Abstract
Background
Away from home (AFH) meals are known to be energy-dense and of poor diet quality. Both direct and indirect exposure (e.g., neighborhood restaurant density) to AFH meals have been implicated as contributors to higher body weight and adverse health outcomes.
Objective
To examine the association of frequency of eating AFH and fast-food meals with biomarkers of chronic disease and dietary intake.
Design
This cross-sectional study used frequency of AFH and fast-food meal and biomarker data from the NHANES 2005-2010. Information on weekly frequency of AFH and fast-food meals was collected via questionnaire during the household interview. The metabolic biomarkers examined included BMI, serum cholesterol (total, HDL, and LDL), triglycerides, glycohemoglobin, and fasting glucose (n=8314, age ≥20, NHANES 2007-2010). Biomarkers of dietary exposure included serum concentrations of vitamins A, D, E, C, B-6, B-12, folate, and carotenoids (n=4162; 2005-2006). Multiple linear and logistic regression methods adjusted for complex survey methodology and covariates.
Results
American adults reported a mean of 3.9 (95% CI 3.7, 4.0) AFH and 1.8 (1.6, 1.9) fast-food meals/week. Over 50% of adults reported ≥3 AFH and >35% reported ≥2 fast-food meals/week. Mean BMI of more frequent AFH or fast-food meal reporters was higher (Ptrend≤0.0004). Serum concentrations of total, LDL, and HDL cholesterol were related inversely with frequency of AFH meals (P<0.05). Frequency of fast-food meals and serum HDL-cholesterol were also related inversely (P=0.0001). Serum concentrations of all examined micronutrients (except vitamin A and lycopene) declined with increasing frequency of AFH meals (P<0.05); women and ≥50 y olds were at higher risk.
Conclusions
Reporters of frequent AFH and fast-food meals had higher BMI and lower concentrations of HDL cholesterol; but profiles of other biomarkers did not indicate higher metabolic risk. However, the serum concentrations of nutrients with mostly plant foods as sources declined with increasing AFH meal frequency.
Keywords: NHANES, restaurant eating, fast food, BMI, metabolic biomarkers, nutritional biomarkers, away-from-home meals
Introduction
The proportion of daily energy contributed by AFH foods to American diets increased from 18% in 1977-78 to 32% in 2005-2008 (1). AFH foods tend to be higher in energy density, fat, and sodium but lower in fruits, vegetables, whole grains and protective nutrients (2-6). Not surprisingly, therefore, AFH consumers are reported to have higher energy intake but poor diet quality (7-11). These nutritional concerns have also been reflected in reports that have implicated frequent AFH meal consumption as a possible contributor to increasing adiposity of the population (7, 11-14). Neighborhood availability of fast-food establishments has also been linked to higher body weight (15-18), and higher risk of all-cause mortality, stroke, and hospitalizations for acute coronary events (19-21).
Given the poor nutritional profile of AFH foods and adverse health outcomes associated with AFH exposure, it is reasonable to posit that established metabolic biomarkers of disease as well as nutritional biomarkers may shed light on how AFH exposure mediates poor health outcomes. Surprisingly, few published reports have asked this question (22-26), and none that have examined nutritional biomarkers or a nationally representative diverse population. Two of the reports to study metabolic risk biomarkers in relation to eating AFH meals are for young adult participants in the CARDIA study (22, 23); another examined adolescent and parent pairs and limited AFH meals to dinners (24); one report each examined take-away foods in Australia (25), and selected fast-foods in Iran (26).
In view of these gaps, we examined the cross-sectional associations of frequency of AFH and fast-food meals with objectively assessed metabolic and nutritional biomarkers in a nationally representative sample of US adults.
Methods
We used public domain data from the continuous National Health and Nutrition Examination Surveys (NHANES) 2005-2006, 2007-2008, and 2009-2010 for this cross-sectional study (27-29). The study protocol was reviewed by the Queens College Institutional Review Board but was not considered human subjects research. The continuous NHANES is an ongoing annual survey, fielded by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention. The NHANES is a multistage, cluster-probability sample of the US population and includes an at-home interview and a physical examination conducted in a mobile examination center (MEC). The MEC visit includes a complete medical examination including, anthropometric measurements, an in-person dietary interview, and collection of blood and urine samples using standardized procedures. The unweighted response rates for the MEC examined samples for these surveys were >75% (30).
Exposure assessment
The frequency of AFH and fast-food meals was determined from questions asked during the household interview administered by trained interviewers using the Computer-Assisted Personal Interviewing system (27-29). For the 2005-2006 survey cycles, the question was: “On average, how many meals per week do you get that were not prepared at home? Please include meals from both dine-in and carry out restaurants, restaurants that deliver food to your home, cafeterias, fast-food places, food courts, food stands, meals prepared at a grocery store, and meals from vending machines.” For survey cycles 2007-2008 and 2009-2010, the question read, “During the past 7 days, how many meals did you get that were prepared away from home in places such as restaurants, fast food places, food stands, grocery stores, or from vending machines?” Based on distribution of frequency of AFH meals in the analytic sample, AFH meal frequency was categorized as 0, 1-2, 3-5, and >6 times per week, for descriptive purposes. The survey cycles 2007-2010 also included one question on fast-food meals: “How many of the meals you ate away from home did you get from a fast-food or pizza place?”, which was categorized as 0, 1, 2-3, and >4 times per week. The NHANES 1999-2004 survey cycles included a question on frequency of “restaurant” prepared meals only, which was not comparable to questions asked in subsequent surveys; therefore data from these surveys was not examined for the current study.
Outcomes examined
For combined survey cycles 2007-2008 and 2009-2010, we examined BMI and serum concentrations of metabolic biomarkers: total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, glycohemoglobin, and fasting glucose. The laboratory assay methods for these biomarkers followed an established protocol as described by the NCHS (27-29). Briefly, total cholesterol and triglycerides were assayed using enzymatic reactions on a Roche/Hitachi Modular P Chemistry Analyzer, and HDL-cholesterol was analyzed via a modification of the traditional multistep precipitation reaction. LDL-cholesterol was computed using the Friedewald calculation. Glycohemoglobin was assayed using the G7 Glycohemoglobin Analyzer, and fasting glucose via a hexokinase-mediated reaction on a Roche/Hitachi Modular P Chemistry Analyzer.
Because of limited nutritional biomarker data in survey years 2007-2010, we used the NHANES 2005-2006 data for serum concentrations of vitamins A, B6 (as pyridoxal- 5'- phosphate), B-12, folate (serum and RBC), C, D, E, α-carotene, trans-β-carotene, β-cryptoxanthin, lutein+zeaxanthin, and lycopene. Assay methodology for various nutritional biomarkers is detailed by the NCHS (27). Briefly, serum concentrations of vitamins A, E and the carotenoids were assayed using high performance liquid chromatography (HPLC) with photodiode array detection; vitamin D (as 25-OH-vitamin D) was assayed via a 2-step procedure using the Diasorin assay; pyridoxal phosphate (co-enzyme form of vitamin B-6) was analyzed via reversed phase HPLC using fluorometric detection; the Bio-Rad Laboratories Quantaphase II radioassay was used for folate/vitamin B12. Although serum and RBC folate concentrations were measured in the 2007-2010 surveys, the assay methodology differed from that used in 2005-2006. All procedures for phlebotomy, handling of blood samples, analysis of samples, laboratory assay methods, and reporting procedures followed a standardized protocol specified by the NHANES (27-29).
The metabolic and nutritional biomarkers were operationalized as continuous variables and as dichotomous variables relative to a risk threshold. Risk threshold cut-offs (31) for the metabolic biomarkers were: total cholesterol (≥200 mg/dL), HDL-cholesterol (<40 mg/dL), LDL-cholesterol-1 (≥100 mg/dL and ≥130 mg/dL), triglycerides (≥150 mg/dL), glycohemoglobin (≥5.7%), and fasting glucose (≥100mg/dL). Risk threshold cut-offs for nutritional biomarkers were the same as those used in prior NCHS publications (32): serum vitamin A (<20 ug/dL), vitamin C (<11.4 umol/L), vitamin D (<20 ng/ml), vitamin E (<500 ug/dl), PLP (<20 nmol/L), vitamin B-12 (<200 pg/ml), serum folate (<2ng/ml), and RBC folate (<95 ng/ml).
Analytic Sample for metabolic biomarkers
All non-pregnant, non-lactating respondents aged ≥20 years, who answered the question about frequency of AFH meals in the NHANES 2007-2010, with measured concentration of at least one of the above mentioned metabolic biomarkers, were eligible for inclusion in the study (n=10953). From this eligible sample, those who reported using hypocholesterolemic and hypoglycemic medications were excluded (n=2639), for a final sample of 4070 men and 4244 women.
According to NCHS recommendations (27-29), we excluded respondents reporting <8.5 hours of fasting before phlebotomy from the analytic sample for serum LDL-cholesterol and triglycerides, and those reporting <9 hours of fasting from the fasting glucose analysis. Respondents with fasting serum triglycerides concentration of >400 mg/dl were also excluded from the LDL-cholesterol analytic sample.
Analytic Sample for nutritional biomarkers
The analytic sample for nutritional biomarkers measured in the NHANES 2005-2006 included all non-pregnant, non-lactating respondents aged ≥20 years, with answer to the frequency of AFH meals question, and at least one measured nutritional biomarker (2159 males and 2003 females).
Covariates
To determine whether the reported weekly frequency of AFH or fast food meals was independently associated with concentrations of biomarkers of chronic disease, we accounted for the effect of other potential confounders of these associations. The covariates were decided apriori based on known associations of disease biomarkers and dietary behaviors. These included age (20-39, 40-59, and ≥60 y), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, others), family income relative to the poverty threshold or poverty income ratio (≤1.3, >1.3 to ≤3.5, >3.5), years of education (<12, 12, some college, and ≥college), serum cotinine, an indicator of nicotine exposure (continuous), alcohol drinking status (never, former, current, unknown), physical activity (tertiles of MET minutes/week of total physical activity), and hours of fasting prior to phlebotomy (continuous).
Regression models for nutritional biomarkers also included history of supplement use, any self-reported disease condition, and BMI in addition to covariates mentioned above. For serum vitamin D, season of MEC exam, and for serum vitamin E and all carotenoids, total cholesterol concentrations were additional covariates.
Statistical Methods
Following NCHS analytic guidelines, the data from NHANES 2007-2008 and 2009-2010 were combined for analysis of metabolic biomarker outcomes. We computed the covariate adjusted mean frequency of AFH and fast-food meals to assess the independent association of each socio- demographic and lifestyle variable with weekly frequency of AFH and fast-food meals.
The independent association of the weekly frequency of AFH meals or fast-food meals with each biomarker outcome was examined using multiple linear and logistic regression methods. The frequency of AFH and fast-food meals variables were operationalized both as categorical variables and as continuous trend variables. The biomarkers were modeled as continuous dependent variables in linear regression and as binary categories that indicate a risk threshold in logistic regression models. . These regression models included the appropriate covariates identified above.
The distributions of serum total cholesterol, HDL-cholesterol, and vitamin C were approximately normal; for these outcomes the results are presented as adjusted means with 95% confidence intervals. All other biomarkers were log-transformed for multiple linear regression analyses and estimates were then back-transformed to obtain adjusted geometric means and 95% confidence intervals. All analyses were performed using SAS (version 9.2), (Cary, NC), and SUDAAN software (33) for analysis of multistage stratified complex survey data. NCHS recommended sample weights were included in the analyses to address differential sample selection, sample nonresponse, and post-stratification adjustments (34, 35). MEC examined sample weights were used for all examined outcomes except serum LDL cholesterol, triglycerides, and glucose. For LDL cholesterol, triglycerides and glucose outcomes, fasting subsample weights were used as recommended (27-29, 34). Per NCHS analytic guidelines, for analysis of metabolic biomarker outcomes in combined surveys from 2007-2008 and 2009-2010, 1/2 MEC weight (WTMEC2YR or WTSAF2YR) was used for each cycle to produce nationally representative estimates (34). Predicted margins were used to compute adjusted means and proportions from regression models (35, 36). All p-values for tests of statistical significance of regression model coefficients used Sattherwaite-adjusted F tests (33). Two-sided p-values of <0.05 were used to indicate significant associations.
Tests for interactions
Tests of interaction were used to examine if the associations of weekly frequency of AFH or fast-food meals with biomarkers differ by gender or age. An AFH (or fast-food) by age or by gender term was included in separate regression models for each biomarker outcome. When a significant interaction was noted (p-value for interaction <0.05), the analyses were stratified by gender or age and we include gender or age-specific results in Tables.
Sensitivity analysis
Because of concerns about BMI potentially being on the causal pathway in AFH or fast-food associations with biomarkers, BMI was not included as a covariate in regression models for metabolic biomarkers. This approach is similar to that used in published reports of AFH eating and biomarker association (22-23, 25). As alternative analyses, we refitted the regression models with addition of BMI as a covariate.
Results
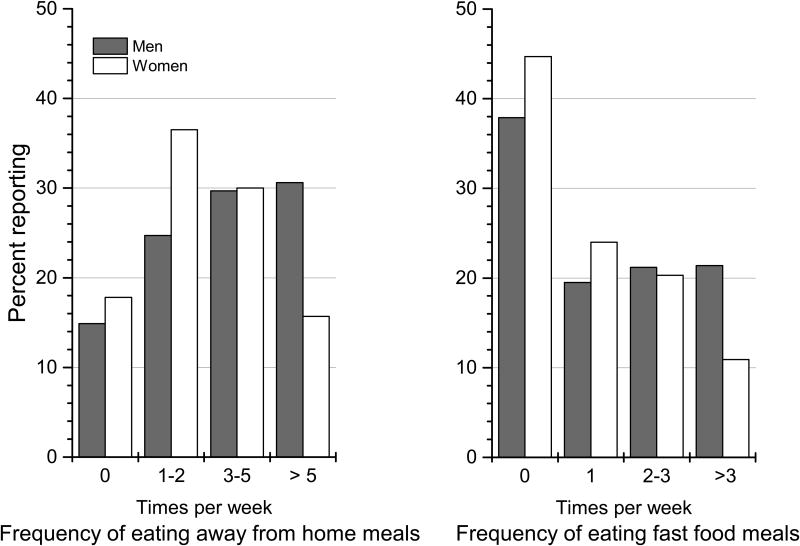
Overall, adult Americans reported a mean of 3.9 AFH and 1.8 fast-food meals/week (Table 1). Male gender, younger age, higher income, and education were independently associated with higher mean AFH and fast-food frequency/week (Table 1). Over 50% of adults reported eating ≥3 AFH meals, and >35% reported ≥2 fast-food meals/week (Figure 1; online supplemental Tables 1 and 2).
Table 1. Unadjusted and adjusted mean1 (95% confidence interval) of weekly frequency of away from home and fast-food meals by socio-demographic and lifestyle characteristics of adult Americans, NHANES 2007-2010.
| Weekly frequency of all away from home meals | Weekly frequency of fast food or pizza meals | |||
|---|---|---|---|---|
| Unadjusted N=8314 | Adjusted N=82972 | Unadjusted N=8314 | Adjusted N=82972 | |
| All | 3.9 (3.7, 4.0) | 3.9 (3.7, 4.0) | 1.8 (1.6, 1.9) | 1.8 (1.6, 1.9) |
| Men (n=4070) | 4.6 (4.3, 4.8) | 4.5 (4.3, 4.7) | 2.2 (2.0. 2.3) | 2.1 (1.9, 2.2) |
| Women (n=4244) | 3.2 (3.1, 3.3) | 3.3 (3.2, 3.4) | 1.4 (1.3, 1.5) | 1.4 (1.3, 1.5) |
| P3 | - | <0.0001 | - | <0.0001 |
| Age (years) | ||||
| 20-39 (n=3340) | 4.6 (4.3, 4.8) | 4.6 (4.3, 4.8) | 2.3 (2.1, 2.5) | 2.3 (2.1, 2.5) |
| 40-59 (n=2926) | 3.7 (3.5, 4.0) | 3.6 (3.4, 3.8) | 1.5 (1.4, 1.7) | 1.5 (1.4, 1.7) |
| ≥60 (n=2048) | 2.5 (2.3, 2.6) | 2.7 (2.5, 2.9) | 0.8 (0.7, 0.9) | 0.9 (0.7, 1.0) |
| P | <0.0001 | <0.0001 | ||
| Race/ethnicity | ||||
| Non-Hispanic white (n=3950) | 4.0 (3.8, 4.2) | 3.9 (3.7, 4.1) | 1.7 (1.5, 1.8) | 1.7 (1.6, 1.9) |
| Non-Hispanic Black (n=1476) | 3.7 (3.4, 4.0) | 3.9 (3.6, 4.1) | 2.3 (2.1-2.4) | 2.2 (2.0, 2.3) |
| Mexican-American (n=1546) | 3.6 (3.3, 3.8) | 3.8 (3.5, 4.1) | 1.9 (1.7, 2.1) | 1.8 (1.6, 2.0) |
| Other (n=1342) | 3.7 (3.3, 4.0) | 3.7 (3.4, 4.0) | 1.6 (1.3, 1.9) | 1.5 (1.2, 1.7) |
| P | -- | 0.4 | - | 0.003 |
| Ratio of family income to poverty threshold | ||||
| ≤1.30 (n=2502) | 3.0 (2.8, 3.2) | 3.1 (2.9, 3.3) | 1.6 (1.5, 1.8) | 1.4 (1.3, 1.6) |
| >1.3 to ≤3.50 (n=2811) | 3.7 (3.5, 4.0) | 3.8 (3.5, 4.1) | 1.9 (1.7, 2.1) | 1.8 (1.6, 2.0) |
| >3.50 (n=2245) | 4.6 (4.3, 4.8) | 4.4 (4.2, 4.7) | 1.8 (1.6, 1.9) | 1.9 (1.7, 2.0) |
| Unknown (n=756) | 3.2 (2.9, 3.5) | 3.4 (3.1, 3.7) | 1.4 (1.2, 1.7) | 1.5 (1.2, 1.7) |
| P | <0.0001 | 0.0009 | ||
| Years of Education | ||||
| <12 (n=2343) | 2.9 (2.6, 3.2) | 3.3 (3.0, 3.6) | 1.6 (1.4, 1.8) | 1.6 (1.4, 1.8) |
| 12 (n=1960) | 3.8 (3.6, 4.1) | 3.9 (3.7, 4.1) | 2.1 (1.9, 2.3) | 2.1 (1.9, 2.3) |
| Some College (n=2300) | 4.1 (3.8, 4.3) | 4.0 (3.7, 4.2) | 1.9 (1.7, 2.0) | 1.8 (1.7, 1.9) |
| ≥ College (n=1700) | 4.4 (4.1, 4.6) | 4.1 (3.9, 4.4) | 1.4 (1.3, 1.6) | 1.5 (1.3, 1.6) |
| P | - | 0.001 | - | <0.0001 |
| Smoking Status | ||||
| Never smoked (n=4507) | 3.9 (3.8, 4.1) | 3.9 (3.8, 4.1) | 1.7 (1.6, 1.8) | 1.7 (1.6, 1.9) |
| Former smoker (n=1774) | 3.7 (3.3, 4.0) | 3.7 (3.4, 4.1) | 1.4 (1.3, 1.6) | 1.6 (1.4, 1.9) |
| Current smoker (n=2029) | 3.9 (3.6, 4.2) | 3.9 (3.6, 4.1) | 2.1 (1.9, 2.4) | 1.9 (1.7, 2.1) |
| P | - | 0.3 | - | 0.1 |
| Alcohol drinking status | ||||
| Never drink (n=951) | 3.0 (2.7, 3.3) | 3.5 (3.2, 3.8) | 1.4 (1.2, 1.5) | 1.6 (1.4, 1.8) |
| Former drinker (n=985) | 3.0 (2.8, 3.3) | 3.5 (3.2, 3.8) | 1.6 (1.4, 1.8) | 1.8 (1.5, 2.0) |
| Current drinker (n=5609) | 4.2 (4.0, 4.3) | 4.0 (3.8, 4.2) | 1.8 (1.7, 2.0) | 1.8 (1.6, 1.9) |
| Unknown (n=769) | 3.6 (3.1, 4.0) | 3.8 (3.4, 4.2) | 1.7 (1.4, 1.9) | 1.7 (1.4, 2.0) |
| P | - | 0.006 | 0.7 | |
| Physical activity as MET minutes/week | ||||
| None (n=2094) | 3.2 (2.9, 3.4) | 3.8 (3.5, 4.0) | 1.6 (1.4, 1.8) | 1.9 (1.7, 2.0) |
| First Tertile (n=2092) | 3.7 (3.5, 3.9) | 3.9 (3.6, 4.1) | 1.6 (1.5, 1.7) | 1.8 (1.6, 1.9) |
| Second tertile (n=2020) | 4.1 (3.9, 4.3) | 3.8 (3.6, 4.1) | 1.5 (1.4, 1.7) | 1.5 (1.4, 1.6) |
| Third Tertile (n=2106) | 4.3 (4.0, 4.7) | 4.0 (3.7, 4.3) | 2.2 (1.9, 2.5) | 1.9 (1.7, 2.2) |
| P | - | 0.5 | - | 0.002 |
Adjusted means were computed from regression models that included frequency/week of all away from home or fast food meals as a continuous dependent variable and all covariates in the table as independent variables. Therefore, the adjusted means column shows the independent association of each covariate adjusted for all other covariates.
Excluded those missing information on education (n=11), smoking status (n=4), and physical activity (n=2).
P value for the Sattherwaite-adjusted F test for frequency of away from home or fast-food meals as a continuous variable.
Figure 1. Weighted percentage of adult American men and women reporting categories of weekly frequency of eating away from home and fast food meals: NHANES 2007-2010.
Frequency of eating AFH meals and metabolic biomarkers
The mean BMI increased with increasing weekly frequency of AFH meals (P=0.0004); the associations were stronger in ≥50 year olds relative to <50 year olds (Table 2). Total cholesterol, HDL-cholesterol, and LDL-cholesterol related inversely with frequency of AFH meals (P<0.05) (Table 2). The inverse associations of AFH meals with total and LDL-cholesterol was significant among women (P=0.001), and the association with HDL-cholesterol was limited to ≥50 y olds (P=0.0001).
Table 2. Adjusted mean1 (95% confidence interval) of body mass index and serum concentration of metabolic biomarkers in American adults by categories of weekly frequency of eating away from home, NHANES 2007-2010.
| Weekly frequency of away from home meals | |||||
|---|---|---|---|---|---|
| BMI or serum biomarker | 0 time | 1-2 times | 3-5 times | ≥6 times | P2 |
| Body mass index3, kg/m2 N=8169 | 27.2 (26.8, 27.6) | 27.8 (27.5, 28.1) | 28.3 (27.9, 28.7) | 28.7 (28.3, 29.1) | 0.0004 |
| <50 y n=4951 | 26.9 (26.3, 27.6) | 28.0 (27.7, 28.2) | 28.2 (27.7, 28.8) | 28.3 (28.0, 28.7) | 0.05 |
| ≥50 y n=3218 | 27.5 (27.0, 28.1) | 27.5 (27.0, 28.0) | 28.4 (27.6, 29.1) | 29.9 (29.1, 30.6) | 0.0004 |
| Total cholesterol4, mg/dL N=8236 | 202 (199, 205) | 199 (197, 201) | 198 (196, 202) | 197 (194, 199) | 0.02 |
| Men (n=4092) | 204 (199, 208) | 197 (194, 200) | 199 (196, 201) | 196 (192, 200) | 0.1 |
| Women (n=4194) | 201 (197, 205) | 201 (199, 204) | 198 (196, 201) | 196 (192, 200) | 0.02 |
| HDL-cholesterol3, mg/dL N=8236 | 54 (53, 56) | 53 (52, 54) | 53 (52, 54) | 52 (51, 53) | 0.03 |
| <50 y n=4973 | 54 (52, 55) | 51 (50, 52) | 52 (50, 53) | 52 (50, 53) | 0.8 |
| ≥50 y n=3263 | 56 (54, 58) | 57 (55, 58) | 55 (54, 57) | 53 (51, 54) | 0.0001 |
| LDL-cholesterol4, mg/dL N=3604 | 117 (114, 120) | 116 (114, 119) | 113 (110, 115) | 110 (107, 113) | 0.002 |
| Men n=1708 | 119 (114, 125) | 116 (112, 120) | 117 (114, 120) | 111 (108, 115) | 0.1 |
| Women n=1896 | 115 (111-119) | 117 (114, 120) | 109 (106, 112) | 109 (105, 114) | 0.001 |
| Triglycerides, mg/dL N=3659 | 108 (103, 112) | 104 (100, 108) | 107 (102, 112) | 105 (100, 110) | 0.8 |
| Fasting glucose, mg/dL N=3668 | 99 (98, 101) | 99 (98, 100) | 98 (97, 99) | 99 (98, 100) | 0.8 |
| Glycohemoglobin3, % N=8234 | 5.41 (5.38,5.44) | 5.41 (5.38, 5.44) | 5.39 (5.37, 5.41) | 5.40 (5.37, 5.44) | 0.4 |
| <50 y n=4966 | 5.30 (5.26, 5.34) | 5.31 (5.28, 5.35) | 5.30 (5.27, 5.32) | 5.29 (5.26, 5.32) | 0.1 |
| ≥50 y n=3268 | 5.59 (5.55, 5.63) | 5.59 (5.54, 5.64) | 5.59 (5.54, 5,64) | 5.68 (5.61, 5.76) | 0.1 |
Adjusted means were computed from multiple linear regression models with each biomarker as a continuous dependent variable. All biomarkers (except BMI, total and HDL cholesterol) were log-transformed for analysis; therefore, the back-transformed values for LDL-cholesterol, triglycerides, fasting glucose, and glycohemoglobin are geometric means and their 95% confidence intervals. Independent variables included: frequency of AFH meals (0, 1-2, 3-5, ≥6 times), age (20-39, 40-59, ≥60), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, other), Poverty income ratio (≤1.3, >1.3 to 3.5, ≥3.5, unknown), years of education (<12, 12, some college, ≥college), serum cotinine (continuous), hours of fasting before phlebotomy, (continuous), physical activity (none, tertiles of MET minutes/week), alcohol drinking status (never drink, former drinker, current drinker, unknown). N refers to observations used in the regression model for each biomarker.
P value for the Sattherwaite-adjusted F test for frequency of away from home meals as a continuous variable.
Significant interaction of frequency of away from home meals with age (Pinteraction<0.05), thus the results are stratified by age categories.
Significant interaction of frequency of away from home meals with sex (Pinteraction<0.05), thus the results are stratified by sex.
A higher proportion of respondents with more frequent AFH meals had a BMI of ≥30 (P=0.002) (Supplemental Table 3). The proportion of all respondents with total cholesterol concentration of ≥200 mg/dl, and women with LDL-cholesterol concentration of ≥130 mg/dl decreased with increasing number of AFH meals (P≤0.01).
Frequency of eating fast-food meals and metabolic biomarkers
The mean BMI of more frequent fast-food meal reporters was higher and the mean serum HDL-cholesterol was lower (P<0.0001); the associations were stronger in women than men (Table 3).
Table 3. Adjusted mean1 (95% confidence interval) of body mass index and serum concentration of metabolic biomarkers in American adults by categories of weekly frequency of fast-food or pizza meals, NHANES 2007-2010.
| Weekly frequency of fast food or pizza meals | |||||
|---|---|---|---|---|---|
| BMI or serum Biomarker | 0 time | 1 time | 2-3 times | ≥4 times | P2 |
| BMI3, kg/m2 | |||||
| All N=8169 | 27.5 (27.1, 27.8) | 27.9 (27.6, 28.2) | 28.9 (28.4, 29.4) | 28.8 (28.3, 29.2) | <0.0001 |
| Men n=4002 | 27.9 (27.4, 28.3) | 28.0 (27.6, 28.4) | 28.5 (28.0, 29.0) | 28.6 (28.2, 29.0) | 0.05 |
| Women n=4167 | 27.2 (26.8, 27.6) | 27.7 (27.3, 28.1) | 29.3 (28.6, 29.9) | 29.0 (28.1, 29.8) | <0.0001 |
| Total cholesterol, mg/dL N=8236 | 199 (197, 202) | 198 (196, 200) | 199 (196, 201) | 198 (196, 201) | 0.5 |
| HDL-cholesterol3, mg/dL | |||||
| All n=8236 | 54 (53, 55) | 53 (52, 54) | 52 (51, 53) | 51 (50, 52) | <0.0001 |
| Men n=4042 | 48 (47, 49) | 48 (47, 49) | 48 (46, 49) | 46 (45, 47) | 0.003 |
| Women n=4194 | 60 (59, 61) | 58 (57, 60) | 56 (55, 57) | 56 (54, 58) | 0.001 |
| LDL-cholesterol4, mg/dL | |||||
| All n=3604 | 113 (111, 116) | 117 (113, 120) | 113 (110, 116) | 114 (110, 118) | 0.6 |
| <50 y n=2151 | 107 (105, 110) | 112 (109, 116) | 111 (107, 114) | 108 (104, 112) | 0.8 |
| ≥50 y n=1453 | 123 (118, 129) | 126 (121, 131) | 118 (113, 123) | 129 (122, 137) | 0.5 |
| Triglycerides, mg/dL n=3659 | 103 (98, 109) | 103 (99, 108) | 110 (106, 115) | 110 (104, 117) | 0.2 |
| Fasting glucose3, mg/dL | |||||
| All n=3668 | 99 (98, 100) | 99 (98, 100) | 99 (98, 100) | 99 (98, 100) | 0.5 |
| Men n=1750 | 102 (101, 104) | 102 (101, 104) | 101 (99, 102) | 101 (99, 102) | 0.1 |
| Women n=1918 | 97 (95, 98) | 95 (94, 97) | 97 (96, 99) | 98 (96, 101) | 0.2 |
| Glycohemoglobin, % N=8234 | 5.42 (5.39, 5.44) | 5.39 (5.36, 5.42) | 5.39 (5.36, 5.42) | 5.40 (5.37, 5.44) | 0.2 |
Adjusted means were computed from multiple linear regression models with each biomarker as a continuous dependent variable. All biomarkers (except BMI, total and HDL cholesterol) were log-transformed for analysis; therefore, the back-transformed values for LDL-cholesterol, triglycerides, fasting glucose, and glycohemoglobin are geometric means and their 95% confidence intervals. Independent variables included: frequency of fast food meals (0, 1, 2-3, ≥4 times), age (20-39, 40-59, ≥60), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, other), Poverty income ratio (≤1.3, >1.3 to 3.5, ≥3.5, unknown), years of education (<12, 12, some college, ≥college), serum cotinine (continuous), hours of fasting before phlebotomy, (continuous), physical activity (none, tertiles of MET minutes/week), alcohol drinking status (never drink, former drinker, current drinker, unknown). N refers to observations used in the regression model for each biomarker.
P value for the Sattherwaite-adjusted F test for frequency of fast food meals as a continuous variable.
Significant interaction of fast-food meals with sex (Pinteraction<0.05), thus the results are stratified by sex
Significant interaction of frequency of fast-food meals with age (Pinteraction<0.05), thus the results are stratified by age categories.
The proportion of Americans with BMI of ≥30 was higher in more frequent reporters of fast-food meals and the association was stronger in women (P=0.0004) relative to men (P=0.01) (Supplemental Table 4). In <50 y olds, with increasing frequency of fast-food meals, serum HDL-cholesterol concentration of <40 mg/dl was more likely (P=0.0002) but glycohemoglobin concentration of ≥5.7% was less likely (P=0.03). The proportion of women with fasting glucose concentration of ≥ 100 mg/dl increased with increasing frequency of fast-food meals (P=0.04).
Frequency of eating AFH meals and nutritional biomarkers
Weekly frequency of eating AFH meals was an independent, inverse correlate of the serum concentrations of vitamins D, E, vitamin B-12, folate (and RBC folate), α-carotene, trans-β-carotene, lutein-zeaxanthin, and β-cryptoxanthin (P<0.05) (Table 4). The inverse associations of frequency of AFH meals with serum vitamin C and pyridoxal-5'-phosphate were significant in women (P=0.001) and in ≥50 year olds (P≤0.001). Serum vitamin A and lycopene concentrations were not related with frequency of AFH meals.
Table 4. Adjusted mean1 (95% confidence interval) of serum concentration of nutritional biomarkers in American adults by categories of weekly frequency of eating away from home, NHANES 2005-2006.
| Weekly frequency of away from home meals | |||||
|---|---|---|---|---|---|
| Serum Biomarker | 0 | 1-2 times | 3-5 times | ≥6 times | P2 |
| Vitamin A, ug/dL N=4022 | 59.3 (58.1, 60.5) | 59.1 (58.1, 60.2) | 58.5 (57.0, 59.9) | 58.3 (56.4, 60.3) | 0.2 |
| Vitamin D, ng/mL N=4062 | 21.9 (20.7, 23.1) | 21.4 (20.3, 22.6) | 21.3 (20.0, 22.7) | 20.8 (19.5, 22.2) | 0.01 |
| Vitamin E, ug/dL N=4021 | 1193 (1160, 1228) | 1192 (1164, 1220) | 1184 (1157, 1211) | 1148 (1114, 1183) | 0.001 |
| Vitamin C3, 4, umol/L All N=4036 | 56.0 (53.8, 58.2) | 54.7 (52.6, 56.8) | 53.5 (51.5, 55.6) | 52.1 (49.4, 54.7) | 0.008 |
| Men n=2101 | 49.7 (46.8, 52.6) | 49.1 (46.4, 51.8) | 49.7 (47.3, 52.0) | 49.9 (47.0, 52.7) | 0.5 |
| Women n=1935 | 61.9 (58.1, 65.6) | 59.9 (56.9, 62.8) | 57.3 (54.5, 60.0) | 52.8 (48.4, 57.2) | 0.001 |
| <50 y n=2107 | 52.3 (50.6, 53.9) | 50.2 (47.1, 53.4) | 50.8 (48.5, 53.1) | 50.2 (46.6, 53.9) | 0.2 |
| ≥50 y n=1929 | 61.0 (57.4, 64.6) | 60.7 (57.1, 64.3) | 56.9 (53.5, 60.3) | 53.7 (50.2, 57.1) | 0.0009 |
| Serum folate, ng/ml n=4029 | 12.5 (11.8, 13.2) | 12.3 (11.7, 13.0) | 12.0 (11.3, 12.7) | 11.0 (10.5, 11.5) | <0.0001 |
| Red blood cell folate, ng/ml n=4041 | 285 (275, 294) | 280 (274, 286) | 274 (267, 282) | 266 (258, 275) | 0.007 |
| Vitamin B-123, pg/ml All n=3972 | 483 (460, 506) | 480 (457, 505) | 464 (443, 485) | 458 (434, 483) | 0.04 |
| Men n=2062 | 470 (436, 507) | 467 (442, 493) | 466 (442, 491) | 459 (440, 478) | 0.9 |
| Women n=1910 | 493 (468, 519) | 495 (464, 527) | 462 (433, 492) | 449 (415, 486) | 0.002 |
| Pyridoxal-5′-phosphate3,4, nmol/L All n=4056 | 52.2 (46.9, 58.1) | 53.8 (50.4, 57.5) | 49.6 (45.6, 54.1) | 46.5 (43.1, 50.1) | 0.002 |
| Men n=2110 | 55.6 (50.3, 61.3) | 57.2 (52.1, 62.8) | 56.1 (50.4, 62.4) | 52.8 (49.6, 56.3) | 0.4 |
| Women n=1946 | 48.6 (42.2, 55.9) | 50.8 (47.8, 53.9) | 43.9 (39.3, 49.1) | 39.9 (35.1, 45.2) | 0.001 |
| <50 y n=2111 | 48.1 (42.0, 55.1) | 53.8 (49.3, 58.8) | 50.3 (46.5, 54.4) | 48.8 (44.1, 54.0) | 0.2 |
| ≥50 y n=1945 | 56.9 (51.7, 62.8) | 54.1 (50.4, 58.6) | 48.0 (42.3, 54.6) | 41.1 (36.8, 46.0) | 0.0001 |
| α-carotene, ug/dL N=4018 | 3.43 (3.08, 3.82) | 3.10 (2.87, 3.35) | 2.75 (2.44, 3.09) | 2.51 (2.25, 2.81) | 0.0001 |
| Trans-β-carotene, ug/dl n=4021 | 14.2 (13.0, 15.6) | 13.5 (12.6, 14.5) | 11.9 (11.2, 12.7) | 10.8 (10.1, 11.5) | <0.0001 |
| Lutein-zeaxanthin, ug/dL n=4021 | 14.9 (14.1, 15.7) | 14.5 (13.9, 15.1) | 14.1 (13.6, 14.7) | 14.0 (13.5, 14.5) | 0.05 |
| β-cryptoxanthin, ug/dL n=3999 | 7.85 (7.01, 8.79) | 7.53 (7.11, 7.97) | 7.65 (7.19, 8.14) | 6.96 (6.51, 7.43) | 0.01 |
| Total lycopene, ug/dL N=3979 | 37.4 (35.5, 39.4) | 39.1 (37.8, 40.4) | 40.5 (39.1, 41.9) | 40.3 (38.9, 41.8) | 0.1 |
Adjusted means were computed from multiple linear regression models with each biomarker as a continuous dependent variable. All nutritional biomarkers (except vitamin C) were log-transformed for analysis; therefore, for all variables except vitamin C, the back-transformed values are geometric means. Independent variables included: frequency of away from home meals (0, 1-2, 3-5, ≥6 times), age (20-39, 40-59, ≥60), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, other), Poverty income ratio (≤1.3, >1.3 to 3.5, ≥3.5, unknown), years of education (<12, 12, some college, ≥college), serum cotinine (continuous), hours of fasting before phlebotomy, (continuous), any self-reported chronic disease (yes, no), supplement user (yes, no), alcohol drinking status (never drink, former drinker, current drinker, unknown), body mass index (continuous). Models for vitamin E and all carotenoids included serum total cholesterol; models for vitamin D included the month of MEC exam (November to April, May to October). N refers to observations used in the regression model for each biomarker.
P value for the Sattherwaite-adjusted F test for frequency of away from home meals as a continuous variable.
Significant interaction of frequency of away from home meals with sex (Pinteraction<0.05), thus the results are stratified by sex.
Significant interaction of frequency of away from home meals with age (Pinteraction<0.05), thus the results are stratified by age categories.
The number of respondents with concentrations of serum vitamin A, vitamin E, folate, and RBC folate below the risk threshold were too small (11, 16, 1, and 15, respectively) for regression modeling. The likelihood of serum vitamin D concentration of <20 ng/ml increased with increasing frequency of AFH meals (P=0.0003) (Supplemental Table 5). The proportion of ≥50 year olds with serum pyridoxal-5'-phosphate concentration of <20 nmol/L increased with more frequent AFH meals (P=0.0006).
Sensitivity analysis
In alternative analyses, results for metabolic biomarkers were essentially unchanged with addition of BMI to regression models (data not shown).
Discussion
Key findings of this study of objectively assessed metabolic and nutritional outcomes in relation to frequency of AFH and fast-food meals are: 1) >50% of American adults reported ≥3 or more AFH and >35% reported ≥2 fast-food meals week; 2) higher BMI and inverse associations of most examined nutritional biomarkers with increasing frequency of AFH meals, with women and older Americans showing greater vulnerability; 3) inverse associations of serum total cholesterol, HDL-cholesterol, and LDL-cholesterol with frequency of AFH meals but no associations with serum triglycerides, glucose, and glycohemoglobin.
The estimated prevalence of >50% of Americans reporting ≥3 AFH meals/week is remarkably higher than the NHANES 1999-2000 estimate of 41% we reported previously (7). However, the questions used to elicit the AFH meal information in 2007-2010 (current study) differed from that used in 1999-2000. Because no information on relative comparability of estimates from these different questions is available, it is not possible to comment on whether this is a real increase or an artifact of the questions used in the surveys.
Given that fruits and vegetables are the best known sources of vitamins C, E, folate, and carotenoids (37, 38), the strong inverse associations of serum concentrations of these nutrients with AFH meals are not surprising. More frequent AFH meals in women and ≥ 50 year olds in particular is of concern as these demographic groups showed significant inverse associations for some biomarkers not noted in men (vitamins C, B-6, and B-12) or <50 y olds (vitamin C and B-6). To our knowledge, however, there are no published reports that have examined nutritional biomarkers in relation to AFH meal intake for corroborative evidence. The fast-food and nutritional biomarker association could not be examined in the current study because the NHANES 2005-2006 did not include questions on fast-food intake.
Except BMI and serum HDL-cholesterol concentration, this study found few significant adverse associations of metabolic biomarkers with weekly frequency of AFH or fast-food meals. Although comparisons with few published papers are limited by the type of evidence, a closer look at three published papers with comparable exposures, presents a mixed picture. In 18-30 year old participants, Pereira et al found no cross-sectional associations of frequency of fast-food intake with body weight or insulin resistance. However, baseline fast-food frequency of >3 times/week was related with higher BMI and insulin resistance at 15 years of followup (22). In another analysis of data from the same cohort mentioned above, higher fast-food (but not restaurant) meal frequency was related with higher BMI, triglycerides, and insulin resistance and lower HDL-cholesterol concentrations at 13 years of followup (23). Serum concentrations of total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides were not related with weekly frequency of “take away” food consumption in Australian adults aged 26-36 years (25); however, women (not men) with frequency of ≥2 “take away” meals/week relative to ≤ 1 time/week were reported to have higher fasting glucose and insulin (25).
The reasons for lack of the expected associations of AFH eating with metabolic biomarkers in the present study are not known, but we can speculate about some possible explanations. First, although our analyses adjusted for several known confounders of the AFH eating and metabolic biomarker associations, residual confounding by these and other unknown confounders remains a possibility. Second, we may consider the possibility that people who eat out more often may try to make healthier selections at these places or try to eat healthfully at home prepared meals. The available evidence is sparse but suggests the opposite, i.e., at least in 2-18 year old children who reported fast-food intake, remainder of the diet was also of poor quality (39). Moreover, the findings of lower nutritional biomarker concentrations in relation to AFH meal frequency in the current study are also counter to this argument. Finally, although the associations of several nutritional biomarker associations with AFH frequency were in the expected inverse direction, the possibility that the frequency of AFH and fast-food meals was misreported must also be considered.
Although recent studies have found little evidence of better nutritional quality of restaurant meals relative to fast-food meals (2, 3), Duffy et al (23), in a prospective study found fast-food meal frequency (but not restaurant meal frequency) to be associated with higher BMI and lower HDL-cholesterol concentration. The frequency of all AFH meals in our study included both fast-food and non-fast-food meals. Therefore, we expected that associations of the two exposures examined in our study (all AFH meals or only fast-food meals) with metabolic biomarkers to be somewhat comparable. Both exposures were indeed found to relate to higher BMI and lower HDL-cholesterol in the present study. The relative importance of the association of AFH and fast-food exposures with lower HDL-cholesterol remains uncertain given recent reports that have questioned the causal role of HDL-cholesterol in increasing cardiovascular risk (40).
The cross-sectional nature of the present study precludes ascertainment of temporality. However use of a large, representative, diverse sample of the US population and objective outcomes (biomarkers) in relation to frequency of AFH and fast-food meals are strengths of this study. The biomarkers assessment in the NHANES was done using standardized assay procedures with uniform quality control protocols. We acknowledge that the exposures (frequency of AFH and fast-food meals) were self-reported and no information on validation of the questions used to collect this information is available.
At present, most self-selected American diets are not in accord with the US dietary guidelines (41). Increasing frequency of AFH meals is likely to make these dietary goals still harder to attain. These facts argue for systemic changes to include smaller portion sizes (42) and expansion of options for whole grains, vegetables, and fruits as part of AFH meals. However, the issue is complex because despite expressed interest in healthy dining out, few American diners order the so-called “healthy” offerings (43), and consumer response to calorie labeling has been disappointing (44, 45).
In conclusion, reporters of frequent AFH and fast-food meals had higher BMI and lower HDL-cholesterol; but profiles of other biomarkers did not show greater metabolic risk. However, lower circulating concentrations of nutrients with mostly plant foods as sources suggest that more frequent AFH meal consumers, especially women and older Americans, need to choose these foods carefully while dining out or at home.
Supplementary Material
Acknowledgments
We thank Lisa L. Kahle for expert SAS and SUDAAN programming support and David Check, NCI, NIH, for graphic support.
Supported in part by the intramural research program of the Department of Health and Human Services, National Cancer Institute, NIH (BIG).
Footnotes
Conflicts of interest: None
Supplementary information is available at International Journal of Obesity's website.
Contributor Information
Ashima K. Kant, Dept. of Family, Nutrition, and Exercise Sciences, Queens College of the City University of New York, Flushing, NY
Melanie I. Whitley, Dept. of Family, Nutrition, and Exercise Sciences, Queens College of the City University of New York, Flushing, NY
Barry I. Graubard, Division of Cancer Epidemiology and Genetics, Biostatistics Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD
References
- 1.Lin B, Guthrie J. Nutritional Quality of Food Prepared at Home and Away From Home, 1977-2008, EIB-105. US Department of Agriculture, Economic Research Service. 2012 Dec; [Google Scholar]
- 2.Scourboutakos MJ, L'Abbé MR. Restaurant menus: calories, caloric density, and serving size. Am J Prev Med. 2012;43:249–55. doi: 10.1016/j.amepre.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Scourboutakos MJ, Semnani-Azad Z, L'Abbe MR. Restaurant meals: almost a full day's worth of calories, fats, and sodium. JAMA Intern Med. 2013;173:1373–4. doi: 10.1001/jamainternmed.2013.6159. [DOI] [PubMed] [Google Scholar]
- 4.Wu HW, Sturm R. What's on the menu? A review of the energy and nutritional content of US chain restaurant menus. Public Health Nutr. 2013;16:87–96. doi: 10.1017/S136898001200122X. Erratum in: Public Health Nutr. 2012; 15:2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer KW, Hearst MO, Earnest AA, French SA, Oakes JM, Harnack LJ. Energy content of U.S. fast-food restaurant offerings: 14-year trends. Am J Prev Med. 2012;43:490–7. doi: 10.1016/j.amepre.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hearst MO, Harnack LJ, Bauer KW, Earnest AA, French SA, Michael Oakes J. Nutritional quality at eight U.S. fast-food chains: 14-year trends. Am J Prev Med. 2013;44(6):589–94. doi: 10.1016/j.amepre.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Kant AK, Graubard BI. Eating out in American, 1987-2000: trends and nutritional correlates. Prev Med. 2004;38:243–9. doi: 10.1016/j.ypmed.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Clemens LH, Slawson DL, Klesges RC. The effect of eating out on quality of diet in premenopausal women. J Am Diet Assoc. 1999;99:442–4. doi: 10.1016/s0002-8223(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 9.Paeratakul S, Ferdinand DP, Champagne CM, Ryan DH, Bray GA. Fast-food consumption among US adults and children: dietary and nutrient intake profile. J Am Diet Assoc. 2003;103:1332–8. doi: 10.1016/s0002-8223(03)01086-1. [DOI] [PubMed] [Google Scholar]
- 10.Todd JE, Mancino L, Lin B. The Impact of Food Away From Home on Adult Diet Quality, ERR-90. U.S. Department of Agriculture, Economic Research Service; 2010. http://www.ers.usda.gov/publications/err-economic-research-report/err90.aspx#.U6CVzvldWiw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowman SA, Vinyard BT. Fast food consumption of U.S. adults: impact on energy and nutrient intakes and overweight status. J Am Coll Nutr. 2004;23:163–168. doi: 10.1080/07315724.2004.10719357. [DOI] [PubMed] [Google Scholar]
- 12.Jeffery RW, French SA. Epidemic obesity in the United States: are fast foods and television viewing contributing? Am J Public Health. 1998;88:277–280. doi: 10.2105/ajph.88.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrory MA, Fuss PJ, Hays NP, Vinken AG, Greenberg AS, Roberts SB. Overeating in America: association between restaurant food consumption and body fatness in healthy adult men and women ages 19–80. Obesity Research. 1999;7:564–571. doi: 10.1002/j.1550-8528.1999.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 14.Binkley JK, Eales J, Jekanowski M. The Relation between Dietary Change and Rising US Obesity. International Journal of Obesity. 2000;24(8):1032–1039. doi: 10.1038/sj.ijo.0801356. [DOI] [PubMed] [Google Scholar]
- 15.Mehta NK, Chang VW. Weight status and restaurant availability: a multilevel analysis. Am J Prev Med. 2008;34:127–133. doi: 10.1016/j.amepre.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Harmer P, Cardinal BJ, Bosworth M, Johnson-Shelton D. Obesity and the built environment: does the density of neighborhood fast-food outlets matter? Am J Health Promot. 2009;23:203–209. doi: 10.4278/ajhp.071214133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore LV, Diez Roux AV, Nettleton JA, Jacobs DR, Franco M. Fast-food consumption, diet quality, and neighborhood exposure to fast food: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2009;170:29–36. doi: 10.1093/aje/kwp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleischhacker SE, Evenson KR, Rodriguez DA, Ammerman AS. A systematic review of fast food access studies. Obes Rev. 2011;12:e460–71. doi: 10.1111/j.1467-789X.2010.00715.x. [DOI] [PubMed] [Google Scholar]
- 19.Alter DA, Eny K. The relationship between the supply of fast-food chains and cardiovascular outcomes. Can J Public Health. 2005;96:173–7. doi: 10.1007/BF03403684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahern M, Brown C, Dukas S. A national study of the association between food environments and county-level health outcomes. J Rural Health. 2011;27:367–79. doi: 10.1111/j.1748-0361.2011.00378.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamano T, Kawakami N, Li X, Sundquist K. Neighbourhood environment and stroke: a follow-up study in Sweden. PLoS One. 2013;8:e56680. doi: 10.1371/journal.pone.0056680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira MA, Kartashov AI, Ebbeling CB, Van Horn L, Slattery ML, Jacobs DR, Jr, Ludwig DS. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet. 2005;365:36–42. doi: 10.1016/S0140-6736(04)17663-0. Erratum in: Lancet. 2005; 365:1030. [DOI] [PubMed] [Google Scholar]
- 23.Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR, Jr, Popkin BM. Regular consumption from fast food establishments relative to other restaurants is differentially associated with metabolic outcomes in young adults. J Nutr. 2009;139:2113–8. doi: 10.3945/jn.109.109520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulkerson JA, Farbakhsh K, Lytle L, Hearst MO, Dengel DR, Pasch KE, Kubik MY. Away-from-home family dinner sources and associations with weight status, body composition, and related biomarkers of chronic disease among adolescents and their parents. J Am Diet Assoc. 2011;111(12):1892–7. doi: 10.1016/j.jada.2011.09.035. Erratum in: J Am Diet Assoc. 2012; 112:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KJ, Blizzard L, McNaughton SA, Gall SL, Dwyer T, Venn AJ. Takeaway food consumption and cardio-metabolic risk factors in young adults. Eur J Clin Nutr. 2012;66:577–84. doi: 10.1038/ejcn.2011.202. [DOI] [PubMed] [Google Scholar]
- 26.Bahadoran Z, Mirmiran P, Golzarand M, Hosseini-Esfahani F, Azizi F. Fast food consumption in Iranian adults; dietary intake and cardiovascular risk factors: Tehran Lipid and Glucose Study. Arch Iran Med. 2012;15(6):346–51. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention: National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: US Department of Health and Human Services, CDC; [Accessed 10/26/13]. Available from: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes05_06.aspx. [Google Scholar]
- 28.Centers for Disease Control and Prevention: National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: US Department of Health and Human Services, CDC; [Accessed 10/26/13]. Available from: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes07_08.aspx. [Google Scholar]
- 29.National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention: National Center for Health Statistics; Hyattsville, MD: US Department of Healthy and Human Services, CDC; [Accessed 10/26/13]. Available from: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes09_10.aspx. [Google Scholar]
- 30.Centers for Disease Control and Prevention: National Center for Health Statistics. NHANES response rates and CPS total. Hyattsville, MD: US Department of Health and Human Services, CDC; [Accessed 12/26/13]. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_cps.htm. [Google Scholar]
- 31.Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. 2nd National report on biochemical indicators of Diet and Nutrition in the US population. Hyattsville, MD: US Department of Health and Human Services, CDC; 2012. National Center for Environmental Health Survey. Available from: http://www.cdc.gov/nutritionreport/pdf/Nutrition_Book_complete508_final.pdf#zoom=100 (cited November 2013) [Google Scholar]
- 33.Sudaan. Release 11.0.RTI International. Research Triangle Park; NC: [Google Scholar]
- 34.Johnson CL, Paulose-Ram R, Ogden CL, et al. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010. National Center for Health Statistics. Vital Health Stat. 2013;2(161) Available from: http://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf. [PubMed] [Google Scholar]
- 35.Korn EL, Graubard BI. Analysis of health surveys. New York, NY: John Wiley and Sons; 1999. [Google Scholar]
- 36.Graubard BI, Korn EL. Predictive margins for survey data. Biometrics. 1999;55:652–9. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 37.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin C, E, Selenium, and carotenoids. National Academy Press; Washington, DC: 2000. [Google Scholar]
- 38.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 39.Poti JM, Duffey KJ, Popkin BM. The association of fast food consumption with poor dietary outcomes and obesity among children: is it the fast food or the remainder of the diet? Am J Clin Nutr. 2014;99:162–71. doi: 10.3945/ajcn.113.071928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80. doi: 10.1016/S0140-6736(12)60312-2. Erratum in: Lancet. 2012; 380: 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.USDA. Center for Nutrition Policy and Promotion. Diet quality of Americans in 2001-2002 and 2007-2008 as measured by the Healthy Eating Index-2010. [Accessed 5/15/2014];Nutrition Insight. 2013 51 Available from: http://www.cnpp.usda.gov/Publications/NutritionInsights/Insight51.pdf. [Google Scholar]
- 42.Cohen DA, Story M. Mitigating the health risks of dining out: the need for standardized portion sizes in restaurants. Am J Public Health. 2014;104:586–90. doi: 10.2105/AJPH.2013.301692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.http://www.huffingtonpost.com/Menuism/do-americans-want-healthy_b_4856158.html Accessed 5/29/14
- 44.Hammond D, Goodman S, Hanning R, Daniel S. A randomized trial of calorie labeling on menus. Prev Med. 2013;57:860–6. doi: 10.1016/j.ypmed.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Kiszko KM, Martinez OD, Abrams C, Elbel B. The Influence of Calorie Labeling on Food Orders and Consumption: A Review of the Literature. J Community Health. 2014 Apr 24; doi: 10.1007/s10900-014-9876-0. [Epub ahead of print] PubMed PMID: 24760208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.