Abstract
Serotonin (5-hydroxytraptamin, 5-HT) has been implicated to play critical roles in early neural development. Recent reports have suggested that perinatal exposure to selective serotonin reuptake inhibitors (SSRIs) resulted in cortical network miswiring, abnormal social behavior, callosal myelin malformation, as well as oligodendrocytes (OL) pathology in rats. To gain further insight into the cellular and molecular mechanisms underlying SSRIs-induced OL and myelin abnormalities, we investigated the effect of 5-HT exposure on OL development, cell death, and myelination in cell culture models. First, we showed that 5-HT receptor 1A and 2A subtypes were expressed in OL lineages, using immunocytochemistry, Western blot, as well as intracellular Ca2+ measurement. We then assessed the effect of serotonin exposure on the lineage development, expression of myelin proteins, cell death, and myelination, in purified OL and neuron-OL myelination cultures. For pure OL cultures, our results showed that 5-HT exposure led to disturbance of OL development, as indicated by aberrant process outgrowth and reduced myelin proteins expression. At higher doses, such exposure triggered a development-dependent cell death, as immature OLs exhibited increasing susceptibility to 5-HT treatment compared to OL progenitor cells (OPCs). We showed further that 5-HT-induced immature OL death was mediated at least partially via 5-HT2A receptor, since cell death could be mimicked by 5-HT2A receptor agonist DOI, but attenuated by pre-treatment with 5-HT2A receptor antagonist ritanserin. Utilizing a neuron-OL myelination co-culture model, our data showed that 5-HT exposure significantly reduced the number of myelinated internodes. In contrast to cell injury observed in pure OL cultures, 5-HT exposure did not lead to OL death or reduced OL density in neuron-OL co-cultures. However, abnormal patterns of contactin-associated protein (Caspr) clustering were observed at the sites of Node of Ranvier, suggesting that 5-HT exposure may affect other axon-derived factors for myelination. In summary, this is the first study to demonstrate that manipulation of serotonin levels affects OL development and myelination, which may contribute to altered neural connectivity noted in SSRIs-treated animals.
Keywords: serotonin, oligodendrocyte, myelination, rat, differentiation, apoptosis
Introduction
The role of serotonin (5-hydrotryptamine, 5-HT) as a major neuromodulator in the adult central nervous system (CNS) has been well established (Barnes and Sharp 1999). During development, however, serotonin plays a critical role in early brain patterning. For example, it has been shown that serotonin is involved in neuronal differentiation and migration, axonal growth and pathfinder, dendritic arborization, synaptogenesis, circuit formation, and neuronal plasticity (Daubert and Condron, 2010). One of the well-replicated observations is that during early embryonic period, there is a transient uptake of serotonin by primary thalamic sensory neurons from their cortical target areas. In particular, for example, mice with either serotonin transporter (Sert) (Esaki et al. 2005) or monoamine oxidase A gene deficiency (Cases et al. 1996) show disrupted primary somatosensory cortical organization. Similarly, manipulation of serotonin levels in rodents with serotonin reuptake inhibitors (SSRIs) (Xu et al. 2004), or serotonin depletion drug PCPA (Persico, et al. 2000) leads to disorganized cortical barrel fields.
Interestingly, we have previously reported that early life exposure to citalopram (one of the most selective serotonin reuptake inhibitors, SSRIs) disrupts not only cortical network wiring but also leads to abnormal neurobehaviors in rats (Simpson et al. 2011). Additional aberrant outcomes are the alterations of oligodendrocytes (OLs) and myelin formation (Simpson et al. 2011). Therefore, we hypothesize that manipulating serotonin levels may disrupt OL development and/or myelination, which might be a contributing factor for neurobehavioral abnormalities observed in SSRI-treated rats. This hypothesis was tested in the current study utilizing two cell culture models. Since very little, if any information, is currently available regarding whether 5-HT receptors are expressed by OL lineage cells, additional experiments have been conducted to investigate the expression of certain 5-HT receptors in OL lineages.
Materials and Methods
The sources of reagents are listed below. Dulbecco’s modified Eagle Medium (DMEM)/Ham’s F12 and F15 medium, Insulin-Transferrin-Selenium, 7.5% bovine serum albumin, platelet derived growth factor (PDGF)-AA, basic fibroblast growth factor (bFGF), penicillin/streptomycin, 2.5% trypsin, neurobasal medium (NBM) and B27 supplement: Invitrogen (Carlsbad, CA). Ciliary neurotrophin factor (CNTF), progesterone, triiodothyronine, biotin, putrescine, cysteine, hydrocortisone, and 5-HT2 receptor agonist 1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride, (±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride (DOI), 5-HT2A receptor antagonist, ritanserin: Sigma (St. Louis, MO). Terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling (TUNEL) kit: Millipore (Billerica, MA). Cell Death ELISA kit: Roche (Indianapolis, IN). Antibodies used in this study are listed in table 1.
Table 1.
Summary of antibodies used in this study
| Antibodies | Source | Host | Target(s) of labeling | Applications |
|---|---|---|---|---|
|
| ||||
| Myelin basic protein (MBP) | Millipore | Ms | myelin; mature OL | IC/WB |
| Neural/Glial antigen 2 (NG2) | Millipore | Rb | early OL progenitor | IC |
| OL marker O4 | Millipore | Ms | late OL progenitor | IC |
| OL marker O1 | Millipore | Ms | Mature OL | IC |
| OL transcription factor 2 (Olig2) | Millipore | Rb | Total OL lineage | IC/WB |
| 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) | Millipore | Ms | Mature OL and myelin | IC/WB |
| Anti-Oligodendrocyte (Rip) | Millipore | Ms | Immature and Mature OL | IC |
| Myelin proteolipid protein (PLP) | Millipore | Ms | Mature OL and myelin | WB |
| CD11b (OX-42) | Millipore | Ms | microglia/macrophage | IC |
| Glial fibrillary acidic protein (GFAP) | Millipore | Ms | astrocyte | IC |
| Contactin-associated protein (Caspr) | Millipore | Ms | paranodal domain | IC |
| Phosphorylated neurofilament H (pNF) | Abcam | Rb | mature axon | IC |
| 5HT1AR | Abcam | Rb | 5HT 1A receptor | IC |
| 5HT1AR | Santa Cruz | Rb | 5HT 1A receptor | WB |
| 5HT2AR | Abcam | Rb | 5HT 2A receptor | IC |
| 5HT2AR | Calbiochem | Rb | 5HT 2A receptor | WB |
| Caspase-3 (cleaved) | Cell Signaling | Rb | Active form of caspase-3 | IC |
Ms: mouse; Rb: rabbit; IC: immunocytochemistry; WB: Western blot.
Animals
Time-pregnant Sprague-Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN). Animals arrived in the laboratory on day 13 (for neuron-OL co-culture) or day 19 (for OL culture) of gestation. For OL cultures using postnatal rat pups, the day of delivery was defined as postnatal day 0 (P0). All procedures for animal care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Primary OL culture
OL progenitor cells (OPCs) were prepared from P1 rat forebrain, as described previously (Pang et al. 2005). Briefly, forebrain tissue was dissociated with 0.0625% trypsin/0.02% EDTA. The dissociated cells were cultured in 25 cm2 uncoated Falcon flasks at 37 °C under 5% CO2 in a humidified incubator. Cells were maintained in a chemically defined medium (CDM) formulated with DMEM/F12 containing 0.1% BSA, 100 μM putrescine, 20 nM progesterone, 10 nM sodium selenium, 20 nM biotin, 5 μg/ml cysteine, 5 nM hydrocortisone, 5 μM insulin, 50 μM transferrin, 2 nM L-glutamine, and penicillin/streptomycin, supplemented with platelet derived growth factor-AA (PDGF-AA) and basic fibroblast growth factor (bFGF) (final concentration 10 ng/ml, each). OPCs were sub-cultured and transferred to poly-L-lysine-coated flasks, and fed with fresh medium and growth factors. For identification of glial cell phenotype, OPCs were seeded onto poly-L-Lysine-coated coverslips and immunostained with antibodies for OL lineage (NG2, O4, O1 and MBP), or antibodies for astrocytes (GFAP) and microglia (OX-42). In the presence of PDGF-AA/bFGF and sub-cultured for 3 passages, more than 95.4% of the cells were positive for NG2 but negative for GFAP, OX-42 and MBP, indicating that the predominant cell population was pure OPCs, while astrocytes, microglia and mature OLs were minimal.
We then enriched stage-specific developing OLs, according to a protocol described previously (Pang, et al. 2005). Based on distinct morphology and expression of stage-specific lineage markers, OL lineage is roughly divided into 4 developmental stages: OPC, pro-OL, immature OL and mature OL. In brief, OPCs were identified as NG2+/O4+ cells when higher concentration of PDGFaa plus bFGF (10 ng/ml, each) was supplemented in the medium. Pro-OLs were identified as NG2−/O4+/O1− cells when OPCs were maintained in the differential medium (CDM with 20 ng/ml of thyroxine and triiodothyronine, 10 ng/ml of CNTF, 0.5ng/ml of PDGF-AA and bFGF) for 1 wk. Immature OLs were identified as O4+/O1+/MBP− cells, which were differentiated from OPCs in CDM supplemented with 10 ng/ml of CNTF, 50 ng/ml of IGF-1, 10 ng/ml NT-3, but without PDGF/bFGF for 5 days. Finally, mature OLs were identified as O4-/MBP+ cells after 5 days in the differentiating medium without any growth factors. Typically, the predominant OL lineage cells at a specific developmental stages were as: 95% of NG2+/O4− cells in OPC cultures, 92% of O4+/O1− cells in pro-OL cultures, 85% of O4+/O1+ cells in immature OL cultures, and 89% of MBP+ cells in mature OL cultures.
Assessment of 5-HT1A and 2A receptors on OL lineage cells
Expression of 5-HT1A and 2A receptors in OL lineage cells was initially assessed by immunofluorescence staining. Cells were seeded onto poly-L-lysine-coated glass coverslips at a density of 2×104 per coverslip. Stage-specific OLs were fixed with 1% paraformaldehyde (PFA) for 10 min at RT, blocked with 10% normal goat serum, and incubated with antibodies against 5-HT1A (1:400) or 2A (1:300) receptor in combination with antibodies against OL lineage markers including NG2 (1:200), O4 (1:400), Rip (1:500), and MBP (1:200), which are sequentially expressed by OL lineages following maturation. Following washing, the coverslips were incubated with Alex fluo-488 or 555 conjugated second antibodies. Nuclei were counter-stained with DAPI.
Next, we verified the specificity of the immunostaining by Western blot. OLs at three developing stages (OPCs, pro-OLs, and immature OLs) were lysed in the lysis buffer (50mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM MgCL2, 1 mM EGTA, 1% Triton X-100 and 10% glycerol) supplemented with protease inhibitors. After centrifugation at 14,000g for 10 min, the supernatant was collected, and total proteins were determined by a BCA Protein Assay Kit (Pierce, IL). Samples were denatured and subjected to 12% SDS-PAGE, and proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat milk/1% BSA in PBS for 2 h at RT, and incubated with the primary antibodies against 5-HT1A and 2A receptor (both at 1:1000) overnight at 4°C. Following washing, membranes were incubated with horseradish peroxidase-conjugated second antibody, and signals were detected using the ECL-prime system (GE healthcare).
Finally, changes of intracellular Ca2+ were measured in immature OLs following 5-HT exposure. OPCs were seeded on poly-L-lysine-coated 24-well plates at a density of 2×104/well, and were differentiated into immature OLs. Intracellular Ca2+ levels were measured dynamically within 16 min using a fluorescence Calcium AM kit (Invitrogen). (RS)-2-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA, used at 1 μM), which is known to increase intracellular Ca2+ levels via Ca2+ channels, was used as a positive control. The optical density (OD) was acquired using a fluorescence multiplate reader (Synergy HT, Biotech) at 492/520 nm.
Assessment of OL development following 5-HT exposure
Next, the effect of 5-HT exposure on OL development was assessed by immunofluorescence. OPCs or immature OLs were treated in the differentiating medium with 5-HT (10 and 100 μM) for 5 consecutive days. Medium containing freshly prepared 5-HT was changed every 48 h. At the end of the treatment, cells were washed with PBS and fixed in 1% PFA. Standard immunocytochemistry was performed with the primary antibodies diluted as following: NG2 (1:200), O4 (1:400), O1 (1:400), Rip (1:500), GalC (1:200) and MBP (1:200). The combination of these markers was aimed to assess cell maturation status as well as morphological characteristics, especially the outgrowth of cell processes.
Immunostaining was visualized using biotin-labeled secondary antibody followed by Alex488- or 555-conjugated streptavidin. Nuclei were counter-stained with either propidium iodide (PI) or DAPI, depending on which type of fluorescence being used for visualization of antigen of interest. Coverslips were viewed under a fluorescence microscope (Olympus BX60), and images were acquired by a monochrome digital camera. To assess the effect of 5-HT on cell morphology, images of Rip immunostained individual cells were analyzed utilizing the Image J software (NIH). The areas occupied by the entire cell surface (the soma and processes, excluding the nucleus) of a single cell were calculated. A total of 50 cells for each condition from three independent experiments, were included for the final data analysis.
Detection of cell death
Cell death was assessed and compared between OPCs and immature OLs following 5-HT exposure. Cells were treated with 5-HT (1, 5, 25, and 100 μM) for 48 h, fixed by 1% PFA, and processed for TUNEL staining, per the manufacture’s instruction. TUNEL+ cells were stained with FITC while nuclei were counter-stained with PI. The number of TUNEL+ cells was counted and the results were presented as a percentage of TUNEL+ to total cells (counter-stained with PI). For each coverslip, cells in 10 randomly selected 40× objective fields were counted and averaged. The final data were from triplicate coverslips per condition of 3 independent experiments. To quantify dose-dependent cell death by DOI exposure (in OPCs and immature OLs, treated for 48 h), a Cell Death ELISA was used, and the results were presented as a percentage over the control. Finally, to test whether 5HT2A receptor is involved in 5-HT-induced cell death, immature OLs were pre-treated with the 5-HT2A receptor antagonist ritanserin (1 μM) for 60 min, followed by exposure to 5-HT (1, 5, 25, 100 μM) for 48 h. Cleaved caspase-3 was detected by immunoblotting.
Quantification of myelin proteins by immunoblotting
Expression of myelin proteins was determined by Immunoblotting. Immature OLs were treated with 5-HT (1~100 μM) for 5 days. Medium (with fresh made 5-HT) was changed every 48 h. At the end of treatment, cells were washed twice with ice-cold PBS, and collected by centrifugation at 4°C. Cell pellets were lysed, denatured, transferred to nitrocellulose membranes, and then blotted against the primary antibodies (MBP at 1:400, PLP at 1:400, CNPase at 1:1000, and Olig2 at 1:200). The membranes were stripped and re-probed for α-tubulin (1:20000). Images were acquired by the ChemiDoc MP Imaging system (Bio-Rad).
Myelination co-culture, serotonin treatment, and myelin quantification
The neuron-OL myelination culture was prepared from E16 rat spinal cord, as described previously (Pang Y, et al. 2012). Briefly, spinal cord tissue was collected, washed in 1 ml of 1×HBSS (without Ca2+ and Mg2+), and dissociated with Trypsin-EDTA for 15 min at 37°C. The enzymatic reaction was stopped by addition of 1.5 ml trypsin inhibitor-DNase I solution and centrifuged at 800g for 5 min. The supernatant was replaced with 5 ml plating medium (50% normal horse serum and 20% 1× HBSS with Ca2+/Mg2+ in DMEM) and tissue was titrated with a 1 ml pipet tips for 10 times. The dissociated cell suspension was then passed through a 40 μm cell strainer. Cells were then seeded on poly-L-lysine-coated coverslips, at a density of 0.4×105/cm2. After 2 h adhesion, the plating medium was replaced with myelination medium (N2 mixed with NBM-B27 at 1:1). NGF (50 ng/ml) and NT-3 (10 ng/ml) were included in the medium for the first week. The medium was changed every three days by replacing 2/3 of the medium with fresh medium. The day of the primary culture was defined as 1 day in vitro (DIV). At DIV10, the culture medium was replaced with a further modified medium mixed with insulin-free N2 and NBM-B27 (at a ratio of 4:1) to prevent cell overgrowth. The final concentrations of individual component in N2 medium (DMEM-F12 based, high glucose, Invitrogen) are listed as following: insulin (10 μg/ml), transferrin (50 μg/ml), sodium selenite (5.2 ng/ml), hydrocortisone (18 ng/ml), putrescine (16 μg/ml), progesterone (6.3 ng/ml), biotin (10 ng/ml), N-acetyl-L-cysteine (5 μg/ml), BSA (0.1%), and penicillin-streptomycin (50 units/ml).
The co-cultures were exposed to 5-HT (10 and 100 μM) starting at DIV10 (just before the onset of myelination in this system which typically starts at DIV13) and were continuously exposed until DIV40 (when abundant myelinated internodes were observed) (Pang Y, et al. 2012). During this relatively long period of exposure, medium containing freshly prepared 5-HT was changed every 3 days. At DIV40, cells were fixed with 1% PFA, permeabilized with 0.2% triton-X 100, and processed for MBP/pNF, or MBP/Caspr double-immunofluorescence staining. Myelination was quantified by Image J software (NIH) as described previously (Pang Y, et al. 2012). Briefly, images of 10 randomly selected 40× objective fields were acquired by a digital camera for each coverslip. The areas of myelinated internodes co-labeled with MBP and pNF (superimposed from green and red fluorescence channel) as well as that of total pNF+ axons (red fluorescence channel) were measured to calculate the myelination index, which was defined as: [areas of myelinated (MBP+/pNF+) axons/total axons (pNF+)]%. The myelination index for each coverslip was an average of 10 individual images, and the final data were from triplicated coverslips per condition of 3 independent experiments.
Statistics
Unless otherwise specified, all data were from three independent experiments. Data were analyzed using SigmaPlot software (version 11.0). One-way ANOVA followed by post-hoc Tukey analysis was performed to compare differences among multiple treatments, and p value <0.05 was considered statistically significant.
Results
1. Expression of 5-HT 1A and 2A receptors in OL lineage
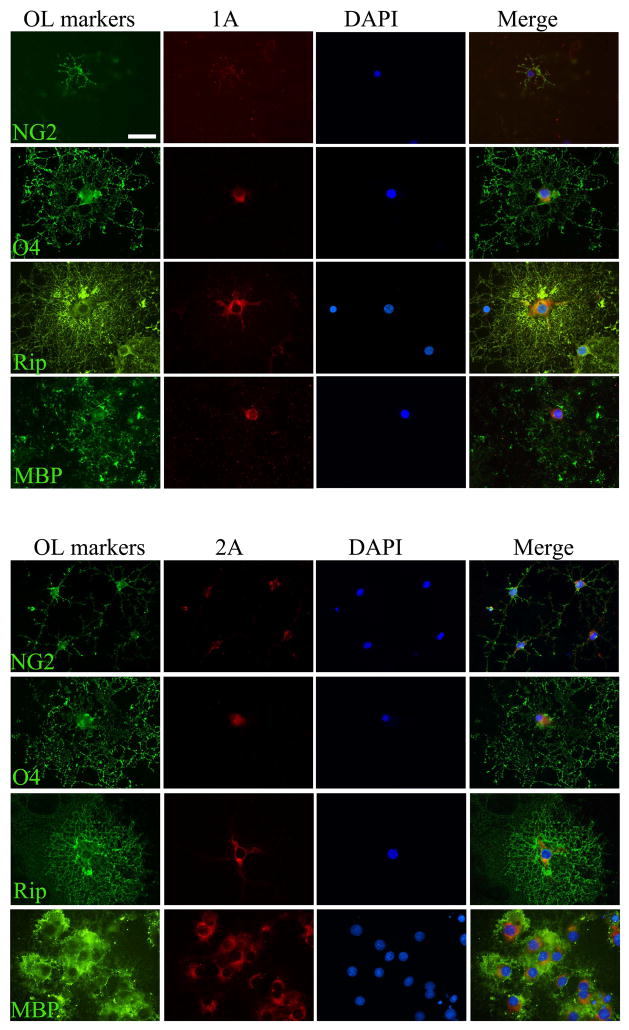
We initially assessed expression patterns of 5-HT1A and 2A receptors in OL lineages by immunostaining. As shown in Fig. 1, 5-HT1A (upper panel) and 2A (lower panel) receptors were detected throughout all developmental stages, indicated by their co-localization with developmental OL markers. The immunoreactivity of both 1A and 2A receptor tended to increase following OL differentiation (weaker staining detected in NG2+ cells, while stronger staining detected in MBP+ cells).
Fig. 1.
Detection of 5-HT1A and 2A receptors in OL lineage cells by immunofluorescence staining. 5-HT1A receptor (upper panel) and 5-HT2A receptor (lower panel) were double-immunolabeled with OLs lineage markers, which are sequentially expressed by OLs following differentiation. Both 5-HT1A and 2A receptors were detected throughout OL lineages; however, the immunoreactivity of both receptor subtypes tended to increase following cell maturation, with the higher levels observed in Rip+ and MBP+ cells. Scale bars: 50 μm.
Next, we validated the specificity of the immunostaining by Western blot. As shown in Fig 2A, the bands with strong signal at predicted molecular sizes (~46 kDa for 5HT1A and ~53 kDa for 5-HT2A), were detected on all stages of developing OLs.
Fig. 2.
Characterization of 5-HT1A and 2A receptors in developing OLs by Western blot and intracellular Ca2+ assay. A: Both 1A and 2A receptors at predicted molecular weight were detected by Western blot in OL developing OLs. B: Intracellular Ca2+ increased in immature OLs upon 5-HT application (1 μM). Date represent a net increase of intracellular Ca2+ levels over 10 min period. N=6 in each group. *p<0.05 vs the control. C. Representative graphs show dynamic changes of intracellular in the control (medium only), 5-HT, and AMPA (used as a control) treated cells in a single well.
To further evaluation the functional property of 5-HT2A receptor, we measured intracellular Ca2+ levels in immature OLs upon 5-HT (1 μM) treatment. A steady increase of intracellular Ca2+ was observed (Fig. 2B). In contrast, AMPA, which is known to open Ca2+-permeable AMPA-GluR in OPCs and immature OLs (Itoh et al, 2002), evoked a sharp increase of intracellular Ca2+ (Figure 2C). This is consistent with the finding that 5-HT2A receptor-mediated intracellular Ca2+ increase is primarily due to liberating intracellular Ca2+ stores (Nagatomo et al., 2004).
2. 5-HT exposure led to morphological aberrance in developing OLs
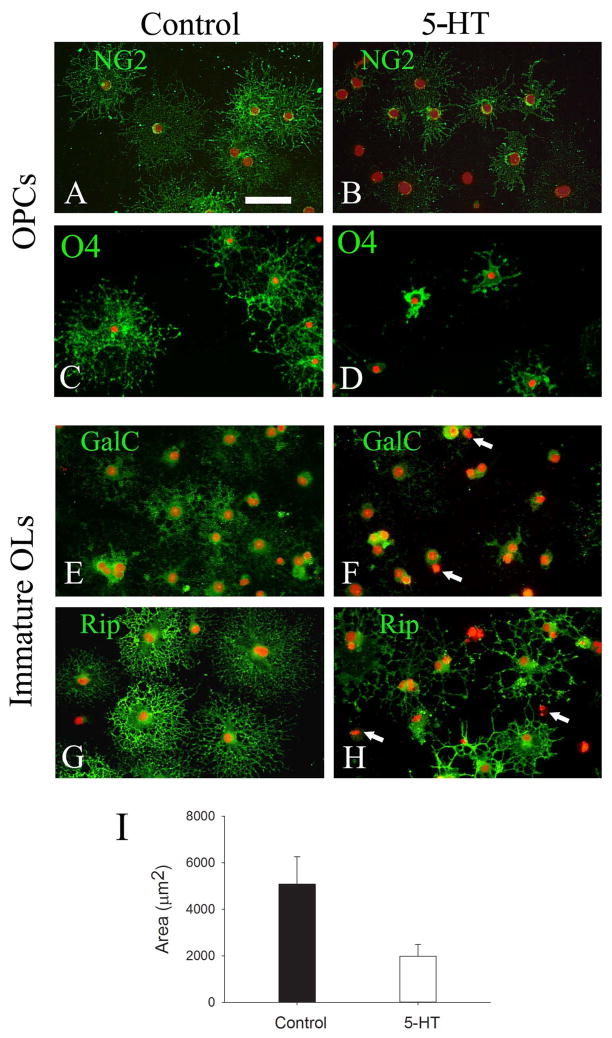
Next, we evaluated the effect of 5-HT exposure on OL development, based on expression patterns of developmental markers as well as morphological criteria. Since the expression of 5-HT1A and 2A receptors appeared to increase following OL lineage maturation, we treated both OPCs and immature OLs with 10 and 100 μM of 5-HT. As shown in Fig 3, the morphology of either OPCs or immature OLs was altered by 5-HT exposure; however the immature OLs showed a greater compromise. The majority of OPCs in the control showed nicely extended processes after 5 days of differentiation, as shown by both NG2 (Fig. 3A) and O4 (Fig. 3C) immunostaining, respectively. In contrast, 5-HT-treated OPCs showed fewer and shorter processes (Fig. 3B&D, respectively). It is worth noting that no significant differences were noted between 10 and 100 μM of 5-HT-treated OPCs, compared to an obvious dose-response of immature OLs to 5-HT treatment. For instance, at 10 μM, 5-HT exposure led to marked morphological alterations of immature OLs. As shown in Fig. 3F, GalC immunoreactivity was exclusively restricted to the somata with essentially no staining on cell processes, as compared to a uniformly immunostained pattern of both the soma and processes of the control cell (Fig. 3E). Similarly, Rip immunostained cells clearly showed a compromised process outgrowth characterized by fewer, distorted, and simpler morphology (Fig. 3H), which was in striking contrast to the control cells exhibiting extensive, elaborating process network (Fig. 3G). Quantitative analysis of cell surface areas showed a significantly reduction in 5-HT-treated OLs, as compared to the controls (Fig. 3I). In addition to these signs of developmental disturbance, a few of 5-HT-treated immature OLs also showed degenerative characteristics (pyknotic nuclei, with little or no processes, marked with arrows in Fig. 3F&2H). At higher concentration (i.e. 100 μM), the deleterious effect of 5-HT on the morphology of immature OLs (as judged by GalC and Rip immunostaining) was further increased, which was concomitant with increased cell death (data not shown).
Fig. 3.
5-HT exposure led to OL developmental disturbance. OPCs (A-D) or Immature OLs (EH) were treated with 5-HT (10 μm) for 5 days, and cells were immunostained with stage-specific OL markers to assess morphological changes. OPCs treated with 5-HT showed significantly reduced NG2 and O4 immunoreactivity (B and D, respectively), as compared to control cells (A and C, respectively). Compared to OPCs, immature OLs showed even greater developmental disturbance, indicated by not only weaker GalC immunostaining in the somata, but also markedly reduced processes in the 5-HT treatment (F), as compared to the control (E). The compromised processes in 5-HT treated cells were more pronounced by Rip immunostaining, which showed fewer, shortened, and distorted processes (H), compared to elaborating, extensive process network in the control (G). Some degenerative cells in 5-HT-treatment are shown by their condensed nuclei (marked as arrows in F and H). Semi-quantitative analysis of Rip immunolabeling showed that 5-HT treatment significant reduced cell surface areas (I). Data were from a total of 50 cells from three independent experiments. *p<0.01 vs the control. Scale bar: 50 μm.
3. 5-HT exposure reduces myelin protein expression in OLs
Next, we quantified several major myelin proteins including MBP, PLP, CNPase and Olig2, by Western blot. As shown in Fig. 4, the expression of MBP and PLP, two major myelin proteins found in the myelin sheath, were markedly decreased in immature OLs following 5 days of exposure to 5-HT (5–100 μM). Furthermore, such decrease was clearly in a dose-dependent manner. In contrast, CNPase, which is expressed at high levels in the cytoplasm, was significantly decreased only when 5-HT concentration reached at 100 μM. The expression of the nuclear transcription factor Olig2 was not affected by 5-HT exposure at any doses.
Fig. 4.
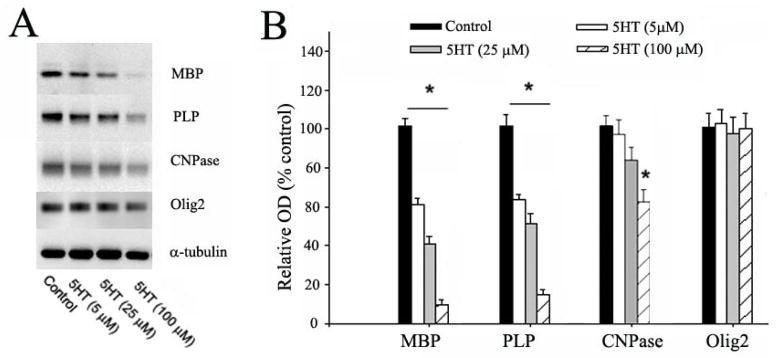
5-HT exposure reduced the expression of myelin proteins in OLs. A: representative Western blots show the expression of MBP, PLP, CNPase, and Olig2 in immature OLs following exposure to 5-HT (5–100 μM) for 5 days. B: The relative expression levels of myelin proteins were determined by normalizing the optical density (OD) of their respective target bands to α-tubulin, expressed as a percentage to the control. The expression of MBP and PLP was significantly reduced by 5-HT exposure in a dose-dependent manner. In contrast, a significant reduction of CNPase expression was only found when 5-HT concentration reached 100 μM, whereas no change of Olig2 expression was detected at all doses. *p<0.05 vs the control. Data are mean ±SD from three independent treatments.
4. 5-HT exposure at high doses results in OL injury
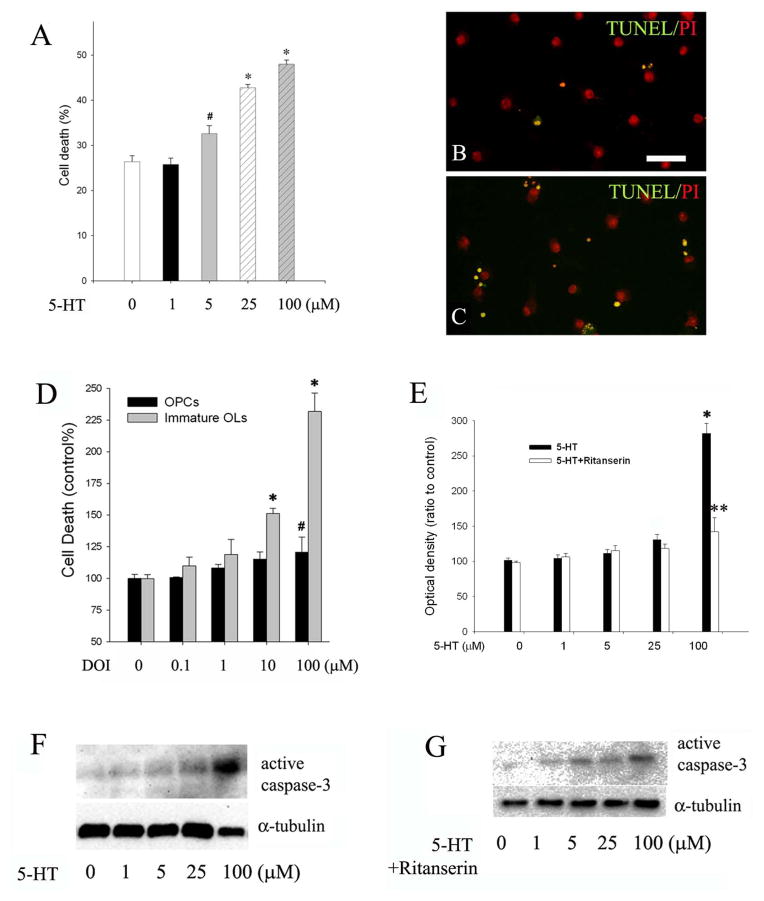
As noted in the immunostaining studies, some immature OLs appeared to be degenerative, indicated by their condensed nuclei following 5-HT exposure. To better characterize 5-HT-mediated cell death, OPCs or immature OLs were treated with increasing concentration of 5-HT (from 1 μM to 250 μM) for 48 h, and cell death was assessed by TUNEL staining. 5-HT treatment did not affect OPC survival, even at 250 μM (data not shown). In contrast, the number of TUNEL+ cells was significantly increased in 5-HT-treated immature OLs at ≥5 μM (Fig 5A–C). At the highest dose we have tested (250 μM), 5-HT essentially killed all immature OLs (data not shown). Since the immunostaining and immunoblotting data suggested that 5-HT2A may be up-regulated following OL differentiation, and that 5-HT2A has been reported to mediate neuronal death following certain insults (Capela et al. 2006, 2007), we then tested whether 5-HT2A receptor was responsible for 5-HT-induced immature OL death by using 5-HT2A receptor agonist DOI as well as its antagonist ritanserin. DOI exposure resulted in both OPCs and immature OLs damage; however, immature OLs showed a markedly increased vulnerability to DOI treatment. As shown in Fig 5D, significant OPC death (~ 20% increase over the control) was observed only when DOI reached 100 μM. In contrast, 5-HT at 100 μM resulted in a 230% increase of immature OL death. Conversely, pre-treatment with ritanserin significantly attenuated 5-HT-induced caspase-3 activation (Fig. 5F–G).
Fig. 5.
5-HT-induced OL death was mediated by 5-HT2A receptor. Immature OLs were exposed to increasing concentration of 5-HT for 48 h, and cell death was determined by TUNEL. A: the percentage of TUNEL+ cells increased significantly in 5-HT treatment, in a dose-dependent manner. B-C: representative micrographs of TUNEL (green fluorescence) in the control (B) and 5-HT treatment (C, shown 5-HT at 25 μM). Nuclei were counter-stained with PI (red). Scale bar: 50 μm. D: exposure to 5-HT2A receptor agonist DOI led to cell death in both OPCs and immature OLs; however, immature OLs showed an increased susceptibility than OPCs. *p<0.01 and #p<0.05 vs their respective controls (shown as 0 μM). E: conversely, 5-HT-induced caspase-3 activation in immature OLs was blocked by pre-treatment with ritanserin (1 μM). **p<0.01 vs 5-HT at 100 μM. F–G: representative western blots show that 5-HT dose-dependently activated caspase-3 in immature OLs (F), which was partially blocked by pre-treatment with ritanserin. Data are presented as mean ±SD from three independent experiments.
5. 5-HT exposure results in myelin malformation
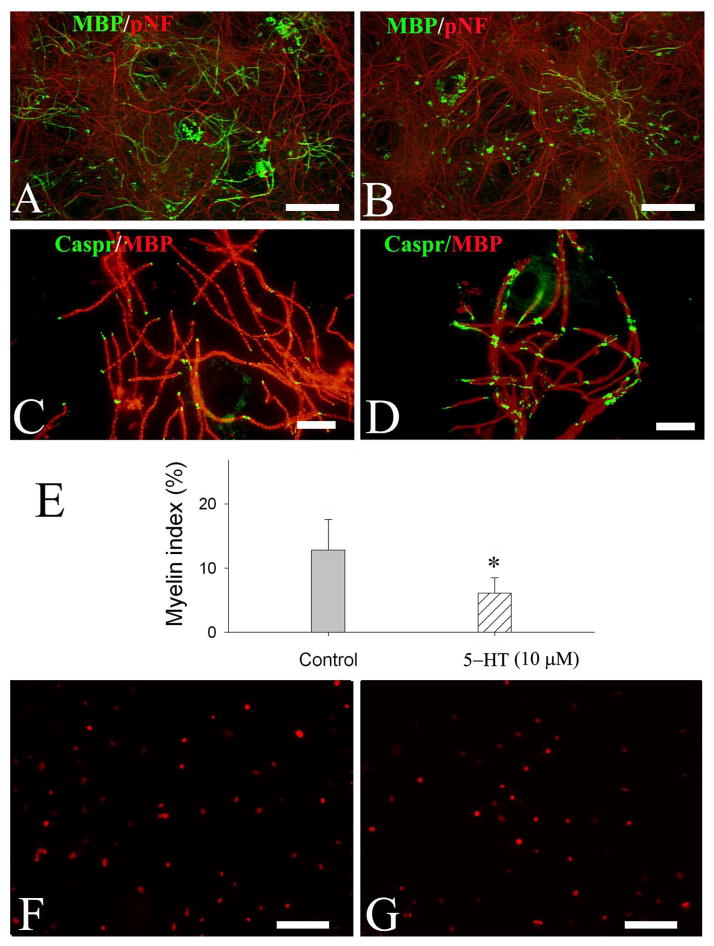
Finally, the effect of 5-HT on myelination was assessed in myelination cell cultures. By quantifying the number of myelinated internodes (double-labeled with MBP/pNF), we showed that 5-HT exposure significantly reduced myelination (5-HT at 10 or 100 μM, p>0.05) (Fig 6B). Interestingly, the clustering of paranodal domain protein, Caspr, was also affected by 5-HT exposure. In the control culture, caspr immunostaining was shown to be restricted at the paranodal domains which were regularly spaced by myelinated internodes (Fig. 6C). In contrast, 5-HT-treated cultures showed scattered, irregular patterns of caspr clustering (Fig. 6D). To test whether 5-HT induced myelination deficit was due to reduced number of OL lineage cells, the cultures were also immunostained with Olig2 antibody to determine the total number of OL lineages. Interestingly, the number of Olig2+ cells in 5-HT treatment (980.3±76.5 cells/mm2) was not significantly different from that of the control (879.9±63.2 cells/mm2) (Fig 6F and 6G, respectively).
Fig. 6.
5-HT exposure resulted in myelin malformation in neuron-OL co-cultures. Neuron-OL myelination cultures were exposed to 5-HT (10 and 100 μM) from DIV10 to DIV40. Myelinated internodes were revealed by co-localization of MBP and pNF immunostaining, and the paranodal domains were demonstrated by Caspr immunofluorescence. Representative micrographs show myelinated internodes (double-labeling with MBP and pNF) in the control (A) and 5-HT-treated cultures (B). The expression of caspr in 5-HT-treated cultures showed a diffuse, enlarged pattern (D), which was in contrast to a more localized, regular spaced pattern in the control culture (C). Quantification of myelinated internodes shows that myelin index was significantly reduced by 5-HT exposure (E); however, no significant difference was noted between 10 and 100 μM 5-HT treatments. There was no significant change of total OL lineage numbers between 5-HT treatment (G) and the control (F). Data were from three independent treatments with triplicate coverslips per condition. Scale bars: 200 μm (A, B, F&G); 50 μm (C&D).
Discussion
Altered serotonin system has long been thought to play a role in the pathogenesis of autism; however, the underlying mechanisms are not well understood (Whitaker-Azmitia 2001; 2005; Pardo 2007). Based on reports from both in vivo and in vitro studies which demonstrated that serotonin can affect many aspects of neural development, it is proposed that early manipulation of serotonin levels, either via genetic or environmental means, can lead to aberrant neural network organization (Levitt 2011). In SSRI-treated neonatal rats, we have previously reported not only altered cortical connectivity, but also pathology in OLs and myelin (Simpson et al. 2011). Here we report that manipulating serotonin levels in vitro affects OL development and myelination, and even triggers cell death at higher concentrations. Our finding suggests that elevated brain serotonin levels following neonatal SSRI exposure may directly target developing OLs leading to myelin malformation. Because the neural information processing requires proper integrity of axons, myelin sheath and the nodal structures, myelin malformation might contribute to, at least partially, the observed neurobehavioral deficits noted in the SSRI-exposed rats.
Although a variety of serotonin receptors in the adult brain are well described and reviewed (Barnes and Sharp 1999), their expression patterns in the developing brain are less clear. Nevertheless, several studies have provided evidence that 5-HT receptors are expressed in early developing rodent brain. For example, Bonnin et al. (2006) showed that mRNAs of 5-HT1 class of receptors (1A, 1B, 1D and 1F subtypes) are expressed in the forebrain of mice as early as E 14.5, in a regional restricted pattern. Lauder et al. (2000) reported that immunoreactivity of 5-HT2 receptors (2A, 2B and 2C) is detected in the neuroepithelia of brain and spinal cord, the notochord, and the cranial neural crest at E9-E12. Additionally, Slaten et al. (2010) reported that 5-HT4 receptor is transiently expressed in the mouse developing thalamocortical projections from E13-E17. While these studies described a spatiotemporal pattern of 5-HT receptor expression in the developing brain, there is a paucity of information regarding cellular localization of these receptors. In vitro, several studies have reported that cultured astrocytes express 5-HT receptors, including 5-HT-1A, 5-HT2A (Merzak 1996), 5-HT5 (Carson, et al., 1996), and 5-HT7 subtypes (Shimizu et al. 1998). In the postmortem human brain, Wu et al. (1999) demonstrated that 5-HT2A receptor was highly expressed by activated astrocytes in patients who suffered from Alzheimer’s disease, Huntington’s disease, frontotemporal dementia, and Creutzfeldt-Jakob disease, but was not detected in astrocytes from normal controls. In line with these findings, it has been shown that astrocytes express serotonin transporter, both in vitro (Kubota, et al. 2001) and in vivo (Pickel, et al. 1999). In contrast, expression of 5-HT receptors in OL lineage cells is less clear. Using immunohistochemistry, Maxishima et al. (2001) reported that 5-HT2A receptor was detected in GFAP+ astrocytes but not Rip+ OLs in the dorsal funiculus of spinal cord in six-week-old rats. Gaietta et al. (2003) demonstrated that 5-HT2A receptor mRNA and protein was expressed in rat sciatic nerves and cultured Schwann cells. The first evidence to suggest that 5-HT receptors are expressed in OL lineage was from a study by Schaumburg and colleagues (2008), who demonstrated that 5-HT2A receptors were expressed in human embryonic stem cell-derived OPCs, and, interestingly, it was the 5-HT2A receptor that mediated JC virus infection of OLs. Nevertheless, whether 5-HT receptors are expressed by OL lineage in vivo and/or in primary cell cultures remain elusive. In the present study, we observed strong positive staining of 1A and 2A receptors on OL lineage especially more differentiated cells. The cytoplasmic staining pattern of both 1A and 2A in most OLs may suggest that these receptors have high turnover rate. Our additional data from Western blot and intracellular calcium measurement provided further support that 5-HT1A and 2A, two important and widely studied 5-HT receptor subtypes, are expressed by OL lineages.
Utilizing 5-HT2A receptor agonist DOI and antagonist ritanserin, our data suggest that 5-HT2A receptor might play a role in 5-HT-mediated immature OL injury. Although 5-HT itself and DOI were able to induce OL death, DOI showed greater potency. A possible explanation for the differences between 5-HT and DOI in immature OL injury is that there might be multiple 5-HT receptor subtypes expressed on OLs, each of which could be simultaneously activated upon 5-HT exposure, while some of these receptor signaling might be pro-survival. For instance, it has been reported that 5-HT1A agonist 8-OH-DPA could activate phosphoinositide 3-kinase (PI3K) signaling pathway (Polter et al., 2012), which is known for its potent pro-survival effect on cells. In contrast, DOI has been shown to activate 5-HT2A receptor and apoptotic cell death signaling pathway (Capela J, et al. 2013).
Although our data strongly suggest that 5-HT-induced OL injury is mediated by 5-HT2A receptor, a non-receptor-mediated mechanism cannot be excluded. Several studies have demonstrated that 5-HT could be uptake into cells by Sert, where it is oxidized by monoamine oxidase-A (MAO-A) to generate oxidative species leading to apoptotic cell death (Serafeim et al., 2002; Bianchi et al., 2005). To address this issue, more studies are needed in the near future.
The observations that 5-HT receptors might be up-regulated following OL maturation led us to further investigate whether OPCs and immature OLs response differently to 5-HT treatment. Not surprisingly, cell death data showed a development-dependent pattern, with immature OLs showing a marked increased susceptibility than OPCs to 5-HT or DOI exposure. Furthermore, 5-HT-induced OL damage appears to be mediated via apoptotic mechanism, as indicated by pyknotic nuclei, positive staining for TUNEL, and cleaved caspase-3. Although our study was conducted in vitro, the implication of functional consequence could be intriguing. The immature OLs are the predominant OL lineage population in the human fetal brain at late gestation (Back et al. 2011), which are intrinsically vulnerable to a range of insults including proinflammatory cytokines, excitotoxicity, and oxidative stress (Volpe et al. 2011). SSRIs are widely prescribed to pregnant women suffering from major depression, while SSRIs have been shown to be able to cross placenta and reach fetal compartments (Loughhead et al. 2006). Thus, it is possible that SSRIs could also reach the fetal brain. In fact, experimental data suggest that extracellular 5-HT levels could arise up to 20-fold upon SSRI exposure in animals (Tao et al. 2000), and such high levels of extracellular serotonin may adversely affect OL development, myelination, and even cause neural degeneration.
Previously, we have reported ultrastructural abnormalities of both white matter OLs and myelin sheath in SSRI-exposed rats. Those pathological changes include vacuole/lysosome inclusions of OL soma, multi-nucleated OLs, as well as loosely packed myelin sheaths around axons, raising the possibility that elevated extracellular serotonin levels upon blockage of Sert may directly affect OLs and/or myelination (Simpson, et al. 2011). The current study supports this notion by demonstrating that high levels of serotonin exposure resulted in OL developmental disturbance, cell injury, and myelination deficit. Given that there were no signs of OL degeneration and/or reduction of OL population in the myelination co-culture, it is possible that the compromised myelination was due to factors other than cell injury. In fact, myelination is a highly orchestrated developmental program that can be affected by many factors, including numbers of mature OLs, axonal surface molecules, as well as trophic factors secreted from neurons and glial cells (Emery 2010). During development, OL process extension is an important step for pre-myelinating OLs to initially survey the local environment in finding suitable axons for myelination (Kirby et al. 2006). The altered process outgrowth, as demonstrated by Rip immunostaining in 5-HT-treated cells (Fig 3H&I), suggests that OL developmental disturbance might be one of the contributing factors for poor myelination. Alternatively, 5-HT exposure might also affect axon-derived factors for myelination. The altered cytoarchitecture at the nodes of Ranvier, as revealed by Caspr immunostaining, suggests this possibility. Caspr, an adhesion molecule diffusely expressed on axonal surface before active myelination, is progressively clustered at paranodal domain upon completion of myelination (Rios et al. 2000). The aberrant pattern of caspr clustering noted in 5-HT-treated cultures suggests that axon-derived myelinating factors might also be affected by high concentration of 5-HT.
In summary, our study provides the first evidence to suggest that elevated extracellular 5-HT levels may adversely affect OL survival, development, as well as myelination. The current findings offer a mechanistic view regarding the consequence in animal model and/or human subjects after early exposure to drugs such as SSRIs.
Acknowledgments
This work is supported by NIH grants 56NS054278, R01MH084194, R01NS080844, and funds from Department of Pediatrics, University of Mississippi Medical Center.
Abbreviations
- CDM
chemically defined medium
- CNPase
2′,3′-cyclic nucleotide 3′-phosphodiesterase
- DOI
1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride, (±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride
- Caspr
contactin-associated protein
- DIV
days in vitro
- Gal C
galactosylceramidase
- MBP
myelin basic protein
- PLP
myelin proteolipid protein
- NBM
neurobasal medium
- pNF
phosphorylated neurofilament H
- GFAP
glial fibrillary acidic protein
- OL
oligodendrocytes
- OPC
OL progenitor cell
- SSRI
selective serotonin reuptake inhibitor
- Sert
serotonin transporter
- TUNEL
terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling
Footnotes
The authors have no conflicts of interest to declare.
References
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2011;21:1302–12. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Peng W, Hewlett W, Levitt P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience. 2006;141:781–94. doi: 10.1016/j.neuroscience.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Capela JP, Ruscher K, Lautenschlager M, Freyer D, Dirnagl U, Gaio AR, Bastos ML, Meisel A, Carvalho F. Ecstasy-induced cell death in cortical neuronal cultures is serotonin 2A-receptor-dependent and potentiated under hyperthermia. Neuroscience. 2006;139:1069–81. doi: 10.1016/j.neuroscience.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Capela JP, Fernandes E, Remião F, Bastos ML, Meisel A, Carvalho F. Ecstasy induces apoptosis via 5-HT (2A)-receptor stimulation incortical neurons. Neurotoxicology. 2007;28:868–75. doi: 10.1016/j.neuro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Capela JP, da Costa Araújo S, Costa VM, Ruscher K, Fernandes E, de Bastos ML, Dirnagl U, Meisel A, Carvalho F. The neurotoxicity of hallucinogenic amphetamines in primary cultures of hippocampal neurons. Neurotoxicology. 2013;34:254–63. doi: 10.1016/j.neuro.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Thomas EA, Danielson PE, Sutcliffe JG. The 5-HT5A serotonin receptor is expressed predominantly by astrocytes in which it inhibits cAMP accumulation: a mechanism for neuronal suppression of reactive astrocytes. Glia. 1996;17:317–26. doi: 10.1002/(SICI)1098-1136(199608)17:4<317::AID-GLIA6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Daubert EA, Condron BG. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci. 2010;33:424–34. doi: 10.1016/j.tins.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci U S A. 2005;102:5582–7. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Gaietta GM, Yoder EJ, Deerinck T, Kinder K, Hanono A, Han A, Wu C, Ellisman MH. 5-HT2a receptors in rat sciatic nerves and Schwann cell cultures. J Neurocytol. 2003;32:373–80. doi: 10.1023/B:NEUR.0000011331.58835.fd. [DOI] [PubMed] [Google Scholar]
- Itoh T, Beesley J, Itoh A, Cohen AS, Kavanaugh B, Coulter DA, Grinspan JB, Pleasure D. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem. 2002;81:390–402. doi: 10.1046/j.1471-4159.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- Kubota N, Kiuchi Y, Nemoto M, Oyamada H, Ohno M, Funahashi H, Shioda S, Oguchi K. Regulation of serotonin transporter gene expression in human glial cells by growth factors. Eur J Pharmacol. 2001;417:69–76. doi: 10.1016/s0014-2999(01)00906-2. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Wilkie MB, Wu C, Singh S. Expression of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors in the mouse embryo. Int J Dev Neurosci. 2000;18:653–62. doi: 10.1016/s0736-5748(00)00032-0. [DOI] [PubMed] [Google Scholar]
- Levitt P. Serotonin and the autisms: a red flag or a red herring? Arch Gen Psychiatry. 2011;68:1093–4. doi: 10.1001/archgenpsychiatry.2011.98. [DOI] [PubMed] [Google Scholar]
- Loughhead AM, Stowe ZN, Newport DJ, Ritchie JC, DeVane CL, Owens MJ. Placental passage of tricyclic antidepressants. Biol Psychiatry. 2006;59:287–90. doi: 10.1016/j.biopsych.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Maxishima MM, Shiga T, Shutoh F, Hamada S, Maeshima T, Okado N. Brain Res. 2001;19:270–273. doi: 10.1016/s0006-8993(00)03150-4. [DOI] [PubMed] [Google Scholar]
- Merzak A, Koochekpour S, Fillion MP, Fillion G, Pilkington GJ. Expression of serotonin receptors in human fetal astrocytes and glioma cell lines: a possible role in glioma cell proliferation and migration. Brain Res Mol Brain Res. 1996;41:1–7. doi: 10.1016/0169-328x(96)00058-7. [DOI] [PubMed] [Google Scholar]
- Nagatomo T, Rashid M, Abul Muntasir H, Komiyama T. Functions of 5-HT2A receptor and its antagonists in the cardiovascular system. Pharmacol Ther. 2004;104:59–81. doi: 10.1016/j.pharmthera.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Effect of tumor necrosis factor-alpha on developing optic nerve oligodendrocytes in culture. J Neurosci Res. 2005;80:226–34. doi: 10.1002/jnr.20450. [DOI] [PubMed] [Google Scholar]
- Pang Y, Zheng B, Kimberly SL, Cai Z, Rhodes PG, Lin RC. Neuron-oligodendrocyte myelination co-culture derived from embryonic rat spinal cord and cerebral cortex. Brain Behav. 2012;2(1):53–67. doi: 10.1002/brb3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17:434–47. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Altamura C, Calia E, Puglisi-Allegra S, Ventura R, Lucchese F, Keller F. Serotonin depletion and barrel cortex development: impact of growth impairment vs. serotonin effects on thalamocortical endings. Cereb Cortex. 2000;10:181–91. doi: 10.1093/cercor/10.2.181. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J. Ultrastructural localization of the serotonin transporter in limbic and motor compartments of the nucleus accumbens. J Neurosci. 1999;19:7356–66. doi: 10.1523/JNEUROSCI.19-17-07356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Yang S, Jope RS, Li X. Functional significance of glycogen synthase kinase-3 regulation by serotonin. Cell Signal. 2012;24:265–71. doi: 10.1016/j.cellsig.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–64. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumburg C, O’Hara BA, Lane TE, Atwood WJ. Human embryonic stem cell-derived oligodendrocyte progenitor cells express the serotonin receptor and are susceptible to JC virus infection. J Virol. 2008;82:8896–99. doi: 10.1128/JVI.00406-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafeim A, Grafton G, Chamba A, Gregory CD, Blakely RD, Bowery NG, Barnes NM, Gordon J. 5-Hydroxytryptamine drives apoptosis in biopsylike Burkitt lymphoma cells: reversal by selective serotonin reuptake inhibitors. Blood. 2002;99:2545–53. doi: 10.1182/blood.v99.7.2545. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Nishida A, Zensho H, Miyata M, Yamawaki S. Agonist-induced desensitization of adenylyl cyclase activity mediated by 5-hydroxytryptamine7 receptors in rat frontocortical astrocytes. Brain Res. 1998;16:57–62. doi: 10.1016/s0006-8993(97)01185-2. [DOI] [PubMed] [Google Scholar]
- Slaten ER, Hernandez MC, Albay R, 3rd, Lavian R, Janusonis S. Transient expression of serotonin 5-HT4 receptors in the mouse developing thalamocortical projections. Dev Neurobiol. 2010;70:165–81. doi: 10.1002/dneu.20775. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RC. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci U S A. 2011;108:18465–70. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential effect of local infusion of serotonin reuptake inhibitors in the raphe versus forebrain and the role of depolarization-induced release in increased extracellular serotonin. J Pharmacol Exp Ther. 2000;294:571–9. [PubMed] [Google Scholar]
- Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. Reprint of “The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci. 2011;29:565–82. doi: 10.1016/j.ijdevneu.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479–85. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Wu C, Singh SK, Dias P, Kumar S, Mann DM. Activated astrocytes display increased 5-HT2a receptor expression in pathological states. Exp Neurol. 1999;158:529–33. doi: 10.1006/exnr.1999.7105. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sari Y, Zhou FC. Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res Dev Brain Res. 2004;150:151–61. doi: 10.1016/j.devbrainres.2003.02.001. [DOI] [PubMed] [Google Scholar]