Abstract
Two-pore domain K+ (K2P) channels are involved in a variety of physiological processes by virtue of their high basal activity and sensitivity to various biological stimuli. One of these processes is secretion of hormones and transmitters in response to stimuli such as hypoxia, acidosis and receptor agonists. The rise in intracellular [Ca2+] ([Ca2+]i) that is critical for the secretory event can be achieved by several mechanisms: (a) Inhibition of resting (background) K+ channels, (b) activation of Na+/Ca2+-permeable channels and (c) release of Ca2+ from intracellular stores. Here, we discuss the role of TASK and TREK in stimulus-secretion mechanisms in carotid body chemoreceptor cells and adrenal medullary/cortical cells. Studies show that stimuli such as hypoxia and acidosis cause cell depolarization and transmitter/hormone secretion by inhibition of TASK or TREK. Subsequent elevation of [Ca2+]i produced by opening of voltage-dependent Ca2+ channels then activates a Na+-permeable cation channel, presumably to help sustain the depolarization and [Ca2+]i. Agonists such as angiotensin II may elevate [Ca2+]i via multiple mechanisms involving both inhibition of TASK/TREK and Ca2+ release from internal stores to cause aldosterone secretion. Thus, inhibition of resting (background) K+ channels and subsequent activation of voltage-gated Ca2+ channels and Na+-permeable non-selective cation channels may be a common ionic mechanism that lead to hormone and transmitter secretion.
Keywords: Hypoxia, carotid body glomus cells, chemoreceptors, adrenal gland, non-selective cation channel, Two-pore domain K+ channels, background K+ channel, hypoxia, acidosis, aldosterone, angiotensin II
Introduction
Excitable and non-excitable cells express two-pore domain K+ (K2P) channels that provide the cell with the ability to maintain a negative resting membrane potential (Em) and to respond to various biological stimuli. Some K2P channels such as TASK1 (K2P3.1), TASK3 (K2P9.1), THIK-1 (K2P13.1) and TRESK (K2P18.1) are basally active across the physiological range of Em and therefore help set the resting Em [17]. These K2P channels also serve to limit depolarization and promote repolarization. K2P channels such as TREK1 (K2P2.1) and TREK2 (K2P10.1) generally exhibit low basal activity at rest but are strongly activated by external stimuli such as free fatty acids and mechanical stretch of the membrane [34]. Other K2P channels such as TWIK1 (K2P1.1), TASK5 (K2P15.1) and THIK2 (K2P12.1) produce little or no measurable plasma membrane currents under physiological conditions but may become active under pathophysiological conditions. Whether they also function as ion channels of intracellular organelles is not yet known, but is a distinct possibility. K2P channels have now been implicated in a number of physiological functions such as chemoreception, hormone secretion, pain perception, fluid and salt balance, and neuroprotection [5]. In this article, we describe and discuss the role of K2P channels in stimulus-secretion coupling in the carotid body and adrenal gland.
Role of K2P channels in stimulus-secretion coupling in the carotid body
The primary function of carotid body chemoreceptors (also known as Type 1 or glomus cells) is to detect a change in arterial O2 pressure and convert this signal to increased or decreased secretion of appropriate transmitters. The transmitters bind to their receptors present at the nerve terminals of afferent carotid sinus nerve, generate action potentials that propagate to the brainstem cardiorespiratory center and increase ventilation and affect the autonomic nervous system function. During hypoxia, this feedback mechanism helps to maintain the arterial O2 pressure close to the normal level. Although the general signaling pathways by which hypoxia increases the transmitter secretion from glomus cells are known, a detailed understanding of the roles of various ion channels in this process remains incomplete and some aspects of signaling by ion channels are highly controversial. TASK1 and TASK3 are highly expressed in glomus cells and inhibited by hypoxia and acidosis, but their role in O2 sensing and subsequent transmitter secretion is still poorly defined, as discussed below.
TASK1/3 as the hypoxia-sensitive background K+ channel in carotid body glomus cells
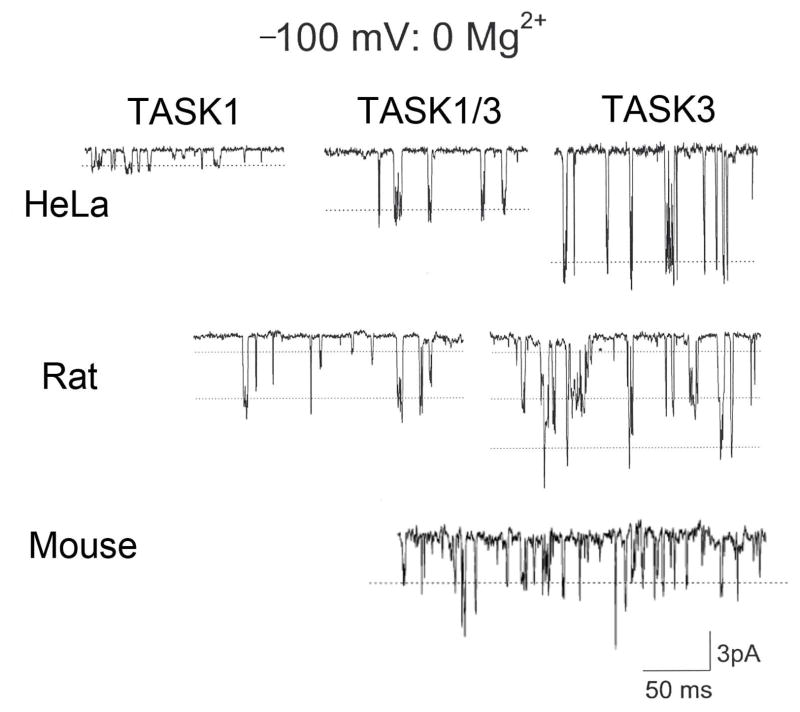
Hypoxia inhibits the background K+ current to cause depolarization that eventually leads to transmitter secretion. Therefore, the first set of studies has dealt with the identification and the hypoxic sensitivity of the background K+ channel expressed in glomus cells. Earlier studies have identified a TASK1-like K+ channel as a target of hypoxia in rat glomus cells [12]. More detailed biophysical and pharmacological analyses based on single channel recording in cell-attached patches showed that a major isoform of TASK expressed in glomus cells was a heteromer of TASK1 and TASK3 and that this heteromer was inhibited by hypoxia [35,60]. This conclusion was made possible because earlier studies showed that TASK1 and TASK3 could form a heteromer and that the single channel conductance of TASK3, but not that of TASK1, was reduced by external divalent cations [15,45]. If the concentration of divalent cations in the external solution is ~3 mM or higher, distinguishing between TASK1 and TASK3 becomes difficult as the kinetic properties of TASK1 and TASK3 are similar (~16-pS). Fig. 1 illustrates this point by showing the differences in channel amplitude when the pipette solution is Ca2+- and Mg2+-free. Fig. 1 also shows that rat and mouse glomus cells express mainly TASK1/3 heteromer with some contribution from TASK-1 and TASK-3 homomers. Recent studies using TASK knockout mice have also provided evidence that TASK1/3 heteromer is the major background O2-sensitive channel in mouse glomus cells [60]. As TASK1/3 is the main ion channel present in cell-attached patches near the resting Em, TASK1/3 should provide a major fraction of the background K+ current that helps set the resting Em in rat and mouse glomus cells.
Figure 1. Single-channel conductance levels of TASK isoforms in divalent-free external solution.
Cell-attached patch recordings from HeLa cells expressing TASK1, TASK3 and TASK1/3 heteromer, and from glomus cells isolated from ~2 week-old rat and mouse are shown. Pipette potential is held at +100 mV. Pipette solution contains (mM): 140 KCl, 5 EGTA, and 10 HEPES (pH 7.3) and the bath perfusion solution contains (mM): 140 KCl, 5 EGTA, 1 MgCl2 and 10 HEPES (pH 7.3). The single-channel conductance of TASK-like channel in glomus cells from both rat and mouse is most close to that of TASK1/3. Channel openings with conductance levels that are similar to those of TASK1 and TASK3 homomers are also observed in glomus cells but at much lower frequencies.
Interestingly, carotid body from human patients expressed TASK1 but not TASK3, as judged by microarray and PCR analyses [24,42]. Because whole carotid body consists of many types of cell, it would be important to confirm such findings by showing that the 16-pS TASK1 but not the 34-pS TASK3 is present in cell-attached patches of human glomus cells. Finding of only TASK1 in human glomus cells would suggest a species-dependent expression of TASK isoforms in the CB. If this were true, what would be the advantage of having TASK1 in human carotid body and TASK1/3 in the rodent? Small differences in pH sensitivity and O2 sensitivity exist between TASK1 and TASK1/3, but they do not suggest any clear explanation.
Although glomus cells from all breathing animal species are believed to possess O2-sensitive K+ channels, whether TASK is expressed and what TASK isoforms are present is largely unknown except for those of rat and mouse. It would be interesting to know which TASK isoform is used as O2-sensing K+ channels in various species. It is also possible that ion channels other than TASK, such as Kv and BK, are used for O2 sensing in some species. In preliminary experiments using dissociated cells from the carotid body, we detected THIK1 mRNA in clusters of rat glomus cells by RT-PCR, suggesting that THIK1 may also contribute to the background K+ conductance. Because the single channel conductance is ~5 pS [32], it is difficult to clearly identify THIK1 when the 34-pS TASK channels are always active in the same patch. Based on our initial observation, THIK-1 activity was low compared to that of TASK.
Chronic hypoxia is well known to be associated with remodeling of the carotid body, enhanced chemoreceptor sensory response and ventilatory acclimatization to hypoxia. This suggests that hypoxia may enhance the level of transmitter secretion to increase the sensory response. It seems quite possible that the level of expression and the hypoxic sensitivity of TASK may be modified in chronic hypoxia to produce such changes in cellular and physiological responses. Cell surface expression of TASK is regulated by a number of cellular signals [51,67], and it seems likely that chronic hypoxia can modify the function of these signals to alter the expression level. Chronic hypoxia can also alter mitochondrial function to sensitize TASK to hypoxia. These are exciting possibilities to test in the future.
Relative roles of TASK and BK in stimulus-secretion coupling in the carotid body
One of the unresolved issues in the topic of O2 sensing by ion channels by glomus cells is the contribution of TASK vs. BK. In addition to TASK, glomus cells express large-conductance Ca2+-activated K+ channels (BK or maxi-K) that are opened by both depolarization and elevation of [Ca2+]i. Some investigators have suggested that BK mediates the hypoxic depolarization and transmitter secretion in glomus cells by showing that BK is active at rest and hypoxia inhibits BK activity [49,50,52]. This would mean that inhibition of BK is responsible for the transmitter secretion. In our own studies using isolated glomus cells with a resting Em of ~ −60 mV, BK was clearly expressed but was always in the closed state, presumably due to a low [Ca2+]i in resting cells [35]. Depolarization of the membrane patch by more than ~30 mV was necessary to observe opening of BK, which presumably occurs as a result of cell depolarization and an increase in [Ca2+]i. Therefore, we feel that in isolated and clustered glomus cells, BK is unlikely to participate in the initial depolarization elicited by hypoxia. This is consistent with the earlier finding in isolated rat glomus cells that application of tetraethylammonium TEA that blocks BK, has no effect on [Ca2+]i [9]. The different views on BK function in O2 sensing remains unresolved at this time, and could be due to different basal resting Em of cells used in different studies. Different isolation procedures could also account for the differences in resting Em levels.
In our studies, we observed low frequency spontaneous depolarizations in a small fraction of isolated glomus cells in normoxia, possibly in part due to perfusion-induced mechanical disturbance of glomus cells [1], but the ionic nature of this phenomenon is not yet clear. TASK does not appear to be involved, as TASK activity is unchanged during such transient depolarizations. Presence of spontaneous oscillation in [Ca2+]i has also been reported earlier [28]. Because glomus cells in the carotid body are believed to be electrically-coupled by gap junctions [2], it is possible that spontaneous [Ca2+]i oscillations occur under normoxic conditions for all glomus cells in vivo, but not in dispersed cells. Such [Ca2+]i oscillations could account for the basal release of catecholamines in normoxia unrelated to any effect on TASK activity. Autocrine actions of transmitters such as ATP and adenosine may also assist in spontaneous fluctuations in Em and [Ca2+]i [59]. Clearly, more definitive studies are needed to understand the relative contributions of TASK and BK to hypoxia-induced depolarization, rise in [Ca2+]i and transmitter secretion in vitro and in vivo. The effect of hypoxia on BK also needs to be reevaluated, as most studies have not tested this under strict physiological conditions in intact cells.
Studies in mice lacking the TASK gene
The role of TASK in hypoxia-induced transmitter secretion has been studied in TASK knockout mice, but the findings from these studies are unexpected and interpretation is somewhat difficult. In the first study, the hypoxia- and hypercapnia-induced increase in ventilation was impaired in TASK1−/− and TASK1/3−/− mice (double knockout), but not in TASK3−/− mice [58]. The carotid sinus nerve activity, a measure of transmitter secretion from the carotid body, was also similarly reduced in TASK1−/− and TASK1/3−/− mice, but not in TASK3−/− mice, leading the authors to conclude that TASK1 is the important hypoxia-sensitive channel in mice. If TASK-1/3 is the major background channel, why do TASK1−/− and TASK3−/− mice show such different hypoxic responses? One possible explanation is that TASK1−/− and TASK3−/− mice express markedly different levels of TASK3 and TASK1, respectively, and thus affect the degree of the hypoxic response.
In another study, the resting Em of glomus cells measured by perforated patch technique was ~3 and ~5 mV more depolarized in TASK1−/− and TASK1/3−/− mice, respectively, than wild type mice whose resting Em was −57 mV [47]. Interestingly, catecholamine secretion in response to hypoxia was maintained in TASK1/3−/− mice even after blockade of BK channels. In support of these findings, [Ca2+] responses to hypoxia and cyanide were similar in TASK knockout and wild type mice, regardless of which TASK isoform was deleted [60]. These studies suggest that TASK has a minor role in pheripheral chemoreception. However, it is difficult to reconcile the findings observed in TASK−/− mice with what we know about TASK1/3 in glomus cells, which is that TASK1/3 is the major background K+ channel and strongly inhibited by hypoxia [10,35]. Could it be that the loss of TASK1/3 is somehow compensated by a change in chemoreceptive mechanism in the carotid body as well as in other respiratory areas of the brainstem? If so, there must be other ion channels that are open near the resting Em and sensitive to inhibition by hypoxia, but such channels have not yet been reported. It would be interesting to determine which non-TASK ion channels are expressed in TASK1/3−/− mice and which of these ion channels are sensitive to hypoxia. Central chemoreception as judged by the ventilatory sensitivity to elevated pCO2 was found to be unchanged in TASK1/3−/− mice compared to wild type mice [43]. Therefore, the remodeling may be specific to the peripheral chemoreceptors. To resolve these important issues, a conditional knockout system with drug-controlled transcriptional activation where transcription of a gene can be reversibly turned on or off would be preferred. This would limit the extent of remodeling of the O2 sensing mechanism and allow one to study the function of an ion channel without involvement of large compensatory mechanisms.
TASK inhibition recruits a Ca2+-activated, Na+-permeable cation channel
When a K+ channel such as TASK is inhibited by hypoxia, it is the resting Na+ influx via Na+-permeable channels that depolarize the cells. Na+-permeable channels can be background Na+ channels or non-selective cation channels (NSCC). NSCC is usually expressed in nearly every cell type, and we have recently identified and characterized a 20-pS NSCC in glomus cells [33]. The 20-pS channel is mostly closed in normoxia and therefore is not a background Na+ channel that is basally active. Interestingly, hypoxia-induced inhibition of TASK1/3 was quickly followed by activation of the 20-pS channel. In addition to hypoxia, stimuli that depolarized glomus cells such as high external KCl and acid (pH 6.0) also activated the 20-pS channel. Further studies showed that the rise in [Ca2+]i was directly responsible for the activation of the NSCC channel. Thus, a part of the hypoxia-induced depolarization is due to Na+ influx via NSCC. Increased Na+ influx should enhance Ca2+ influx via voltage-dependent Ca2+ channels and contribute to the elevation of [Ca2+]i until a steady state level is reached for a given level of hypoxia. Although the properties of the 20-pS NSCC channel are similar to those of TRPM4 and TRPM5 that are also Ca2+-activated monovalent cation channels, TRPM4−/− and TRPM5−/− mice still expressed the 20-pS channel. Therefore, the molecular identity of the 20-pS NSCC is yet to be determined.
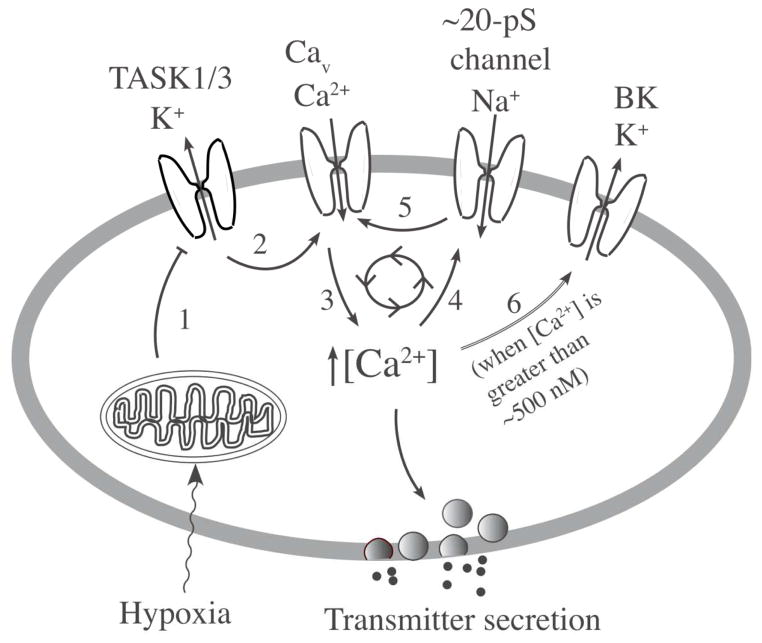
The threshold level of [Ca2+]i that activated NSCC was about ~100 nM higher than the basal [Ca2+] level of ~100 nM measured and calculated with our protocol. Studies in isolated rat glomus cells showed that a mild level of hypoxia (>5% O2) inhibits TASK activity up to 50%, and produces a small increase (~100 nM above basal) in [Ca2+]i. However, this low level of increase in [Ca2+] was generally ineffective in activating NSCC. A moderate to severe hypoxia (0–5% O2) inhibited TASK activity by 5080%, increased [Ca2+]i above ~200 nM, and activated NSCC. As the reversal potential of the 20-pS channel is ~ −28 mV, its activation should lead to Na+ influx and further depolarization up to −28 mV. These findings suggest that the transmitter secretion produced by mild hypoxia is probably due to inhibition of TASK and a small rise in [Ca2+]i without involvement of the NSCC. On the other hand, transmitter secretion produced by moderate to severe hypoxia is likely to involve TASK inhibition and enhanced Na+ influx via the NSCC due to the higher level of [Ca2+]i. Thus, NSCC may provide a mechanism to enhance depolarization and the rise in [Ca2+]i during moderate to severe hypoxia. These ionic mechanisms are schematically illustrated in Fig. 2, which shows a model of O2 sensing that incorporates both TASK and the 20-pS channel. The contribution of TASK and the NSCC to the excitation of glomus cells in response to hypoxia needs to be further demonstrated using a selective inhibitor. In the future, it would be important to show that the 20-pS cation channel is also expressed in human glomus cells. Defining the function of the 20-pS channel in glomus cells in more intact system (i.e., in undissociated carotid body) would also be important to further understand its role in hypoxia-induced transmitter secretion from glomus cells.
Figure 2. A model showing a critical role of TASK in hypoxia-induced secretion of transmitters.
Hypoxia acts on the O2 sensor (probably mitochondria) that generates a signal that inhibits TASK activity (step 1). Inhibition of TASK leads to cell depolarization due to continuous influx of Na+ via background Na+ channels and this opens Ca2+ channels (step 2). Ca2+ influx increases intracellular [Ca2+] in the cell (step 3) stimulating the secretion of transmitters. Ca2+ acts on the 20-pS cation channel (NSCC) to increase Na+ influx (step 4) and enhance Ca2+ influx (step 5). High [Ca2+]i activates BK to limit further depolarization (step 6). These mechanisms maintain the level of depolarization and [Ca2+]i in response to a given level of hypoxia.
Role of TASK in autoregulation of transmitter secretion in glomus cells
Hypoxia causes secretion of many transmitters from glomus cells and these include dopamine, acetylcholine (ACh), ATP, endothelin, nitric oxide and serotonin [44,46]. Among these, ACh and ATP are thought to be the primary transmitters that increase afferent carotid sinus nerve activity [66]. These agonists are thought to “autoregulate” transmitter secretion via autocrine and paracrine pathways, where they bind to receptors present on glomus cells and modify glomus cell excitability. The effects of transmitters on the carotid body function are very complex, where some transmitters suppress and others enhance the initial response to hypoxia. The effect of each secreted transmitter on glomus cell excitability, the net effect of all secreted transmitters on glomus cell excitability, and the concentrations reached at the nerve terminal are yet to be clearly defined.
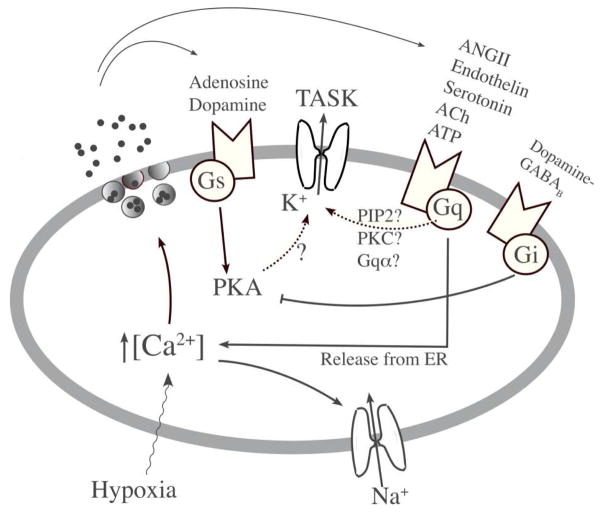
Although we now know that transmitters bind to the receptors on glomus cells, the signaling pathways that modulate the transmitter secretion are not well defined. Most transmitters secreted by glomus cells bind to receptors that are coupled to Gq, Gs and Gi/o whose cellular targets include TASK (Fig. 3). The reported mechanisms by which an agonist that binds to a Gq-coupled receptor inhibits TASK include a direct action of Gqα on TASK, depletion of membrane phosphatidylinositol-4,5-bisphosphate, phosphorylation by protein kinase C and a PLC-mediated pathway unrelated to PKC [13,41,54,61]. Because most of the studies on signaling pathways involving G proteins have been performed in heterologous expression systems, it is difficult to know which of these mechanisms are functional in native cells. Regardless of the mechanisms involved, agonists such as angiotensin II (AngII), endothelin-1, ATP, serotonin and dopamine that can bind to Gq-coupled receptors are predicted to inhibit TASK and thus augment transmitter secretion. In our own study, 0.1 αM AngII applied to cell-attached patches of rat glomus cells did not significantly affect TASK activity but elevated [Ca2+]i (unpublished). This finding suggests that the AT1 receptor-Gq is coupled to ER Ca2+ release but not to TASK in glomus cells. In the future, it will be necessary to test the effect of each transmitter as well as receptor antagonists on TASK and cell function in glomus cells to determine which transmitters inhibit TASK and which signaling mechanism is involved. For transmitters such as AngII and ACh that augment [Ca2+]i in glomus cells presumably via release of Ca2+ from internal stores as well as influx from extracellular space [19,27], it would also be interesting to know whether they also activate the Ca2+-sensitive non-selective cation channel to help sustain the [Ca2+]i response and transmitter secretion.
Figure 3. Putative auto-regulatory mechanisms involving TASK in glomus cells.
Glomus cells express a large number of different types of receptors coupled to Gs, Gi and Gq. Transmitters secreted from glomus cells by hypoxia probably bind to such receptors and modulate cell excitability via autocrine and paracrine pathways. Although it has been suggested that agonists linked to PKA modulate TASK activity, this remains to be proven. Agonists coupled to Gq are predicted to inhibit TASK activity, but they have not yet been tested directly in glomus cells. Which of these transmitters produce a significant feedback on hypoxia-induced depolarization is not clear and this topic remains an area of future study.
Interestingly, baclofen, a γ-aminobutyric acid (GABAB) receptor agonist coupled to Gi/o, was found to increase TASK-like current in glomus cells and this was via protein kinase A (PKA) [25]. On the other hand, adenosine acting on Gs-coupled A2a receptor inhibited TASK-like current and caused depolarization and elevation of [Ca2+]i via the PKA pathway [64]. These results suggest that TASK activity is regulated by phosphorylation by PKA. As whole-cell recordings cannot really identify TASK, it is unclear whether the recorded current affected by PKA was truly TASK. It has become customary to call a voltage-independent leak current as TASK, but one has to keep in mind that there are other non-TASK leak channels. A more definitive study is needed to address the PKA-TASK coupling by recording TASK channels only and also show that phosphorylation of TASK by PKA modulates TASK activity in carotid body glomus cells. In future studies, it would make sense to record single identifiable TASK channels in response to inhibitors and activators of PKA using glomus cells or other native cells that express TASK.
Insensitivity of cloned TASK to hypoxia in mammalian cell lines
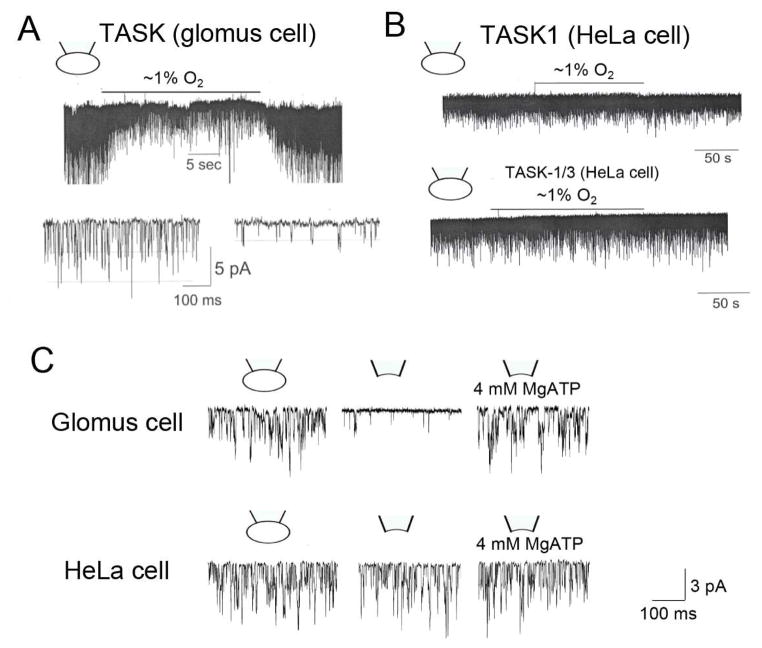
There are two important differences in TASK function in glomus cells and in cell lines made to express TASK by transfection. First, endogenous TASK expressed in glomus cells is highly sensitive to hypoxia (Fig. 4A), but TASK1, TASK3 or TASK1/3 expressed in mammalian cell lines such as HEK293, COS-7 or HeLa cells show very low or no sensitivity to hypoxia (Fig. 4B). Second, TASK in glomus cells is highly sensitive to intracellular [ATP] such that washout of ATP (by forming an inside-out patch) results in a large decrease (~90%) in TASK activity [62]. However, removal of ATP from these mammalian cell lines expressing a TASK by forming an inside-out patch has a minimal effect on TASK activity (Fig. 4C). In fact, one potential proposed mechanism by which hypoxia inhibits TASK in glomus cells is a decrease in cytosolic [ATP] near the plasma membrane due to reduced generation of ATP by mitochondria [11,62]. Therefore, one reason that hypoxia does not inhibit TASK expressed in the cell lines may be that cloned TASK is insensitive to ATP.
Figure 4. O2 and ATP sensitivity of TASK expressed in glomus cells and HeLa cells.
(A) Tracing shows a rapid and reversible inhibition of TASK activity in response to hypoxia in glomus cells. Channel currents are recorded from cell-attached patches with pipette potential at 0 mV. Pipette solution contains (mM): 140 KCl, 5 EGTA, 1 MgCl2 and 10 HEPES (pH 7.3) and the bath perfusion solution contains (mM): 117 NaCl, 5 KCl, 23 NaHCO3, 1 MgCl2, 1 CaCl2, 10 HEPES and 11 glucose (pH 7.3). Expanded current tracings show that hypoxia reduces TASK activity as well as the amplitude. (B) Same experiment as in A except that HeLa cells expressing TASK1 or TASK1/3 are used for channel current recording. Hypoxia has no effect on TASK activity. (C) Cell-attached patch recording from glomus cells shows robust TASK activity. TASK activity decreases markedly upon formation of inside-patch in glomus cells but not in HEK293 cells. Application of MgATP restores TASK activity in glomus cells.
The different sensitivities of TASK in glomus cells and cell lines to hypoxia and ATP pose an interesting dilemma because TASK in glomus cells and HEK/COS/HeLa cells is expected to be structurally and functionally identical. Consistent with this notion, TASK1/3 expressed in cell lines shows biophysical properties and sensitivities to various modulators (pHo, ruthenium red, divalent cation) similar to those observed with TASK in glomus cells [35]. The finding that TASK expressed in cell lines is insensitive to ATP is intriguing, as it suggests the presence of an ATP-sensitive accessory protein in glomus cells that is not present elsewhere. Identifying the mechanism of the difference in ATP sensitivity should reveal an important aspect of TASK function that we do not yet know.
For hypoxia signaling, it seems likely that intracellular signaling components for hypoxia-TASK coupling is either missing in cell lines or that the sensitivity of the O2 sensing system is low compared to that in glomus cells. As the hypoxic inhibition of TASK is thought to be via inhibition of the mitochondrial oxidative phosphorylation process, perhaps the mitochondrial O2 sensing hemeproteins are far less sensitive to hypoxia in cell lines than those in glomus cells. Indeed, it was found that the mitochondria of glomus cells are unusually sensitive to hypoxia compared to those of sympathetic neurons, based on measurement of mitochondrial Em and [NADH] [11]. Differences in the O2 affinity of mitochondrial enzymes could also explain why glomus cells from newborn animals are less sensitive to hypoxia than those from older (>3 weeks) animals with respect to both TASK inhibition and rise in [Ca2+]i [38,56]. Numerous studies have shown that mitochondria undergo postnatal maturation of enzymatic activities, coupling between enzymes and enzyme synthesis. Whether such changes occur during postnatal maturation of carotid body would be important to know.
Role of TASK in hypoglycemia-secretion coupling
The role of carotid body as a chemosensor for hypoxia, hypercapnia and acidosis is well established, but its role as a glucosensor is controversial. In a study, the carotid body (or receptors anatomically close by) was required for insulin-induced counter-regulatory response to mild hypoglycemia in dogs [39]. In support of this finding, low glucose stimulated the secretion of catecholamines in rat carotid body slices, the secretion of ACh in whole cat carotid bodies, and the sensory discharge in co-cultured glomus cell-petrosal neuron preparations [26,48,65]. In other studies using isolated preparations where chemoafferent nerve discharge or catecholamine secretion was measured, low glucose failed to elicit a response [6,28]. In isolated glomus cells from ~2–3 week old Sprague-Dawley rats, removal of glucose did not elicit an increase in [Ca2+]i or inhibit TASK activity [37]. In glomus cells isolated from adult Wistar rats, removal of glucose from the perfusion solution produced a small increase in [Ca2+]i, and potentiated the hypoxia-induced increase in [Ca2+]i [28]. The rise in [Ca2+]i produced by glucose-free solution was attributed to inhibition of sodium pump and modulation of Na/Ca exchanger.
Thus, evidences for and against the role of carotid body as a glucosensor have been presented, but it is difficult to resolve the opposite findings. In experiments where low glucose excites glomus cells, could TASK be involved? Our own studies showed no acute (~5 min) effect of removal of glucose on TASK activity or the resting Em in isolated rat glomus cells, and therefore, we are led to conclude that the carotid body is unlikely to be a direct glucosensor that rapidly detects the level of arterial glucose concentration. Rather, it may be an indirect effect of metabolic changes produced by hypoglycemia that modulates the carotid body function, as suggested previously [6]. Further studies are needed to study the relationship between various metabolic conditions and the function of the carotid body.
Role of K2P channels in stimulus-secretion coupling in the adrenal gland
Adrenal gland is another organ that expresses a high level of TASK. Both adrenal medulla and cortex show abundant expression of TASK1 and TASK3 with high basal activity. TREK-1 is also expressed in the adrenal gland [21]. TASK5 mRNA is detected in human adrenal gland, but cloned TASK5 does not form a functional channel at the plasma membrane [36]. Thus, cells of the adrenal gland use TASK and TREK to regulate their resting Em and excitability.
Chromaffin cells of adrenal medulla secrete catecholamines in response to ACh released from sympathetic preganglionic neurons within the splanchnic nerve. Catecholamines regulate vascular resistance and cardiorespiratory function in preparation for physical activity (fight-or-flight response). In the newborn, hypoxia and acidosis stimulate the secretion of catecholamines directly from adrenal medulla, as a part of the physiological response to adapt to the new atmospheric environment. In adrenal glomerulosa cells, angiotensin II (AngII) causes secretion of aldosterone that regulates blood pressure by increasing reabsorption of sodium and increased excretion of both potassium (by principal cells) and hydrogen ions (by intercalated cells of the collecting duct) in the kidney. There is now evidence indicating that stimulus-induced secretion of these hormones involves TASK and TREK.
TASK and secretion of catecholamines
Innervation of adrenal medulla by the sympathetic nervous system is absent in the newborn and occurs during the first few weeks of life [7,57]. When the sympathetic innervation is present, the secretion of catecholamines from the adrenal chromaffin cells in response to increased sympathetic activity is due to ACh binding to nicotinic receptors whose opening results in Na+ influx, cell depolarization, opening of the Ca2+ channel, elevation of [Ca2+]i and exocytosis of vesicles containing catecholamines. In the newborn, where the innervation of the adrenal gland is not present, the secretion of catecholamines that occurs in response to stress such as hypoxia and acidosis is also produced as a result of elevation of [Ca2+]i, but this does not involve Na+ influx via nicotinic receptors.
In adrenal medullary cells of newborn animals, studies show that hypoxia-induced elevation of [Ca2+]i is mediated by inhibition of mitochondrial oxidative phosphorylation [8]. Could the mechanism in adrenal chromaffin cells therefore be similar to that in carotid body glomus cells and occur via inhibition of TASK? Although TASK is expressed in adrenal chromaffin cells, the role of this K+ channel in hypoxia-induced secretion of catecholamines is not clear. To address this question, we recorded single channels from cell-attached patches from adrenal medullary chromaffin cells isolated from newborn rats. The most abundant channel was identified to be TASK1/3 with properties similar to that in carotid body glomus cells (Fig. 5A). TASK1/3 in adrenal cells was moderately sensitive to hypoxia, with 30–40% reduction in channel activity. Therefore, the inhibition of TASK may partly underlie the hypoxia-induced depolarization, elevation of [Ca2+]i, and secretion of catecholamines from adrenal medullary cells. Non-neurogenic response of adrenal chromaffin cells to hypoxia and hypercapnia observed in newborn animals disappears after ~two weeks, as the adrenal gland begins to be innervated. After innervation, the medullary cells lose their sensitivity to hypoxia. It would be interesting to know whether this is due to a loss of hypoxic sensitivity of TASK.
Figure 5. Basally active channels in isolated rat adrenal medullary cells.

Tracings show single-channel openings from cell-attached patches with pipette and bath solutions containing 140 mM KCl. TASK (A) and Type 4 K+ (B) channels were most frequently observed, followed by TREK (C). In the last tracing (D), a non-selective cation channel with long-lasting opening (see arrow) was also observed in some patches. This cation channel was activated by a rise in intracellular [Ca2+] and is most likely TRPM4. These four types of ion channels were also present in adrenal cortical cells.
In adrenal medulla, hypercapnia and acidosis also augment secretion of catecholamines, but this does not involve mitochondria. Although the mechanism is not yet known, it seems likely that an acid-sensitive K+ channel such as TASK is the target of hypercapnia and acidosis. Our own study of ion channels in isolated rat adrenal medullary cells shows that there are two types of acid-sensitive K+ channels. One is TASK1/3 with a single channel conductance of 34-pS and a mildly inward rectifying current-voltage relationship, similar to that in carotid body glomus cells. The other is a ~36-pS K+ channel but with single channel kinetics different from those of TASK1/3 (see Fig. 5B). The 36-pS K+ channel was inhibited by 1 mM tetraethylammonium (TEA) applied to the extracellular side of the outside-out patches. It also showed a strong voltage-dependence and a high external pH sensitivity, similar to that previously observed in cerebellar granule cells where it was designated “Type 4 K+ channel” [30]. The 36-pS K+ channel was the second most frequently recorded channel in adrenal medullary cells. Therefore, both TASK and the 36-pS channel are likely to convey the acid signal to catecholamine secretion not only in the newborn but also in adult animals.
In isolated adrenal medullary cells from newborn rats, TREK channels with low basal activity were also observed (Fig. 5C). Human TREK-1 has been reported to be sensitive to low external pH [53], and therefore could participate in regulating the secretion of catecholamines, especially if TREK activity is elevated in certain pathological conditions. Adrenal medullary cells also express a non-selective cation channel with properties similar to those of TRPM4 that is activated by a rise in [Ca2+]I (Fig. 5D). This TRPM4-like channel could be involved in hypoxia-induced depolarization and elevation of [Ca2+]i. Surprisingly, adult TRPM4−/− mice show enhanced catecholamine secretion in response to ACh and increased sympathetic tone and hypertension [40]. A possibility exists that deletion of TRPM4 elicits a compensatory response to enhance the sensitivity of the cells to ACh.
TASK and secretion of aldosterone
In this Issue, Bandulik et al. discuss the topic of aldosterone secretion by the adrenal gland from their studies using TASK knockout mice. Here we focus on intracellular signaling pathways involving TASK/TREK. In adrenal glomerulosa cells of the cortex, AngII binds to AT1 receptors and promotes secretion of aldosterone, a mineralocorticoid that regulates fluid and salt balance (Fig. 6). As AT1 receptor is coupled to Gq, AngII is predicted to inhibit TASK in glomerulosa cells and cause cell depolarization leading to aldosterone secretion. Indeed, AngII was found to inhibit both TASK in glomerulosa cells as well as in oocytes expressing AT1 receptor and TASK [16,18]. As predicted, deletion of TASK genes causes various types of hyperaldosteronism, but the phenotypes produced by knockout of TASK are not easily explained simply based on the function of TASK as a background K+ channel. For example, the same TASK gene deletion produced different remodeling of the adrenal gland in male and female mice and the studies also suggest different roles for TASK-1 and TASK-3 in adrenal cortical function [3,4].
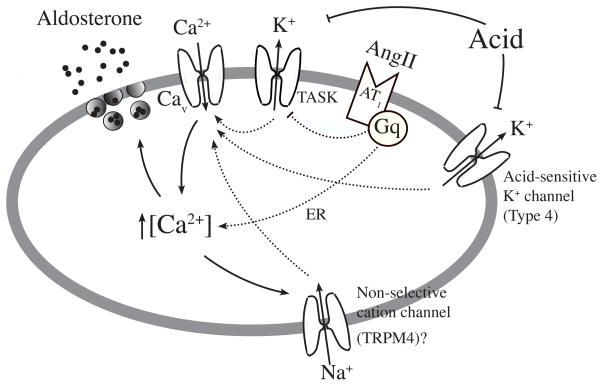
Figure 6. A model depicting pathways by which AngII stimulates aldosterone secretion in adrenal glomerulosa cells.
Adrenal glomerulosa cells express TASK, Type 4 K+ channel, and TRPM4-like cation channel. TREK is also expressed but not shown. AngII elevates [Ca2+] presumably by inhibiting TASK (via Gq) and/or by releasing Ca2+ from ER. Acid is expected to act on two acid-sensing K+ channels (TASK and the Type 4 channel) to elevate [Ca2+]. TRPM4-like cation channel, by allowing Na+ influx, is also predicted to assist in the depolarization and elevation of [Ca2+].
Stimulation of aldosterone production by metabolic acidosis enhances proton excretion and serves to limit disturbances in systemic acid-base equilibrium. Hyperkalemia is another well-known stimulus for aldosterone secretion. Hyperkalemia should cause depolarization by reducing efflux of K+ through background K+ channels and enhance Ca2+ influx via voltage-dependent Ca2+ channels. For acidosis, one would predict that inhibition of the acid-sensitive K+ channels such as TASK would cause depolarization. However, a recent study shows that in the presence of AT1R blockade, mice lacking TASK1/3 unexpectedly show a larger aldosterone secretion in response to acid loading compared to wild type control [29]. In the presence of AT1R blockade, acid loading in TASK−/− mice induced a marked increase in aldosterone production that was of greater magnitude than in WT mice. Although TASK is probably involved to a certain extent, this finding in TASK1/3−/− mice indicates that there must be another target for acid.
Our own single channel studies show that there is in fact another type of K+ channel that is highly pHo-sensitive and abundantly expressed in adrenal cortical cells. This acid-sensitive (Type 4) K+ channel is identical in biophysical properties to those described above for adrenal medullary cells and cerebellar granule neurons. The strong acid sensitivity (~65% inhibition by changing pHo from 7.3 to 6.8) suggests that this K+ channel is likely to be a target for acid. The relative importance of TASK and the Type 4 channel in acid-induced production of aldosterone will need to be studied further. It seems quite possible that both K+ channels are necessary for transduction of the acid signal into depolarization.
Interestingly, a high level of expression of TREK1 was reported in bovine adrenal glomerulsa cells with no detection of TASK3 mRNA [23]. Both AngII and vasopressin strongly inhibited (~70–80%) TREK1 and depolarized the cells by ~30 mV [21]. Gq-coupled receptor agonists inhibited TREK1 and TREK2 in heterologous expression system, and this effect was observed in bovine adrenal glomerulosa cells. Inhibition of TREK1 by AngII was found to be due to a rise in [Ca2+]i rather than PIP2 depletion [22]. The inhibition of TREK by Ca2+ is somewhat curious, because TREK in general is not thought of as a Ca2+-sensitive channel. Perhaps, the inhibition occurs indirectly via Ca2+-mediated signals in this particular cell type. In our own experiments, both TASK and TREK were observed in rat adrenal medullary and cortical cells, but mainly TASK was active. Formation of inside-out patches or applying arachidonic acid caused activation of TREK-like channels. Therefore, questions arise as to why bovine adrenal glomerulosa cells express only TREK1 and what makes TREK1 active at rest in these cells. It would be interesting to determine the expression of TASK and TREK in adrenal glands of other animal species, and identify which of the two is involved in aldosterone secretion.
Stimulus-secretion coupling in other cell types
The stimulus-secretion coupling mechanisms described for the carotid body and adrenal gland resemble those described for pancreatic β cells. For example, glucose stimulates the release of insulin from pancreatic β cells. This involves ATP-sensitive K+ channel that is inhibited by glucose-generated ATP in the cell, and TRPM2/4 that is activated by a rise in [Ca2+]i and further modulates insulin secretion. A few other examples of stimulus-secretion coupling processes involving K2P channels can also be identified. In hippocampal astrocytes, stimulation of the cannabinoid receptor induced a fast glutamate secretion that was dependent on binding of Gβγ to the N-terminus of TWIK1/TREK1 heterodimer [31,63]. Authors have suggested an intriguing possibility that Gβγ binding induces a pore dilation to allow permeation of large molecules such as glutamate, but this remains to be experimentally demonstrated. In MLE-12 murine alveolar epithelial cells, chronic hyperoxia and mechanical stretch reduced the expression of TREK1 [55]. Authors have suggested that prolonged hyperoxia and stretch promote secretion of inflammatory mediators (cytokines) in this tissue as a result of reduced TREK1 function. Pancreatic exocrine cells express TALK1 and TALK2 that are modulated by nitric oxide and reactive oxygen species that regulate the secretory function [20]. Cardiac myocytes express TASK1 and TREK1, and secretion of atrial natriuretic peptide may be modulated by pH- and stretch-induced changes in these K+ channel function. These examples suggest that K2P channels are involved in stimulus-secretion coupling processes in many types of cells.
Future directions
K2P channels such as TASK serve as background K+ channels that provide the necessary K+ efflux pathway to maintain a negative resting Em and aid in repolarization when cells depolarize. Studies in the carotid body and adrenal gland show that K2P channels also participate in the secretion of transmitters and hormones in response to biological stimuli. This is accomplished by inhibition of a basally active K2P channel, which leads to cell depolarization and Ca2+ influx via voltage-dependent Ca2+ channels. The rise in [Ca2+]i then promotes the exocytosis of vesicles containing transmitters and hormones. There are several important questions that still need to be addressed to better understand the role of K2P channels in stimulus-secretion coupling. (1) How much contribution does each type of K2P channel make to the stabilization of the resting Em in a given cell type? In other words, is the stimulus-induced inhibition of a K2P channel alone sufficient to elicit the depolarization necessary for transmitter/hormone secretion, or does the K2P channel work together with other ion channels to achieve the physiological response? These questions address the lack of effect of TASK deletion on hypoxic excitation of carotid body glomus cells and acid-induced secretion of aldosterone on adrenal cells. (2) How does a stimulus inhibit a K2P channel such as TASK or TREK? At present, we do not fully understand how hypoxia inhibits TASK1/3 in chemoreceptor cells of the carotid body, how AngII modulates TASK in adrenal cortical cells, and how cannabinoids enhance glutamate secretion through TWIK1/TREK1 heterodimers in astrocytes. These are difficult questions to answer and probably require new experimental techniques. (3) Are there ways to directly modulate the function of TASK or TREK to depress or enhance secretion of transmitters and hormones? For example, doxapram is a respiratory stimulant that inhibits TASK in the carotid body and possibly at other sites and helps in ventilation [14]. Development of drugs that acts on these K2P channels would not only help to better understand the role of these K2P channel, but may also help to treat conditions such as dyspnea and hyperaldosteronism.
Acknowledgments
This work was made possible by a grant from NIH (HL-111497).
References
- 1.Abudara V, Eyzaguirre C. Mechanical sensitivity of carotid body glomus cells. Respir Physiol Neurobiol. 2008;161(2):210–213. doi: 10.1016/j.resp.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abudara V, Jiang RG, Eyzaguirre C. Behavior of junction channels between rat glomus cells during normoxia and hypoxia. J Neurophysiol. 2002;88(2):639–649. doi: 10.1152/jn.2002.88.2.639. [DOI] [PubMed] [Google Scholar]
- 3.Bandulik S, Penton D, Barhanin J, Warth R. TASK1 and TASK3 potassium channels: determinants of aldosterone secretion and adrenocortical zonation. Horm Metab Res. 2010;42(6):450–457. doi: 10.1055/s-0029-1243601. [DOI] [PubMed] [Google Scholar]
- 4.Bandulik S, Tauber P, Penton D, Schweda F, Tegtmeier I, Sterner C, Lalli E, Lesage F, Hartmann M, Barhanin J, Warth R. Severe hyperaldosteronism in neonatal Task3 potassium channel knockout mice is associated with activation of the intraadrenal renin-angiotensin system. Endocrinology. 2013;154(8):2712–2722. doi: 10.1210/en.2013-1101. [DOI] [PubMed] [Google Scholar]
- 5.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29(11):566–575. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bin-Jaliah I, Maskell PD, Kumar P. Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physiol. 2004;556(Pt 1):255–266. doi: 10.1113/jphysiol.2003.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bournaud R, Hidalgo J, Yu H, Girard E, Shimahara T. Catecholamine secretion from rat foetal adrenal chromaffin cells and hypoxia sensitivity. Pflugers Arch. 2007;454(1):83–92. doi: 10.1007/s00424-006-0185-z. [DOI] [PubMed] [Google Scholar]
- 8.Brown ST, Buttigieg J, Nurse CA. Divergent roles of reactive oxygen species in the responses of perinatal adrenal chromaffin cells to hypoxic challenges. Respir Physiol Neurobiol. 2010;174(3):252–258. doi: 10.1016/j.resp.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498(Pt 3):649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157(1):55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Buckler KJ, Turner PJ. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol. 2013;591(Pt 14):3549–3563. doi: 10.1113/jphysiol.2013.257741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525(Pt 1):135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, Viana F, Garrison JC, Bayliss DA. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci U S A. 2006;103(9):3422–3427. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotten JF, Keshavaprasad B, Laster MJ, Eger EI, 2nd, Yost CS. The ventilatory stimulant doxapram inhibits TASK tandem pore (K2P) potassium channel function but does not affect minimum alveolar anesthetic concentration. Anesth Analg. 2006;102(3):779–785. doi: 10.1213/01.ane.0000194289.34345.63. [DOI] [PubMed] [Google Scholar]
- 15.Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002;277(7):5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- 16.Czirjak G, Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol. 2002;16(3):621–629. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- 17.Czirjak G, Enyedi P. TRESK background K(+) channel is inhibited by phosphorylation via two distinct pathways. J Biol Chem. 2010;285(19):14549–14557. doi: 10.1074/jbc.M110.102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol. 2000;14(6):863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- 19.Dasso LL, Buckler KJ, Vaughan-Jones RD. Muscarinic and nicotinic receptors raise intracellular Ca2+ levels in rat carotid body type I cells. J Physiol. 1997;498(Pt 2):327–338. doi: 10.1113/jphysiol.1997.sp021861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duprat F, Girard C, Jarretou G, Lazdunski M. Pancreatic two P domain K+ channels TALK-1 and TALK-2 are activated by nitric oxide and reactive oxygen species. J Physiol. 2005;562(Pt 1):235–244. doi: 10.1113/jphysiol.2004.071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enyeart JJ, Danthi SJ, Liu H, Enyeart JA. Angiotensin II inhibits bTREK-1 K+ channels in adrenocortical cells by separate Ca2+- and ATP hydrolysis-dependent mechanisms. J Biol Chem. 2005;280(35):30814–30828. doi: 10.1074/jbc.M504283200. [DOI] [PubMed] [Google Scholar]
- 22.Enyeart JJ, Liu H, Enyeart JA. Calcium-dependent inhibition of adrenal TREK-1 channels by angiotensin II and ionomycin. Am J Physiol Cell Physiol. 2011;301(3):C619–629. doi: 10.1152/ajpcell.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enyeart JJ, Xu L, Danthi S, Enyeart JA. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J Biol Chem. 2002;277(51):49186–49199. doi: 10.1074/jbc.M207233200. [DOI] [PubMed] [Google Scholar]
- 24.Fagerlund MJ, Kahlin J, Ebberyd A, Schulte G, Mkrtchian S, Eriksson LI. The human carotid body: expression of oxygen sensing and signaling genes of relevance for anesthesia. Anesthesiology. 2010;113(6):1270–1279. doi: 10.1097/ALN.0b013e3181fac061. [DOI] [PubMed] [Google Scholar]
- 25.Fearon IM, Zhang M, Vollmer C, Nurse CA. GABA mediates autoreceptor feedback inhibition in the rat carotid body via presynaptic GABAB receptors and TASK-1. J Physiol. 2003;553(Pt 1):83–94. doi: 10.1113/jphysiol.2003.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald RS, Shirahata M, Chang I, Kostuk E. The impact of hypoxia and low glucose on the release of acetylcholine and ATP from the incubated cat carotid body. Brain Res. 2009;1270:39–44. doi: 10.1016/j.brainres.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 27.Fung ML, Lam SY, Chen Y, Dong X, Leung PS. Functional expression of angiotensin II receptors in type-I cells of the rat carotid body. Pflugers Arch. 2001;441(4):474–480. doi: 10.1007/s004240000445. [DOI] [PubMed] [Google Scholar]
- 28.Gallego-Martin T, Fernandez-Martinez S, Rigual R, Obeso A, Gonzalez C. Effects of low glucose on carotid body chemoreceptor cell activity studied in cultures of intact organs and in dissociated cells. Am J Physiol Cell Physiol. 2012;302(8):C1128–1140. doi: 10.1152/ajpcell.00196.2011. [DOI] [PubMed] [Google Scholar]
- 29.Guagliardo NA, Yao J, Bayliss DA, Barrett PQ. TASK channels are not required to mount an aldosterone secretory response to metabolic acidosis in mice. Mol Cell Endocrinol. 2011;336(1–2):47–52. doi: 10.1016/j.mce.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542(Pt 2):431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang EM, Kim E, Yarishkin O, Woo DH, Han KS, Park N, Bae Y, Woo J, Kim D, Park M, Lee CJ, Park JY. A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat Commun. 2014;5:3227. doi: 10.1038/ncomms4227. [DOI] [PubMed] [Google Scholar]
- 32.Kang D, Hogan JO, Kim D. THIK-1 (K2P13.1) is a small-conductance background K(+) channel in rat trigeminal ganglion neurons. Pflugers Arch. 2014;466(7):1289–1300. doi: 10.1007/s00424-013-1358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang D, Wang J, Hogan JO, Vennekens R, Freichel M, White C, Kim D. Increase in cytosolic Ca2+ produced by hypoxia and other depolarizing stimuli activates a non-selective cation channel in chemoreceptor cells of rat carotid body. J Physiol. 2014;592(Pt 9):1975–1992. doi: 10.1113/jphysiol.2013.266957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim D. Fatty acid-sensitive two-pore domain K(+) channels. Trends Pharmacol Sci. 2003;24(12):648–654. doi: 10.1016/j.tips.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587(Pt 12):2963–2975. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D, Gnatenco C. TASK-5, a new member of the tandem-pore K(+) channel family. Biochem Biophys Res Commun. 2001;284(4):923–930. doi: 10.1006/bbrc.2001.5064. [DOI] [PubMed] [Google Scholar]
- 37.Kim D, Kim I, Papreck JR, Donnelly DF, Carroll JL. Characterization of an ATP-sensitive K(+) channel in rat carotid body glomus cells. Respir Physiol Neurobiol. 2011;177(3):247–255. doi: 10.1016/j.resp.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim D, Papreck JR, Kim I, Donnelly DF, Carroll JL. Changes in oxygen sensitivity of TASK in carotid body glomus cells during early postnatal development. Respir Physiol Neurobiol. 2011;177(3):228–235. doi: 10.1016/j.resp.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49(9):1434–1442. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- 40.Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londono JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest. 2010;120(9):3267–3279. doi: 10.1172/JCI41348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578(Pt 2):377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mkrtchian S, Kahlin J, Ebberyd A, Gonzalez C, Sanchez D, Balbir A, Kostuk EW, Shirahata M, Fagerlund MJ, Eriksson LI. The human carotid body transcriptome with focus on oxygen sensing and inflammation--a comparative analysis. J Physiol. 2012;590(Pt 16):3807–3819. doi: 10.1113/jphysiol.2012.231084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27(51):14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murali S, Zhang M, Nurse CA. Angiotensin II mobilizes intracellular calcium and activates pannexin-1 channels in rat carotid body type II cells via AT1 receptors. J Physiol. 2014;592(Pt 21):4747–4762. doi: 10.1113/jphysiol.2014.279299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musset B, Meuth SG, Liu GX, Derst C, Wegner S, Pape HC, Budde T, Preisig-Muller R, Daut J. Effects of divalent cations and spermine on the K+ channel TASK-3 and on the outward current in thalamic neurons. J Physiol. 2006;572(Pt 3):639–657. doi: 10.1113/jphysiol.2006.106898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nurse CA. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J Physiol. 2014;592(Pt 16):3419–3426. doi: 10.1113/jphysiol.2013.269829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortega-Saenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, Lopez-Barneo J. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010;135(4):379–392. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pardal R, Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5(3):197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- 49.Pardal R, Ludewig U, Garcia-Hirschfeld J, Lopez-Barneo J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc Natl Acad Sci U S A. 2000;97(5):2361–2366. doi: 10.1073/pnas.030522297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peers C, Wyatt CN. The role of maxiK channels in carotid body chemotransduction. Respir Physiol Neurobiol. 2007;157(1):75–82. doi: 10.1016/j.resp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Renigunta V, Fischer T, Zuzarte M, Kling S, Zou X, Siebert K, Limberg MM, Rinne S, Decher N, Schlichthorl G, Daut J. Cooperative endocytosis of the endosomal SNARE protein syntaxin-8 and the potassium channel TASK-1. Mol Biol Cell. 2014;25(12):1877–1891. doi: 10.1091/mbc.E13-10-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riesco-Fagundo AM, Perez-Garcia MT, Gonzalez C, Lopez-Lopez JR. O(2) modulates large-conductance Ca(2+)-dependent K(+) channels of rat chemoreceptor cells by a membrane-restricted and CO-sensitive mechanism. Circ Res. 2001;89(5):430–436. doi: 10.1161/hh1701.095632. [DOI] [PubMed] [Google Scholar]
- 53.Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci U S A. 2009;106(34):14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schiekel J, Lindner M, Hetzel A, Wemhoner K, Renigunta V, Schlichthorl G, Decher N, Oliver D, Daut J. The inhibition of the potassium channel TASK-1 in rat cardiac muscle by endothelin-1 is mediated by phospholipase C. Cardiovasc Res. 2013;97(1):97–105. doi: 10.1093/cvr/cvs285. [DOI] [PubMed] [Google Scholar]
- 55.Schwingshackl A, Teng B, Ghosh M, West AN, Makena P, Gorantla V, Sinclair SE, Waters CM. Regulation and function of the two-pore-domain (K2P) potassium channel Trek-1 in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2012;302(1):L93–L102. doi: 10.1152/ajplung.00078.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sterni LM, Bamford OS, Tomares SM, Montrose MH, Carroll JL. Developmental changes in intracellular Ca2+ response of carotid chemoreceptor cells to hypoxia. Am J Physiol. 1995;268(5 Pt 1):L801–808. doi: 10.1152/ajplung.1995.268.5.L801. [DOI] [PubMed] [Google Scholar]
- 57.Thompson RJ, Farragher SM, Cutz E, Nurse CA. Developmental regulation of O(2) sensing in neonatal adrenal chromaffin cells from wild-type and NADPH-oxidase-deficient mice. Pflugers Arch. 2002;444(4):539–548. doi: 10.1007/s00424-002-0853-6. [DOI] [PubMed] [Google Scholar]
- 58.Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28(35):8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tse A, Yan L, Lee AK, Tse FW. Autocrine and paracrine actions of ATP in rat carotid body. Can J Physiol Pharmacol. 2012;90(6):705–711. doi: 10.1139/y2012-054. [DOI] [PubMed] [Google Scholar]
- 60.Turner PJ, Buckler KJ. Oxygen and mitochondrial inhibitors modulate both monomeric and heteromeric TASK-1 and TASK-3 channels in mouse carotid body type-1 cells. J Physiol. 2013;591(Pt 23):5977–5998. doi: 10.1113/jphysiol.2013.262022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veale EL, Kennard LE, Sutton GL, MacKenzie G, Sandu C, Mathie A. G(alpha)q-mediated regulation of TASK3 two-pore domain potassium channels: the role of protein kinase C. Mol Pharmacol. 2007;71(6):1666–1675. doi: 10.1124/mol.106.033241. [DOI] [PubMed] [Google Scholar]
- 62.Williams BA, Buckler KJ. Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type 1 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286(1):L221–230. doi: 10.1152/ajplung.00010.2003. [DOI] [PubMed] [Google Scholar]
- 63.Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY, Oh SJ, Hwang EM, Marmorstein AD, Bae YC, Park JY, Lee CJ. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151(1):25–40. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Xu F, Xu J, Tse FW, Tse A. Adenosine stimulates depolarization and rise in cytoplasmic [Ca2+] in type I cells of rat carotid bodies. Am J Physiol Cell Physiol. 2006;290(6):C1592–1598. doi: 10.1152/ajpcell.00546.2005. [DOI] [PubMed] [Google Scholar]
- 65.Zhang M, Buttigieg J, Nurse CA. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J Physiol. 2007;578(Pt 3):735–750. doi: 10.1113/jphysiol.2006.121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol. 2000;525(Pt 1):143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuzarte M, Heusser K, Renigunta V, Schlichthorl G, Rinne S, Wischmeyer E, Daut J, Schwappach B, Preisig-Muller R. Intracellular traffic of the K+ channels TASK-1 and TASK-3: role of N- and C-terminal sorting signals and interaction with 14-3-3 proteins. J Physiol. 2009;587(Pt 5):929–952. doi: 10.1113/jphysiol.2008.164756. [DOI] [PMC free article] [PubMed] [Google Scholar]