Abstract
STUDY QUESTION
Do endometrial stromal fibroblasts (eSF) in women with polycystic ovary syndrome (PCOS) (eSFpcos) exhibit altered estrogen and/or progesterone (P4) responses, which may explain some of the adverse reproductive outcomes and endometrial pathologies in these women?
SUMMARY ANSWER
In vitro, eSF from women with PCOS exhibit an aberrant decidualization response and concomitant changes in pro-inflammatory cytokine, chemokine and matrix metalloproteinase (MMP) release and immune cell chemoattraction. In vivo these aberrations may result in suboptimal implantation and predisposition to endometrial cancer.
WHAT IS KNOWN ALREADY
The endometrium in women with PCOS has several abnormalities including progesterone (P4) resistance at the gene expression level, likely contributing to subfertility, pregnancy complications and increased endometrial cancer risk in PCOS women.
STUDY DESIGN, SIZE, DURATION
Prospective, university-based, case–control, in vitro study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Cultures of eSFPCOS (n = 12, Rotterdam and NIH criteria) and eSFControl (Ctrl) (n = 6, regular cycle length, no signs of hyperandrogenism) were treated with vehicle, estradiol (E2, 10 nM) or E2P4 (10 nM/1 μM) for 14 days. Progesterone receptor (PGR) mRNA was assessed with quantitative real-time PCR (qRT–PCR) and eSF decidualization was confirmed by insulin-like growth factor-binding protein-1 (IGFBP-1) transcript and protein expression. Fractalkine (CX3CL1), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL) 6, 8 and 11, macrophage chemoattractant protein (MCP) 1 and 3, CCL5 (RANTES) and MMPs (MMP1, 2, 3, 7, 9, 10 and 12) were measured in conditioned media by Luminex multiplex assays, and chemotactic activity of the conditioned media was tested in a migration assay using CD14+ monocyte and CD4+ T-cell migration assay. Effects of IL-6 (0.02, 0.2, 2 or 20 ng/ml) or IL-8 (0.04, 0.4, 4, or 40 ng/ml) or combination (0.2 ng/ml IL-6 and 4.0 ng/ml IL-8) on 14-d decidualization were also tested. ANOVA with pre-planned contrasts was used for statistical analysis.
MAIN RESULTS AND THE ROLE OF CHANCE
Hormonal challenge with E2P4 to induce decidualization revealed two distinct subsets of eSFPCOS. Eight eSFPCOS (dPCOS) and all eSFCtrl (dCtrl) cultures showed a normal decidualization response to E2P4 as determined by morphology and IGFBP-1 secretion. However, 4 eSFPCOS cultures showed blunted decidualization (ndPCOS) in morphological assessment and low IGFBP-1 levels even though all three groups exhibited normal estrogen-mediated increase in PGR expression. Interestingly dPCOS had decreased IL-6 and GM-SCF secretion compared with dCtrl, whereas the ndPCOS cultures showed increased IL-6 and 8, MCP1, RANTES and GM-CSF secretion at base-line and/or in response to E2 or E2P4 compared with dCtrl and/or dPCOS. Furthermore, even though PGR expression was similar in all three groups, P4 inhibition of MMP secretion was attenuated in ndPCOS resulting in higher MMP2 and 3 levels. The conditioned media from ndPCOS had increased chemoattractic activity compared with dCtrl and dPCOS media. Exogenously added IL-6 and/or 8 did not inhibit decidualization in eSFCtrl indicating that high levels of these cytokines in ndPCOS samples were not likely a cause for the aberrant decidualization.
LIMITATIONS, REASONS FOR CAUTION
This is an in vitro study with a small sample size, utilizing stromal cell cultures from proliferative and secretory phase endometrium. The effect of PCOS on endometrial epithelium, another major histoarchitectural cell compartment of the endometrium, was not evaluated and should be considered in future studies. Furthermore, results obtained should also be confirmed in a larger data set and with mid/late secretory phase in vivo samples and models.
WIDER IMPLICATIONS OF THE FINDINGS
The alterations seen in ndPCOS may contribute to endometrial dysfunction, subfertility and pregnancy complications in PCOS women. The results emphasize the importance of understanding immune responses related to the implantation process and normal endometrial homeostasis in women with PCOS.
STUDY FUNDING/COMPETING INTEREST(S)
Sigrid Juselius Foundation, Academy of Finland, Finnish Medical Foundation, Orion-Farmos Research Foundation (to T.T.P.), the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) U54HD 055764-07 Specialized Cooperative Centers Program in Reproduction and Infertility Research (to L.C.G.), the NICHD the Ruth L. Kirschstein National Research Service Awards grant 1F32HD074423-03 (to J.C.C.). The authors have no competing interests.
Keywords: PCOS, endometrium, decidualization, cytokines, MMP
Introduction
In humans decidualization is a progesterone (P4) driven differentiation process essential to prepare the endometrium for successful embryo implantation and maintenance of pregnancy (Cha et al., 2012). Decidualization occurs in the secretory phase when endometrium undergoes vast and complex cellular differentiation dependent on progesterone receptor (PGR) up-regulation by estradiol (E2), activation of P4 signaling and convergence of cyclic adenosine monophosphate (cAMP) signaling (Aghajanova et al., 2009; Zhu et al., 2014). Glandular secretory transformation occurs in response to P4, and perivascular endometrial stromal fibroblasts (eSF) initiate decidualization throughout the endometrium that continues to progress if conception occurs (Cha et al., 2012; Zhu et al., 2014). Decidualized eSFs undergo morphological and functional changes with glycogen and lipid accumulation in the cytoplasm, providing a source of nutrition for the developing embryo prior to placental development (Cha et al., 2012; Zhu et al., 2014). In response to P4-mediated activation of transcription factors forkhead box protein O1 (FOXO1) and homeobox A10 (HOXA10), decidualized eSFs produce insulin-like growth factor-binding protein 1 (IGFBP-1), commonly used as a marker of decidualization. IGFBP-1 restricts trophoblast invasion and endometrial growth, in part, by inhibiting insulin growth factor 1 (IGF-1) action (Irwin and Giudice, 1998).
Several growth factors, cytokines and matrix metalloproteinases (MMPs) play a role in the decidualization process to optimize the endometrial environment for implantation where balanced trophoblast invasion, outgrowth and vasculature establishment are achieved (Staun-Ram and Shalev, 2005). Several cytokines, e.g. IL-11, LIF, IL-6 and transient immune cells, are present during decidualization and participate in the communication between differentiated endometrium and the embryo (Kojima et al., 1994; Cork et al., 2001). In addition to cytokines, MMPs, a family of zinc-dependent proteases, are also highly expressed in decidua as they proteolyze the extracellular matrix, allowing tissue growth and remodeling in response to normal proliferative and differentiative signals (Godbole et al., 2011). MMPs allow for histoarchitectural modifications necessary for embryo attachment and invasion where suppression of endometrial MMPs is necessary to maintain endometrial stability preventing excessive invasion of extravillus trophoblasts and abnormal placental development (Visse and Nagase, 2003; Licht et al., 2007; Godbole et al., 2011). In endometrium P4 normally suppresses MMP expression directly, up-regulates tissue inhibitors of metalloproteinases (TIMPs) and modulates cytokine activity (Higuchi et al., 1995; Bischof et al., 1998; Wissink et al., 1998)—all of which control MMP actions, although in P4-resistant disorders, this suppression of MMPs is not observed.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in reproductive aged women, affecting around 10% of this population and characterized as menstrual dysfunction, anovulatory infertility, hyperandrogenism and insulin resistance (Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group 2012). In PCOS impaired follicle maturation and consequent anovulation cause a chronic P4-deficient state that affects the endometrial milieu. Furthermore, even with ovulation (and thus P4 acting on endometrium), the PCOS endometrium has several abnormalities including altered steroid receptor and glucose transporter 4 (GLUT4) expression as well as P4-resistance (Apparao et al., 2002; Villavicencio et al., 2006; Savaris et al., 2011; Carvajal et al., 2013). Moreover, we as well as others have recently reported an inflammatory milieu in PCOS endometrium with an increased inflammatory profile in the proliferative phase and decreased uterine natural killer cell influx in late secretory phase. This is likely to contribute to subfertility, increased prevalence of pregnancy complications and endometrial cancer in these women (Matteo et al., 2010; Haoula et al., 2012; Piltonen et al., 2013; Barry et al., 2014; Naver et al., 2014)
Since P4-regulation of endometrium during the secretory phase involves a complex network of differentiative cues with growth and pro-inflammatory factors and MMPs, we hypothesized that eSF from women with PCOS may show aberrant P4-responsiveness during in vitro decidualization and concomitant altered production of pro-inflammatory cytokines and MMPs.
Materials and Methods
Study subjects and tissues
Endometrial tissue biopsies were obtained through the National Institute of Health (NIH)/University of California, San Francisco (UCSF), Human Endometrial Tissue and DNA Bank in accordance with the guidelines of the Declaration of Helsinki. Informed consent was obtained from all participants in the UCSF Center for Reproductive Health, and the study was approved by the UCSF Committee on Human Research. For the cytokine challenge, tissue samples were acquired from the Department of Obstetrics and Gynecology, University of Oulu, Finland. The tissue collection was approved by the hospital ethics committee and informed consent was obtained from all participants. Endometrial biopsies (Pipelle, Cooper Surgical, Shelton, CT) were collected, and eSF were isolated from 12 women with PCOS fulfilling both Rotterdam and NIH criteria and 11 control women (Table I). All PCOS subjects had normal 17-hydroxyprogesterone, prolactin and thyroid hormone levels. Control samples were obtained from healthy volunteers and women undergoing benign gynecological surgery. All controls reported menstrual cycles with regular interval (25–35 days) and no indication of having PCOS. Only one control subject was a smoker. None of the subjects was exposed to hormonal medications for at least 3 months prior to tissue sampling and were confirmed not pregnant during the time of study participation. The clinical summary of the study participants and tissue samples is shown in Table I.
Table I.
Clinical characteristics of the study subjects.
| ID | Group | Age | BMI | Histology | PCOS | Diagnosis | Procedure | Medication | Smoking | Ethnicity |
|---|---|---|---|---|---|---|---|---|---|---|
| C1a | Ctrl | 37 | 32.36 | PE | No | Undesired fertility | Pipelle, tubal ligation | None | No | Asian |
| C2a | Ctrl | 39 | 24.31 | PE | No | Undesired fertility | Pipelle, tubal ligation | None | No | Asian |
| C3a | Ctrl | 41 | 24 | SE | No | Ovarian cyst | Pipelle, cystectomy | None | No | Caucasian |
| C4a | Ctrl | 43 | 31.22 | PE | No | Menorrhagia, ut. fibroids, adenomyosis | Pipelle, hysterectomy | None | No | Black |
| C5a | Ctrl | 24 | 18.8 | SE | No | Volunteer | Pipelle | None | No | Asian |
| C6a | Ctrl | 32 | 24.9 | PE | No | Volunteer | Pipelle | None | No | Caucasian |
| C7b | Ctrl | 48 | 25.06 | PE | No | Fibroids, rectocele | Pipelle, hysterectomy, kolporafia posterior | None | No | Caucasian |
| C8b | Ctrl | 29 | 21.48 | SE | No | Cystocele, descensus uteri | Pipelle, hysterectomy, kolporafia anterior | None | No | Caucasian |
| C9b | Ctrl | 42 | 28.7 | PE | No | Abdominal pain | Pipelle, diagnostic laparoscopy | Omepratzol | Yes | Caucasian |
| C10b | Ctrl | 43 | 32 | SE | No | Rectocele | Pipelle, kolporafia posterior | Lamictal, melatonin, mirtazapin | No | Caucasian |
| C11b | Ctrl | 41 | 25.6 | PE | No | Volunteer | Pipelle | Seloken | No | Caucasian |
| dPC1a | PCOS | 29 | 41.15 | PE | HA, A, PCO | PCOS, reflux, sleep apnea, IGT | Pipelle | Metformin | No | Caucasian |
| dPC2a | PCOS | 23 | 18.21 | SE | Hirsutism, OA, PCO | PCOS | Pipelle | None | No | Middle eastern |
| dPC3a | PCOS | 40 | 39.2 | SE | HA, OA, PCO | PCOS | Pipelle | None | No | Caucasian |
| dPC4a | PCOS | 28 | 21.8 | Interval | HA, OA, PCO | PCOS | Pipelle | None | No | More than one |
| dPC5a | PCOS | 26 | 22.24 | SE | HA, acne, OA, PCO | PCOS | Pipelle | None | No | Caucasian |
| dPC6a | PCOS | 27 | 33.6 | PE | HA, hirsutism, OA, PCO | PCOS, IGT | Pipelle | Metformin | No | N/A |
| dPC7a | PCOS | 25 | 24.3 | PE | HA, acne, OA, PCO | PCOS | Pipelle | None | No | More than one |
| dPC8a | PCOS | 36 | 33 | PE | HA, OA, PCO | PCOS | Pipelle | Salbutamol inh. | No | Asian |
| ndPC1a | PCOS | 30 | 26.26 | PE | HA, hirsutism, acne, A, PCO | PCOS, Factor V Leiden heterozygote | Pipelle | None | No | Caucasian |
| ndPC2a | PCOS | 25 | 30.37 | PE | HA, acne, A, PCO | PCOS | Pipelle | None | No | Caucasian |
| ndPC3a | PCOS | 38 | 39.76 | PE | HA, hirsutism, acne, OA, PCO | PCOS, migrane, IGT | Pipelle | None | No | Black |
| ndPC4a | PCOS | 33 | 34.8 | N/A | HA, hirsutism, OA, PCO | PCOS | Pipelle | None | No | Hispanic |
PE, proliferative phase; S, secretory phase; HA, biochemical hyperandrogenism; OA, oligo-amenorrhea; A, amenorrhea; PCO, polycystic ovaries; IGT, impaired glucose tolerance in OGTT; N/A, not acquired.
aSamples used in the primary decidualization study.
bSamples used in cytokine challenge test.
Primary eSF cultures and decidualization experiments
eSF isolation and culture
Endometrial samples were digested with collagenase (6.4 mg/ml collagenase type I and 100 U/ml hyaluronidase in Hanks Buffered Salt Solution with Ca2+ and Mg2+) following filtration using an optimized protocol as previously described (Aghajanova et al., 2009; Chen et al., 2013). Stromal cell cultures we established as reported earlier with purity validated by vimentin and e-cadherin immunohistochemistry (Chen et al., 2013). Cells were cultured in growth medium (phenol red-free medium of 3:1 high-glucose Dulbecco's Modified Eagle's Medium [DMEM)/MCDB-105 (a fibroblast-lineage supplement medium containing trace elements and amino acids), 0.676 mM sodium pyruvate, 10% (v/v) charcoal-stripped fetal bovine serum (FBS), 1% (w/v) penicillin–streptomycin mix, 50 µg/ml gentamycin, 5 µg/ml insulin] which was renewed approximately every 2–3 days for up to the third passage and thereafter the cells were used in experiments.
Decidualization
One hundred thousand eSFs were plated into 12-well plates in duplicate and cultured in growth medium. When confluent, cells were incubated for 24 h in low-serum medium (3:1 high-glucose DMEM/MCDB-105, 0.75 mM sodium pyruvate, 50 µg/ml gentamycin, 2% FBS) prior to hormone treatment. For decidualization, cultures of eSFPCOS (n = 12) and eSFCtrl (n = 6) were treated with 0.1% ethanol vehicle (veh), estradiol (E2,10 nM) or E2P4 (10 nM/1 µM) in low-serum medium for 14 days, with feeding every other day as previously described (Aghajanova et al., 2009). At day 14 conditioned media were collected and stored at −80°C, and cells were trypsinized, counted (TC20 Automated Cell Counter, Bio-Rad, to adjust the cell counts for cytokine and MMP measurements) and stored at –80 °C for RNA isolation.
Morphological assessment
Cell morphology was assessed at every media change using inverted phase contrast light microscopy. At day 14, prior to cell harvesting all wells were imaged (Zeiss Thornwood, NY) and the morphological changes of eSF were estimated by two different observers. Decidualized eSF cultures were classified based on cell shape as decidualized (spindle-like) or non-decidualized (polygonal/cobblestone).
RNA isolation, cDNA synthesis and quantitative real-time polymerase chain reaction
Total RNA was isolated from cultured eSF (Nucleospin RNA purification kit, Machery Nagel, Bethlehem, PA) following the manufacturer's protocol, including DNAse treatment. Purity of all RNA samples was confirmed (Nanodrop, Wilmington, DE) and cDNA were synthesized utilizing the Taqman Preamp Master mix (Life Technologies, Grand Island, NY), utilizing a 14-cycle enrichment protocol, as previously described (Piltonen et al., 2013).
To determine the hormonally-regulated gene expression in eSF in different study groups, PGR (a measure of estrogen responsiveness) and IGFBP-1 (a measure of progesterone responsiveness) mRNA expression was measured using 20 ng cDNA, and 1 µM for each primer pair. Amplification was performed using the Stratagene MX3005P (Agilent, Santa Clara, CA) Thermocycler with variables previously described (Aghajanova et al., 2009). Dissociation curves for both target and housekeeping genes were utilized to ensure the absence of primer dimers and other non-specific amplification. Primers were designed by Fluidigm and optimized for quantitative real-time polymerase chain reaction (qRT–PCR) following the Fluidigm Biomark guidelines on mRNA amplification, including primer amplification efficiency, amplicon size and appropriate dissociation temperatures governing mRNA amplification. The amplification conditions were compliant with the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) (Johnson et al., 2014). All target gene expressions were normalized to YWHAZ, a highly conserved gene in PCOS endometrium (Sadek et al., 2012), coding the 14-3-3 signal transduction protein. No significant changes in YWHAZ expression were observed across dCtrl, dPCOS, ndPCOS, suggesting high stability in expression across treatment groups. The comparative (delta-delta) Ct method was used to measure relative gene expression (ABI User bulletin 2).
Protein measurements
ELISA
IGFBP-1 in conditioned media was measured using ELISA (Alpha Diagnostics, San Antonio, TX). Samples were assayed in duplicate and both standard curve and pre-measured IGFBP-1 recombinant protein controls were run in each experiment. Levels of IGFBP-1 for each sample were normalized to total cell count for each sample.
Luminex multiplex assays
A custom multiplex Luminex kit (EMD Millipore, Billerica, MA) was utilized to assay the quantity of cytokines and MMPs secreted into the conditioned media. Targeted cytokines included fractalkine (CX3CL1), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL), 8, 11, monocyte chemoattractant protein 1 (MCP-1; CCL2) and 3 (CCL7), and Regulated upon Activation, Normal T-cell Expressed, and Secreted Chemokine (c-c motif) ligand 5 (CCL5; RANTES). In addition, MMP 1, 2, 3, 7, 9, 10 and 12 were also measured. All protocols were based on the manufacturer's specifications and performed as previously described (Chen et al., 2013). All experiments were conducted in duplicate. To determine inter-assay variability, several samples were run on two or more kits and showed an inter-assay variability <5%.
Migration assays
Immune cells utilized for migration assays were isolated from buffy coats purchased from the Stanford Blood Bank (Palo Alto, CA). Buffy coats were processed by Ficoll-density gradient centrifugation to separate peripheral blood mononuclear cells from red blood cells, granulocytes and platelets. Monocytes were positively selected with CD14+ and T-cells with CD4+ specific microbeads (Miltenyi Biotec, Auburn, CA). Both CD14+ monocytes and CD14−/CD4+ T cells were allowed to equilibrate in Roswell Park Memorial Institute medium (RPMI) 1640 with 10% FBS overnight. Cells were then treated with serum-free media for an additional 12 h before initiation of migration experiments.
CD14+
CD14+ monocyte migration was conducted using the Millipore QCM 5 μm migration assay (EMD Millipore). Attached monocytes in culture were detached using Accutase (EMD Millipore), pelleted and resuspended in PBS with 1% BSA, and 105 cells were added to the upper migration chamber. Then, 500 μl of low-serum medium with E2P4 (same concentrations as utilized for the decidualization experiment) were added to the bottom chamber as negative controls. Standard curves were established with medium containing 2% FBS, a known chemoattractant for monocytes and T cells, and 6 h was determined as the equilibrium period during which maximal migratory activity occurred. Conditioned media pooled from eSF cultures (dCtrl, dPCOS and ndPCOS) treated with or without hormones were added to the lower chamber. Cells that migrated across the membrane and adhered to the opposite side were stained with the provided staining solution and counted. Four random non-overlapping fields at ×200 were counted and averaged. Experiments were conducted in triplicate and repeated twice.
CD4+
CD4+ T cell migration was conducted using the QCM 3 µm non-adherent migration assay (EMD Millipore), designed to measure cell migration of non-adherent cells, including T cells. As with monocyte experiments, cells were treated with low-serum media prior to the experiment. 105 cells were added to the upper migration chamber while E2P4-treated conditioned media from different eSF cultures (dCtrl, dPCOS and ndPCOS) were added to the lower chamber. After 6 h migrated cells were collected in the bottom chamber. The provided WST reagent was added to measure the metabolic activity of migrated T cells to provide a quantification of T-cell migration (Chen et al., 2014). After an additional 2 h for viability signal development, 50 μl of the WST medium containing cells were measured for optical density at 450 nm.
Cytokine challenge during decidualization
During the cytokine challenge 0.02, 0.2, 2 or 20 ng/ml IL-6 or 0.04, 0.4, 4, or 40 ng/ml of IL-8 or combination of 0.2 ng/ml IL-6 and 4.0 ng/ml IL-8 were added to E2P4 treatments during 14-d decidualization experiments in eSFCtrl, as described above. Morphology was assessed as in the primary decidualization study, and condition media were collected, stored, and the cells were counted in each well, as described above. WST assays were performed to confirm the non-toxicity of the cytokine concentrations added (data not shown).
Statistics
R-commander (2012) was used to determine differences in IGFBP-1 levels in conditioned media, using ANOVA with Tukey's posthoc analysis in both decidualization and cytokine challenge studies. To determine the effects of hormones and disease on patterns of cytokine and MMP secretion by eSF, data were analyzed using preconceived orthogonal contrasts with pairwise comparisons of specific experimental groups with the Statistical Analysis System software (SAS, 2011) (Chen et al., 2011). Pairwise contrasts included the effects of hormones (veh versus E2, veh versus E2P4 and E2 versus E2P4) and the effects of disease [veh or E2 or E2P4 for dCtrl versus respective treatment groups in PCOS (dPCOS and ndPCOS)]. Pairwise contrasts were also utilized to determine the effects of the conditioned media on inducing CD14+ monocyte and CD4+ T cell migration.
Results
Clinical characteristics
Participants in the ndPCOS group tended to be more obese than the dCtrl, although this did not reach statistical significance by ANOVA. The mean age across dCtrl, dPCOS and ndPCOS groups was comparable. In the dPCOS group, two patients were on Metformin therapy. These subjects were obese and were tested for impaired glucose tolerance in OGTT (Table I).
Impaired decidualization in eSFPCOS
Morphology
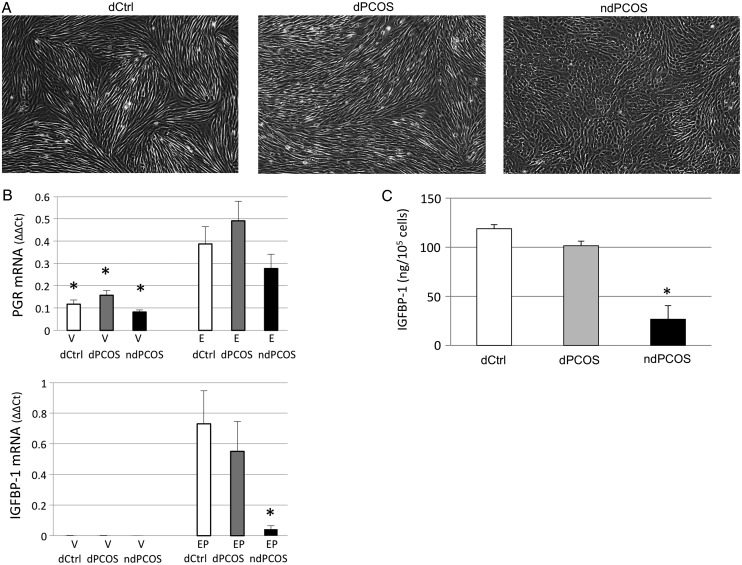
All cell cultures were monitored throughout the decidualization experiment for morphological changes, and the images were captured from all cultures and treatments at day 14 prior harvesting. According to the morphological assessment none of the vehicle and E2 cultures showed any sign of change in morphology at day 14 (Fig. 1A). However, in E2P4 treated wells all six control samples and eight PCOS samples showed distinct morphological changes from typical cobblestone eSF morphology into spindle-like cells beginning from day 10 (data shown at day 14, Fig. 1A). Alternatively, four PCOS samples showed no change in morphology suggesting compromised decidualization (Fig. 1A).
Figure 1.
(A) Morphological assessment of eSF after 14-days treatment with 10 nM estradiol + 1 µM progesterone (E2P4). eSF from controls (dCtrl) and 8/12 women with PCOS (dPCOS) decidualized but eSF from 4/12 women with PCOS did not (ndPCOS). (B) PGR and insulin-like growth factor binding protein 1 (IGFBP-1) mRNA expression after vehicle (V) or steroid hormone (10 nM E2 or E2P4) treatment. PGR expression was comparable between the study groups whereas ndPCOS presented with decreased IGFBP-1 mRNA expression. (C) IGFBP-1 response was also decreased at the protein level (ELISA) in ndPCOS compared with other study groups. Only cells treated with E2P4 are shown. No IGFBP-1 was detected after incubation with V or E2.
qRT–PCR analysis of hormone-responsive genes
To determine estradiol (E2) responsiveness of cells, PGR expression was measured during E2 treatment. The results showed significantly increased (3-fold, P < 0.05) expression of PGR transcripts in dCtrl, dPCOS and ndPCOS during E2 treatment compared with vehicle. Expression of PGR did not differ statistically significantly among the three groups, indicating that PCOS did not affect E2-induced PGR expression (although on average the relative expression of PGR was lower for ndPCOS in veh and E2P4 treated groups compared with dCtrl or dPCOS; F) (Fig. 1B). To validate the morphological findings and to confirm whether the decidualization process was successful or impaired, IGFBP-1 mRNA was also measured after vehicle or E2P4 treatment. The data showed that the IGFBP-1 mRNA expression in response to E2P4 in ndPCOS was significantly decreased (P < 0.05) compared with both dCtrl and dPCOS (Fig. 1B).
IGFBP-1
Decidualization was also assessed by measuring secreted IGFBP-1 protein (ELISA) into conditioned media. For vehicle and E2 treated cells no IGFBP-1 was detected, as expected (data not shown). In contrast, all six control samples treated with E2P4 showed high IGFBP-1 concentration (mean ± SD; 118.94 ± 4.12 ng/105 cells) validating the morphological findings (Fig. 1C). The IGFBP-1 concentration in E2P4 treated morphologically decidualized PCOS samples (dPCOS) was equivalent to the dCtrl samples (101.58 ± 4.65 ng/105 cells, P > 0.05) (Fig 1C). As expected, PCOS samples that showed aberrant decidualization morphology, also had concomitantly low IGFBP-1 secretion (26.67 ± 13.74 ng/105 cells, P < 0.05) compared with dCtrl and dPCOS, and the difference remained after correcting for cell counts (Fig. 1C).
Altered cytokine secretion in eSFPCOS
IL-6 and 8, 11, RANTES, MCP-1 and 3, GM-CSF and Fractalkine were measured in the conditioned media (Supplementary data, Table SI).
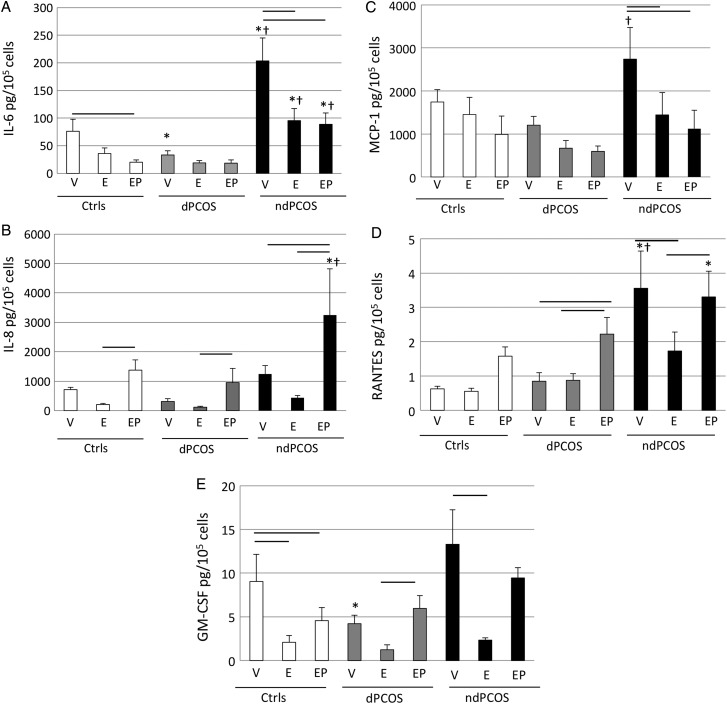
IL-6 and 8
The basal IL-6 secretion decreased in response to E2 and/or E2P4 administration (Fig. 2A and Supplementary data, Table SI) within dCtrl (E2P4, P = 0.03) and ndPCOS groups (E2 and E2P4, P < 0.001), but not in dPCOS. Interestingly, basal IL-6 secretion was lower in dPCOS compared with dCtrl (P = 0.03); whereas, basal IL-6 secretion was increased in ndPCOS versus dCtrl (P < 0.0001). Furthermore the steroid hormone response of IL-6 was blunted in ndPCOS as E2 and E2P4 treated cells presented with increased IL-6 secretion compared with dCtrl and dPCOS (E2 and E2P4, P < 0.01) (Fig. 2A, Supplementary data, Table SI). Basal IL-8 levels were comparable between the study groups; however, the E2P4 treated IL-8 levels were higher in ndPCOS than in dCtrl and dPCOS (P < 0.05, P < 0.01, Fig. 2B).
Figure 2.
Multiplex protein analysis (Luminex) of different cytokines (pg/105 cells) after 14-days treatment with vehicle (V) or 10 nM estradiol (E2), or 10 nM E2 plus 1 µM progesterone (P4). (A) Interleukin 6 (IL-6). (B) Interleukin 8 (IL-8). (C) Monocyte chemoattractant protein-1 (MCP-1). (D) Regulated on Activation, Normal T Cell Expressed (RANTES). (E) Granulocyte-macrophage colony-stimulating factor (GM-CSF). DCtrl, eSF from controls: dPCOS eSF from women with PCOS (8/12) that exhibit decidualization; ndPCOS, eSF from women with PCOS (4/12) that did not decidualize. The significant differences (P < 0.05) are marked as follows: − (line) between the different treatment groups within the study group (dCtrl or dPCOS or ndPCOS) and * (asterisk) compared with dCtrl and † (cross) compared with dPCOS in the same treatment group between different study groups.
MCP-1, RANTES and GM-CSF
Basal MCP-1 secretion was increased in ndPCOS versus dPCOS (P < 0.01, Fig. 2C, Supplementary data, Table SI) and a similar trend was observed compared with dCtrl (P = 0.06). Treatment with E2 and E2P4 decreased MCP-1 secretion in ndPCOS (P < 0.05, P < 0.01). A similar trend was also observed in dCtrl and dPCOS (Fig. 2C). Similar to IL-6, basal ndPCOS RANTES secretion was increased versus dCtrl and dPCOS (both P < 0.0001, Supplementary data, Table SI). In dPCOS and ndPCOS RANTES secretion was induced by P4 compared with basal and/or E2 treatment (P < 0.05), and a similar trend was also observed in the dCtrl group although the increase did not reach statistical significance (Fig. 2D). Moreover, the E2P4-treated ndPCOS samples had increased RANTES levels compared with dCtrl (P < 0.01) (Fig. 2D). Interestingly basal GM-CSF levels were decreased in dPCOS compared with dCtrl (Fig. 2E). E2 treatment decreased basal GM-CSF levels in dCtrl (P ≤ 0.01), but not in the PCOS groups. E2P2 treatment decreased GM-CSF levels in dCtrl compared with basal levels; however, this decrease was not observed in dPCOS and there was only a trend in ndPCOS. On the other hand the E2P4 treatment resulted in GM-CSF levels that were statistically comparable among the groups (Fig 2E).
No statistically significant differences were observed in IL-11, MCP-3 and fractalkine levels among the study groups (Supplementary data, Table SI).
Impaired decidualization results in high secreted MMPs by ndPCOS
Matrix metalloproteinase 1, 2, 3, 7, 9, 10 and 12 were measured in the condition media (Supplementary data, Table SII).
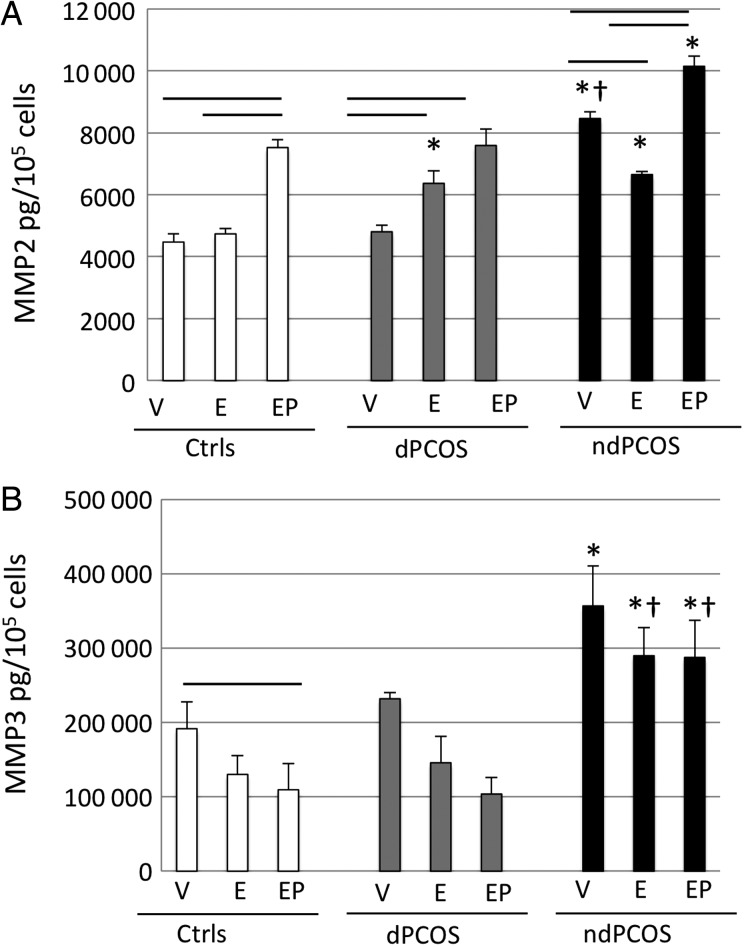
MMP2 and 3
Basal MMP2 levels were higher in ndPCOS compared with dCtrl and dPCOS (Fig. 3A). Interestingly, regulation of MMP2 by E2 differed in all three groups: E2 treatment did not alter, increased and decreased MMP2 levels in dCtrl, dPCOS, and ndPCOS, respectively (Fig. 3A). Overall, in E2-treated cells, MMP2 levels were increased in PCOS samples compared with dCtrl. E2P4 treatment increased MMP2 levels in all groups, and the levels were higher in the ndPCOS group versus dCtrl (P < 0.001, Fig. 3A). MMP3 levels did not differ between dCtrl and dPCOS. However, basal MMP3 levels were decreased by E2P4 treatment only in the dCtrl group; whereas, levels were increased in all treatment groups in ndPCOS versus dCtrl (Fig. 3B)
Figure 3.
(A) MMP2 and (B) MMP3 multiplex protein concentration (pg/105 cells) after 14-days treatment with vehicle (V) or 10 nM estradiol (E2), or 10 nM E2 plus 1 µM progesterone (E2P4). DCtrl, eSF from controls; dPCOS, eSF from women with PCOS (8/12) that exhibit decidualization; ndPCOS, eSF from women with PCOS (4/12) that did not decidualize. The significant differences (P < 0.05) are marked as follows: − (line) between the different treatment groups within the study group (dCtrl or dPCOS or ndPCOS) and * (asterisk) compared with dCtrl and † (cross) compared with dPCOS in the same treatment group between different study groups.
No statistically significant differences were found in secreted levels of MMP1, 7, 10 and 12 between the study or treatment groups. MMP9 was not detected.
Impaired decidualization promotes immune cell migration in PCOS
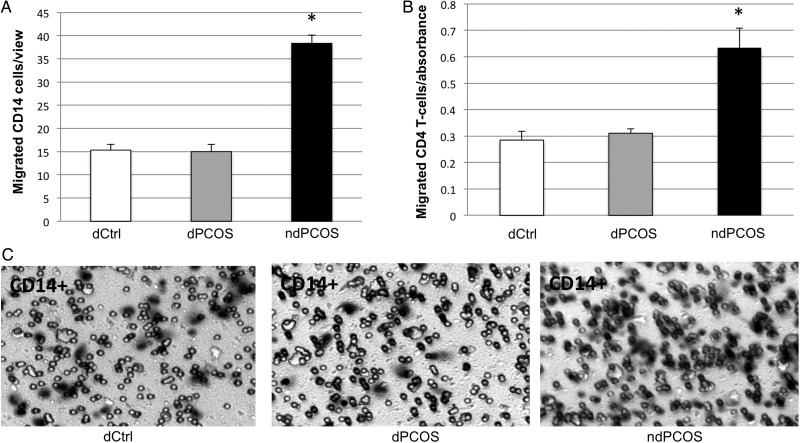
To test whether increased cytokines secreted by ndPCOS affected the migration potential of different immune cells, CD14+ monocytes and CD4+ T-cells were exposed to pooled dCtrl, dPCOS or ndPCOS condition media (Fig. 4). Both cell types showed increased migratory potential in a presence of ndPCOS media compared with the media of dCtrl or dPCOS, consistent with high chemotactic cytokine concentrations (IL-8, MCP-1 and RANTES) in ndPCOS.
Figure 4.
Migration response of (A) CD14+ monocytes and (B) CD4+ T-cells to conditioned media [14-d treatment with 10 nM estradiol (E2) + 1 µM progesterone (E2P4)]. DCtrl, eSF from controls; dPCOS, eSF from women with PCOS (8/12) that exhibit decidualization; ndPCOS, eSF from women with PCOS (4/12) that did not decidualize. (C) Microsocpic analysis of CD14+ monocyte migration in dCtr, dPCOS and ndPCOS.
IL-6 or IL-8 did not inhibit decidualization
As IL-6 and IL-8 levels were increased in ndPCOS samples during decidualization compared with dCtrl and dPCOS, we investigated whether IL-6 and/or 8 could inhibit decidualization. All control stromal cell samples showed typical decidualization morphology after 14-d E2P4 + cytokine challenge test regardless of concentration or cytokine(s) used. IGFBP-1 in condition media was assessed, and all samples had comparable IGFBP-1 concentrations to decidualizing control eSF (data not shown).
Discussion
To our knowledge, this is the first in vitro study showing impaired endometrial differentiation in response to steroid hormones, in women with PCOS. These novel data report decidualization failure in a subset of eSFs obtained from PCOS endometrium with concomitant altered cytokine and MMP profiles. We postulate that these abnormalities may contribute to endometrial pathologies common in PCOS women, i.e. subfertility, pre-eclampsia and endometrial cancer.
Steroid hormone response of eSFPCOS
The present data revealed a subpopulation of women with PCOS whose eSFs exhibited a blunted decidualization response to E2P4 treatment. Interestingly, the non-decidualizing phenotype was not related to any specific clinical characteristics of the patients. Although, all ndPCOS samples were from the proliferative phase and thus may reflect their hormonal (P4) response profile. The altered E2P4 response was also unrelated to PGR expression, as all study groups (dCtrl, dPCOS and ndPCOS) exhibited a robust increase in PGR gene expression in response to E2 even though expression of decidualization marker IGFBP-1 was compromised in PCOS samples. Thus, it is possible that blunted P4 response in eSFPCOS is rooted elsewhere. DNA methylation or microRNAs have been implicated in the governing of hormonal-responsive genes, including P4 mediated pathways (Lam et al., 2012; Houshdaran et al., 2014), and in this context warrant further investigation.
Decidualization and concomitant endometrial IGFBP-1 production from differentiating stromal cells are essential for embryo attachment, and thus decreased IGFBP-1 may compromise normal implantation as it regulates IGF II bioavailability at the feto-maternal interface and restricts trophoblast invasion (Giudice et al., 1993; Irwin et al., 1993; Irwin et al., 1999). In this study, the IGFBP-1 levels in dPCOS tended to be lower than in dCtrl, although this did not reach statistical significance. On the other hand, ndPCOS with failed decidualization, demonstrated morphologically as well as by low IGFBP-1 production, may explain implantation abnormalities in some women with PCOS (Gratton et al., 2002; Palomba et al., 2012). In addition to its role in embryo implantation, IGFBP-1 may play a crucial role in the pathogenesis of endometrial hyperplasia/cancer, known risks in women with PCOS, by inhibiting IGF-1 and E2-stimulated epithelial proliferation (Rutanen et al., 1988; Rajkumar et al., 1996).
Cytokines and chemokines secreted by eSFPCOS
Cytokines and chemokines are secreted by endometrial epithelial cells and stromal fibroblasts and by stationary and transient immune cells. They participate in the dialogue between maternal and embryonic tissues and also between epithelial and stromal compartments (Cha et al., 2012; Chen et al., 2013). During the window of implantation (WOI) and the decidualization process, cytokines including the glycoprotein 130 (gp130) family members IL-11, LIF, IL-6 facilitate implantation; whereas, the chemokines IL-8, MCP-1 and RANTES recruit leucocyte cohorts to the implantation site (Dimitriadis et al., 2005). Disturbances in cytokine and chemokine expression result in absolute or partial implantation failure and abnormal placental formation in mice and humans (Cha et al., 2012; Banerjee et al., 2013).
IL-6
IL-6 is a multifunctional cytokine with a wide range of biological activities. In addition to epithelium IL-6 is expressed in endometrial stromal cells and in decidua and has an important role coordinating placental morphogenesis and trophoblast invasion (Lockwood et al., 2008; Piltonen et al., 2013). IL-6-deficient mice have reduced fertility and fewer implantation sites and in vitro IL-6 decreases mice embryo attachment and growth (Jovanovic and Vicovac, 2009; Prins et al., 2012). Women experiencing recurrent miscarriage have decreased endometrial IL-6 levels, and IL-6 expression is elevated in decidua in women with pre-eclampsia (Demir et al., 2009; Prins et al., 2012). In this study eSF IL-6 secretion was decreased in response to E2 and E2P4 in dCtrl and ndPCOS groups, demonstrating that eSF that fail to decidualize can respond in other ways to steroid hormones. Interestingly, in dPCOS basal IL-6 levels were decreased compared with controls, and they did not decrease in response to steroid hormones, similar to other groups, which may indicate cytokine secretion aberrancy also in the decidualized PCOS samples. On the other hand, ndPCOS with blunted decidualization presented with increased basal IL-6 secretion and in response to steroid hormones, versus dCtrl and dPCOS. We have recently shown that PCOS women with hyperandrogenism and oligo/amenorrhea have increased IL-6 secretion in proliferative phase endometrium compared with controls (Piltonen et al., 2013). The data herein may imply that this inherent altered cytokine secretion persists later in secretory phase in the cycle, and if so, it may contribute to altered endometrial immune profile in these women (Piltonen et al., 2013). As eSF communicate with endometrial epithelium and endometrial leukocytes, aberrant production of IL-6 could result in altered paracrine signaling throughout the endometrium, resulting in disrupted signaling in luminal epithelium and affecting the crosstalk at the maternal–fetal interface.
IL-8
IL-8 is a potent chemoattractant of migrating immune cells and is involved in implantation as well as in the pathogenesis of endometrial cancer (Caballero-Campo et al., 2002; Ewington et al., 2012). In this study P4 increased IL-8 levels compared with E2 treatment, although in ndPCOS IL-8 levels were increased compared with dCtrl and dPCOS. The increase in IL-8 may partly explain the chemoattractant profile of ndPCOS media in the migration assays and may relate to imbalanced leukocyte migration that has been suggested to be related to implantation failure (Tuckerman et al., 2010; Ramos-Medina et al., 2013).
As both IL-6 and 8 were increased in E2P4 treated ndPCOS samples, we also tested whether they were able to inhibit decidualization in eSFCtrl. After treatment, all samples showed high IGFBP-1 levels and typical morphology for decidualization, suggesting that the high cytokine levels are an adverse outcome of altered cell function in PCOS rather than a cause for the aberrant decidualization per se.
MCP-1 and RANTES
Basal levels of other chemoattractants (MCP-1 and RANTES) were also increased in ndPCOS compared with dCtrl and dPCOS. MCP-1 and RANTES are associated with monocyte and activated T-cell chemoattraction during implantation, and their actions are tightly linked to the inflammatory cascade and NFκB activity (Zhao et al., 2002; Li et al., 2011). Interestingly, up-regulation of RANTES and MCP-1 and activation of NFκB and MAPK signaling in first trimester decidua have been associated with pre-eclampsia, a condition with increased prevalence in PCOS (Li et al., 2011). MCP-1 may also alter immune cell maturation. Previous studies have reported that Th1 cells (CD4 T-cells) are necessary during the implantation process to promote an immune-tolerant environment. High MCP-1 levels increase terminal differentiation of CD4T cells into Th2 helper cells, which theoretically should improve embryonic tolerance (He et al., 2012). Also, RANTES modulates the immune balance. In in vivo studies in mice the placental RANTES expression is tightly regulated by P4, and high levels lead to embryo resorption (Ramhorst et al., 2007). That E2P4 treatment stimulated high levels of RANTES secreted by ndPCOS compared with dCtrl and dPCOS may imply impaired inhibitory P4 action on RANTES regulation in ndPCOS. This finding is similar to that seen in endometriosis, a condition also presenting with P4-resistance and subfertility (Hornung et al., 2001; Wieser et al., 2005).
GM-CSF
GM-CSF is a chemoattractant for migratory dendritic cells (DCs) and macrophages in endometrium and also promotes their maturation (Robertson et al., 1999; Moldenhauer et al., 2010). Interestingly, GM-CSF-null mice have reproductive defects with increased prevalence of fetal loss (Robertson et al., 2000). That GM-CSF was down-regulated basally in dPCOS versus dCtrl may relate to poor endometrial receptivity and previous findings of altered DC migration in these women (Matteo et al., 2010). However, as the hormone response patterns were similar in all groups, even though GM-CSF levels in ndPCOS tended to be higher compared with other groups, the clinical significance remains uncertain and warrants additional functional studies in PCOS endometrium. Altogether, high chemoattractant cytokine secretion in ndPCOS is consistent with our in vitro findings that ndPCOS condition media possess high chemoattractant properties for migratory immune cells.
MMPs
MMPs comprise a family of zinc-containing endopeptidases that degrade extracellular matrix, crucial for tissue growth and expansion and blood vessel development (Visse and Nagase 2003). MMPs are generally inhibited by P4 (Henriet et al., 2002), and eSF-derived MMPs are up-regulated upon P4 withdrawal, facilitating tissue shedding (Irwin et al., 1996). Furthermore, epithelial MMP2 and 9 have been implicated in endometrial cancer pathogenesis (Karahan et al., 2007). During the implantation process, MMPs, secreted by the epithelium, stroma and trophoblast cells, participate in this complex process (Cha et al., 2012). Interestingly, endometrium in women with endometriosis has increased MMP2 and 3 expression, reflecting P4-resistance (Chung et al., 2002; Jana et al., 2013), as in this study. Basal MMP2 and 3 levels were increased in ndPCOS but also in response to E2 or E2P4 compared with dCtrl, consistent with a blunted response of P4 despite normal PGR expression and suggesting a post-receptor defect. Increased MMP action has also been linked to pre-eclampsia and implantation abnormalities, supporting the inflammatory data presented herein (Lockwood et al., 2014). Despite these compelling results, further studies with additional MMPs, as well as measurement of TIMPs warrant further investigation in PCOS endometrium to understand more fully how MMPs are involved in the PCOS-related endometrial abnormalities.
Limitations of this study
There are several limitations to our study. First, the sample size (especially in the ndPCOS group) was limited. The limitation of PCOS samples was partly due to the fact that only women fulfilling all Rotterdam criteria (and NIH criteria) (hyperandrogenism, oligo-amenorrhea, PCO) for PCOS were included. Furthermore, due to limited amount of samples, we had to include samples both from proliferative and secretory phase. However, we have previously demonstrated that by the third passage, the cycle phase from which eSF are derived does not influence their response to E2 or P4 (Aghajanova et al., 2009). Also, in this study there were no signs of in vivo E2 exposure (reflected in induced PGR gene expression) or E2P4 exposure (e.g. induced IGFBP-I protein and transcripts), and the patterns of secreted proteins were similar among samples obtained in different cycle phases. As all experiments were conducted beyond passage 3, we included samples from different cycle phases.
Another limitation is that not all subjects were on Metformin. As Metformin has anti-inflammatory properties (Morin-Papunen et al., 2003), it is possible that it could affect the cytokine response values. However, our statistical analysis indicated little variability of the dPCOS group average of cytokines with or without those two samples. Furthermore, after excluding these two samples no changes were observed with regard to the secreted proteins, PGR transcript expression (E2 sensitivity) or IGFBP-I transcript expression (E2P4 sensitivity), suggesting that using our end-points, the use of Metformin™ did not affect the readouts.
Other limitations include that our study focused on only one cell type even though previously, we have shown that freshly isolated endometrial epithelium of women with PCOS also exhibits a pro-inflammatory phenotype (Piltonen et al., 2013). Moreover, as our previous study showed inflammatory crosstalk between stromal cells and epithelial cells (Chen et al., 2013), future studies including several cell types should be undertaken. Lastly due to selection of the absolute anovulatory phenotype of PCOS patients in this study, this led to limited access of PCOS samples in the secretory phase (for the more common oligo-ovulatory PCOS phenotype), thus preventing more rigorous histological analysis under natural P4 action in vitro.
Conclusion
In conclusion, the study provides novel in vitro data showing that a subset of women with PCOS have an aberrant decidualization response of their eSF to E2 and P4, with concomitant increased pro-inflammatory cytokine, chemokine and MMP release—creating a microenvironment conducive to recruiting migratory immune cells. These data support the idea that the endometrium of women with PCOS may present a compromised endometrial environment for implantation and also abnormal endometrial function, resulting in sub-optimal implantation, and predisposition to endometrial cancer.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
T.T.P.: Original study design, conducted sample collection, sample preparation and experiments, data analysis and drafted the manuscript, workload equal to J.C.C. J.C.C.: Improved study design, conducted sample preparation and experiments, data analysis and drafted the manuscript, workload equal to T.T.P. M.Kh.: Conducted sample preparation and experiments, data analysis and helped drafting the manuscript. M.Ka.: Conducted sample collection and preparation and helped drafting the manuscript. A.L.: Conducted sample preparation and histology readings and helped improving the manuscript. T.S.: Sample collection. N.T.: Sample collection. H.H.: Anthropometric clinical data query, helped improving the manuscript. J.C.I.: Histology readings and helped improving the manuscript. L.C.G. Principal investigator, study design, improved the manuscript.
Funding
The Sigrid Juselius Foundation, Academy of Finland, Finnish Medical Foundation, Orion-Farmos Research Foundation (to T.T.P.), the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) U54HD 055764-07 Specialized Cooperative Centers Program in Reproduction and Infertility Research (to L.C.G.) and the NICHD the Ruth L. Kirschstein National Research Service Awards grant 1F32HD074423-03 (to J.C.C.)
Conflict of interest
None declared.
Supplementary Material
References
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis . Biol Reprod 2009;80:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Consensus on women's health aspects of polycystic ovary syndrome (PCOS) . Hum Reprod 2012;27:14–24.22147920 [Google Scholar]
- Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome . Biol Reprod 2002;66:297–304. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Jana SK, Pasricha P, Ghosh S, Chakravarty B, Chaudhury K. Proinflammatory cytokines induced altered expression of cyclooxygenase-2 gene results in unreceptive endometrium in women with idiopathic recurrent spontaneous miscarriage . Fertil Steril 2013;99:179–187. [DOI] [PubMed] [Google Scholar]
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis . Hum Reprod Update 2014;20:748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof P, Meisser A, Campana A, Tseng L. Effects of decidua-conditioned medium and insulin-like growth factor binding protein-1 on trophoblastic matrix metalloproteinases and their inhibitors . Placenta 1998;19:457–464. [DOI] [PubMed] [Google Scholar]
- Caballero-Campo P, Dominguez F, Coloma J, Meseguer M, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation . Mol Hum Reprod 2002;8:375–384. [DOI] [PubMed] [Google Scholar]
- Carvajal R, Rosas C, Kohan K, Gabler F, Vantman D, Romero C, Vega M. Metformin augments the levels of molecules that regulate the expression of the insulin-dependent glucose transporter GLUT4 in the endometria of hyperinsulinemic PCOS patients . Hum Reprod 2013;28:2235–2244. [DOI] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy . Nat Med 2012;18:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Frankshun AL, Wiley AA, Miller DJ, Welch KA, Ho TY, Bartol FF, Bagnell CA. Milk-borne lactocrine-acting factors affect gene expression patterns in the developing neonatal porcine uterus . Reproduction 2011;141:675–683. [DOI] [PubMed] [Google Scholar]
- Chen JC, Erikson DW, Piltonen TT, Meyer MR, Barragan F, McIntire RH, Tamaresis JS, Vo KC, Giudice LC, Irwin JC. Coculturing human endometrial epithelial cells and stromal fibroblasts alters cell-specific gene expression and cytokine production . Fertil Steril 2013;100:1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Johnson BA, Erikson DW, Piltonen TT, Barragan F, Chu S, Kohgadai N, Irwin JC, Greene WC, Giudice LC, et al. Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts . Hum Reprod 2014;29:1255–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HW, Lee JY, Moon HS, Hur SE, Park MH, Wen Y, Polan ML. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium . Fertil Steril 2002;78:787–795. [DOI] [PubMed] [Google Scholar]
- Cork BA, Li TC, Warren MA, Laird SM. Interleukin-11 (IL-11) in human endometrium: expression throughout the menstrual cycle and the effects of cytokines on endometrial IL-11 production in vitro . J Reprod Immunol 2001;50:3–17. [DOI] [PubMed] [Google Scholar]
- Demir B, Guven S, Guven ES, Atamer Y, Gul T. Serum IL-6 level may have role in the pathophysiology of unexplained infertility . Am J Reprod Immunol 2009;62:261–267. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation . Hum Reprod Update 2005;11:613–630. [DOI] [PubMed] [Google Scholar]
- Ewington L, Taylor A, Sriraksa R, Horimoto Y, Lam EW, El-Bahrawy MA. The expression of interleukin-8 and interleukin-8 receptors in endometrial carcinoma . Cytokine 2012;59:417–422. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Dsupin BA, Jin IH, Vu TH, Hoffman AR. Differential expression of messenger ribonucleic acids encoding insulin-like growth factors and their receptors in human uterine endometrium and decidua . J Clin Endocrinol Metab 1993;76:1115–1122. [DOI] [PubMed] [Google Scholar]
- Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion . Fertil Steril 2011;95:1278–1283. [DOI] [PubMed] [Google Scholar]
- Gratton RJ, Asano H, Han VK. The regional expression of insulin-like growth factor II (IGF-II) and insulin-like growth factor binding protein-1 (IGFBP-1) in the placentae of women with pre-eclampsia . Placenta 2002;23:303–310. [DOI] [PubMed] [Google Scholar]
- Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome . Hum Reprod 2012;27:1327–1331. [DOI] [PubMed] [Google Scholar]
- He YY, He XJ, Guo PF, Du MR, Shao J, Li MQ, Li DJ. The decidual stromal cells-secreted CCL2 induces and maintains decidual leukocytes into Th2 bias in human early pregnancy . Clin Immunol 2012;145:161–173. [DOI] [PubMed] [Google Scholar]
- Henriet P, Cornet PB, Lemoine P, Galant C, Singer CF, Courtoy PJ, Eeckhout Y, Marbaix E. Circulating ovarian steroids and endometrial matrix metalloproteinases (MMPs) . Ann N Y Acad Sci 2002;955:119–138; discussion 157–158, 396–406. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Kanzaki H, Nakayama H, Fujimoto M, Hatayama H, Kojima K, Iwai M, Mori T, Fujita J. Induction of tissue inhibitor of metalloproteinase 3 gene expression during in vitro decidualization of human endometrial stromal cells . Endocrinology 1995;136:4973–4981. [DOI] [PubMed] [Google Scholar]
- Hornung D, Bentzien F, Wallwiener D, Kiesel L, Taylor RN. Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid . Mol Hum Reprod 2001;7:163–168. [DOI] [PubMed] [Google Scholar]
- Houshdaran S, Zelenko Z, Irwin JC, Giudice LC. Human endometrial DNA methylome is cycle-dependent and is associated with gene expression regulation . Mol Endocrinol 2014;28:1118–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin JC, Giudice LC. Insulin-like growth factor binding protein-1 binds to placental cytotrophoblast alpha5beta1 integrin and inhibits cytotrophoblast invasion into decidualized endometrial stromal cultures . Growth Horm IGF Res 1998;8:21–31. [DOI] [PubMed] [Google Scholar]
- Irwin JC, de las Fuentes L, Dsupin BA, Giudice LC. Insulin-like growth factor regulation of human endometrial stromal cell function: coordinate effects on insulin-like growth factor binding protein-1, cell proliferation and prolactin secretion . Regul Pept 1993;48:165–177. [DOI] [PubMed] [Google Scholar]
- Irwin JC, Kirk D, Gwatkin RB, Navre M, Cannon P, Giudice LC. Human endometrial matrix metalloproteinase-2, a putative menstrual proteinase. Hormonal regulation in cultured stromal cells and messenger RNA expression during the menstrual cycle. J Clin Invest 1996;97:438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin JC, Suen LF, Martina NA, Mark SP, Giudice LC. Role of the IGF system in trophoblast invasion and pre-eclampsia . Hum Reprod 1999;14(Suppl 2):90–96. [DOI] [PubMed] [Google Scholar]
- Jana SK, Banerjee P, Mukherjee R, Chakravarty B, Chaudhury K. HOXA-11 mediated dysregulation of matrix remodeling during implantation window in women with endometriosis . J Assist Reprod Genet 2013;30:1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G, Nour AA, Nolan T, Huggett J, Bustin S. Minimum information necessary for quantitative real-time PCR experiments . Methods Mol Biol 2014;1160:5–17. [DOI] [PubMed] [Google Scholar]
- Jovanovic M, Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in HTR-8/SVneo cell line . Placenta 2009;30:320–328. [DOI] [PubMed] [Google Scholar]
- Karahan N, Guney M, Baspinar S, Oral B, Kapucuoglu N, Mungan T. Expression of gelatinase (MMP-2 and MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinoma . Eur J Gynaecol Oncol 2007;28:184–188. [PubMed] [Google Scholar]
- Kojima K, Kanzaki H, Iwai M, Hatayama H, Fujimoto M, Inoue T, Horie K, Nakayama H, Fujita J, Mori T. Expression of leukemia inhibitory factor in human endometrium and placenta . Biol Reprod 1994;50:882–887. [DOI] [PubMed] [Google Scholar]
- Lam EW, Shah K, Brosens JJ. The diversity of sex steroid action: the role of micro-RNAs and FOXO transcription factors in cycling endometrium and cancer . J Endocrinol 2012;212:13–25. [DOI] [PubMed] [Google Scholar]
- Li M, Wu ZM, Yang H, Huang SJ. NFkappaB and JNK/MAPK activation mediates the production of major macrophage- or dendritic cell-recruiting chemokine in human first trimester decidual cells in response to proinflammatory stimuli . J Clin Endocrinol Metab 2011;96:2502–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocrinol 2007;269:85–92. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Yen CF, Basar M, Kayisli UA, Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G, Schatz F. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells . Am J Pathol 2008;172:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Basar M, Kayisli UA, Guzeloglu-Kayisli O, Murk W, Wang J, De Paz N, Shapiro JP, Masch RJ, Semerci N, et al. Interferon-gamma protects first-trimester decidual cells against aberrant matrix metalloproteinases 1, 3, and 9 expression in preeclampsia . Am J Pathol 2014;184:2549–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteo M, Serviddio G, Massenzio F, Scillitani G, Castellana L, Picca G, Sanguedolce F, Cignarelli M, Altomare E, Bufo P, et al. Reduced percentage of natural killer cells associated with impaired cytokine network in the secretory endometrium of infertile women with polycystic ovary syndrome . Fertil Steril 2010;94:2222–2227, 2227.e1–3. [DOI] [PubMed] [Google Scholar]
- Moldenhauer LM, Keenihan SN, Hayball JD, Robertson SA. GM-CSF is an essential regulator of T cell activation competence in uterine dendritic cells during early pregnancy in mice . J Immunol 2010;185:7085–7096. [DOI] [PubMed] [Google Scholar]
- Morin-Papunen L, Rautio K, Ruokonen A, Hedberg P, Puukka M, Tapanainen JS. Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome . J Clin Endocrinol Metab 2003;88:4649–4654. [DOI] [PubMed] [Google Scholar]
- Naver KV, Grinsted J, Larsen SO, Hedley PL, Jorgensen FS, Christiansen M, Nilas L. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia . BJOG 2014;121:575–581. [DOI] [PubMed] [Google Scholar]
- Palomba S, Russo T, Falbo A, Di Cello A, Amendola G, Mazza R, Tolino A, Zullo F, Tucci L, La Sala GB. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case–control study . J Clin Endocrinol Metab 2012;97:2441–2449. [DOI] [PubMed] [Google Scholar]
- Piltonen TT, Chen J, Erikson DW, Spitzer TL, Barragan F, Rabban JT, Huddleston H, Irwin JC, Giudice LC. Mesenchymal stem/progenitors and other endometrial cell types from women with polycystic ovary syndrome (PCOS) display inflammatory and oncogenic potential . J Clin Endocrinol Metab 2013;98:3765–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders . J Reprod Immunol 2012;95:1–14. [DOI] [PubMed] [Google Scholar]
- Rajkumar K, Dheen ST, Murphy LJ. Hyperglycemia and impaired glucose tolerance in IGF binding protein-1 transgenic mice . Am J Physiol 1996;270:E565–E571. [DOI] [PubMed] [Google Scholar]
- Ramhorst R, Gutierrez G, Corigliano A, Junovich G, Fainboim L. Implication of RANTES in the modulation of alloimmune response by progesterone during pregnancy . Am J Reprod Immunol 2007;57:147–152. [DOI] [PubMed] [Google Scholar]
- Ramos-Medina R, Garcia-Segovia A, Leon JA, Alonso B, Tejera-Alhambra M, Gil J, Caputo JD, Seyfferth A, Aguaron A, Vicente A, et al. New decision-tree model for defining the risk of reproductive failure . Am J Reprod Immunol 2013;70:59–68. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Roberts CT, Farr KL, Dunn AR, Seamark RF. Fertility impairment in granulocyte-macrophage colony-stimulating factor-deficient mice . Biol Reprod 1999;60:251–261. [DOI] [PubMed] [Google Scholar]
- Robertson SA, O'Connell AC, Hudson SN, Seamark RF. Granulocyte-macrophage colony-stimulating factor (GM-CSF) targets myeloid leukocytes in the uterus during the post-mating inflammatory response in mice . J Reprod Immunol 2000;46:131–154. [DOI] [PubMed] [Google Scholar]
- Rutanen EM, Pekonen F, Makinen T. Soluble 34 K binding protein inhibits the binding of insulin-like growth factor I to its cell receptors in human secretory phase endometrium: evidence for autocrine/paracrine regulation of growth factor action . J Clin Endocrinol Metab 1988;66:173–180. [DOI] [PubMed] [Google Scholar]
- Sadek KH, Cagampang FR, Bruce KD, Shreeve N, Macklon N, Cheong Y. Variation in stability of housekeeping genes in endometrium of healthy and polycystic ovarian syndrome women . Hum Reprod 2012;27:251–256. [DOI] [PubMed] [Google Scholar]
- Savaris RF, Groll JM, Young SL, DeMayo FJ, Jeong JW, Hamilton AE, Giudice LC, Lessey BA. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles . J Clin Endocrinol Metab 2011;96:1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staun-Ram E, Shalev E. Human trophoblast function during the implantation process . Reprod Biol Endocrinol 2005;3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckerman E, Mariee N, Prakash A, Li TC, Laird S. Uterine natural killer cells in peri-implantation endometrium from women with repeated implantation failure after IVF . J Reprod Immunol 2010;87:60–66. [DOI] [PubMed] [Google Scholar]
- Villavicencio A, Bacallao K, Avellaira C, Gabler F, Fuentes A, Vega M. Androgen and estrogen receptors and co-regulators levels in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia . Gynecol Oncol 2006;103:307–314. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry . Circ Res 2003;92:827–839. [DOI] [PubMed] [Google Scholar]
- Wieser F, Vigne JL, Ryan I, Hornung D, Djalali S, Taylor RN. Sulindac suppresses nuclear factor-kappaB activation and RANTES gene and protein expression in endometrial stromal cells from women with endometriosis . J Clin Endocrinol Metab 2005;90:6441–6447. [DOI] [PubMed] [Google Scholar]
- Wissink S, van Heerde EC, vand der Burg B, van der Saag PT. A dual mechanism mediates repression of NF-kappaB activity by glucocorticoids . Mol Endocrinol 1998;12:355–363. [DOI] [PubMed] [Google Scholar]
- Zhao D, Lebovic DI, Taylor RN. Long-term progestin treatment inhibits RANTES (regulated on activation, normal T cell expressed and secreted) gene expression in human endometrial stromal cells . J Clin Endocrinol Metab 2002;87:2514–2519. [DOI] [PubMed] [Google Scholar]
- Zhu H, Hou CC, Luo LF, Hu YJ, Yang WX. Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions . Gene 2014;551:1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.