Abstract
Objective
Pulmonary dead-space fraction is one of few lung-specific independent predictors of mortality from acute respiratory distress syndrome (ARDS). However, it is not measured routinely in clinical trials and thus altogether ignored in secondary analyses that shape future research directions and clinical practice. This study sought to validate an estimate of dead-space fraction for use in secondary analyses of clinical trials.
Design
Analysis of patient-level data pooled from ARDS clinical trials. Four approaches to estimate dead-space fraction were evaluated: three required estimating metabolic rate; one estimated dead-space fraction directly.
Setting
U.S. academic teaching hospitals.
Patients
Data from 210 patients across three clinical trials were used to compare performance of estimating equations with measured dead-space fraction. A second cohort of 3,135 patients from six clinical trials without measured dead-space fraction was used to confirm whether estimates independently predicted mortality.
Interventions
None.
Measurements and Main Results
Dead-space fraction estimated using the unadjusted Harris-Benedict equation for energy expenditure was unbiased (mean ± SD Harris-Benedict 0.59 ± 0.13; measured 0.60 ± 0.12). This estimate predicted measured dead-space fraction to within ± 0.10 in 70% of patients and ± 0.20 in 95% of patients. Measured dead-space fraction independently predicted mortality (OR 1.36 per 0.05 increase in dead-space fraction, 95% CI 1.10–1.68; p < .01). The Harris-Benedict estimate closely approximated this association with mortality in the same cohort (OR 1.55, 95% CI 1.21–1.98; p < .01) and remained independently predictive of death in the larger ARDSNet cohort. Other estimates predicted measured dead-space fraction or its association with mortality less well.
Conclusions
Dead-space fraction should be measured in future ARDS clinical trials to facilitate incorporation into secondary analyses. For analyses where dead-space fraction was not measured, the Harris-Benedict estimate can be used to estimate dead-space fraction and adjust for its association with mortality.
Keywords: Respiratory Distress Syndrome, Adult, Acute Lung Injury, Respiratory Dead Space, Capnography, Clinical Trials as Topic
INTRODUCTION
Few lung-specific predictors of mortality from acute respiratory distress syndrome (ARDS) exist. Impaired oxygenation assessed by PaO2:FiO2 is a defining feature of ARDS, but severity inconsistently correlates with clinical outcomes (1–3). Oxygenation index, an alternative measure of oxygenation that includes mean airway pressure, may correlate with outcomes more reliably (2). In addition to measures of oxygenation, respiratory system compliance and pulmonary dead-space fraction have been found in multiple studies to predict mortality from ARDS (4–7). Yet while hypoxemia and compliance are commonly reported, dead-space fraction is rarely assessed in clinical trials.
Increased dead-space fraction occurs within hours of ARDS onset and independently predicts mortality, even after accounting for overall illness severity, hypoxemia, and compliance (4). Sustained elevation of dead-space fraction over the first week additionally identifies patients less likely to survive hospitalization (5, 8). Microvascular endothelial injury, microvascular thrombi, and derangements in pulmonary blood flow are characteristic features of ARDS that lead to increased dead-space fraction (9–11). Hyperinflation with excessive applied or intrinsic positive end-expiratory pressure (PEEP) may further increase dead-space fraction (12–14). Lowering tidal volume also increases dead-space fraction (15), an effect that may be offset partially by a brief end-inspiratory pause with each breath (16).
Determination of dead-space fraction requires measurement of expired carbon dioxide (CO2) in a volume of expired gas. This volume is either derived by integrating flow or measured directly by collection in a Douglas bag. Expired CO2 is measured only infrequently in routine clinical care and clinical trials of ARDS, contributing to underreporting of dead-space fraction.
Absent routine measurement or a reliable estimate, most studies do not account for dead-space fraction. Yet, such secondary analyses of clinical trials influence future research directions and, at times, even clinical decisions. Secondary analyses should account for known independent predictors of the outcome of interest to calculate a valid effect estimate, particularly when the analysis is not performed according to the groups to which patients were originally randomized. Otherwise, the calculated effect—benefit or harm—might be attributable to residual confounding, i.e. to other differences between groups that would explain the findings.
To address this gap, we evaluated the validity of four approaches to estimating dead-space fraction. Three approaches required predicting energy expenditure: the unadjusted Harris-Benedict estimate, which employs the eponymous formula for resting energy expenditure; the Siddiki estimate, which adjusts Harris-Benedict to account for hypermetabolic conditions often encountered in critical illness; and the Penn State estimate, which was derived specifically for use in critically ill patients. We also derived a novel approach to estimate dead-space fraction directly without requiring estimation of energy expenditure as an intermediate step. Dead-space fraction estimates were evaluated for their prediction of measured dead-space fraction and prediction of the association between measured dead-space fraction and mortality. We hypothesized that estimating dead-space fraction directly would yield the highest predictive validity.
METHODS
Study Design
De-identified patient-level data pooled from three randomized controlled trials of early ARDS (VD/VT cohort) were used to compare directly measured dead-space fraction with four methods for estimating dead-space fraction. To evaluate the association between estimated dead-space fraction and mortality in a larger population, a second cohort was created by pooling data from completed NHLBI ARDS Network trials in which dead-space fraction was not measured (ARDSNet Cohort). The study was exempt from review by the Institutional Review Board.
Subjects
Patients eligible for the VD/VT cohort had baseline measured dead-space fraction obtained within 24 hours of study enrollment and prior to any study interventions. Patients were enrolled in one of three randomized controlled trials testing therapies for early ARDS. These trials were chosen because dead-space fraction was reported in their primary publications. The ARDSNet ALTA trial (17) was a multicenter randomized, placebo-controlled trial in which patients with early ARDS were assigned to receive aerosolized albuterol (5 mg) or saline placebo every 4 hours for up to 10 days. The trial was stopped early for futility, with no significant difference in mortality or ventilator-free days between groups. EPVent (18) was a single-center randomized, controlled trial in which patients with early ARDS were assigned to undergo mechanical ventilation with PEEP adjustment guided by esophageal pressure or according to the ARDSNet PEEP titration table. The trial was stopped early for reaching the primary endpoint of improved PaO2:FiO2, with no significant difference in mortality or ventilator-free days in the unadjusted primary analysis. The APC trial (19) was a multicenter randomized, placebo-controlled trial in which patients with early ARDS were assigned to receive activated protein C (APC) 24 µg/kg/h or placebo for 96 hours. No significant difference in mortality or ventilator-free days was found.
For the ARDSNet cohort without measured dead-space fraction available, data were pooled from the ARMA (1) (low tidal volume group only), ALVEOLI (20), FACTT (21, 22), ALTA (17), OMEGA (23), and EDEN (24) trials. Inclusion and exclusion criteria for each trial are described in the original referenced publications.
Measurement of Physiological Dead-space Fraction
In the VD/VT cohort, physiological dead-space fraction was calculated by measuring mean expired CO2 using volumetric capnography according to a validated protocol (25). An arterial blood gas was obtained at the time of expired gas analysis. Measured dead-space fraction was calculated using the Enghoff modification to the Bohr equation (26):
where PaCO2 and PECO2 represent partial pressure of CO2 in arterial blood and expired gas, respectively. All measurements were made prior to study interventions.
Estimating Equations for Physiological Dead-space Fraction
Methods for estimating dead-space fraction that do not require measurement of expired CO2 typically depend on the alveolar ventilation equation:
where PaCO2 is measured in mmHg, V̇CO2 represents CO2 production (mL/min), and V̇A represents alveolar minute ventilation (L/min). Because V̇A is defined as the difference between total minute ventilation and dead-space minute ventilation, this equation can be rewritten and, after solving for VD/VT, yields:
where RR is respiratory rate (breaths/minute) and VT is tidal volume (liters). In this rearranged equation for dead-space fraction, the only variable not routinely available is V̇CO2, which may be calculated from the resting energy expenditure (REE) using the rearranged Weir equation (27):
where RQ is the respiratory quotient, assumed to be 0.8 for this analysis.
In this study, four different strategies for estimating dead-space fraction were considered. All physiological measurements required for dead-space fraction estimates were obtained prior to study interventions associated with the clinical trial.
- Unadjusted Harris-Benedict estimate: The original sex-specific Harris-Benedict equations (28) were used to estimate REE:
with weight (Wt) in kg, height (Ht) in cm, and age in years. The value for REEHB was inserted into the rearranged Weir equation to calculate V̇CO2, which was then used to calculate dead-space fraction. - Siddiki estimate: Siddiki et al. (29) proposed using a modified Harris-Benedict equation to estimate REE. In this approach, REEHB is adjusted to account for the hypermetabolic state resulting from certain clinical conditions:
where hf is a unitless multiplier term for hypermetabolic factors with potential values of 1.13 per °C above 37°C, 1.2 for minor surgery, 1.35 for major trauma, and 1.6 for severe infection. The hypermetabolic factor yielding the highest value for hf is selected to calculate REESiddiki. The value for REESiddiki was inserted into the rearranged Weir equation to calculate V̇CO2, which was then used to calculate dead-space fraction. In the original report of Siddiki et al. (29), the rearranged Weir equation for V̇CO2 differs trivially from that above due to rounding. - Penn State estimate: An alternative formulation for estimating REE was derived previously by Frankenfield et al. (30, 31) specifically for critically ill patients. This approach uses the Mifflin-St. Jeor equation (32) to estimate REE for the patient in good health:
with weight (Wt) in kg, height (Ht) in cm, and age in years. Additional clinical variables are then incorporated to yield the Penn State equations for REE in critical illness (30, 31):
where BMI is body mass index, RR is respiratory rate (breaths/minute), VT is tidal volume (liters), and Tmax is the maximum temperature (°C) over the last 24 hours. Direct estimate from physiological variables: A novel alternative approach to estimate dead-space fraction directly was developed using least angle regression to derive a prediction model. Only variables with physiological plausibility were considered for inclusion in the model: anthropometrics (height, measured body weight, predicted body weight, body mass index, body surface area, sex, age, and race/ethnicity), respiratory variables (tidal volume, tidal volume per predicted body weight, respiratory rate, minute ventilation, minute ventilation per predicted body weight, PaCO2, PaO2:FiO2, respiratory system compliance, PEEP, number of quadrants with infiltrates on chest imaging, and Murray lung injury score (33)), hemodynamic variables that may affect ventilation/perfusion matching (systolic blood pressure, mean arterial pressure, shock as defined by the Brussels criteria (34), heart rate, and rate-pressure product), primary cause of lung injury, and maximum temperature over the previous 24 hours. Clinically relevant multiplicative interaction terms were also considered, consisting of PaCO2 and each of: minute ventilation, minute ventilation per predicted weight, body mass index, body surface area, measured body weight, height, sex, age, and temperature.
Model building employed least angle regression with five-fold cross-validation to minimize cross-validated mean squared prediction error. For parsimony, only the first five variables were retained in the final model since mean squared prediction error improved minimally with additional variables. The final model was refit using ordinary least squares to derive the reported coefficients:
where RR is respiratory rate (breaths/minute), PEEP represents set PEEP (cmH2O) on the mechanical ventilator, LIS is Murray lung injury score (33), PaCO2 is measured in mmHg, and V̇E represents total minute ventilation (liters/minute).
Comparison of Approaches
Dead-space fraction estimates were evaluated based on two overarching criteria: prediction of measured dead-space fraction and prediction of the association between measured dead-space fraction and mortality. Measured and estimated dead-space fraction were compared graphically using the Bland-Altman approach for assessing agreement between methods of clinical measurement (35). Quantitatively, methods were compared according to bias and accuracy. Bias describes whether the estimate systematically under-predicts or over-predicts measured dead-space fraction and was determined by comparing the difference in means between measurement and each estimating equation. A one-sample t-test was performed to determine if the mean difference was significantly different from zero. Accuracy describes how close each estimated value for dead-space fraction is to the true measured value and was calculated in two ways. First, the 95th percentile of the absolute difference between measured and estimated values was calculated; the absolute difference was used to avoid canceling effects of negative and positive values. Second, the proportion of estimated dead-space fraction values that fell within ± 0.10 or ± 0.20 of measured dead-space fraction was calculated.
Measured and estimated dead-space fraction were also compared for their ability to predict 28-day mortality. Mortality per dead-space fraction quintile was used to evaluate whether the predictive ability of each dead-space fraction estimate varied by level. Simple logistic regression was used to compare the unadjusted association between each dead-space fraction estimate and mortality. In a sensitivity analysis, measured dead-space fraction was then added to each model to determine whether the association between estimated dead-space fraction and mortality included effects beyond that explained by measured dead-space fraction. Multivariable logistic regression was performed to evaluate the relationship between each dead-space fraction estimate and mortality. First, backward elimination (threshold p ≤ .05 to remain in model) was used to construct the best-fitting model of mortality with measured dead-space fraction, selecting from the following candidate predictors: age, shock, APACHE II, tidal volume per predicted body weight, PEEP, primary cause of lung injury, and respiratory system compliance. Berlin ARDS severity (3) and clinical trial enrolled were forced into the model as categorical variables for face validity. The selected covariates were then used to fit logistic models for each estimate of dead-space fraction. Finally, the area under the receiver operating curve (AUROC) for logistic regression models of mortality was used to determine whether measured dead-space fraction and the best-performing estimate improved predictive validity of the Berlin definition. Statistical analyses were performed using SAS 9.3 (Cary, NC). A two-sided p ≤ .05 was considered statistically significant.
RESULTS
A total of 210 patients in the VD/VT cohort were included in the primary analysis. In this cohort, measured dead-space fraction was markedly elevated (mean ± SD 0.60 ± 0.12). An additional 3,135 patients enrolled in the ARDSNet trials did not have measured dead-space fraction and were included in the second cohort. Patient characteristics for both cohorts are described in Table 1. Overall, mortality at 28 days was significantly lower in the VD/VT cohort (16% vs. 22%; p = .05).
Table 1.
Patient characteristics
| VD/VT Cohort | ARDSNet Cohort (n = 3,135) |
||||
|---|---|---|---|---|---|
| ALTA (n = 92) |
EPVent (n = 57) |
APC (n = 61) |
Total (n = 210) |
||
| Demographics | |||||
| Age | 50 ± 17 | 54 ± 19 | 52 ± 18 | 52 ± 18 | 51 ± 16 |
| Female | 46 (50%) | 22 (39%) | 26 (43%) | 94 (45%) | 1,439 (46%) |
| Non-white race | 28 (30%) | 7 (12%) | 18 (30%) | 53 (25%) | 871 (28%) |
| Body mass index (kg/m2) | 29.0 ± 9.0 | 30.7 ± 9.3 | 28.3 ± 7.4 | 29.3 ± 8.7 | 28.7 ± 7.6 |
| Clinical Characteristics | |||||
| APACHE II | 26 ± 7 | 26 ± 6 | 20 ± 7 | 25 ± 7 | 27 ± 7 |
| Shock | 59 (64%) | 33 (58%) | 16 (26%) | 108 (51%) | 1,733 (55%) |
| Primary cause of lung injury | |||||
| Aspiration or pneumonia | 50 (54%) | 8 (14%) | 44 (72%) | 102 (49%) | 1,952 (62%) |
| Sepsis | 24 (26%) | 14 (25%) | 9 (15%) | 47 (22%) | 659 (21%) |
| Multiple transfusions | 2 (2%) | 0 | 0 | 2 (1%) | 65 (2%) |
| Trauma | 8 (9%) | 15 (26%) | 0 | 23 (11%) | 223 (7%) |
| Other | 8 (9%) | 20 (35%) | 8 (13%) | 36 (17%) | 235 (8%) |
| Respiratory Characteristics | |||||
| Tidal volume (mL) | 390 ± 70 | 493 ± 96 | 415 ± 92 | 425 ± 94 | 408 ± 94 |
| Tidal volume (mL/kg PBW) | 6.3 ± 0.9 | 7.7 ± 1.4 | 6.7 ± 1.4 | 6.8 ± 1.3 | 6.5 ± 1.4 |
| Respiratory rate (breaths/min) | 28 ± 7 | 25 ± 6 | 27 ± 7 | 27 ± 7 | 27 ± 7 |
| PEEP (cmH2O) | 9 ± 3 | 13 ± 4 | 9 ± 3 | 10 ± 4 | 10 ± 4 |
| PaO2/FiO2 | 184 ± 63 | 148 ± 57 | 160 ± 63 | 167 ± 63 | 172 ± 76 |
| PaCO2 (mmHg) | 40 ± 8 | 41 ± 8 | 41 ± 11 | 41 ± 9 | 41 ± 10 |
| Respiratory system compliance (mL/cmH2O) | 32 ± 17 | 36 ± 11 | 31 ± 22 | 33 ± 17 | 32 ± 17 |
| Murray lung injury score | 2.4 ± 0.6 | 2.8 ± 0.6 | 2.6 ± 0.6 | 2.6 ± 0.6 | 2.6 ± 0.7 |
| VD/VT measured | 0.57 ± 0.12 | 0.67 ± 0.10 | 0.59 ± 0.12 | 0.60 ± 0.12 | N/A |
| Clinical Outcomes | |||||
| Mortality | 12 (13%) | 15 (26%) | 7 (11%) | 34 (16%) | 693 (22%) |
| Vent-free days through day 28 | 16 ± 10 | 10 ± 9 | 15 ± 10 | 14 ± 10 | 14 ± 11 |
Data are reported as mean ± standard deviation or number (%).
Abbreviations: PEEP = positive end-expiratory pressure; VD/VT = dead-space fraction.
Performance of Estimating Equations
Bias was evaluated by comparing mean differences between estimated and measured dead-space fraction (Table 2). Both the Siddiki and Penn State estimates were significantly biased toward underestimation of measured dead-space fraction (mean difference −0.32 ± 0.35 and −0.08 ± 0.12, respectively; p < .01 for both comparisons). The unadjusted Harris-Benedict and direct estimates were unbiased (mean difference −0.01 ± 0.12 and 0 ± 0.09, respectively; p = .30 for unadjusted Harris-Benedict estimate; direct estimate unbiased by design).
Table 2.
Performance of estimating equations compared to measured dead-space fraction in VD/VT Cohort
| Method of Dead-space Estimation | ||||
|---|---|---|---|---|
| Harris-Benedict | Siddiki | Penn State | Direct | |
| VD/VT mean ± SD, all patients | 0.59 ± 0.13 | 0.28 ± 0.38 | 0.52 ± 0.14 | 0.60 ± 0.08 |
| Survivors | 0.58 ± 0.14 | 0.25 ± 0.39 | 0.50 ± 0.14 | 0.59 ± 0.07 |
| Non-survivors | 0.67 ± 0.11 | 0.47 ± 0.28 | 0.59 ± 0.11 | 0.66 ± 0.08 |
| Bias: difference between measured and estimated VD/VT, mean ± SD | −0.01 ± 0.12 | −0.32 ± 0.35* | −0.08 ± 0.12* | 0 ± 0.09*† |
| Accuracy: 95th percentile of absolute difference between measured and estimated VD/VT | 0.20 | 1.03 | 0.30 | 0.17 |
| VD/VT accuracy threshold of ± 0.10 | ||||
| Accuracy (%) | 70.1 | 26.5 | 52.5 | 73.0 |
| Values overestimating (%) | 15.7 | 3.4 | 4.9 | 13.7 |
| Values underestimating (%) | 14.2 | 70.1 | 42.6 | 13.2 |
| VD/VT accuracy threshold of ± 0.20 | ||||
| Accuracy (%) | 95.1 | 44.6 | 85.3 | 98.5 |
| Values overestimating (%) | 1.5 | 1.0 | 0.5 | 0.5 |
| Values underestimating (%) | 3.4 | 54.4 | 14.2 | 1.0 |
| Correlation coefficient | 0.58* | 0.45* | 0.56* | 0.67* |
Abbreviations: VD/VT = dead-space fraction
p< .01 between estimated and measured dead-space fraction
Direct estimate was unbiased by design.
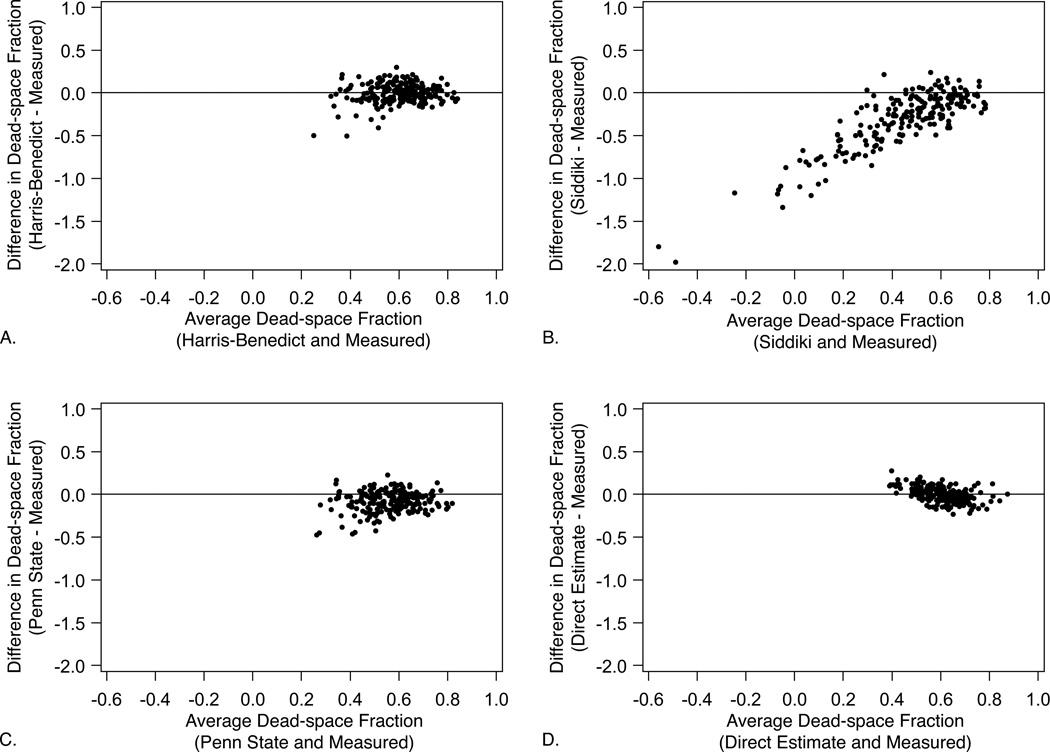
Accuracy was evaluated first as the 95th percentile of the absolute difference between measured and estimated dead-space fraction (Table 2). The unadjusted Harris-Benedict and direct estimates were the most accurate estimates of dead-space fraction (95th percentile 0.20 and 0.17, respectively). The Penn State estimate displayed intermediate accuracy relative to the other formulations (95th percentile 0.30). The Siddiki estimate was the least accurate (95th percentile 1.03).
Accuracy was also evaluated as the proportion of dead-space fraction estimates within two pre-specified thresholds of measured dead-space fraction: ± 0.10 and ± 0.20 (Table 2). Again, the unadjusted Harris-Benedict and direct estimates displayed the best performance, with 70.1% and 73.0% of estimates within ± 0.10 of measured dead-space fraction, respectively, and > 95% of estimates within ± 0.20 for both formulations. By contrast, the Siddiki and Penn State estimates were considerably less accurate, predicting dead-space fraction to within ± 0.10 of the measured value only 26.5% and 52.5% of the time. The Siddiki estimate alone yielded negative values for dead-space fraction, while no estimate produced values ≥ 1.00. Graphical comparison of each estimate with measured dead-space fraction using Bland-Altman plots confirmed these findings (Fig. 1).
Figure 1.
Bland-Altman analyses comparing measured dead-space fraction with that determined by each estimating equation: (a) Harris-Benedict, (b) Siddiki, (c) Penn State, (d) direct estimate. Plots consist of the difference between estimated and measured dead-space fraction formulations (vertical axis) versus the average value of the two approaches (horizontal axis). Differences greater than zero indicate overestimation.
Prediction of Mortality
Measured dead-space fraction was significantly higher in non-survivors compared to survivors (0.67 ± 0.12 vs. 0.59 ± 0.12; p < .01). Estimated dead-space fraction also was significantly higher among non-survivors for all estimating equations (p < .01 for all comparisons) (Table 2).
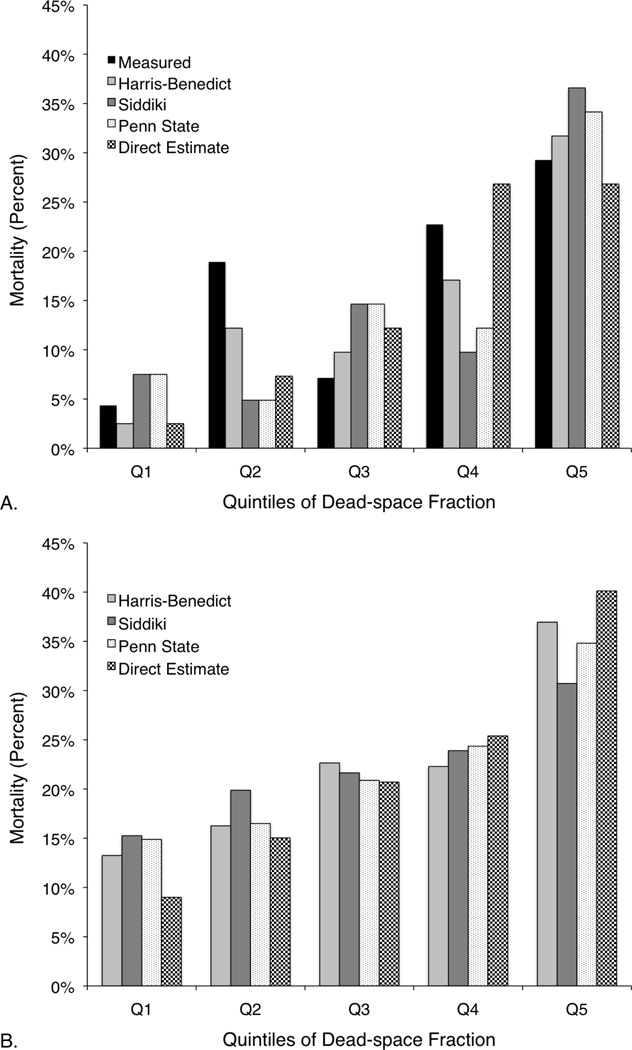
Mortality by quintile of measured and estimated dead-space fraction is reported in Figure 2. Quintiles were calculated separately for each formulation of dead-space fraction to account for potential differences in scaling. Mortality per unadjusted Harris-Benedict quintile was within ± 6.7% of that per measured dead-space fraction for all quintiles (Fig. 2A). No other estimating equation as closely approximated the measured dead-space fraction per-quintile mortality. In the ARDSNet cohort, mortality similarly increased with successive quintiles of each dead-space fraction estimate (Fig. 2B).
Figure 2.
Observed mortality by quintile of measured or estimated dead-space fraction: (a) among patients with measured dead-space fraction (VD/VT Cohort); (b) among patients without measured dead-space fraction (ARDSNet Cohort). Quintiles were calculated separately for each formulation of dead-space fraction.
In the unadjusted analysis, higher measured dead-space fraction was significantly associated with increased risk of death (Table 3). The multivariable model-building process identified measured dead-space fraction, APACHE II, and PEEP as statistically significant independent predictors of death, with clinical trial enrolled and Berlin ARDS severity forced into the model for face validity but not reaching statistical significance. In the multivariable analysis, for every 0.05 increase in measured dead-space fraction, odds of death increased by 36% (OR 1.36, 95% CI 1.10–1.68; p < .01).
Table 3.
Odds ratios (95% CIs) for death per 0.05 increase inmeasured or estimated dead-space fraction*
| Measured | Harris-Benedict | Siddiki | Penn State | Direct | |
|---|---|---|---|---|---|
| VD/VTCohort | |||||
| Unadjustedanalysis | 1.36 (1.14–1.62) | 1.40 (1.14–1.71) | 1.12 (1.04–1.22) | 1.36 (1.13–1.63) | 1.74 (1.34–2.27) |
| Multivariable analysis† | 1.36 (1.10–1.68) | 1.55 (1.21–1.98) | 1.19 (1.07–1.31) | 1.53 (1.21–1.95) | 1.80 (1.26–2.56) |
| ARDSNet Cohort | |||||
| Unadjustedanalysis | Not measured | 1.16 (1.11–1.20) | 1.04 (1.02–1.05) | 1.14 (1.10–1.18) | 1.47 (1.39–1.56) |
| Multivariable analysis† | Not measured | 1.10(1.05–1.14) | 1.03(1.02–1.05) | 1.09(1.04–1.13) | 1.41 (1.30–1.53) |
Odds ratios for all formulations of dead-space fraction differ significantly from 1.0 with p< .01 for all analyses.
Multivariable model adjusted for APACHE, Berlin ARDS severity, PEEP, and clinical trial enrolled.
Each estimate of dead-space fraction also was significantly associated with mortality on unadjusted and multivariable analyses in both cohorts (p < .01 for all analyses). However, odds ratios for death varied considerably (Table 3) due to differences in scaling and accuracy of dead-space fraction estimates and variation in predicting the relationship between measured dead-space fraction and mortality. In the VD/VT cohort, the Harris-Benedict and Penn State odds ratios for death were most similar to that of measured dead-space fraction in both the unadjusted and multivariable analyses (multivariable model: ORdeath per 0.05 increase in dead-space fraction, Harris-Benedict OR 1.55, 95% CI 1.21–1.98; Penn State OR 1.53, 95% CI 1.21–1.95). The Siddiki odds ratio was considerably lower (OR 1.19, 95% CI 1.07–1.31), while that of the direct estimate was higher (OR 1.80, 95% CI 1.26–2.56). In the ARDSNet cohort, the Harris-Benedict and Penn State odds ratios for death were similar to each other, while the Siddiki and direct estimate odds ratios differed considerably.
In the sensitivity analysis, only the direct estimate remained significantly associated with mortality after adding measured dead-space fraction to the model, indicating the direct estimate had an association with mortality that was independent of measured dead-space fraction.
Compared with modeling mortality using the Berlin definition alone, adding measured dead-space fraction significantly improved predictive validity in the VD/VT cohort, with an AUROC of 0.689 (95% CI 0.587–0.791) vs. 0.534 (95% CI 0.440–0.628; p = .02). Similarly, adding the unadjusted Harris-Benedict estimate to the Berlin definition significantly improved predictive validity for mortality in both the VD/VT cohort (AUROC 0.714, 95% CI 0.616–0.813 vs. 0.543; 95% CI 0.449–0.637; p < .01) and the ARDSNet cohort (AUROC 0.644, 95% CI 0.617–0.672 vs. 0.592, 95% CI 0.566–0.618; p < .01).
Prediction of Outlying Estimates
A post-hoc analysis was conducted to identify the subset of patients in whom the unadjusted Harris-Benedict estimate did not predict measured dead-space fraction accurately, to within ± 0.10. Among baseline characteristics, only PaCO2 differed significantly between patients with inaccurate compared to accurate estimates (39 ± 9 vs. 41 ± 9, respectively; p = .04). Nearly half (43%) of patients with PaCO2 < 30 mmHg had an inaccurate Harris-Benedict estimate, compared with just 28% of patients with PaCO2 ≥ 30 mmHg (p = .17). Considering only underestimates, PaCO2 was < 30 mmHg in 24% of patients; by contrast, only 8% of patients in whom the Harris-Benedict approach did not underestimate dead-space fraction had PaCO2 < 30 mmHg (p < .01). Such marked hypocapnia occurred infrequently in our cohorts (in 10% and 9% of patients in the VD/VT and ARDSNet cohorts, respectively), as would be expected during lung-protective ventilation for ARDS. No comparable pattern was seen with overestimation.
DISCUSSION
The present study demonstrates that dead-space fraction is best estimated using the unadjusted Harris-Benedict equation for energy expenditure to predict measured dead-space fraction and its association with mortality. The Harris-Benedict and direct estimates predicted measured dead-space fraction most accurately. However, the direct estimate was associated with death independent of measured dead-space fraction, indicating a relationship with mortality beyond that explained by measured dead-space fraction. Still, even the Harris-Benedict approach, the best-performing estimate, predicted measured dead-space fraction to within ± 0.10 in only 70% of patients and ± 0.20 in 95% of patients – a considerable range given the scale of measure. Therefore, estimates of dead-space fraction should not replace prospective measurement in future clinical trials of ARDS. For secondary analyses of existing clinical trials data where dead-space fraction was not measured, the unadjusted Harris-Benedict estimate can be used to estimate the independent association between dead-space fraction and mortality.
Increased dead-space fraction is a clinical hallmark of ARDS that independently predicts patient outcomes (3, 4). For this reason, dead-space fraction was considered in formulating the 2012 Berlin definition of ARDS to lend further face validity to the definition (36). However, dead-space fraction was not included in the final Berlin definition because it is not routinely measured (36). The present study found that adjusting for measured dead-space fraction in the Berlin definition significantly improved predictive validity for mortality. Adjusting for estimated dead-space fraction using the Harris-Benedict approach similarly improved the Berlin definition’s predictive validity for mortality (3). These findings reinforce the need to adjust for dead-space fraction as a marker of ARDS severity that independently predicts mortality.
Secondary analyses of epidemiological and clinical trials data are a mainstay of ARDS research due to the expense and resources required to conduct clinical trials (37). Because such secondary analyses play a central role in shaping future directions of ARDS research and clinical practice, it is essential they incorporate known independent predictors of ARDS mortality. Oxygenation, respiratory system compliance, and dead-space fraction have been identified repeatedly as predictors of clinical outcomes from ARDS (2, 4, 7). While oxygenation and respiratory system mechanics are frequently considered, dead-space fraction is rarely measured in such studies and thus altogether ignored in secondary analyses.
Few prior reports have attempted to estimate dead-space fraction in ARDS. The Siddiki estimate (29) was shown previously in a study of just thirteen patients to substantially underestimate measured dead-space fraction (38), a finding confirmed here in a much larger cohort. In fact, the Siddiki estimate produced negative values for dead-space fraction in some patients because its use of hypermetabolic factors led to overestimating V̇CO2 (39). Frankenfield et al. derived a predictive equation for dead-space fraction requiring measurement of end-tidal CO2 in a heterogeneous population of critically ill patients (40). Their study excluded patients with FiO2 > 0.60 due to equipment limitations, limiting generalizability to ARDS cohorts. Moreover, end-tidal CO2 is not routinely captured in clinical trials or epidemiological data, limiting utility of this approach for the purpose of secondary analyses of existing data.
Alternative surrogates for dead-space fraction have been proposed. The ARDS Berlin Definition Task Force considered total minute ventilation standardized to a PaCO2 of 40 mmHg (3, 41). Post-hoc analysis of the Berlin cohort found that stratifying patients with severe ARDS (PaO2:FiO2 ≤ 100) by standardized minute ventilation significantly improved mortality risk prediction (3). Sinha et al. proposed a similar formulation, the ventilatory ratio, which includes predicted minute ventilation and predicted PaCO2 (42). Both approaches may be useful for bedside contemplation of minute ventilation requirements, but neither has been validated as a surrogate for measured dead-space fraction to support use in clinical research.
Important limitations to this study exist. First, estimated dead-space fraction is not intended for use in place of direct measurement in clinical practice or future prospective studies. Rather, this report highlights the need to incorporate measurement of dead-space fraction in future clinical trials and prospectively collected observational data. When measured dead-space fraction is unavailable, the unadjusted Harris-Benedict estimate may be considered for research purposes. The unadjusted Harris-Benedict estimate tends to underestimate dead-space fraction when PaCO2 is < 30 mmHg. Thus, caution should be used when a large proportion of the study population has marked hypocapnia.
Second, the best-performing estimate here relies on the unadjusted Harris-Benedict equation for energy expenditure and assumes a respiratory quotient of 0.8 for all patients. Respiratory quotient fluctuates with feeding, nutritional status, and anaerobiosis (43, 44), factors not considered here. The unadjusted Harris-Benedict equation has been shown previously to be unreliable in predicting energy expenditure in critically ill patients (39), which in part may be due to variation in ventilator settings (15, 45, 46), metabolic stress (47, 48), and fasting status (44) in prior studies. By contrast, a singular focus on early ARDS and protocolized low tidal volume ventilation here likely improved its performance in estimating dead-space fraction. It is unclear how the unadjusted Harris-Benedict dead-space fraction estimate would perform in cohorts managed with different ventilator settings, prone positioning, or experimental interventions.
Finally, differences in mechanical ventilation practices directly affect measured dead-space fraction and may limit its utility as a marker of disease severity absent comparable settings. Tidal volume, PEEP titration, dynamic hyperinflation, and use of a brief end-inspiratory pause all have been shown to affect measured dead-space fraction irrespective of underlying disease severity (12–16). In this study, these effects likely were mitigated in part by use of protocolized lung-protective ventilation for all included patients, though between-patient variation still occurred (Table 1).
CONCLUSIONS
Dead-space fraction is one of few lung-specific independent predictors of mortality from ARDS. As such, dead-space fraction should be measured whenever possible in clinical trials and prospective epidemiological studies to permit adjustment for its effects in secondary analyses. For analyses of existing data where dead-space fraction was not measured, the unadjusted Harris-Benedict estimate should be considered to estimate the association between dead-space fraction and mortality.
Acknowledgments
FINANCIAL SUPPORT
This work was supported by NIH T32-HL007633 (Beitler), N01-HR56179 (Thompson), R37-HL51856 (Matthay), SCCOR HL-74005 (Matthay), UM1-HL108724 (Talmor), and K24-HL093218 (Malhotra).
Dr. Beitler received support for article research from the National Institutes of Health (NIH). His institution received grant support from the NHLBI (Grant: T32-HL007633). Dr. Thompson received support for article research from the NIH. His institution received grant support from the NIH/NHLBI. Dr. Matthay received support for article research from the NIH, served as a board member for Roche DSMB, and consulted for Cerus Inc and GSK. His institution received grant support from the NHLBI and NIAID. Dr. Talmor received grant support from the NHLBI and received support for article research from the NIH. Dr. Liu served as a board member for Astute Biomedical (Clinical Events Adjudication Committee), Complexa (Scientific Advisory Board), Cytopheryx (Data Safety Monitoring Board), and Roche DSMB. She received support for travel from the American Thoracic Society (Member of publications committee; trip to planning meeting paid for by ATS), American Society of Nephrology (Reimbursement for travel to national meeting for presentation), Continuous Renal Replacement Therapies Meeting (Reimbursement for travel to national meeting for presentation). She consulted for Chemocentryx, Cerus Inc, and GSK; lectured for the NIH; has stock in Amgen; disclosed other support from Abbott (Gift of reagents for biomarker assays) and CMIC (Gift of reagents for biomarker assays); and received support for article research from the NIH. Her institution received grant support from the NIH (listed as funding source in manuscript), NHLBI, and NIAID. Dr. Hayden is employed by the NIH-NHLBI National Heart Award (#: HHSN268200536179C, Fund Number: 219579) and received support for article research from the NIH. His institution received grant support from the NIH-NHLBI National Heart Award (#: HHSN268200536179C, Fund Number: 219579). Dr. Malhotra received support for article research from the NIH. His institution consulted for Pfizer, SHC, SGS, Apnex, and Apnicure (relinquished all outside personal income in 2012).
Footnotes
CONFLICTS OF INTEREST
All authors report that they have no potential conflicts of interest to declare.
Copyright form disclosures:
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Seeley E, McAuley DF, Eisner M, et al. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63:994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranieri VM, Rubenfeld GD, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 5.Kallet RH, Alonso JA, Pittet J-F, et al. Prognostic value of the pulmonary dead-space fraction during the first 6 days of acute respiratory distress syndrome. Respir Care. 2004;49:1008–1014. [PubMed] [Google Scholar]
- 6.Cepkova M, Kapur V, Ren X, et al. Pulmonary dead space fraction and pulmonary artery systolic pressure as early predictors of clinical outcome in acute lung injury. Chest. 2007;132:836–842. doi: 10.1378/chest.07-0409. [DOI] [PubMed] [Google Scholar]
- 7.Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raurich JM, Vilar M, Colomar A, et al. Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respir Care. 2010;55:282–287. [PubMed] [Google Scholar]
- 9.Tomashefski JF, Davies P, Boggis C, et al. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 10.Calfee CS, Gallagher D, Abbott J, et al. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med. 2012;40:1731–1737. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Günther A, Mosavi P, Heinemann S, et al. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:454–462. doi: 10.1164/ajrccm.161.2.9712038. [DOI] [PubMed] [Google Scholar]
- 12.Suter PM, Fairley B, Isenberg MD. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med. 1975;292:284–289. doi: 10.1056/NEJM197502062920604. [DOI] [PubMed] [Google Scholar]
- 13.Vieillard-Baron A, Prin S, Augarde R, et al. Increasing respiratory rate to improve CO2 clearance during mechanical ventilation is not a panacea in acute respiratory failure. Crit Care Med. 2002;30:1407–1412. doi: 10.1097/00003246-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Tusman G, Suárez-Sipmann F, Böhm SH, et al. Monitoring dead space during recruitment and PEEP titration in an experimental model. Intensive Care Med. 2006;32:1863–1871. doi: 10.1007/s00134-006-0371-7. [DOI] [PubMed] [Google Scholar]
- 15.Kiiski R, Kaitainen S, Karppi R, et al. Physiological effects of reduced tidal volume at constant minute ventilation and inspiratory flow rate in acute respiratory distress syndrome. Intensive Care Med. 1996;22:192–198. doi: 10.1007/BF01712236. [DOI] [PubMed] [Google Scholar]
- 16.Devaquet J, Jonson B, Niklason L, et al. Effects of inspiratory pause on CO2 elimination and arterial PCO2 in acute lung injury. J Appl Physiol. 2008;105:1944–1949. doi: 10.1152/japplphysiol.90682.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178:618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network: Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 21.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network: Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 23.Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallet RH, Daniel BM, Garcia O, et al. Accuracy of physiologic dead space measurements in patients with acute respiratory distress syndrome using volumetric capnography: comparison with the metabolic monitor method. Respir Care. 2005;50:462–467. [PubMed] [Google Scholar]
- 26.Sinha P, Flower O, Soni N. Deadspace ventilation: a waste of breath! Intensive Care Med. 2011;37:735–746. doi: 10.1007/s00134-011-2194-4. [DOI] [PubMed] [Google Scholar]
- 27.Weir JB, de V. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol (Lond.) 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddiki H, Kojicic M, Li G, et al. Bedside quantification of dead-space fraction using routine clinical data in patients with acute lung injury: secondary analysis of two prospective trials. Crit Care. 2010;14:R141. doi: 10.1186/cc9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frankenfield DC, Coleman A, Alam S, et al. Analysis of estimation methods for resting metabolic rate in critically ill adults. JPEN J Parenter Enteral Nutr. 2009;33:27–36. doi: 10.1177/0148607108322399. [DOI] [PubMed] [Google Scholar]
- 31.Frankenfield D. Validation of an equation for resting metabolic rate in older obese, critically ill patients. JPEN J Parenter Enteral Nutr. 2011;35:264–269. doi: 10.1177/0148607110377903. [DOI] [PubMed] [Google Scholar]
- 32.Mifflin MD, St Jeor ST, Hill LA, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 33.Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 34.Bernard G. The Brussels score. Sepsis. 1997;1:43–44. [Google Scholar]
- 35.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 36.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 37.Spragg RG, Bernard GR, Checkley W, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181:1121–1127. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charron C, Repesse X, Bouferrache K, et al. PaCO2 and alveolar dead space are more relevant than PaO2/FiO2 ratio in monitoring the respiratory response to prone position in ARDS patients: a physiological study. Crit Care. 2011;15:R175. doi: 10.1186/cc10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frankenfield D, Hise M, Malone A, et al. Prediction of resting metabolic rate in critically ill adult patients: results of a systematic review of the evidence. J Am Diet Assoc. 2007;107:1552–1561. doi: 10.1016/j.jada.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Frankenfield DC, Alam S, Bekteshi E, et al. Predicting dead space ventilation in critically ill patients using clinically available data. Crit Care Med. 2010;38:288–291. doi: 10.1097/CCM.0b013e3181b42e13. [DOI] [PubMed] [Google Scholar]
- 41.Wexler HR, Lok P. A simple formula for adjusting arterial carbon dioxide tension. Can Anaesth Soc J. 1981;28:370–372. doi: 10.1007/BF03007805. [DOI] [PubMed] [Google Scholar]
- 42.Sinha P, Sanders RD, Soni N, et al. Acute respiratory distress syndrome: the prognostic value of ventilatory ratio-a simple bedside tool to monitor ventilatory efficiency. Am J Respir Crit Care Med. 2013;187:1150–1153. doi: 10.1164/rccm.201211-2037LE. [DOI] [PubMed] [Google Scholar]
- 43.Kan M-N, Chang H-H, Sheu W-F, et al. Estimation of energy requirements for mechanically ventilated, critically ill patients using nutritional status. Crit Care. 2003;7:R108–R115. doi: 10.1186/cc2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zauner C, Schneeweiss B, Kranz A, et al. Resting energy expenditure in short-term starvation is increased as a result of an increase in serum norepinephrine. Am J Clin Nutr. 2000;71:1511–1515. doi: 10.1093/ajcn/71.6.1511. [DOI] [PubMed] [Google Scholar]
- 45.Kiiski R, Takala J, Kari A, et al. Effect of tidal volume on gas exchange and oxygen transport in the adult respiratory distress syndrome. Am Rev Respir Dis. 1992;146:1131–1135. doi: 10.1164/ajrccm/146.5_Pt_1.1131. [DOI] [PubMed] [Google Scholar]
- 46.Brandi LS, Bertolini R, Santini L, et al. Effects of ventilator resetting on indirect calorimetry measurement in the critically ill surgical patient. Crit Care Med. 1999;27:531–539. doi: 10.1097/00003246-199903000-00030. [DOI] [PubMed] [Google Scholar]
- 47.van Lanschot JJ, Feenstra BW, Vermeij CG, et al. Calculation versus measurement of total energy expenditure. Crit Care Med. 1986;14:981–985. doi: 10.1097/00003246-198611000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Kiiski R, Takala J. Hypermetabolism and efficiency of CO2 removal in acute respiratory failure. Chest. 1994;105:1198–1203. doi: 10.1378/chest.105.4.1198. [DOI] [PubMed] [Google Scholar]