Abstract
Cardiorespiratory fitness (CRF) is an objective measure of habitual physical activity (PA), and has been linked to increased brain structure and cognition. The gold standard method for measuring CRF is graded exercise testing (GXT), but GXT is not feasible in many settings. The objective of this study was to examine whether a non-exercise estimate of CRF is related to gray matter (GM) volumes, white matter hyperintensities (WMH), cognition, objective and subjective memory function, and mood in a middle-aged cohort at risk for Alzheimer’s disease (AD). Three hundred and fifteen cognitively healthy adults (mean age = 58.58 years) enrolled in the Wisconsin Registry for Alzheimer’s Prevention underwent structural MRI scanning, cognitive testing, anthropometric assessment, venipuncture for laboratory tests, and completed a self-reported PA questionnaire. A subset (n=85) underwent maximal GXT. CRF was estimated using a previously validated equation incorporating sex, age, body-mass index, resting heart rate, and self-reported PA. Results indicated that the CRF estimate was significantly associated with GXT-derived peak oxygen consumption, validating its use as a non-exercise CRF measure in our sample. Support for this finding was seen in significant associations between the CRF estimate and several cardiovascular risk factors. Higher CRF was associated with greater GM volumes in several AD-relevant brain regions including the hippocampus, amygdala, precuneus, supramarginal gyrus, and rostral middle frontal gyrus. Increased CRF was also associated with lower WMH and better cognitive performance in Verbal Learning & Memory, Speed & Flexibility, and Visuospatial Ability. Lastly, CRF was negatively correlated with self- and informant-reported memory complaints, and depressive symptoms. Together, these findings suggest that habitual participation in physical activity may provide protection for brain structure and cognitive function, thereby decreasing future risk for AD.
Keywords: cardiorespiratory fitness, preclinical Alzheimer’s disease, MRI, white matter hyperintensities, cognition, mood
Introduction
Cardiorespiratory fitness (CRF) is an objective measure of habitual physical activity that reflects the overall capacity of the cardiopulmonary system (American College of Sports Medicine., 2014; Mailey et al., 2010). Increasing evidence has shown that higher levels of CRF are related to better brain health (Colcombe et al., 2003; Colcombe et al., 2004; Gordon et al., 2008; Johnson, Kim, Clasey, Bailey, & Gold, 2012) and cognition (Baker et al., 2010; Brown et al., 2010; Newson & Kemps, 2006; Prakash et al., 2011), as well as lower risk of Alzheimer’s disease (AD) in older adults (Defina et al., 2013; Liu et al., 2012; Vidoni, Honea, Billinger, Swerdlow, & Burns, 2012). Specifically, higher CRF is associated with preservation of critical brain areas in cognitively healthy older adults (Colcombe et al., 2003) and persons with AD (Honea et al., 2009), as well as reduced brain atrophy in those with early-stage AD (Burns et al., 2008). More recently, CRF has been associated with preserved white matter microstructure (Johnson et al., 2012). Additionally, Prakash et al. (2011) showed a relationship between CRF and executive function using the Stroop Task, and DeFina et al. (2013) indicated that higher CRF levels in midlife were linked with lower risk of dementia later in life.
Currently, the gold standard method for measuring CRF is graded exercise testing (GXT) with gas exchange measurement. Unfortunately, there are several barriers to GXT, especially among older adults. These include the amount of time required for test completion, equipment costs, need for medical oversight, and the risk of injury and cardiac events (American College of Sports Medicine., 2014). These barriers make it difficult for many health care and research facilities to implement GXT measurements of CRF. Submaximal approaches to assessing CRF, such as the Rockport 1-mile walk test (Kline et al., 1987) and the Astrand and Rhyming Cycle Ergometer Test (Astrand & Ryhming, 1954), have been proposed as reliable and valid alternatives to GXT. However, these submaximal methods are not entirely free of the constraints associated with the gold standard GXT measure.
Recognizing the impact of CRF on pertinent health-related outcomes, yet faced with the infeasibility of obtaining GXT measurements on a large number of individuals, researchers have turned to developing non-exercise estimates of CRF (Jackson et al., 1990; Jackson et al., 2012; Lakoski et al., 2011; Nes et al., 2011). These low-cost, low-risk estimates of CRF are typically formed by regressing maximal oxygen uptake (VO2max) on variables that are both clinically-available and thought to have an impact on CRF, such as age, sex, body mass index (BMI), resting heart rate (RHR), and self-reported physical activity. Jurca et al. (2005) established one such non-exercise CRF estimate and found that it correlated highly with GXT-assessed VO2max (.76 ≤ r ≤ .81) within three large, independent cohorts. This study was later validated in an older sample (mean age = 66.73 years) by Mailey et al. (2010), who also found high concordance (r = .67) between the CRF estimate, VO2max, and the Rockport 1-mile walk protocol. Using a modified version of the Jurca et al. (2005) CRF estimate, McAuley et al. (2011) found that CRF was associated with increased hippocampal volume, processing speed, and working memory in a sample of older adults. However, no studies to date have examined whether such non-exercise CRF estimates are predictive of neural health, cognition, and mood using a diverse array of outcome measures in a relatively younger cohort with specific risk factors for AD.
Therefore, the objective of this study was to determine whether a non-exercise estimate of CRF is associated with measures of brain health, cognitive function, subjective and informant-reported memory complaints, and mood in an asymptomatic, middle-aged, cohort at risk for AD. We hypothesized that individuals with greater CRF would exhibit larger grey matter (GM) volumes in AD-relevant regions of interest (ROIs), reduced white matter hyperintensities (WMH), better objective and subjective cognition, and better mood compared with less fit individuals.
Methods
Participants
The Wisconsin Registry for Alzheimer’s Prevention (WRAP) is a longitudinal cohort of approximately 1500 cognitively healthy, middle-aged adults between the ages of 40 and 65 at study entry (Sager, Hermann, & La Rue, 2005). For the present study, 315 participants were selected based on completion of a WRAP wave 2 visit and co-enrollment in ongoing brain imaging studies. Similar to the larger WRAP cohort, this study’s sample was enriched with persons who had a positive family history for AD (72.4%) and were apolipoprotein E4 (APOE4) positive (38.7%). Table 1 shows participants’ relevant background characteristics. The University of Wisconsin Institutional Review Board approved all study procedures and informed consent was obtained from all individual participants included in the study.
Table 1.
Participant Characteristics
| Characteristic | Value* |
|---|---|
| Demographics | |
| Age | 58.58 (6.33) |
| Female, % | 67.9 |
| Years of education | 16.03 (2.35) |
| Family history positive, % | 72.4 |
| APOE4 gene, % | 38.7 |
| CRF Estimate Components | |
| Amount of moderate exercise (minutes/week) | 48.29 (63.31) |
| Body mass index (kg/m2) | 27.96 (5.54) |
| Resting heart rate (beats/minute) | 64.72 (9.33) |
| Cognitive and Mood Measures | |
| Mini Mental State Exam | 29.47 (0.88) |
| Self-reported memory problems, %† | 26.2 |
| IQCODE † | 48.38 (3.01) |
| CES-D | 5.69 (5.61) |
| Vascular Risk Indices | |
| Total cholesterol (mg/dL) | 200.30 (35.21) |
| HDL cholesterol (mg/dL) | 62.39 (18.80) |
| Homocysteine (µmol/L) | 9.06 (2.95) |
| hs C-reactive protein (mg/L) | 2.12 (3.29) |
| Glucose (mg/dL) | 96.38 (13.75) |
| Insulin (µU/mL) | 8.77 (6.26) |
| Interleukin-6 (pg/mL) | 1.85 (1.84) |
| Systolic blood pressure (mmHg) | 124.05 (15.56) |
| Diastolic blood pressure (mmHg) | 73.90 (9.06) |
| Waist circumference (cm) | 90.08 (15.03) |
| Hip circumference (cm) | 106.47 (12.13) |
| Central adiposity (men > 102 cm; women > 88 cm), % | 33.7 |
| HOMA-IR | 5.24 (27.49) |
| Hypertension, % | 78.9 |
| Diabetes, % | 3.2 |
| Smoker (ever), % | 46.3 |
All values are mean (SD) unless otherwise indicated. HDL= high-density lipoprotein; HOMA-IR = Homeostasis Model Assessment of Insulin Resistance; CES-D = Center for Epidemiologic Studies Depression Scale; IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly
N=244 for Self-reported memory problems due to exclusion of the response “I don’t know” from analyses; N=281 for IQCODE because 34 informants did not return/complete the questionnaire
Non-exercise CRF Measure
Our non-exercise estimate of CRF was based on the formula proposed by Jurca et al. (2005) i.e., CRF = 18.07+ Sex (2.77) – Age (.10) – BMI (.17) – RHR (.03) + Self-Reported Physical Activity, with sex coded as female = 0 and male = 1. BMI was calculated using the standard equation [weight (kg) / height (m)2]. RHR was determined using a GE Dinamap Pro 400 V2 Vital Signs Monitor with a GE Critikon Blood Pressure Cuff. In the Jurca et al. (2005) study, self-reported physical activity was assessed with one question that required participants to rate on a five-point scale how many minutes they engaged in physical activity per week. In contrast, for our study, we used the moderate intensity physical activity question from the Women’s Health Initiative physical activities questionnaire (McTiernan et al., 2003). This question inquired into the frequency (range from none to ≥5 days/week) and duration (range from < 30 minutes to ≥ 1 hour/session) of engagement in moderate, “not exhausting,” physical activity (e.g. calisthenics, easy swimming) per week. Following established protocol (McTiernan et al., 2003), frequency and duration were multiplied to create a “minutes/week” measure of moderate physical activity. This measure was then dichotomized to indicate whether (“1”) or not (“0”) an individual attained 150 minutes of moderate physical activity per week. The decision to focus on moderate physical activity, and at a threshold of 150 minutes/week, was informed by current national guidelines that recommend older adults engage in a minimum 150 minutes per week of moderate physical activity for maintenance of health (Nelson et al., 2007; U.S. Department of Health & Human Services, 2008).
Graded Exercise Testing
In order to validate the CRF estimate within our own sample, a subset of participants (n=85) performed physician-supervised GXT using a modified Balke protocol (Balke & Ware, 1959). Comfortable brisk walking speeds were determined prior to testing as a safety precaution and to ensure a valid test. For participants who were capable of walking at 3.5 miles per hour comfortably, this speed was used throughout the test. For participants who found this walking speed uncomfortable, a slower speed was chosen. The grade of the treadmill was increased by 2.5% every two minutes until the participant reached volitional exhaustion or the physician stopped the test due to safety concerns. Continuous measurements of oxygen uptake (VO2), carbon dioxide production, minute ventilation, heart rate, and work rate were obtained using a metabolic cart and two-way non-rebreathing valve (TrueOne® 2400 metabolic cart, ParvoMedics, Sandy, UT). The system was calibrated prior to each test using standard gases with known concentrations and with a calibrated three-liter syringe. Peak effort was determined based on meeting at least two of the following criteria: 1) respiratory exchange ratio ≥ 1.1, 2) change in VO2 < 200 ml with an increase in work, 3) rating of perceived exertion (RPE) of 17 or greater and 4) achieving 90% of age predicted maximal heart rate. Of the 85 participants who underwent GXT, 64 met peak effort criteria. Peak oxygen consumption (VO2) during exercise was used as the index of cardiorespiratory capacity.
Cardiovascular Risk Factors
Participants completed a health history questionnaire that included questions about history of various cardiovascular diseases. They also underwent a clinic visit at the UW Institute for Clinical and Translational Research that included anthropometric measurements, blood pressure readings, and blood draw for assaying a comprehensive panel of laboratory tests implicated in vascular disease (see Table 1).
Neuroimaging Protocol
MRI images were acquired on a GE ×750 3.0T scanner with an eight-channel phased array head coil (General Electric, Waukesha, WI). A 3D T1-weighted inversion recovery prepared SPGR anatomical sequence was collected using the following parameters: TI/TE/TR = 450ms/3.2/8.2ms, flip angle = 12°, slice thickness = 1mm no gap, FOV = 256, matrix size = 256×256, yielding a voxel resolution of 1 mm × 1 mm × 1 mm. Additionally, a 3D T2-weighted fluid attenuated inversion recovery (FLAIR) sequence was acquired using the following parameters: TI/TE/TR = 1868ms/123ms/6000ms, flip angle = 90°, slice thickn ess = 2mm no gap, FOV = 256 mm, matrix size = 256 × 256, yielding a voxel resolution of 1 mm × 1 mm × 2 mm. Scanning was completed after a minimum 4-hour fast from food, tobacco, caffeine, and medications with vasomodulatory properties. Fifteen participants did not have useable imaging data and hence did not contribute data to the brain imaging analyses. Time between WRAP wave 2 visit and MRI scan was on average 1.85 years (SD=1.21 years), with 94% of MRI scans occurring after WRAP wave 2 visit, 3.7% occurring before WRAP wave 2 visit, and 2.3% occurring concurrently.
FreeSurfer image analysis suite version 5.1.0 (http://surfer.nmr.mgh.harvard.edu/) was used to derive GM regions of interest (ROIs) from the T1 images, as described in prior publications (Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999). While the automated procedure is in principle 100% reproducible, user inspection and iterative control point editing is often required, and thus was implemented in this study in order to ensure proper cortical reconstruction. In our hands, intra- and inter-rater reliability is excellent (ICC > .99), based on a training sample of 10 brains of varying age and scan quality, rated by three technicians twice in blind fashion. This study focused on 14 a priori ROIs associated with memory, executive function, or AD, namely, the hippocampus, amygdala, posterior cingulate, cingulate isthmus, parahippocampal gyrus, entorhinal cortex, fusiform, caudal and rostral anterior cingulate, caudal and rostral middle frontal gyrus, inferior parietal cortex, precuneus, and supramarginal cortex. Volumetric measurements from each hemisphere were averaged to obtain a single value for each ROI.
Lesion Segmentation Tool (LST) version 1.2.3 in SPM8 (P. Schmidt et al., 2012) was used to calculate total volume of WMH. This toolbox is open source and uses automated segmentation with high reliability. For lesion segmentation, LST seeds lesions based on spatial and intensity probabilities from T1 images and hyperintense outliers on T2FLAIR images. Visual inspection was conducted for all segmentation maps. Additional details have been previously described (Birdsill et al., 2014; P. Schmidt et al., 2012).
Neuropsychological Assessment
All participants completed a comprehensive neuropsychological battery (Sager et al., 2005) that included psychometric measures spanning cognitive domains such as memory, attention, executive function, language, and visuospatial ability. Earlier factor analytic studies (Dowling, Hermann, La Rue, & Sager, 2010; Koscik et al., 2014) of these measures within the larger WRAP cohort indicated that they map on to six cognitive factors. These factors and their corresponding psychometric tests include Immediate Memory: Rey Auditory Verbal Learning Test (RAVLT) learning trials 1 and 2 (M. Schmidt, 1996); Verbal Learning & Memory: RAVLT learning trials 3–5, and Delayed Recall (M. Schmidt, 1996); Working Memory: Digit Span and Letter-Number Sequencing subtests from the Wechsler Adult Intelligence Scale, 3rd edition (Wechsler, 1997); Speed & Flexibility: Stroop Color-Word Test Interference Trial (Trenerry, Crosson, DeBoe, & Leber, 1989) and Trail-Making Test A and B (Reitan & Wolfson, 1993); Visuospatial Ability: Block Design and Matrix Reasoning subtests from the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) and Judgment of Line Orientation Test (Benton, 1994); and Verbal Ability: Reading subtest of the Wide-Range Achievement Test, 3rd edition, (Wilkinson, 1993) Vocabulary and Similarities subtests from the WASI (Wechsler, 1999), and the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983). The Mini Mental State Exam (MMSE) was also administered as a measure of global cognitive function.
Self- and Informant-Reported Memory Complaints and Mood
Presence of self-reported memory complaints was determined via a question that asked, “Do you think you have a problem with your memory?” Response options were “yes”, “no”, and “I don’t know”. Informant-reported memory complaints were based on ratings provided by each participant’s study partner on the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (Jorm & Jacomb, 1989). The IQCODE is a 16-item questionnaire that asks about a person’s (in this case, the study participant’s) cognitive function at present compared with the prior 10 years. Responses range from 1 (much improved) to 5 (much worse), with a score of 3 indicating “not much change.” Mood symptoms were assessed via the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977).
Data Analyses
To validate the CRF measure as an indicator of aerobic fitness within our sample, we used Pearson correlation to assess its relationship with VO2 among the subset of participants (n=85) who underwent GXT. To affirm these findings within the full sample (n=315), Pearson (for continuous measures) and point-biserial (for dichotomous measures) correlations were used to assess the CRF measure’s relationship with both laboratory-based and self-reported measures of cardiovascular risk. Additionally, by regressing VO2 on sex, age, BMI, RHR, and self-reported physical activity, we re-created the CRF measure within our GXT subsample; and then used Pearson correlations to examine relationships between this re-created CRF measure and both VO2 and the original CRF measure that was computed using the Jurca equation (2005).
Multivariate analysis of covariance (MANCOVA) was used to examine relationships between the CRF measure and the GM ROIs, with intracranial volume as a covariate. Age and sex were not included as covariates because they are intrinsically controlled for in the CRF estimate. MANCOVA was used in order to reduce alpha inflation given the number of ROIs investigated. A significant omnibus MANCOVA would provide justification for examining each ROI with follow-up univariate analyses of covariance (ANCOVAs). The WMH measure was severely skewed, and various transformations (e.g., log) failed to satisfactorily normalize it. Therefore, we used a median split to divide values into 0 (low WMH) and 1 (high WMH). Logistic regression, adjusted for intracranial volume, was then used to test for associations between CRF and WMH. Linear regression was used to evaluate the association between the CRF measure and each of the six cognitive factors, adjusting for education. As noted above, age and sex were not included as covariates due to their presence in the CRF estimate. Finally, we assessed the relationships between CRF and self- and informant-reported memory complaints, as well as depressive symptoms, using Pearson and point-biserial correlations as applicable. For self-reported memory complaints, only “yes” (n = 64) and “no” (n = 180) responses were considered in the analysis.
Secondary analyses were conducted to determine whether any observed associations between CRF and neuroimaging or cognitive measures were moderated by APOE4, the primary genetic risk factor for AD. This was accomplished by re-fitting the models described above while including a term for the interaction between APOE4 and CRF. All analyses were conducted using IBM SPSS, version 21.0. Only findings with p ≤ .05 (2-tailed) were considered to be significant.
Results
Background Characteristics
Table 1 details the background characteristics of study participants. Participants were predominately female (67.9%) with a mean age of 58.58 ± 6.33 years. Overall, participants were slightly overweight (mean BMI = 27.96 ± 5.54) and reported engaging in 48.29 ± 63.31 minutes of moderate intensity physical activity per week. Participants’ scores on the MMSE (29.47 ± .88) and the IQCODE (48.38 ± 3.01) corroborate the normocognitive status of the cohort.
Associations between CRF estimate, VO2, and Measures of Cardiovascular Risk
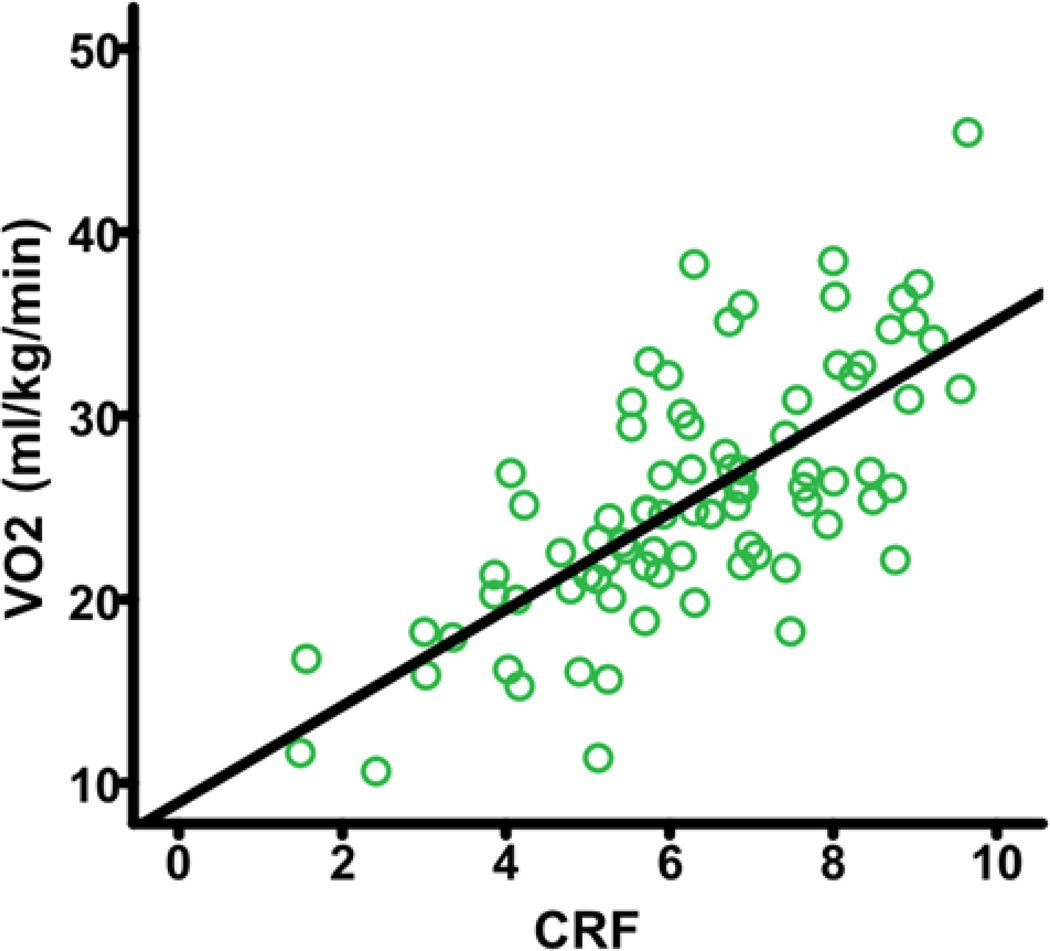
The mean (SD) VO2 was 25.44 ± 6.70 ml/kg/min. The Jurca-based CRF measure was significantly associated with VO2 (r = .71, p < .001; see Figure 1) within our GXT subsample, thus validating the use of this non-exercise CRF measure for further analyses within the full sample. Additionally, the regression of VO2 on sex, age, BMI, RHR, and self-reported physical activity within our GXT subsample yielded the following equation for estimating CRF: CRF = 65.70 + Sex (5.81) – Age (.42) – BMI (.49) – RHR (.02) + Self-Reported Physical Activity (.98), with sex coded as female = 0 and male = 1. The re-created CRF estimate was significantly associated with VO2 (r = .76, p < .001) and the original, Jurca-based CRF estimate (r = .91, p < .001).
Fig. 1.
Higher non-exercise CRF estimate is positively correlated with VO2. CRF=cardiorespiratory fitness; VO2=peak oxygen consumption.
The CRF measure was also significantly associated with several measures of cardiovascular risk, including hypertension, hs C-reactive protein, history of tobacco use, and total cholesterol (see Table 2). Correlations for significant findings were all negative, indicating that higher CRF was associated with lower cardiovascular risk. These findings within the full sample supplemented the correlation found between the Jurca-based CRF measure and VO2 within the GXT subsample, further supporting the use of the non-exercise CRF measure for our substantive analyses.
Table 2.
Correlation between cardiorespiratory fitness and measures of vascular risk
| Vascular Indices | r value | p value |
|---|---|---|
| Total cholesterol | −0.19 | .001 |
| HDL cholesterol | −0.07 | .242 |
| Homocysteine | 0.06 | .302 |
| hs C-reactive protein | −0.32 | <.001 |
| Glucose | −0.02 | .764 |
| Insulin | −0.36 | <.001 |
| Interleukin-6 | −0.18 | .002 |
| Systolic blood pressure | −0.18 | .001 |
| Diastolic blood pressure | 0.09 | .108 |
| Waist circumference | −0.25 | <.001 |
| Hip circumference | −0.61 | <.001 |
| Central adiposity | −0.48 | <.001 |
| HOMA-IR | −0.02 | .794 |
| Hypertension | −0.22 | <.001 |
| Diabetes mellitus | −0.05 | .363 |
| Smoking (ever) | −0.19 | .001 |
HDL= high-density lipoprotein; HOMA-IR = Homeostasis Model Assessment of Insulin Resistance
Relationship between CRF and Brain Structure
The MANCOVA that assessed the relationship between CRF and the GM ROIs revealed a significant omnibus effect of CRF: ^ = .88, F (14, 284) = 2.874, p < .001, partial η2 = .124. Follow up ANCOVAs found significant effects of the CRF measure on the hippocampus, amygdala, fusiform, rostral middle frontal gyrus, inferior parietal cortex, precuneus, and supramarginal cortex with a trend to significance in the entorhinal cortex (see Table 3). The parameter estimates for all significant findings were positive, demonstrating that increased CRF is associated with increased GM volume in these ROIs.
Table 3.
Cardiorespiratory fitness is associated with brain volume in AD-related regions of interest
| Regions of Interest | β (SE) | t value | p value | partial η2 |
|---|---|---|---|---|
| Hippocampus | 37.87 (14.65) | 2.59 | .010 | 0.022 |
| Amygdala | 16.52 (7.41) | 2.23 | .026 | 0.016 |
| Posterior cingulate | −4.59 (13.64) | −0.34 | .737 | 0.000 |
| Cingulate isthmus | 11.58 (11.25) | 1.03 | .304 | 0.004 |
| Parahippocampal | 13.21 (8.86) | 1.49 | .137 | 0.007 |
| Entorhinal | 16.63 (9.46) | 1.76 | .080 | 0.010 |
| Fusiform | 164.41 (32.28) | 5.09 | <.001 | 0.080 |
| Caudal anterior cingulate | 4.07 (12.73) | 0.32 | .749 | 0.000 |
| Rostral anterior cingulate | 5.73 (12.17) | 0.47 | .638 | 0.001 |
| Caudal middle frontal | 21.31 (33.28) | 0.64 | .522 | 0.001 |
| Rostral middle frontal | 113.31 (51.35) | 2.21 | .028 | 0.016 |
| Inferior parietal | 108.92 (51.04) | 2.13 | .034 | 0.015 |
| Precuneus | 71.89 (33.51) | 2.15 | .033 | 0.015 |
| Supramarginal | 162.17 (41.21) | 3.94 | <.001 | 0.050 |
AD=Alzheimer’s disease; η2=eta squared. All models were adjusted for intracranial volume
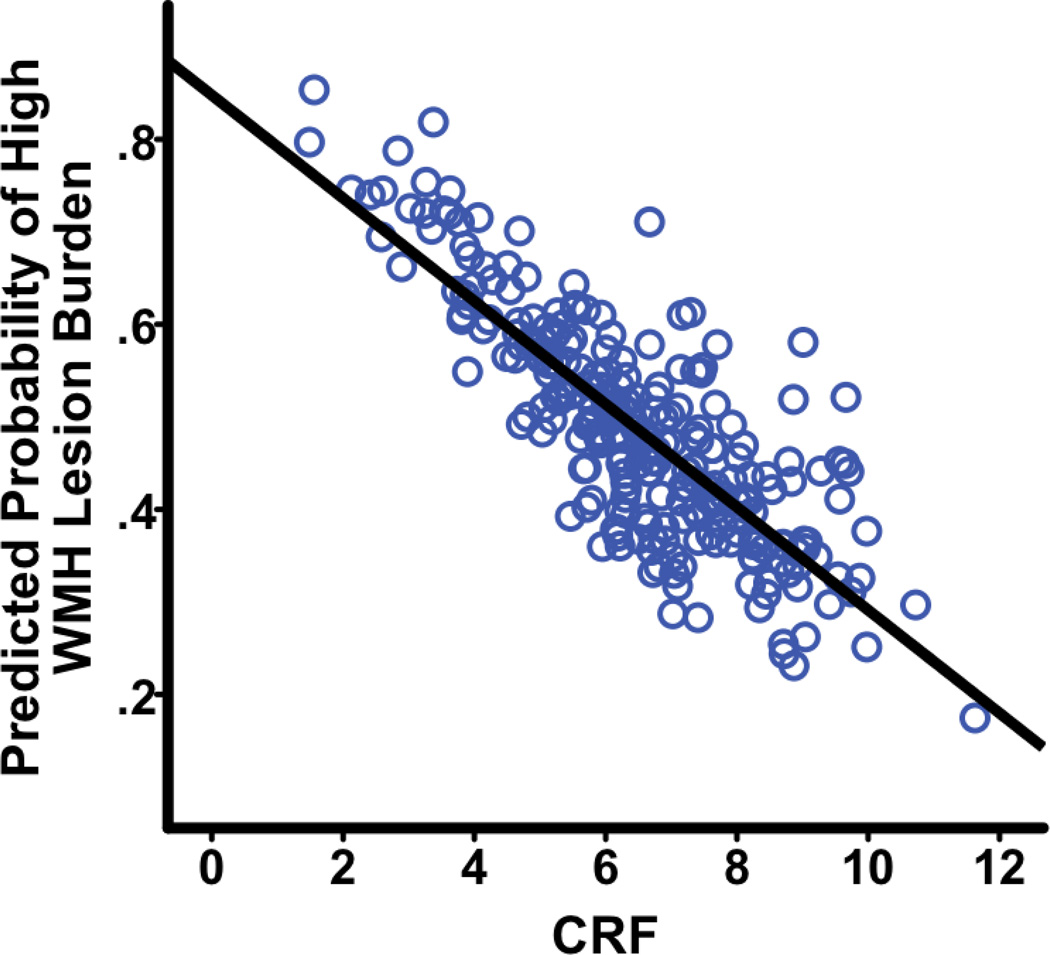
As discussed above, logistic regression was used to assess the relationship between CRF and WMH, dichotomized into low vs. high WMH. The analysis revealed that there was a significant negative relationship between CRF and lesion burden: β(SE) = −.33(.09), Waldχ2 = 13.79, OR = .72, p < .001 (see Figure 2). The odds ratio of .72 indicates that for a unit increase in CRF, there is a corresponding 28% decrease in likelihood of being in the high lesion burden group.
Fig. 2. Higher CRF is associated with lower WMH lesion burden.
CRF=cardiorespiratory fitness; WMH=white matter hyperintensities.
Relationship between CRF and Cognitive Measures
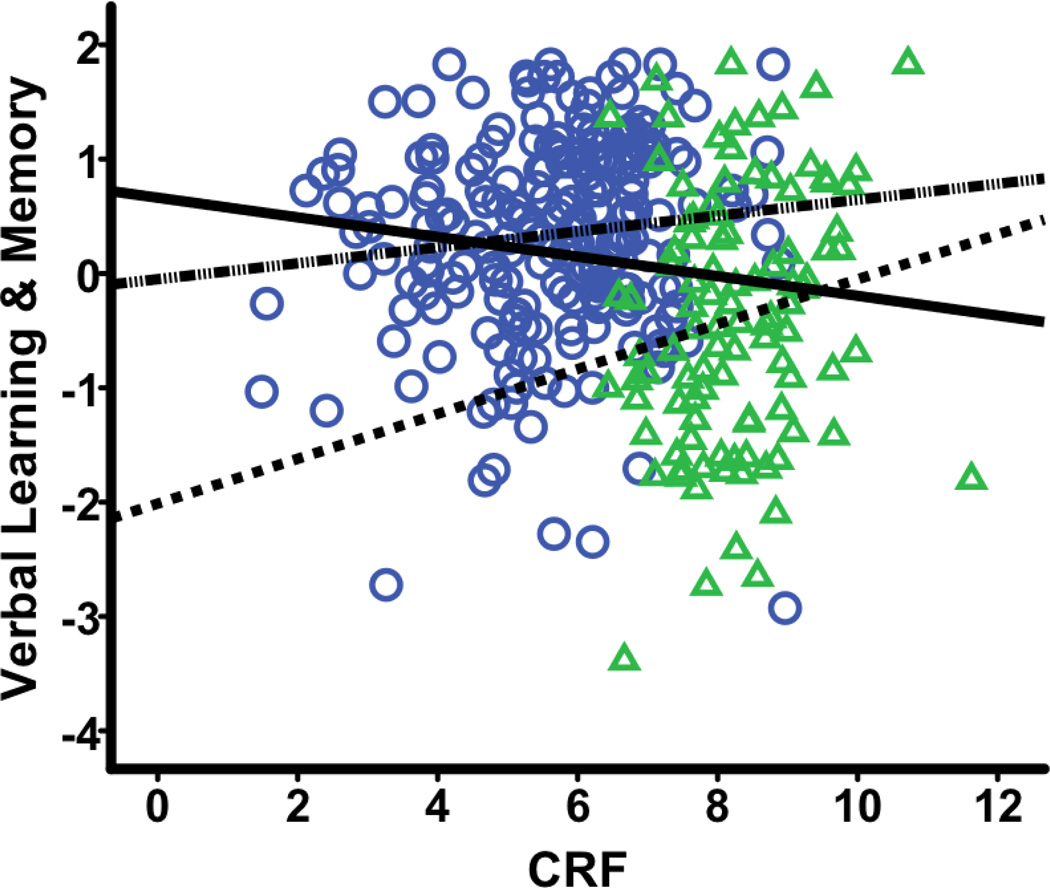
Regression analyses revealed significant associations between CRF and Visuospatial Ability, Speed & Flexibility, and Verbal Learning & Memory. The parameter estimates for both Visuospatial Ability and Speed & Flexibility were positive, indicating that higher CRF is associated with better cognitive performance on these domains. In contrast, the parameter estimate for Verbal Learning & Memory was negative (β = −.09, SE = .03, t = −2.80, p = .005, sr2 = .025), suggesting that higher CRF is associated with worse scores on this measure. This counterintuitive finding warranted further investigation.
Our intuition was that this might be an instantiation of a reversal paradox, specifically Simpson’s paradox, which refers to the phenomenon where the relationship between two variables has a different sign within subgroups compared to the sign observed in the pooled sample (Julious & Mullee, 1994; Simpson, 1951; Tu, Gunnell, & Gilthorpe, 2008). Accordingly, using graphical analyses, we investigated the relationship between CRF and Verbal Learning & Memory within subgroups defined by each of the five variables (i.e., sex, age, BMI, RHR, and Self-Reported Physical Activity) that went into the CRF equation. The subgroups were defined as follows: sex (men vs. women), age (dichotomized at the sample mean of 58.98 years), BMI (dichotomized at 25, the threshold for “overweight”) (U.S. Department of Health & Human Services, 1998), RHR (dichotomized at the sample mean of 64.72 beats/minute), and Self-Reported Physical Activity (dichotomized at 150 minutes/week) (Nelson et al., 2007; U.S. Department of Health & Human Services, 2008).
Given that the relationship between CRF and Verbal Learning & Memory in the pooled sample was negative, Simpson’s paradox would be indicated if the graphical analyses revealed that this association was positive within the subgroups formed by any of the variables, say BMI. Of the 5 variables examined, sex was the only one where this reversal was observed. Specifically, for men as well as for women, the relationship between CRF and Verbal Learning & Memory was positive (r = .17 and .11, respectively) whereas, as already noted, it was negative in the pooled sample (r = −.15; see Figure 3). The recommended fix for Simpson’s paradox is to re-fit the statistical model while including the variable causing the reversal as an additional covariate (Julious & Mullee, 1994). Therefore, we retested the association between CRF and Verbal Learning & Memory while including sex as an additional covariate. This revised analysis now revealed the expected positive relationship between CRF and Verbal Learning & Memory, thus affirming the implemented correction (see Table 4).
Fig. 2. Simpson’s paradox in the association between CRF and Verbal Learning & Memory.
This plot depicts the association between CRF and Verbal Learning & Memory, with the pooled fit line ( ) as well as the subgroup fit lines (
) as well as the subgroup fit lines (  = female,
= female,  = male) overlaid. Within the pooled sample, sex confounds the positive association between the non-exercise CRF estimate and Verbal Learning & Memory seen in the subgroups. Female = circle; Male = triangle; CRF=cardiorespiratory fitness.
= male) overlaid. Within the pooled sample, sex confounds the positive association between the non-exercise CRF estimate and Verbal Learning & Memory seen in the subgroups. Female = circle; Male = triangle; CRF=cardiorespiratory fitness.
Table 4.
Association between cardiorespiratory fitness and cognitive function
| Factor Score | β(SE) | t value | p value | sr2 |
|---|---|---|---|---|
| Verbal Ability | −0.02 (0.03) | −0.62 | .534 | 0.001 |
| Visuospatial Ability | 0.12 (0.03) | 4.51 | <.001 | 0.060 |
| Speed & Flexibility | 0.07 (0.03) | 2.14 | .033 | 0.015 |
| Working Memory | 0.01 (0.03) | 0.24 | .807 | 0.000 |
| Verbal Learning & Memory | 0.10 (0.04) | 2.30 | .022 | 0.015 |
| Immediate Memory | −0.03 (0.03) | −0.91 | .361 | 0.003 |
sr2=squared semi-partial correlation. All models were adjusted for education, except Verbal Learning & Memory, which was adjusted for both education and sex, as discussed in text
Associations between CRF, Self- and Informant-Reported Memory Complaints and Mood
Correlation analyses revealed that the CRF measure was significantly associated with self-reported memory complaints (r = −.13, p = .045), informant-reported memory complaints as measured by the IQCODE (r = −.13, p = .032), and depressive symptoms (r = −.13, p = .017). All correlation coefficients were negative, suggesting that increased CRF was associated with fewer self- and informant-reported memory complaints, as well as fewer depressive symptoms.
Secondary Analyses
The respective models that investigated associations between CRF and GM volumes, WMH, and cognition were refitted after including an APOE4*CRF term. In no model was this interaction term significant (all p’s ≥ .139). This suggests that APOE4 status does not have any modulatory effects on the observed relationship between CRF and GM volumes, WMH or cognition.
Discussion
This study found that a non-exercise estimate of CRF is related to multiple components of brain and cognitive health, including greater GM volume in regions associated with AD, lower total WM lesion burden, and better cognitive ability. These findings were not modified by APOE4 status. Interestingly, we also found that higher CRF was associated with fewer self- and informant-reported memory complaints, and reduced depressive symptoms, although these latter findings were modest in effect. Although we used a non-exercise estimate of CRF in this paper, as opposed to the gold standard GXT, our results are validated by the significant positive correlations observed between the CRF measure and VO2 within a subset of our sample, and further supported by the negative correlations between CRF and multiple measures of vascular risk within the full sample. Furthermore, outside groups have also validated this non-exercise CRF estimate, including Mailey et al. (2010) and McAuley et al. (2011) whose studies found significant correlations between the CRF estimate, maximal GXT, and a submaximal 1-mile walk test. Together, our results, which span a wide variety of imaging, cognitive, and mood measures, suggest that higher CRF could be protective of brain structure, cognitive function, and mood in adults who are at risk for AD.
The literature on the relationships between CRF and AD-relevant brain structures is rapidly expanding. Specifically, Colcombe and colleagues showed in two different studies (2003; 2004) that older adults with higher CRF as measured by GXT had significantly greater GM volumes in regions such as the prefrontal, superior parietal, and medial temporal cortices, as well as the middle frontal gyrus and anterior cingulate cortex. Similarly, another study (Gordon et al., 2008) showed that CRF was related to GM volumes in various brain regions including medial temporal, anterior parietal, and inferior frontal cortices. Other studies have chosen to assess global brain volume as opposed to regional volumes, and found significant decreases in whole brain volume in early AD patients with less CRF (Burns et al., 2008). Interestingly, McAuley et al. (2011), who previously validated the CRF estimate used in the present study, found that CRF was positively correlated with hippocampal volume. Our results, in concordance with the McAuley et al. study (2011) linked CRF to volumetric changes in the hippocampus, but with additional findings in six other areas, including other medial temporal areas such as the amygdala and posterior cortical areas such as the supramarginal gyrus and precuneus. Overall, our GM findings are consistent with these prior studies and strengthen current understanding of the implications of CRF for GM structure. Furthermore, the correspondence between our findings and these prior investigations, which were mostly conducted using GXT, suggest that non-exercise CRF measures might provide meaningful assessments of aerobic fitness.
Additionally, to our knowledge, our study is one of the first to assess WMH burden and its relationship to CRF on a large scale. The only preexisting study assessed the relationship between CRF and WMH in a group of ten former athletes (mean age = 72.4 years) compared to ten sedentary controls (mean age = 74.6 years) and found an 83% reduction in deep WMH in the athletes compared to controls (p = .002), as well as a strong negative correlation between CRF and deep WMH (p < .001) (Tseng et al., 2013). However, there were no significant differences in total WMH or periventricular WMH. Our study, with a much larger sample size, did find significant decreases in total WMH burden in participants with higher CRF. In addition, our study also demonstrated significant negative associations between CRF and measures of cardiovascular risk, including hypertension, tobacco use, and total cholesterol. As previously shown (Debette & Markus, 2010), besides age, such cardiovascular risk factors constitute major risk factors for WMH. By separately assessing the relationship between CRF and both cardiovascular risk factors and WMH, our study was able to show links between these three variables, thus adding evidence to this emerging area of research. However, it is important to note that other studies have assessed white matter integrity in relation to CRF using other techniques, such as diffusion tensor imaging (DTI). For example, Johnson et al. (2012) used DTI to detect increased fractional anisotropy and decreased radial diffusivity in the corpus callosum of older adults with higher CRF, suggesting higher WM structural integrity in these areas. The CRF–white matter relationship, especially in regards to WMH, will be important to investigate further in future studies, potentially leading to increased understanding of the implications of cardiovascular health on WMH.
In our study, we found that higher CRF was associated with increased performance in cognitive domains of Visuospatial Ability, Speed & Flexibility, and Verbal Learning & Memory. Previous research has also linked CRF to cognitive ability and cognitive decline. For example, Brown et al. (2010) showed that both global cognition and specific cognitive domains such as verbal ability, perception, processing speed, and executive function were significantly higher in older women with higher CRF. Our study assessed cognitive function across similar cognitive domains as Brown et al. (2010) and found relationships between CRF and cognition in corresponding areas among a cohort at increased risk for developing AD. In a study of older diabetic adults by Baker et al. (2010), it was shown that increases in VO2max as a result of completion of six months of aerobic exercise training were correlated with improvements on Trails B, Task Switching, and Stroop Interference trial. However, the patients did not show improvements in memory. Unlike the Baker et al. study (2010), our research did show that memory was impacted by CRF, in addition to tests of Speed & Flexibility, such as Trails B. Using a non-exercise CRF estimate, McAuley et al. (2011) found that CRF was associated with better spatial working memory, reaction time, and processing speed. Our study extends this report by interrogating a wider array of cognitive domains in relation to CRF within a cohort of individuals with known risk for AD.
Interestingly, our study also found that in addition to these associations between CRF and objective cognition, subjective memory complaints showed significant associations with CRF. Specifically, both self- and informant-reported memory complaints were lower in people with increased CRF. This is noteworthy, because other groups have indicated that subjective memory complaints may correspond with the earliest signs of incipient AD (Amariglio et al., 2012; Jessen et al., 2006). Additionally, we observed that higher CRF was also associated with fewer depressive symptoms in our study group, which is consistent with previous reports of a link between physical activity and positive affect (Conn, 2010). However, these findings were modest in effect, with relatively small r-values. Therefore, they should be interpreted with discretion. Further investigation of these specific measures is needed to more fully understand the impact CRF may have on subjective memory function and depressive symptoms.
This study was not without limitations, the most significant being the anomalous negative finding in Verbal Learning & Memory. Although sex was included in the regression equation that was used to derive the CRF estimate, it appears that there remained some residual confounding by sex, which then drove the anomalous negative association initially observed between CRF and Verbal Learning & Memory. This suggests that there is need for caution when examining the association between CRF and certain health-related outcomes (e.g., memory performance), as such associations might be confounded by measured and/or unmeasured variables, even after statistical control has been implemented. Another potential limitation to this study was the modification made to the original Jurca CRF equation (2005). It is possible that this modification might have affected the results of the study. However, previous studies have also modified the Jurca equation with no known impacts on their findings (Mailey et al., 2010; McAuley et al., 2011). Additionally, our sample was not demographically representative of the U.S. population, which could influence the generalizability of our results. It also bears noting that the amount of variance in our outcome measures accounted for by CRF was rather modest; reflecting the reality that multiple factors contribute to inter-individual variability in brain health and cognition. This study was cross-sectional in design, as well, which limits our ability to determine causality in CRF’s effect on brain structure and cognition. Future longitudinal studies within our group will examine the influence of CRF on prospective brain/cognitive changes. Intervention studies would also be helpful for establishing causal links between CRF and health-related outcomes.
In conclusion, this study found that higher CRF is related to preserved cerebral GM and WM, better cognitive abilities and mood, and decreased self- and informant-reported memory complaints in a cohort of middle-aged adults at risk for AD. These findings suggest that participation in habitual physical activity could provide protection for brain structure and cognition, thus possibly delaying onset of AD in at-risk older adults.
Acknowledgements
This work was supported by National Institute on Aging grants K23 AG045957 (OCO), R01 AG027161 (MAS), R01 AG021155 (SCJ), P50 AG033514 (SA), and P50 AG033514-S1 (OCO); by a Veterans Administration Merit Review Grant I01CX000165 (SCJ); and by a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison. Portions of this research were supported by the Wisconsin Alumni Research Foundation, the Helen Bader Foundation, Northwestern Mutual Foundation, Extendicare Foundation, and from the Veterans Administration including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI.
Special thanks to James H. Stein, MD, Claudia Korcarz, DVM RDCS, Jean Einerson, MS, Jessica Horn, BS CEP, and the rest of the Artherosclerosis Imaging Research Program for facilitating graded exercise tests; Caitlin A. Cleary, BS, Sandra Harding, MS, Jennifer Bond, BA, Janet Rowley, BA, and the WRAP psychometrists for helping with study data collection; researchers and staff at the Waisman Center, University of Wisconsin–Madison, where the brain scans took place; and participants in the Wisconsin Registry for Alzheimer’s Prevention for their continued dedication.
Footnotes
Disclosures
Elizabeth A. Boots, Stephanie A. Schultz, Jennifer M. Oh, Jordan Larson, Dorothy Edwards, Dane Cook, Rebecca L. Koscik, Maritza N. Dowling, Catherine L. Gallagher, Cynthia M. Carlsson, Howard A. Rowley, Barbara B. Bendlin, Asenath LaRue, Sanjay Asthana, Bruce P. Hermann, Mark A. Sager, Sterling C. Johnson, and Ozioma C. Okonkwo declare no conflicts of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
References
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. 9th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- Astrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. Journal of Applied Physiology. 1954;7(2):218–221. doi: 10.1152/jappl.1954.7.2.218. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Craft S. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. J Alzheimers Dis. 2010;22(2):569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10(6):675–688. [PubMed] [Google Scholar]
- Benton AL. Neuropsychological assessment. Annual Review of Psychology. 1994;45:1–23. doi: 10.1146/annurev.ps.45.020194.000245. [DOI] [PubMed] [Google Scholar]
- Birdsill AC, Koscik RL, Jonaitis EM, Johnson SC, Okonkwo OC, Hermann BP, Bendlin BB. Regional white matter hyperintensities: aging, Alzheimer's disease risk, and cognitive function. Neurobiology of Aging. 2014;35(4):769–776. doi: 10.1016/j.neurobiolaging.2013.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, Poulin MJ. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiology of Aging. 2010;31(12):2047–2057. doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS. Depressive symptom outcomes of physical activity interventions: meta-analysis findings. Annals of Behavioral Medicine. 2010;39(2):128–138. doi: 10.1007/s12160-010-9172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter yperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defina LF, Willis BL, Radford NB, Gao A, Leonard D, Haskell WL, Berry JD. The association between midlife cardiorespiratory fitness levels and later-life dementia: a cohort study. Annals of Internal Medicine. 2013;158(3):162–168. doi: 10.7326/0003-4819-158-3-201302050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling NM, Hermann B, La Rue A, Sager MA. Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer's disease. Neuropsychology. 2010;24(6):742–756. doi: 10.1037/a0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45(5):825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2009;23(3):188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AS, Blair SN, Mahar MT, Wier LT, Ross RM, Stuteville JE. Prediction of functional aerobic capacity without exercise testing. Medicine and Science in Sports and Exercise. 1990;22(6):863–870. doi: 10.1249/00005768-199012000-00021. [DOI] [PubMed] [Google Scholar]
- Jackson AS, Sui X, O'Connor DP, Church TS, Lee DC, Artero EG, Blair SN. Longitudinal cardiorespiratory fitness algorithms for clinical settings. American Journal of Preventive Medicine. 2012;43(5):512–519. doi: 10.1016/j.amepre.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Scheef L. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiology of Aging. 2006;27(12):1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59(2):1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychological Medicine. 1989;19(4):1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Julious SA, Mullee MA. Confounding and Simpson's paradox. BMJ. 1994;309(6967):1480–1481. doi: 10.1136/bmj.309.6967.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurca R, Jackson AS, LaMonte MJ, Morrow JR, Jr, Blair SN, Wareham NJ, Laukkanen R. Assessing cardiorespiratory fitness without performing exercise testing. American Journal of Preventive Medicine. 2005;29(3):185–193. doi: 10.1016/j.amepre.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kline GM, Porcari JP, Hintermeister R, Freedson PS, Ward A, McCarron RF, Rippe JM. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Medicine and Science in Sports and Exercise. 1987;19(3):253–259. [PubMed] [Google Scholar]
- Koscik RL, La Rue A, Jonaitis EM, Okonkwo OC, Johnson SC, Bendlin BB, Sager MA. Emergence of Mild Cognitive Impairment in Late Middle-Aged Adults in the Wisconsin Registry for Alzheimer's Prevention. Dementia and Geriatric Cognitive Disorders. 2014;38(1-2):16–30. doi: 10.1159/000355682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoski SG, Barlow CE, Farrell SW, Berry JD, Morrow JR, Jr, Haskell WL. Impact of body mass index, physical activity, and other clinical factors on cardiorespiratory fitness (from the Cooper Center longitudinal study) American Journal of Cardiology. 2011;108(1):34–39. doi: 10.1016/j.amjcard.2011.02.338. [DOI] [PubMed] [Google Scholar]
- Liu R, Sui X, Laditka JN, Church TS, Colabianchi N, Hussey J, Blair SN. Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Medicine and Science in Sports and Exercise. 2012;44(2):253–259. doi: 10.1249/MSS.0b013e31822cf717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailey EL, White SM, Wojcicki TR, Szabo AN, Kramer AF, McAuley E. Construct validation of a non-exercise measure of cardiorespiratory fitness in older adults. BMC Public Health. 2010;10:59. doi: 10.1186/1471-2458-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E, Szabo AN, Mailey EL, Erickson KI, Voss M, White SM, Kramer AF. Non-Exercise Estimated Cardiorespiratory Fitness: Associations with Brain Structure, Cognition, and Memory Complaints in Older Adults. Ment Health Phys Act. 2011;4(1):5–11. doi: 10.1016/j.mhpa.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL Women's Health Initiative Cohort, S. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;290(10):1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- Nes BM, Janszky I, Vatten LJ, Nilsen TI, Aspenes ST, Wisloff U. Estimating V.O 2peak from a nonexercise prediction model: the HUNT Study, Norway. Medicine and Science in Sports and Exercise. 2011;43(11):2024–2030. doi: 10.1249/MSS.0b013e31821d3f6f. [DOI] [PubMed] [Google Scholar]
- Newson RS, Kemps EB. Cardiorespiratory fitness as a predictor of successful cognitive ageing. Journal of Clinical and Experimental Neuropsychology. 2006;28(6):949–967. doi: 10.1080/13803390591004356. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Voss MW, Erickson KI, Lewis JM, Chaddock L, Malkowski E, Kramer AF. Cardiorespiratory fitness and attentional control in the aging brain. Front Hum Neurosci. 2011;4:229. doi: 10.3389/fnhum.2010.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. Journal of Geriatric Psychiatry and Neurology. 2005;18(4):245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Torrance, CA: Western Psychological Services; 1996. [Google Scholar]
- Schmidt P, Gaser C, Arsic M, Buck D, Forschler A, Berthele A, Muhlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Simpson EH. The interpretation of interaction in contingency tables. Journal of the Royal Statistical Society. Series B (Methodological) 1951:238–241. [Google Scholar]
- Trenerry M, Crosson B, DeBoe J, Leber L. Stroop Neuropsychological Screening Test. Odessa, FL: Psychological Assessment Resources, Inc; 1989. [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, Zhang R. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–516. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YK, Gunnell D, Gilthorpe MS. Simpson's Paradox, Lord's Paradox, and Suppression Effects are the same phenomenon--the reversal paradox. Emerg Themes Epidemiol. 2008;5:2. doi: 10.1186/1742-7622-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services. [Accessed May 22, 2014];Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. 1998 Available at http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.htm. [PubMed]
- U.S. Department of Health & Human Services. [Accessed February 21, 2014];2008 Physical Activity Guidelines for Americans. 2008 Available at http://www.health.gov/paguidelines/guidelines/default.aspx.
- Vidoni ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM. Cardiorespiratory fitness is associated with atrophy in Alzheimer's and aging over 2 years. Neurobiology of Aging. 2012;33(8):1624–1632. doi: 10.1016/j.neurobiolaging.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. 3rd edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wilkinson G. Wide Range Achievement Test Administration Manual. 3rd ed. Wilmington, Delaware: Wide Range Incorporated; 1993. [Google Scholar]