates since the tissue would appear less tissue dense in the ultrasound image.27 Use of HFUS in the evaluation of tissue injury then is dependent on accurate measures (dermal and hypodermal layer thickness, dermal and hypodermal layer density) that may reflect changes or injury in the dermis or hypodermis layers.
Procedure and Analysis Process
The Medical Center Wound Care Program nurse practitioner and co-investigator (VL) or another study nurse (RSB) who were both certified in EPISCAN evaluation trained all study personnel to obtain EPISCAN sacral images for the parent study. Training continued until the study personnel achieved full accuracy in following all recommended image processes (correct patient position, accurate probe location, identification of a good scan at the bedside) and performed return demonstrations. On a weekly basis, one expert investigator (RSB) reviewed the scans obtained by each study personnel and if any individual person submitted poor quality images remedial training was immediately begun. In addition both EPISCAN expert investigators (VL, RSB) continuously monitored study personnel during onsite visits and retraining was conducted every 3 months or more frequently if needed.
HFUS images of the sacrum were obtained daily on each study subject in the parent study up to 7 days or until hospital discharge whichever occurred first. Multiple images were obtained and reviewed at the bedside until the operator determined that a good image was obtained; however all images were stored for later review and analysis. Images were obtained in the early morning whenever possible but were also coordinated with the patient’s usual care activities so as to not disturb the patients unnecessarily. Subsequent statistical analysis incorporated these multiple observations as patient-specific repeated measurements.
Because there were no available guidelines to evaluate image quality at the beginning of study processes all images (obtained in chronological order) from all 150 subjects were evaluated by the two expert team members (RSB, VL) using a basic global rating scale of image quality (1.0 through 4.0) described in Table 1 (bolded criteria). After analysis of agreement of image quality between evaluators, all unacceptable images (rated as 1.0 or 2.0) were eliminated.
After all images were evaluated as described great variation in images rated as 3.0 was evident. Therefore, greater detail using subcategories of 3.0 (i.e. 3.1 through 3.9) was created to identify images with acceptable quality. Images from the first 40 subjects originally evaluated as a 3.0 were then re-evaluated independently by both expert team members (RSB, VL) using the 3.1 through 3.9 criteria and agreement analysis was conducted for overall image quality. In this iterative process, images from the first 20 subjects (127 images) were re-evaluated and an analysis of agreement was determined, refinements made in the 3.1 through 3.9 criteria and subsequent re-evaluation conducted for subjects 21 through 40 (114 images), as well as a combined evaluation of subjects 1 through 40 (241 images). During this process the additional components to evaluate tissue changes (dermal and hypodermal layer thickness, dermal and hypodermal layer density) were also evaluated. Three measurements each of dermal and hypodermal layer thickness and one measurement of dermal and hypodermal density were also documented for each image and agreement analysis was conducted at these same time periods.
HFUS CRITERIA EVALUATION
Subjects and Images
For this evaluative process, HFUS images (N= 241) from 40 subjects, a subset of the parent study, were evaluated. Subjects were from all three ICUs and overall image quality was assessed using the expanded 3.0 to 4.0 scale. The subjects were primarily male and white and equivalently divided among the MRICU, STICU, NSICU. Subjects had a mean Braden Scale score of 13.08 (range 9–18) and mean BMI of 27.94 (± 6.24). Complete characteristics of the sample for the analysis presented here are shown in Table 2.
Table 2.
Subject Characteristics (N=40)
| Mean | SD | Range | |
|---|---|---|---|
| Age | 52.55 | 17.98 | 18–90 |
| BMI | 27.94 | 6.24 | 12.4–42.5 |
| APACHE | 78 | 25.55 | 35–139 |
| Braden Scale Score | 13.08 | 1.89 | 9–18 |
| Median | 25th, 75th percentiles | Range | |
| ICU LoS | 9.05 | 5.9, 17.6 | 1.7–40.1 |
| Hospital LoS | 19.40 | 8.7, 28.9 | 1.8–78.1 |
| Duration of Intubation | 4.21 | 1.9, 6.6 | 0.7–14.4 |
| Count | Percent | ||
| Gender | |||
| Male | 23 | 57.50 | |
| Female | 17 | 42.50 | |
| Race | |||
| White | 21 | 52.50 | |
| Black/AA | 17 | 42.50 | |
| Unknown | 2 | 2.00 | |
| Ethnicity | |||
| Hispanic | 1 | 2.50 | |
| Non-hispanic | 39 | 97.50 | |
| Critical Care Unit | |||
| MRICU | 16 | 40.00 | |
| STICU | 11 | 27.50 | |
| NSICU | 13 | 32.50 |
LoS = Length of Stay
Overall HFUS Image Quality
Evaluations of the HFUS images were conducted in three analyses (subjects 1–20; subjects 21–40; subjects 1–40). Comparisons focused on the extent of agreement between the evaluators. In the first analysis (subjects 1–20) concordance between the evaluators occurred in 58 of 127 images (45.67%); in the second analysis (subjects 21–40) in 38 of 114 images (33.33%) and in the third analysis (subjects 1–40) in 96 of 241 (39.83%). There was agreement in both groups and for the combined sample (p < 0.0001). However, the 95% confidence intervals for the kappa coefficients among all groups demonstrated poor agreement and the 2nd analysis did not show improvement in agreement (Table 3).
Table 3.
Kappa coefficients evaluating agreement between evaluators for overall image quality
| Analysis of agreement using criteria 3.1 through 3.9 | ||||||
|---|---|---|---|---|---|---|
| Subjects 1–20 | Subjects 21–40 | Subjects 1–40 | ||||
| Kappa | 95% CI | Kappa | 95% CI | Kappa | 95% CI | |
| Simple Kappa | 0.3264 | (0.2247, 0.4281) | 0.1488 | (0.0417, 0.2558) | 0.2457 | (0.1706, 0.3207) |
| Weighted Kappa | 0.4981 | (0.4003, 0.5960) | 0.4422 | (0.3441, 0.5404) | 0.4762 | (0.4057, 0.5468) |
| Analysis of agreement using criteria collapsed into 4 categories* | ||||||
| Subjects 1–20 | Subjects 21–40 | Subjects 1–40 | ||||
| Kappa | 95% CI | Kappa | 95% CI | Kappa | 95% CI | |
| Simple Kappa | 0.4142 | (0.2899, 0.5385) | 0.3635 | (0.2254, 0.5015) | 0.3925 | (0.2990, 0.4860) |
| Weighted Kappa | 0.5729 | (0.4691, 0.6766) | 0.4986 | (0.3686, 0.6286) | 0.5421 | (0.4597, 0.6245) |
| Analysis of agreement using criteria collapsed into 2 categories: < 3.5 and ≥ 3.5 | ||||||
| Subjects 1–20 | Subjects 21–40 | Subjects 1–40 | ||||
| Kappa | 95% CI | Kappa | 95% CI | Kappa | 95% CI | |
| Simple Kappa | 0.6176 | (0.5187, 0.7165) | 0.6132 | (0.4968, 0.7295) | 0.6223 | (0.5481, 0.6966) |
Category I (3.0–3.3), II (3.4), III (3.5–3.7) and IV (3.8–4.0).
Since the agreement remained poor even after criteria refinement we theorized that the individual levels (3.1 through 3.9) were too subjective and difficult to distinguish from each other. Therefore based on similar image characteristics, we collapsed the criteria into 4 categories: Category I (3.0–3.3), II (3.4), III (3.5–3.7) and IV (3.8–4.0). Collapsing categories improved concordance for all analyses (1st 79 of 127 = 62.20%; 2nd 71 of 114 = 62.28%; 3rd 150 of 241 = 62.24%). However, the 95% confidence intervals for the kappa coefficients among all analyses again demonstrated poor agreement although the increased concordance is reflected in the higher kappa values using the collapsed 4 categories. Because the agreement remained poor and our original intent was to identify images that were a “good 3.0” compared to a “poor 3.0”, we analyzed agreement using two categories - those below 3.5 and those at 3.5 and above and found good to substantial agreement (Table 3).28;29
Evaluation of Tissue Changes
Dermal and Hypodermal Layer Thickness
The three thickness measurements were averaged within each evaluator and compared by layer (dermis and hypodermis). In the first analysis for the dermis (subjects 1–20) the average difference between evaluators was 0.10mm (± 0.35mm). In the second analysis (subjects 21–40) the difference was 0.16mm (± 0.51mm), and when combined (subjects 1–40) the average difference was 0.12mm (± 0.43mm). No changes in the measurement process occurred between any analyses (Table 4).
Table 4.
Agreement among evaluators for layer thickness and layer density by dermis, hypodermis layer
| Analysis of agreement for layer thickness (mm) | |||
|---|---|---|---|
| Subjects 1–20 | Subjects 22–40 | Subjects 1–40 | |
| Dermal Thickness – Mean Difference (SD) | −0.10 (0.35) | −0.16 (0.51) | −0.12 (0.43) |
| Limits of Agreement | −0.80, 0.60 | −1.18, 0.86 | −0.98, 0.74 |
| Hypodermal Thickness – Mean Difference (SD) | −0.24 (0.50) | −0.03 (0.45) | −0.14 (0.49) |
| Limits of Agreement | −1.24, 1.24 | 0.93, 0.87 | −1.12, 0.84 |
| Analysis of agreement for layer density (median signal intensity distribution) | |||
| Subjects 1–20 | Subjects 22–40 | Subjects 1–40 | |
| Dermal Density – Mean Difference (SD) | −0.16 (3.35) | 1.13 (5.12) | 0.45 (4.31) |
| Limits of Agreement | −6.86, 6.54 | −11.37, 9.11 | −9.07, 8.17 |
| Hypodermal Density – Mean Difference (SD) | −1.52 (4.24) | −0.24 (2.62) | −0.91 (3.62) |
| Limits of Agreement | −10.00, 6.96 | −5.48, 5.00 | −10.86, 6.33 |
SD – standard deviation
For the hypodermis, in subjects 1–20, the average difference between evaluators was 0.24mm (± 0.50mm), in the second analysis (subjects 21–40) only 0.03mm (± 0.44mm) and when combined (subjects 1–40) 0.14 (± 0.49mm). No changes in the measurement process occurred between any analyses (Table 4)
Dermal and Hypodermal Layer Density
Differences in the layer were larger between evaluators for the hypodermis than the dermis layer (Table 4). There were noticeable improvements in the second analysis for the hypodermis layer with respect to reduced standard deviation and a mean difference closer to 0.00, however, standard deviation and mean difference for the dermal layer increased in the second assessment.
DISCUSSION
HFUS imaging has been gaining attention as a potential method of early identification of patients at risk for tissue damage. Although a variety of clinicians may collect and evaluate these images, there are few data about the reliability of the HFUS evaluation process, especially related to obtaining the best image. In addition, because the HFUS technology and software may provide image quantification data, e.g. layer thickness and signal density calculations, it is important that optimal images are identified for ideal patient evaluation.
Although we attempted to develop a very specific scoring system to identify the best image using very specific criteria, we found that the level of subjectivity was so great that the ability of our expert evaluators to achieve agreement using these criteria was poor. However in using a more simplified scoring system (less than or greater than 3.5) we found that reliability for identifying the best image was good. These data suggest that a more global rating is adequate for identifying a good scan. Beyond that global rating, analysis of variations in the image that may be detectable, may be too subjective to create good reliability among scan evaluators.
Differences in measures of dermal thickness were smaller compared to those of hypodermal thickness. This is not surprising since layer detail discrimination decreases with increased depth of the tissue. Images show layers of tissue closer to the epidermis in greater detail and clarity than that of deeper tissues due to the attenuation of the HFUS signal. Overall, when evaluating differences for all subjects, layer thickness differences were minimal in both the dermis and hypodermis (0.14 mm or less).
Similar to the layer thickness measurements, differences in layer density measures were larger between raters for the hypodermis than the dermis layer. There were noticeable improvements in the second assessment for the hypodermis layer with respect to reduced standard deviation and a mean difference closer to 0.00, however, standard deviation and mean difference for the dermis layer increased in the second assessment.
Overall measurements for dermal/hypodermal thickness and dermal/hypodermal density were found to be more reliable than overall image quality. These measurements are obtained directly from the HFUS software limiting the level of subjectivity that may occur. However, the measurements were also dependent on choosing a similar area of the image to conduct the measurements. Even though there were no set patterns in the areas chosen, the overall agreement for both of these measurements was good, indicating that HFUS software-generated measurements for layer thickness and layer density may be reliable measures. These measures may be useful to identify early changes in tissue integrity that may lead to tissue injury or ulceration. Although the association of changes in layer thickness and layer density to eventual tissue injury has yet to be clearly delineated, our documentation of the reliability for these measures is a needed first step for their future use and may aid the design and planning of future studies including determining power and sample size for other researchers seeking to use this modality.
IMPLICATIONS FOR PRACTICE.
The development of pressure ulcers is a costly complication of illness and patient care and the best preventive care strategies have been unable to completely eradicate them.
Early identification of tissue changes that may eventually lead to tissue injury is critical to further reduce the incidence of pressure ulcers.
HFUS technology may identify tissue edema, a potential first sign of changes in tissue integrity, however quality scans and accurate measurements are required.
The criteria developed here may provide a standard method for clinician evaluation of image quality.
HFUS generated measures of layer thickness and density can also be reliably obtained.
Acknowledgments
Supported by funding from NIH, R01 NR010381 (Grap PI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schmid-Wendtner MH, Burgdorf W. Ultrasound scanning in dermatology. Arch.Dermatol. 2005;141:217–224. doi: 10.1001/archderm.141.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Timar-Banu O, Beauregard H, Tousignant J, Lassonde M, Harris P, Viau G, et al. Development of noninvasive and quantitative methodologies for the assessment of chronic ulcers and scars in humans. Wound.Repair Regen. 2001;9:123–132. doi: 10.1046/j.1524-475x.2001.00123.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn C, Angehrn F. Use of high-resolution ultrasound to monitor the healing of leg ulcers: a prospective single-center study. Skin Res.Technol. 2009;15:161–167. doi: 10.1111/j.1600-0846.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- 4.Fornage BD, McGavran MH, Duvic M, Waldron CA. Imaging of the skin with 20-MHz US. Radiology. 1993;189:69–76. doi: 10.1148/radiology.189.1.8372222. [DOI] [PubMed] [Google Scholar]
- 5.Bessonart MN, Macedo N, Carmona C. High resolution B-scan ultrasound of hypertrophic scars. Skin Res.Technol. 2005;11:185–188. doi: 10.1111/j.1600-0846.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 6.Jasaitiene D, Valiukeviciene S, Linkeviciute G, Raisutis R, Jasiuniene E, Kazys R. Principles of high-frequency ultrasonography for investigation of skin pathology. J Eur.Acad.Dermatol.Venereol. 2011;25:375–382. doi: 10.1111/j.1468-3083.2010.03837.x. [DOI] [PubMed] [Google Scholar]
- 7.Cuddigan J, Berlowitz DR, Ayello EA. Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv.Skin Wound.Care. 2001;14:208–215. doi: 10.1097/00129334-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Bours GJ, De Laat E, Halfens RJ, Lubbers M. Prevalence, risk factors and prevention of pressure ulcers in Dutch intensive care units. Results of a cross-sectional survey. Intensive Care Med. 2001;27:1599–1605. doi: 10.1007/s001340101061. [DOI] [PubMed] [Google Scholar]
- 9.Whittington K, Patrick M, Roberts JL. A national study of pressure ulcer prevalence and incidence in acute care hospitals. J.Wound.Ostomy.Continence.Nurs. 2000;27:209–215. doi: 10.1067/mjw.2000.107879. [DOI] [PubMed] [Google Scholar]
- 10.Beckrich K, Aronovitch SA. Hospital-acquired pressure ulcers: a comparison of costs in medical vs. surgical patients. Nurs.Econ. 1999;17:263–271. [PubMed] [Google Scholar]
- 11.Allman RM, Laprade CA, Noel LB, Walker JM, Moorer CA, Dear MR, et al. Pressure sores among hospitalized patients. Ann.Intern.Med. 1986;105:337–342. doi: 10.7326/0003-4819-105-3-337. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services. Healthy People 2010. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- 13.Roadmap for safety: National Quality Forum officially releases 30 safe practices for better healthcare. Qual.Lett.Healthc.Lead. 2003;15:12–14. 1. [PubMed] [Google Scholar]
- 14.Black JM. Moving toward consensus on deep tissue injury and pressure ulcer staging. Adv.Skin Wound Care. 2005;18:415–421. doi: 10.1097/00129334-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 15.NPUAP. National Pressure Ulcer Advisory Panel: Updated Staging System. internet. 2008 [Google Scholar]
- 16.Black J, Baharestani M, Cuddigan J, Dorner B, Edsberg L, Langemo D, et al. National Pressure Ulcer Advisory Panel's updated pressure ulcer staging system. Dermatol.Nurs. 2007;19:343–349. [PubMed] [Google Scholar]
- 17.Andersen ES, Karlsmark T. Evaluation of four non-invasive methods for examination and characterization of pressure ulcers. Skin Res.Technol. 2008;14:270–276. doi: 10.1111/j.1600-0846.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 18.Quintavalle PR, Lyder CH, Mertz PJ, Phillips-Jones C, Dyson M. Use of high-resolution, high-frequency diagnostic ultrasound to investigate the pathogenesis of pressure ulcer development. Adv.Skin Wound.Care. 2006;19:498–505. doi: 10.1097/00129334-200611000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Gniadecka M, Quistorff B. Assessment of dermal water by high-frequency ultrasound: comparative studies with nuclear magnetic resonance. Br.J.Dermatol. 1996;135:218–224. [PubMed] [Google Scholar]
- 20.Porter-Armstrong AP, Adams C, Moorhead AS, Donnelly J, Nixon J, Bader DL, et al. Do high frequency ultrasound images support clinical skin assessment? ISRN.Nurs. 2013;2013:314248. doi: 10.1155/2013/314248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helvig EI, Nichols LW. Use of high-frequency ultrasound to detect heel pressure injury in elders. J Wound.Ostomy.Continence.Nurs. 2012;39:500–508. doi: 10.1097/WON.0b013e3182652648. [DOI] [PubMed] [Google Scholar]
- 22.Yabunaka K, Iizaka S, Nakagami G, Aoi N, Kadono T, Koyanagi H, et al. Can ultrasonographic evaluation of subcutaneous fat predict pressure ulceration? J Wound.Care. 2009;18:192–194. 196. doi: 10.12968/jowc.2009.18.5.42173. [DOI] [PubMed] [Google Scholar]
- 23.Moghimi S, Miran Baygi MH, Torkaman G, Mahloojifar A. Quantitative assessment of pressure sore generation and healing through numerical analysis of high-frequency ultrasound images. J.Rehabil.Res.Dev. 2010;47:99–108. doi: 10.1682/jrrd.2009.04.0045. [DOI] [PubMed] [Google Scholar]
- 24.Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for Predicting Pressure Sore Risk. Nurs.Res. 1987;36:205–210. [PubMed] [Google Scholar]
- 25.Burk RS, Lucas VS, Grap MJ. Measuring Skin Integrity Using High Frequency Ultrasound – A Validation Procedure. SNRS Conference Proceedings. 2013;2014:10. [Google Scholar]
- 26.Longport EPISCAN I-200. Training Guide Quick Reference. 4.0. Longport, Inc; 2007. [Google Scholar]
- 27.Lucas VS, Burk RS, Creehan S, Grap MJ. Utility of high-frequency ultrasound: moving beyond the surface to detect changes in skin integrity. Plast.Surg.Nurs. 2014;34:34–38. doi: 10.1097/PSN.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleiss J, Levin B, Paik M. Statistical methods for rates and proportions. 3rd ed. New York: John Wiley; 2003. [Google Scholar]
- 29.Landis J, Kock G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]