Abstract
Background
Group A streptococcus (GAS) pharyngitis is associated with high rates of rheumatic heart disease (RHD) in developing countries. We sought to identify guidelines for empiric treatment of pharyngitis in low resource settings. To inform the design of GAS vaccines, we determined the emm types associated with pharyngitis among African schoolchildren.
Methods
Surveillance for pharyngitis was conducted among children 5 to 16 years of age attending schools in Bamako, Mali. Students were encouraged to visit a study clinician when they had a sore throat. Enrollees underwent evaluation and throat swab for isolation of GAS. Strains were emm typed by standard methods.
Results
GAS was isolated from 449 (25.5%) of the 1,759 sore throat episodes. Painful cervical adenopathy identified 403 children (89.8%) with GAS infection and was absent in 369 uninfected children (28.2%). Emm type was determined in 396 (88.2%) of the 449 culture-positive children; 70 types were represented and 14 types accounted for 49% of isolates. Based on the proportion of the 449 isolates bearing emm types included in the 30-valent vaccine (31.0%) plus non-vaccine types previously shown to react to vaccine-induced bactericidal antibodies (44.1%), the vaccine could protect against almost 75% of GAS infections among Bamako schoolchildren.
Conclusions
Two promising strategies could reduce RHD in low resource settings. Administering antibiotics to children with sore throat and tender cervical adenopathy could treat most GAS-positive children while reducing use of unnecessary antibiotics for uninfected children. Broad coverage against M types associated with pharyngitis in Bamako schoolchildren might be achieved with the 30-valent GAS vaccine under development.
Keywords: Group A Streptococcus, Streptococcal pharyngitis, emm, Mali
INTRODUCTION
Left untreated, pharyngitis due to Streptococcus pyogenes, or group A streptococcus (GAS), can lead to suppurative complications and acute rheumatic fever (ARF). Whereas ARF and its sequela, rheumatic heart disease (RHD), were important causes of morbidity and mortality in the U.S. and other high income countries in the late 1800s,1, 2 a combination of factors including economic development and specific antibiotic therapy for overt GAS pharyngitis have been credited with subsequent reductions in cases to the point of near-elimination, even in the face of a stable incidence in GAS pharyngitis.3
In stark contrast, RHD afflicts an estimated 1.9 million children aged 5 to 14 years residing in developing countries.4 Many of these children live in conditions with crowding and poor sanitation. In addition, effective prevention of ARF in developing countries is impeded by the poor availability of public education to teach parents when to seek medical care for a child with sore throat, diagnostic tests to guide the practitioners’ decisions regarding initiation of antibiotic treatment, and skills in cardiac auscultation and syndrome recognition that enable a clinician to detect persons with RHD in need of antibiotic prophylaxis. Whereas many studies have been performed to construct clinical algorithms for identification of GAS pharyngitis in developed countries,5–9 there is a need for data to guide clinicians in developing countries, in particular those in sub-Saharan Africa, as the relative risks and benefits of empiric treatment are quite different in these settings with high ARF incidence. Ultimately, development of a safe and effective GAS vaccine that could reduce or supplant the need for primary and secondary antibiotic prophylaxis for RHD offers many advantages. The candidate most advanced in clinical development includes the N-terminal regions of 30 GAS M proteins, designed to evoke type-specific opsonizing antibodies, a correlate of protective immunity.10 The composition of this vaccine was designed based on data from North America and Europe. However, the predicted efficacy for the prevention of RHD will depend on the vaccine’s ability to prevent infections with emm types that cause pharyngitis in developing regions of the world.
We describe herein a prospective cohort study of the epidemiology of GAS pharyngitis among schoolchildren living in Bamako, Mali. This study was conducted to address two aims: 1) to identify clinical features associated with sore throat that are capable of identifying children in this population with GAS infection while minimizing unnecessary treatment of uninfected children, and 2) to characterize the emm types of GAS associated with pharyngitis in this African setting to inform the development of M protein-based vaccines.
METHODS
Sampling Frame and Recruitment Activities
The study was conducted at four public schools in Djikoroni-para and Sébénikoro, two adjacent quartiers (neighborhoods) in Commune IV of Bamako, Mali. Between 1,600 and 5,000 students were enrolled in each school. Children at two of the schools attended either a morning or afternoon session to alleviate over-crowding. Medical care was available at each school for children who became ill during the school day; three schools maintained an on-site infirmary while the fourth utilized a neighboring health center. Study clinicians manned all four facilities to enroll eligible children on weekdays year round.
The study was introduced at the start of each school year by inviting the community to an informational session. Thereafter, study personnel visited the classrooms at least once per week and encouraged students to seek care from study personnel if they developed a sore throat. The school year ran from October to May, with a 3-week break. When school was not in session, local criers visited the quartiers several times per week chanting reminders to parents and children via loudspeaker that the study was ongoing.
Ethical Approvals and Informed Consent
The study was approved by the institutional review boards at the Faculté de Médecine, Pharmacie et Odontostomatologie in Bamako, Mali, the University of Maryland, Baltimore, University of Tennessee Health Science Center and the Memphis Veterans Affair Medical Center. Prior to beginning study activities, the investigators met with the local school and community authorities and obtained community consent. Parental consent was obtained for all participants either at the beginning of the school year or at the time of a pharyngitis episode. In addition, written assent was obtained from all participants 13 to 16 years of age.
Detection and management of pharyngitis cases and sampling
Students aged 5 to 16 years who presented to study personnel at the school infirmary or school-associated health center complaining of sore throat were invited to participate. Enrolled children provided clinical information solicited using a standardized interview to determine the presence of headache, rhinorrhea, chills, cough, difficulty swallowing, hoarseness, nausea, vomiting, malaise, abdominal pain, diarrhea, or a history of feverishness. Thereafter, a study clinician systematically measured the child’s oral temperature, examined the conjunctiva, mouth, pharynx, tonsils, palate, uvula, cervical lymph nodes, and skin, and recorded the findings on a standardized case report form. A swab of the posterior pharynx and tonsillar fossae was collected, placed in Amies charcoal media (CultureSwab™, Becton Dickinson) and transported to the laboratory at ambient temperature for plating within 6 hours of collection.
All participants were given a 3-day supply of acetaminophen at weight-appropriate dosages free of charge. If GAS was isolated, they were given a 10-day supply of penicillin VK and encouraged to complete the treatment course. At the end of the treatment period, a field worker visited the home to recover and record any remaining doses.
Microbiology and emm typing
Throat swabs were used to inoculate 5% sheep’s blood agar media by isolation streaking. A bacitracin disk (0.04 U) was placed in the area of the primary inoculum and the plate was incubated at 35–37°C. Each plate was checked for growth at 24 and 48 hours. Beta-hemolytic, catalase-negative, bacitracin-sensitive, Gram positive cocci in pairs and chains that tested positive for GAS antigen agglutination (Remel, Lenexa) were designated as GAS. Isolates were stored at −80°C in trypticase soy broth with 15% glycerol.
DNA from the GAS isolates was extracted and the emm gene was amplified using standard methodology (Centers for Disease Control (CDC), Atlanta, GA).11 The amplified DNA product was sent to the University of Maryland Biopolymer Laboratory for 5’ sequencing. Trimmed emm sequences containing 150 nucleotide bases encoding the mature protein were aligned with the CDC database of defined emm-types (downloaded September 27, 2012) using FASTA 36.3.5d.12 Each sequence was assigned an emm-type based on parameters as described (http://www.cdc.gov/ncidod/biotech/strep/assigning.htm).
Data analysis
Data were collected on case report forms (Teleform version 8.2.0) that were scanned and used to populate an Access database (Microsoft). Data were analyzed using EpiInfo (CDC, Atlanta GA), NCSS (Number Cruncher Statistical Systems, Kaysville, Utah), and SAS version 9.3 (SAS Institute Inc., Cary NC). Associations between symptoms and GAS were assessed by logistic regression models, allowing for within-individual correlation due to multiple episodes of pharyngitis in an individual participant. A two-sided p-value < 0.05 was considered statistically significant.
RESULTS
From May 2006 to September 2009, 1,418 students at the participating schools contributed 1,759 episodes of pharyngitis (Table 1). There were 244 students who experienced more than 1 episode of pharyngitis; of these, 19 students had 4 or more episodes, 50 had 3 episodes and 175 had 2 episodes. GAS was isolated in 449 episodes (25.5%) experienced by 421 (29.7%) students. GAS was isolated 3 times from 5 students and twice from 18 students. GAS was more commonly isolated from girls with pharyngitis than boys (290/1042 vs 159/717; odds ratio 1.4 (95% CI 1.1–1.7), p = 0.008). There was no evidence of an association between age and GAS isolation (p = 0.88). Pill counts taken at the follow-up visits suggested that a total of 429 (95.5%) children with GAS pharyngitis took all doses of penicillin prescribed; no medication-related adverse events were observed. The percentage of cases positive for GAS was similar from season to season (26.8% in the cold season, 23.6% in the hot season and 25.3% in the rainy season).
Table 1.
Frequency of group A streptococcal pharyngitis among Bamako schoolchildren.
| Age Group | Number of cases of sore throat |
GAS positive cases | |
|---|---|---|---|
| N | % | ||
| 5 to 7 years old | 160 | 45 | 28.1 |
| 8 to 10 years old | 517 | 120 | 23.2 |
| 11 to 13 years old | 817 | 220 | 26.9 |
| 14 to 16 years old | 265 | 64 | 24.1 |
| Total | 1759 | 449 | 100.0 |
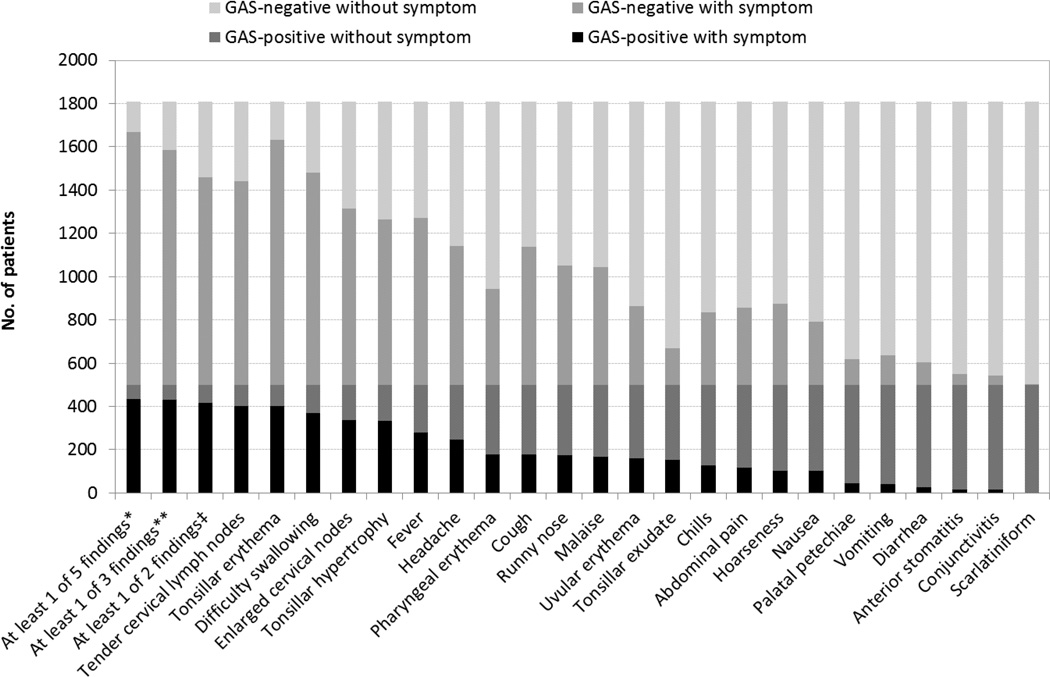
The relative frequency of clinical findings was similar among GAS-positive and GAS-negative children, although there were differences in the absolute proportions (Figure 1). The most frequent findings among GAS-positive and GAS-negative patients, respectively, were tender cervical lymph nodes (89.8% vs 71.8%) and tonsillar erythema (89.3% vs 86.4%). No suppurative or non-suppurative complications of GAS were identified. In multiple logistic regression analysis, the presence of tender cervical lymph nodes, tonsillar exudate, chills, tonsillar hypertrophy, and uvular erythema were positively associated with the isolation of GAS whereas complaints of cough, malaise, and hoarseness were negatively associated, i.e., the absence of these symptoms was significantly more common when GAS was present (Table 2). We evaluated the predictive value of these eight signs and symptoms, when present alone or in combination, for GAS isolation (Table 3). Tender cervical lymph nodes alone conferred a sensitivity of 89.8%. Inclusion of additional signs increased the sensitivity to a maximum of 96.4%. The maximum specificity associated with a sensitivity of 89.8% or higher was seen with tender cervical lymph nodes alone; using this single parameter, 30.0% of episodes classified as GAS are truly infected, and 88.9% of episodes without this sign are truly uninfected, and unnecessary antibiotics could be avoided for 28.2% of truly uninfected children who lack this finding.
Figure 1.
The number of patients with and without various clinical findings, alone or in combination, according to the presence of group A streptococcus (GAS) by throat culture. *5 findings denotes: tender cervical lymph nodes, tonsillar exudate, tonsillar hypertrophy, chills, or uvular erythema; **3 findings denotes: tender cervical lymph nodes, tonsillar exudate, or tonsillar hypertrophy; ‡2 findings denotes: tender cervical lymph nodes or tonsillar exudate.
Table 2.
Multiple logistic regression model to determine the signs and symptoms which were positively or negatively (i.e. odds ratio <1) associated with the isolation of group A streptococcus (GAS) from Bamako schoolchildren with sore throat.
| Number (%) with symptom who have GAS |
Number (%) without symptom who have GAS |
Multivaria te Odds Ratio |
95% Confiden ce Limit |
p-value in multiple regressi on |
|
|---|---|---|---|---|---|
| Tender cervical lymph nodes | 403 (30.0) | 96 (20.6) | 3.06 | 2.17–4.33 | <0.0001 |
| Tonsillar exudate | 152 (47.1) | 347 (23.4) | 2.69 | 2.04–3.54 | <0.0001 |
| Tonsillar hypertrophy | 334 (30.4) | 165 (23.2) | 1.49 | 1.16–1.93 | 0.002 |
| Chills | 129 (27.9) | 370 (27.5) | 1.37 | 1.03–1.81 | 0.030 |
| Uvular erythema | 159 (30.5) | 340 (26.4) | 1.32 | 1.02–1.70 | 0.034 |
| Cough | 177 (21.7) | 322 (32.5) | 0.72 | 0.57–0.91 | 0.005 |
| Malaise | 168 (23.5) | 331(30.2) | 0.67 | 0.52–0.87 | 0.003 |
| Hoarseness | 103 (21.5) | 396 (29.8) | 0.65 | 0.48–0.87 | 0.003 |
Table 3.
Calculated sensitivity, specificity and positive and negative predictive values for individual signs and symptoms significantly associated with isolation of group A streptococcus (GAS) in multivariate logistic regression, when considered alone or in combination.
| Clinical Finding | Sensitivity | Specificity | Positive predictive value |
Negative predictive value |
Treatment ratio‡ |
|---|---|---|---|---|---|
| Individual signs | |||||
| Tender cervical lymph nodes (LN) | 89.8 | 28.2 | 30.0 | 88.9 | 3.0 |
| Tonsillar hypertrophy | 74.4 | 41.7 | 30.4 | 82.6 | 2.8 |
| Uvular erythema | 35.4 | 72.3 | 30.5 | 76.6 | 3.3 |
| Tonsillar exudate | 33.9 | 86.9 | 47.1 | 79.3 | 3.2 |
| Chills | 28.7 | 86.9 | 27.9 | 75.3 | 3.6 |
| Cough | 39.4 | 51.1 | 21.7 | 71.1 | 2.6 |
| Malaise | 37.4 | 58.3 | 23.5 | 73.1 | 2.3 |
| Hoarseness | 22.9 | 71.4 | 21.5 | 73.0 | 2.6 |
| Combinations of signs | |||||
| Either tender cervical LN or tonsillar exudate | 92.9 | 26.8 | 30.3 | 91.6 | 2.3 |
| ≥1 of 3 findings* | 95.5 | 17.2 | 28.3 | 91.8 | 2.5 |
| ≥1 of 5 findings** | 96.4 | 10.8 | 27.0 | 89.8 | 2.7 |
| ≥2 of 5 findings** | 84.0 | 35.7 | 30.9 | 86.7 | 2.2 |
| Either tender cervical LN or tonsillar exudate, and either cough, hoarseness, or malaise | 65.3 | 56.4 | 33.9 | 82.6 | 2.5 |
| ≥ 1 of 3 findings* and ≤ 2 of: cough, hoarseness, malaise | 67.5 | 48.1 | 30.8 | 81.2 | 2.4 |
| ≥ 1 of 3 findings* and no cough | 88.2 | 28.0 | 29.6 | 87.4 | 2.0 |
| ≥ 1 of 3 findings* and no hoarseness | 58.4 | 59.1 | 32.8 | 80.5 | 2.3 |
| ≥ 1 of 3 findings* and no malaise | 73.1 | 42.3 | 30.3 | 82.1 | 2.3 |
3 findings denotes: tender cervical LN, tonsillar exudate, or tonsillar hypertrophy
5 findings denotes: tender cervical LN, tonsillar exudate, tonsillar hypertrophy, chills, or uvular erythema
Based on the presence of the clinical finding, the number of GAS-negative episodes that would be treated for every GAS-positive episode treated
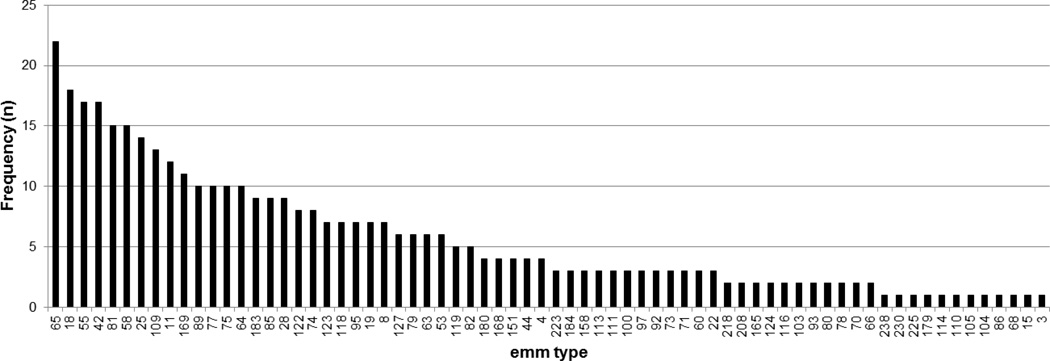
All 449 GAS strains isolated during the surveillance period were subjected to emm typing. An emm type was identified in 396 (88.2%) of the isolates. The sequences did not yield an interpretable result in the remaining 53 isolates. Although 70 emm types were represented, 49% of isolates belonged to 14 emm types (Figure 1). The most common emm type, emm65, accounted for 5.6% of isolates. Twelve emm types were represented only once. Only one of the students who had more than one episode of GAS positive pharyngitis had the same emm type isolated twice (emm 75.1) and these episodes occurred greater than 9 months apart.
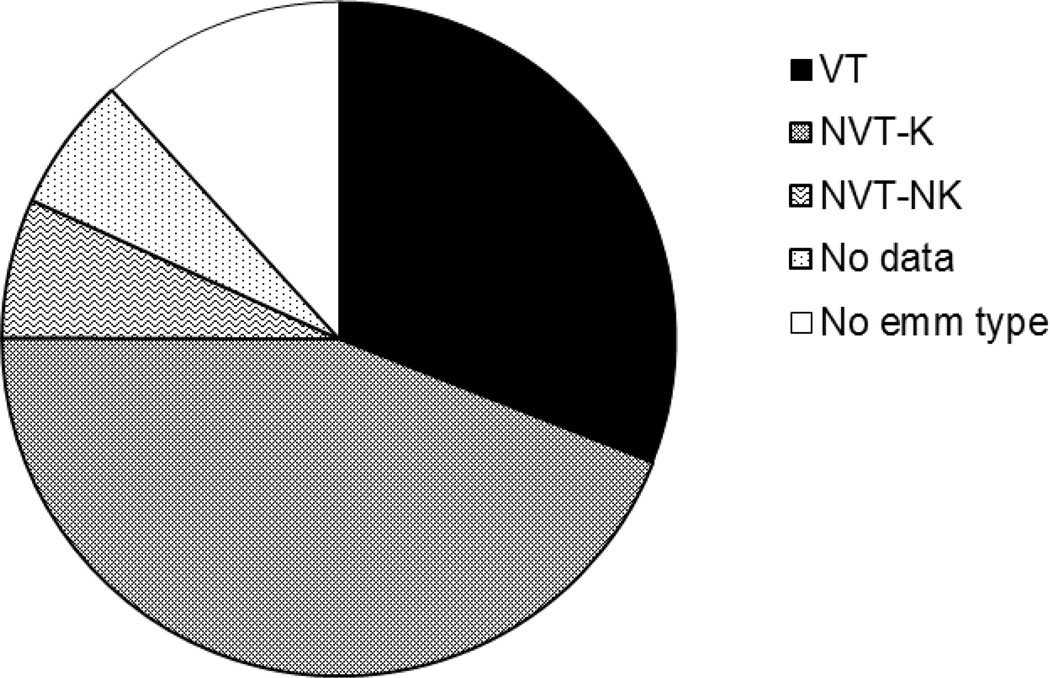
We examined the proportion of emm types that are represented in the 30-valent GAS vaccine currently under development (Figures 2 and 3).10 A total of 139 isolates (31.0%) belonged to 19 of the emm types included in the vaccine. An additional 198 isolates (44.1%), representing 30 emm types, were non-vaccine type isolates but have been shown to exhibit more than 50% bactericidal killing in the presence of rabbit antisera generated after vaccination with the 30-valent vaccine. The most commonly isolated emm type (emm65) was among these. The vaccine did not induce bactericidal activity against 5 non-vaccine types (6.7%), and there were insufficient data to assess whether the vaccine induced cross-reactive bactericidal antibodies for the remaining 15 types (6.5%) isolated. The most commonly isolated type not covered by the vaccine was emm55, of which there were 17 isolates (3.8% of the total). The proportion of isolates that might be covered either directly or by cross-reactive antibodies induced by vaccination varied from year to year (81.5% in 2006, 65.3% in 2007, 75.4% in 2008 and 93.1% in 2009, chi-square, p=0.022).
Figure 2.
The frequency of each emm type among the 396 isolates for which typing data was available. The first 14 types represent 49% of the isolates.
Figure 3.
Of the 449 GAS isolates, 31% (indicated as VT or vaccine type) are included in the 30-valent vaccine presently under development. Based on data indicating that cross-coverage of certain emm types has been observed in-vitro (indicated as non-vaccine type killed, NVT-K), this vaccine could cover almost 75% of the Mali isolates. The vaccine does not cover 6.7% of isolates and there is no information regarding potential coverage of the remaining 18.3% (indicated as no data or no emm type).
DISCUSSION
To our knowledge, this is the first study to systematically quantify the frequency and clinical presentation of GAS pharyngitis among school-aged children in a country designated by the United Nations as least developed. We found that approximately 25% of sore throat episodes among children 5 to 16 years of age attending school in Bamako, Mali were positive for GAS, which is similar to that observed in upper and middle income countries.13–16 Several clinical scoring systems have been developed for use in low resource settings in an attempt to reduce unnecessary antibiotic treatment of the 75% of children with sore throat who are not infected with GAS. The goal is to identify a subset of children who are likely to be GAS positive for microbiologic confirmation, or if unavailable, for empiric treatment. These scoring systems, which generally include multiple clinical parameters, have been evaluated in middle income countries such as Brazil,13, 14, 17, Egypt,13, Latvia and Croatia,15 but it is not known whether they are applicable, feasible, or practical for use in the less developed countries of sub-Saharan Africa where overall implementation of recommended guidelines for clinical care is known to be inadequate.18, 19 After searching for a simplified approach, we found that a single finding, tender cervical lymph nodes, could identify 89.8% of GAS-positive children with sore throat and when absent could avoid unnecessary antibiotics administration in 28.2% of GAS-negative children. Cervical adenopathy (variously defined as enlargement and/or tenderness) has improved the sensitivity of most decision rules (although not to the extent that we observed) and has been incorporated into numerous models.20 Our findings suggest that if Bamako schoolchildren with sore throat were screened for cervical tenderness, then 2.3 GAS-negative children would be treated for every GAS-positive child treated, compared to a treatment ratio of 3.5:1 associated with the standard practice of treating all children with sore throat in our population.
Use of clinical algorithms is driven by the belief that antibiotic cost, toxicity, and treatment-induced antibiotic resistance outweigh the benefits of empiric treatment of all episodes of pharyngitis, and that the 9–16% of GAS-positive children who do not meet criteria have more subtle symptoms and are likely carriers who are at low risk for ARF. However, data are insufficient to draw the same conclusion for low income countries such as Mali. Even if future studies validate the feasibility and performance characteristics of our clinical rule in Mali and other low income settings, the benefits of administering antibiotics to all children with sore throat might still prevail when one considers factors such as the high prevalence of RHD, the difficulty implementing secondary prophylaxis to reduce the risk of chronic valvular damage,21 the absence of penicillin resistance despite widespread utilization, the relatively low cost and low risk of penicillin, and the high cost of RHD, both direct (medical costs) and indirect (loss of productivity).
Based on over 3 years of surveillance among Bamako schoolchildren with sore throat, our findings support and extend observations that considerable emm type diversity occurs in numerous low and middle income countries such as India,22 Brazil,23 and Fiji;24 in these settings, many emm types were identified, but each accounted for a relatively small proportion of the total infections. We found that 25 emm types accounted for 70.2% of the 70 types identified, which is similar to a recent report from Africa where 25 emm types accounted for 62.5% of the 90 types identified.25 By comparison, in high income countries 25 emm types accounted for the vast majority (90.3%) of the 171 types found.25 There are also substantial geographic differences in the relative frequency of the emm types that occur most commonly. Whereas in high income countries the most common emm types are emm1, emm12, emm28, emm3 and emm4, accounting for about half of strains isolated,25 in Bamako these represented only 14 (3.5%) of the 396 typed isolates. The emm type diversity observed in the regions most in need of a vaccine to prevent RHD has driven the development of polyvalent type-specific vaccines, as well as the search for vaccine components that contain conserved regions of the M protein or other highly conserved GAS antigens that might provide protection against a large proportion of the >200 emm types that have been identified.10, 26–29
Several GAS vaccines are undergoing preclinical development and two have previously been evaluated in early-stage clinical trials.26, 27 The latter vaccines incorporate fusion proteins containing peptides of the N-terminal region of M proteins which have been shown to elicit type-specific opsonic antibodies that Lancefield originally associated with protective immunity.30 In addition, there is evidence that these vaccines may provide cross-protection which is thought to be based upon bactericidal antibodies that recognize shared epitopes in the N-terminal region of various emm types.10, 31 Type-specific vaccines containing 6 and 26 M types have been well-tolerated and immunogenic when administered intramuscularly with alum adjuvant in clinical trials.26, 27 A 30-valent vaccine has now been constructed that contains emm types responsible for more than 90% of GAS infections occurring in North America and Europe but has far less homology with the broad spectrum of emm types seen in many developing countries.10 Consequently, an important finding in our study is that bactericidal activity against 44.1% of the non-vaccine emm types circulating in Bamako in addition to 31.0% coverage by vaccine types could translate into nearly 75% protective efficacy by the 30-valent vaccine for Malian children.10, 31 Since we conducted surveillance over a 3-year period, we were able to observe the emm type distribution prospectively and found that significant variations occurred from year-to-year and had a minimum coverage of 65.3%.
A limitation of our study is that asymptomatic children were not included for comparison, so it is difficult to ascertain whether certain emm types were more strongly associated with pharyngitis. Moreover, we cannot determine whether the strain identified in an individual child represents clinical infection and therefore poses a risk for ARF, or asymptomatic colonization, which does not. Therefore, we must infer with caution that the distribution of emm types identified among children with sore throat resemble those associated with ARF in our population. Of note, emm3 and emm18, which accounted for 4.8% of our isolates, have been previously isolated in cases of ARF.32–39 In addition, our data may not be generalizable to children in the same neighborhoods who do not attend school or in less densely populated areas of Mali and where more heterogeneous populations may live.23 From local demographic surveillance data collected over the study period, we know that only 70% of the children living in Djikoroni, one of the study quartiers, attended school.
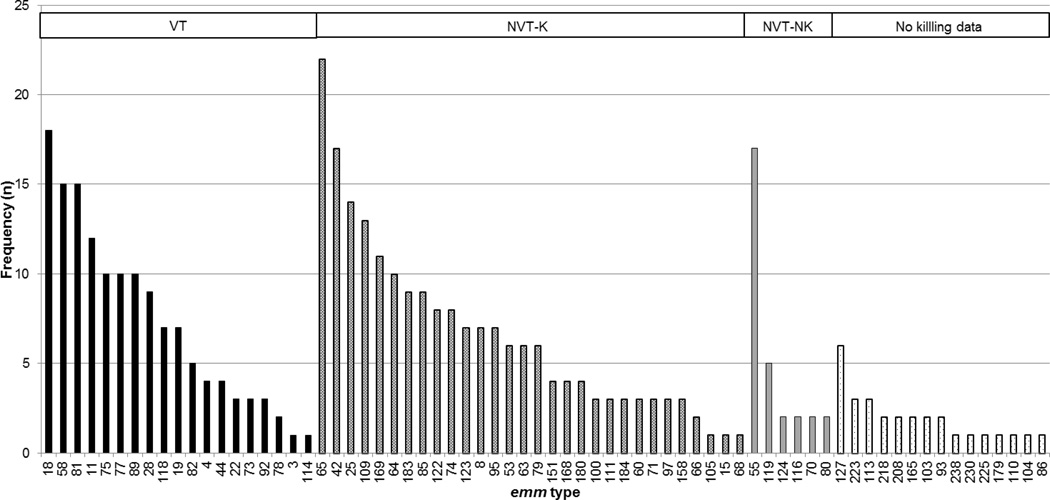
Figure 4.
Of the 70 emm types observed in Malian school children with pharyngitis, 19 types (VT) were among those covered by the 30-valent vaccine under development. Based on in vitro data, the 30-valent vaccine could induce cross-coverage of an additional 30 non-vaccine types (NVT-K). Six types (NVT-NK) would not be covered; there is no data on the coverage of the remaining 15 types observed.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of the student bodies at the participating schools, school administrators, parent-teacher organizations and health care personnel. We thank Joshua Orvis for his assistance in emm typing of GAS isolates. We also offer special thanks to Fran Rubin, PhD and Robin Mason for their dedication to this project and their invaluable guidance and support.
Source of Funding
Dr. Dale is the inventor of certain technologies related to the development of group A streptococcal vaccines, which have been licensed by the University of Tennessee Research Foundation to Vaxent, LLC. Dr. Dale serves as the Chief Scientific Officer of Vaxent and is a member.
Source of Support: National Institutes of Health, UO1AI060592 awarded to JB Dale.
Footnotes
Conflicts of interest
The remaining authors have no conflicts of interest to declare.
REFERENCES
- 1.Stamler J. Cardiovascular diseases in the United States. Am J Cardiol. 1962;10:319–340. doi: 10.1016/0002-9149(62)90320-x. [DOI] [PubMed] [Google Scholar]
- 2.Collins SD. The Incidence of Rheumatic Fever as recorded in General Morbidity Surveys of Families. Supplement No. 198 to Pub.Health Rep. ed. 1947 [Google Scholar]
- 3.Gordis L. The virtual disappearance of rheumatic fever in the United States: lessons in the rise and fall of disease. T. Duckett Jones memorial lecture. Circulation. 1985;72(6):1155–1162. doi: 10.1161/01.cir.72.6.1155. [DOI] [PubMed] [Google Scholar]
- 4.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 5.Attia M, Zaoutis T, Eppes S, Klein J, Meier F. Multivariate predictive models for group A beta-hemolytic streptococcal pharyngitis in children. Acad Emerg Med. 1999;6(1):8–13. doi: 10.1111/j.1553-2712.1999.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 6.Attia MW, Zaoutis T, Klein JD, Meier FA. Performance of a predictive model for streptococcal pharyngitis in children. Arch Pediatr Adolesc Med. 2001;155(6):687–691. doi: 10.1001/archpedi.155.6.687. [DOI] [PubMed] [Google Scholar]
- 7.Breese BB. A simple scorecard for the tentative diagnosis of streptococcal pharyngitis. Am J Dis Child. 1977;131(5):514–517. doi: 10.1001/archpedi.1977.02120180028003. [DOI] [PubMed] [Google Scholar]
- 8.Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239–246. doi: 10.1177/0272989X8100100304. [DOI] [PubMed] [Google Scholar]
- 9.Funamura JL, Berkowitz CD. Applicability of a scoring system in the diagnosis of streptococcal pharyngitis. Clin Pediatr (Phila) 1983;22(9):622–626. doi: 10.1177/000992288302200906. [DOI] [PubMed] [Google Scholar]
- 10.Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine. 2011;29(46):8175–8178. doi: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34(4):953–958. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinhoff MC, bd el Khalek MK, Khallaf N, et al. Effectiveness of clinical guidelines for the presumptive treatment of streptococcal pharyngitis in Egyptian children. Lancet. 1997;350(9082):918–921. doi: 10.1016/s0140-6736(97)03317-5. [DOI] [PubMed] [Google Scholar]
- 14.Smeesters PR, Campos D, Jr, Van ML, de AE, Vanderpas J, Vergison A. Pharyngitis in low-resources settings: a pragmatic clinical approach to reduce unnecessary antibiotic use. Pediatrics. 2006;118(6):e1607–e1611. doi: 10.1542/peds.2006-1025. [DOI] [PubMed] [Google Scholar]
- 15.Rimoin AW, Walker CL, Hamza HS, et al. The utility of rapid antigen detection testing for the diagnosis of streptococcal pharyngitis in low-resource settings. Int J Infect Dis. 2010;14(12):e1048–e1053. doi: 10.1016/j.ijid.2010.02.2269. [DOI] [PubMed] [Google Scholar]
- 16.McIsaac WJ, Kellner JD, Aufricht P, Vanjaka A, Low DE. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA. 2004;291(13):1587–1595. doi: 10.1001/jama.291.13.1587. [DOI] [PubMed] [Google Scholar]
- 17.Joachim L, Campos D, Jr, Smeesters PR. Pragmatic scoring system for pharyngitis in low-resource settings. Pediatrics. 2010;126(3):e608–e614. doi: 10.1542/peds.2010-0569. [DOI] [PubMed] [Google Scholar]
- 18.Pariyo GW, Gouws E, Bryce J, Burnham G. Improving facility-based care for sick children in Uganda: training is not enough. Health Policy Plan. 2005;20(Suppl 1):i58–i68. doi: 10.1093/heapol/czi051. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen DT, Leung KK, McIntyre L, Ghali WA, Sauve R. Does integrated management of childhood illness (IMCI) training improve the skills of health workers? A systematic review and meta-analysis. PLoS One. 2013;8(6):e66030. doi: 10.1371/journal.pone.0066030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer Walker CL, Rimoin AW, Hamza HS, Steinhoff MC. Comparison of clinical prediction rules for management of pharyngitis in settings with limited resources. J Pediatr. 2006;149(1):64–71. doi: 10.1016/j.jpeds.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Karthikeyan G, Mayosi BM. Is primary prevention of rheumatic fever the missing link in the control of rheumatic heart disease in Africa? Circulation. 2009;120(8):709–713. doi: 10.1161/CIRCULATIONAHA.108.836510. [DOI] [PubMed] [Google Scholar]
- 22.Dey N, McMillan DJ, Yarwood PJ, et al. High diversity of group A Streptococcal emm types in an Indian community: the need to tailor multivalent vaccines. Clin Infect Dis. 2005;40(1):46–51. doi: 10.1086/426443. [DOI] [PubMed] [Google Scholar]
- 23.Tartof SY, Reis JN, Andrade AN, Ramos RT, Reis MG, Riley LW. Factors associated with Group A Streptococcus emm type diversification in a large urban setting in Brazil: a cross-sectional study. BMC Infect Dis. 2010;10:327. doi: 10.1186/1471-2334-10-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steer AC, Jenney AW, Kado J, et al. Prospective surveillance of streptococcal sore throat in a tropical country. Pediatr Infect Dis J. 2009;28(6):477–482. doi: 10.1097/INF.0b013e318194b2af. [DOI] [PubMed] [Google Scholar]
- 25.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9(10):611–616. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 26.Kotloff KL, Corretti M, Palmer K, et al. Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: phase 1 trial. JAMA. 2004;292(6):709–715. doi: 10.1001/jama.292.6.709. [DOI] [PubMed] [Google Scholar]
- 27.McNeil SA, Halperin SA, Langley JM, et al. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41(8):1114–1122. doi: 10.1086/444458. [DOI] [PubMed] [Google Scholar]
- 28.Steer AC, Dale JB, Carapetis JR. Progress toward a global group a streptococcal vaccine. Pediatr Infect Dis J. 2013;32(2):180–182. doi: 10.1097/INF.0b013e318281da11. [DOI] [PubMed] [Google Scholar]
- 29.Moreland NJ, Waddington CS, Williamson DA, et al. Working towards a group A streptococcal vaccine: report of a collaborative Trans-Tasman workshop. Vaccine. 2014;32(30):3713–3720. doi: 10.1016/j.vaccine.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Lancefield RC. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 31.Dale JB, Penfound TA, Tamboura B, et al. Potential coverage of a multivalent M protein-based group A streptococcal vaccine. Vaccine. 2013;31(12):1576–1581. doi: 10.1016/j.vaccine.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brundage JF, Gunzenhauser JD, Longfield JN, et al. Epidemiology and control of acute respiratory diseases with emphasis on group A beta-hemolytic streptococcus: a decade of U.S. Army experience. Pediatrics. 1996;97(6 Pt 2):964–970. [PubMed] [Google Scholar]
- 33.Richardson LJ, Towers RJ, Cheng AC, et al. Diversity of emm sequence types in group A beta-haemolytic streptococci in two remote Northern Territory Indigenous communities: implications for vaccine development. Vaccine. 2010;28(32):5301–5305. doi: 10.1016/j.vaccine.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 34.Smoot JC, Korgenski EK, Daly JA, Veasy LG, Musser JM. Molecular analysis of group A Streptococcus type emm18 isolates temporally associated with acute rheumatic fever outbreaks in Salt Lake City, Utah. J Clin Microbiol. 2002;40(5):1805–1810. doi: 10.1128/JCM.40.5.1805-1810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veasy LG, Tani LY, Daly JA, et al. Temporal association of the appearance of mucoid strains of Streptococcus pyogenes with a continuing high incidence of rheumatic fever in Utah. Pediatrics. 2004;113(3 Pt 1):e168–e172. doi: 10.1542/peds.113.3.e168. [DOI] [PubMed] [Google Scholar]
- 36.Miner LJ, Petheram SJ, Daly JA, et al. Molecular characterization of Streptococcus pyogenes isolates collected during periods of increased acute rheumatic fever activity in Utah. Pediatr Infect Dis J. 2004;23(1):56–61. doi: 10.1097/01.inf.0000105180.76624.33. [DOI] [PubMed] [Google Scholar]
- 37.Bisno AL. Group A streptococcal infections and acute rheumatic fever. N Engl J Med. 1991;325(11):783–793. doi: 10.1056/NEJM199109123251106. [DOI] [PubMed] [Google Scholar]
- 38.Shulman ST, Stollerman G, Beall B, Dale JB, Tanz RR. Temporal changes in streptococcal M protein types and the near-disappearance of acute rheumatic fever in the United States. Clin Infect Dis. 2006;42(4):441–447. doi: 10.1086/499812. [DOI] [PubMed] [Google Scholar]
- 39.Stollerman GH. The relative rheumatogenicity of strains of group A streptococci. Mod Concepts Cardiovasc Dis. 1975;44(7):35–40. [PubMed] [Google Scholar]