Abstract
How neoplastic cells respond to therapy is not solely dependent on the complexity of genomic aberrations they harbor, but is also regulated by numerous dynamic properties of the tumor microenvironment. Identifying and targeting critical pathways that improve therapeutic efficacy by bolstering anti-tumor immune responses holds great potential for improving outcomes and impacting long-term patient survival. Macrophages are key regulators of homeostatic tissue and tumor microenvironments; thus therapeutics impacting macrophage presence and/or bioactivity have shown promise in preclinical models, and are now being evaluated in the clinic. This review discusses the molecular/cellular pathways thus far identified whereby macrophages mediate therapeutic responses.
Keywords: macrophages, cancer, tumor, metastasis, chemotherapy, immunotherapy, resistance
Introduction
Macrophages are represented in all tissues by functionally and phenotypically distinct resident populations that are critical for development and homeostasis (Wynn et al., 2013). Under non-pathological conditions, most resident macrophage populations derive from embryonic progenitors and are maintained through local proliferation (Epelman et al., 2014). Exceptions to this include intestinal, dermal and alveolar macrophages at barrier sites (Bain et al., 2014; McGovern et al., 2014; Perdiguero et al., 2014; Yona et al., 2013), and macrophages in the adult heart that are replaced by circulating bone marrow-derived Ly6C+ inflammatory monocytes over a time scale of several weeks (Molawi et al., 2014). Under pathological conditions, there is evidence for both local proliferation and recruitment, with differences observed by tissue location and type of inflammatory insult (Epelman et al., 2014).
Solid tumors appear to be unique; preclinical studies indicate absence of macrophage proliferation and shorter half-lives as compared to resident macrophages in counterpart homeostatic tissues, measurable in days to weeks (Movahedi et al., 2010; Strachan et al., 2013). That said, proliferating CD68+ cells, also positive for proliferating cell nuclear antigen (PCNA) expression, have been observed in breast cancers where they are associated with poor clinical outcome (Campbell et al., 2011). Whether macrophage life span in this context is reflecting diminished tissue integrity, extent of damage/inflammation, or instead represents an adaptive process engaged by tumors to support growth is unclear, but production of the C-C chemokine ligand 2 (CCL2) and/or colony stimulating factor-1 (CSF-1) are necessary to sustain their numbers (Noy and Pollard, 2014). With the critical role for CCL2 and CSF-1 in recruiting macrophages to neoplastic tissue there is growing interest in therapeutics targeting these ligands and/or their respective receptors in an effort to ablate pro-tumorigenic properties of macrophages. This therapeutic approach has led to improved outcomes in a range of pre-clinical models — particularly for agents targeting CSF-1 or the CSF-1 receptor (CSF-1R) — results of which have spurred several clinical trials (Table 1).
Table 1.
Macrophage therapeutic targeting.
| Pathway | Target1 | Efficacy in Murine Models | Clinical Compounds | Clinical Trials in Solid Tumors2 |
|---|---|---|---|---|
| Recruitment | CD11b | Radiation, Chemotherapy | Rovelizumab | |
| CSF-1R | Single Agent (GBM, PDAC), Chemotherapy, Radiation, Angiogenesis Inhibitors | PLX3397, AMG820 IMC-CS4/LY3022855, RG7155/RO5509554 | NCT01596751 (O); NCT01444404 (C); NCT01349036 (O); NCT01004861 (O); NCT01346358 (O); NCT02265536 (O); NCT01494688 (O); NCT02323191 (O) | |
| CCL2 | Single Agent (metastasis, PDAC) | Carlumab | NCT00992186 (C); NCT01204996 (C) | |
| Neuropilin-1 | Angiogenesis inhibitors | MNRP1685A | NCT00747734 (C); NCT00954642 (C) | |
| ANG2 | Single Agent (mammary), Chemotherapy, Angiogenesis Inhibitors | Nesvacumab | NCT01271972 (O); NCT01688960 (O) | |
| Polarization | IL-4 | Single Agent (metastasis), Chemotherapy, Radiation | Pascolizumab | |
| IL4Rα | Dupilumab | |||
| IL-13 | Chemotherapy | Lebrikizumab, Tralokinumab, GSK679586, | ||
| FcγR | Chemotherapy | Rituximab (CD20), Ibrutinib (BTK), R788 (Syk) | ||
| Function | IL-6 | Clazakizumab, Olokizumab, Siltuximab, Sirukumab | NCT00433446 (C); NCT00385827 (C) NCT00841191 (C) | |
| IL-6R | Tocilizumab, Sarilumab | |||
| TNF-α | MAPK inhibitors | Adalimumab, Certolizumab, Etanercept, Golimumab, Infliximab | ||
| Activation | CD40 | Single Agent (PDAC), Chemotherapy | CP-870,893 | NCT00711191 (C); NCT01456585 (C) NCT02157831 (C); NCT01008527 (O) NCT02225002 (C); NCT00607048 (C) NCT01103635 (O) |
Only targets with clinical compounds are listed.
O: ongoing; C: completed. Data obtained from clinicaltrials.gov
As monotherapy, CSF-1R inhibition alone impedes growth of orthotopically implanted pancreatic ductal adenocarcinoma (PDAC) cell lines (Mitchem et al., 2013), prevents cervical carcinogenesis (Strachan et al., 2013), and induces regression of glioblastoma multiforme (GBM) (Pyonteck et al., 2013). In other tumor models, CSF-1R inhibition is without consequence as monotherapy; however, synergism with other modalities, including chemotherapy (DeNardo et al., 2011; Mitchem et al., 2013; Paulus et al., 2006; Ruffell et al., 2014), radiation therapy (Shiao et al., 2015; Xu et al., 2013), angiogenic inhibitors (Priceman et al., 2010), adoptive cell transfer (Mok et al., 2014), and immune checkpoint blockade (Zhu et al., 2014) have been revealed. Together, these findings implicate macrophages in regulating therapeutic responses, and indicate that durable responses may be more likely by augmenting standard-of-care or emerging therapies with “macrophage antagonists”. This review will focus on the mechanisms underpinning these observations, and conclude with a discussion of targeting approaches that extend beyond inhibiting macrophage recruitment.
Clinical Significance of Macrophages
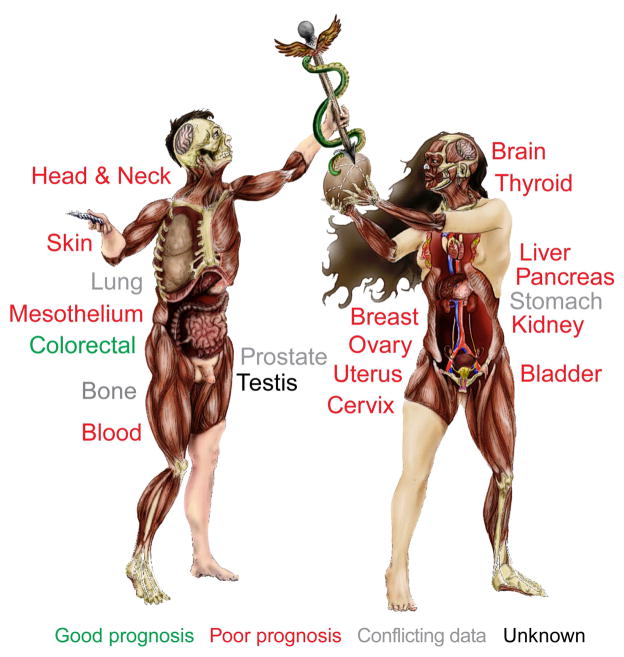
For many solid tumor types, high densities of cells expressing macrophage-associated markers have generally been found to associate with poor clinical outcome (Figure 1) (Komohara et al., 2014; Zhang et al., 2012). There is conflicting data for lung, stomach, prostate and bone, where both positive and negative outcome associations have been reported (Zhang et al., 2012), possibly related to the type/stage of cancer evaluated, (e.g., Ewings sarcoma versus osteosarcoma) (Buddingh et al., 2011; Fujiwara et al., 2011), or to the type of analysis performed (e.g. quantitation of stromal versus intratumoral macrophages). Some discrepancy may also reflect use of different macrophage markers. CD68, a glycoprotein predominantly resident in intracellular granules, represents a fairly specific marker for murine macrophages, and in combination with F4/80, identifies a majority of tumor-associated macrophages. In humans however, CD68 expression is widespread, and includes granulocytes, dendritic cells, fibroblasts, endothelial cells, and some lymphoid subsets (Gottfried et al., 2008; Hameed et al., 1994; Ruffell et al., 2012b); use of CD68 for association studies in this context is thus of variable utility. A clear example of this is non-small cell lung cancer, where detection of macrophage scavenger receptors CD163 and CD204, but not CD68, yielded clear correlations with negative outcome (Chung et al., 2012; Hirayama et al., 2012; Ohri et al., 2011; Quatromoni and Eruslanov, 2012).
Figure 1. Clinical implications of macrophage density.
Organs where tumor progression and/or clinical outcome are negatively associated with increased macrophage density are shown in red. Green indicates a positive association. Organs where the implications of macrophages density are unclear or unknown are shown in gray and black, respectively. Image created by Tarot Walker.
In addition to potentially representing more selective macrophage biomarkers, both CD163 and CD204 are associated with activation of macrophages towards an alternative or tumor-promoting and immunosuppressive phenotype, and accordingly, significant correlations between CD163/CD204 and negative outcomes have been reported across multiple tumor types (Komohara et al., 2014). This correlation may indicate that macrophage polarization can direct clinical outcome, also supported by the positive association between presence of CD68+ cells and survival in colorectal adenocarcinoma (Roxburgh and McMillan, 2012). Unlike most populations of tumor-associated macrophages that possess pro-tumor and immunosuppressive properties (Biswas and Mantovani, 2010), macrophages in human colorectal cancer have been found to be functionally (and phenotypically) anti-tumor (Edin et al., 2012; Ong et al., 2012; Zhang et al., 2014). Together, these data collectively support the tenet that repolarizing macrophages towards an anti-tumor phenotypic state, either by impeding activities/signals that drive pro-tumor polarization, or delivering exogenous signals that enhance anti-tumor polarization, could act as an alternative and perhaps more efficacious approach to blocking macrophage recruitment, even though these activities and responses are all dynamically regulated in vivo. Indeed, an agonist monoclonal antibody against CD40, a co-stimulatory protein found on professional antigen-presenting cells, has demonstrated efficacy in mouse models of pancreatic ductal adenocarcinoma (PDAC) (Beatty et al., 2011) and patients with PDAC (Beatty et al., 2013) when delivered in combination with the chemotherapeutic gemcitabine, ostensibly via the anti-tumor activities of macrophages and CD8+ T cells (Vonderheide et al., 2013). In addition to use of an anti-CSF-1R depleting antibody in diffuse-type giant cell tumors (Ries et al., 2014), these are the first clinical studies to demonstrate potential efficacy of macrophage-targeted agents.
Polarization and Macrophage Function
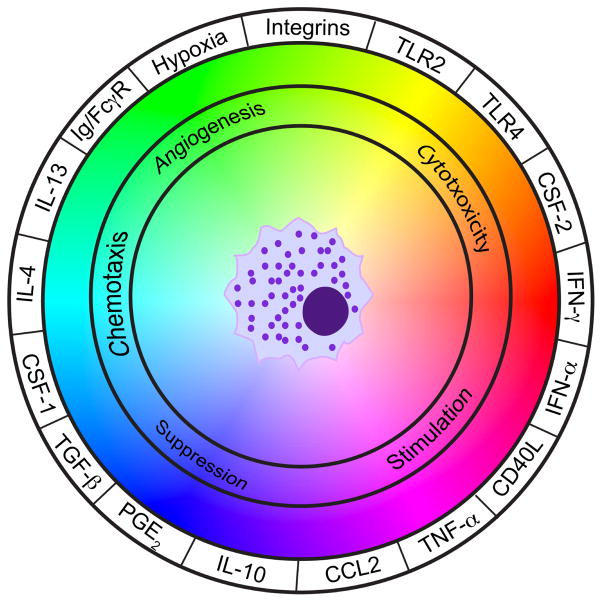
Macrophages produce an array of cytokines, chemokines, polypeptide growth factors, hormones, matrix remodeling proteases and metabolites, many of which possess tumor-promoting activities (De Palma and Lewis, 2013; Noy and Pollard, 2014; Ruffell et al., 2012a). A caveat to some of these reported activities is that many findings originate from cell culture studies utilizing neoplastic myeloid cell lines or bone marrow-derived macrophages, and thus cannot account for the complex milieu of polarization signals that macrophages would be exposed to in vivo (Figure 2). This includes the aforementioned CSF-1 and CCL2, prostaglandin E2 (PGE2), and damage-associated molecular patterns (DAMPs) such as high-mobility group box 1 protein (HMGB1), extracellular ATP, and degraded extracellular matrix components (Ruffell et al., 2012a; Zelenay and Reis e Sousa, 2013).
Figure 2. Macrophage polarization as a dynamic system.
The integration of multiple signals emanating from the tumor microenvironment (outer circle) dictates the functional role of macrophages (inner circle). Integrins and toll-like receptors (TLRs) will be engaged by multiple ligands.
Stabilization of hypoxia inducible factor (HIF)-1α and -2α are also important in mediating the pro-tumor properties of macrophages, as evidenced from the use of LysM-cre mice to induce myeloid-specific loss of either factor (Doedens et al., 2010; Imtiyaz et al., 2010). As might be expected, hypoxic conditions drive an angiogenic phenotype in macrophages, and in vivo this occurs specifically in a subpopulation of macrophages found within hypoxic regions of tumors that express low levels of major histocompatability complex (MHC) II (Laoui et al., 2014; Movahedi et al., 2010). The recruitment of macrophages (presumably MHCIILO) into hypoxic regions through Neuropilin-1 also supports an immunosuppressive phenotype (Casazza et al., 2013) that is likely dependent upon HIF-1α (Doedens et al., 2010). Surprisingly however, while hypoxia can induce HIF-1α-dependent expression of arginase-1 in macrophages (Doedens et al., 2010), neither improving tumor oxygenation, nor preventing macrophage recruitment into hypoxic areas alters arginase-1 expression (Casazza et al., 2013; Laoui et al., 2014). This discrepancy might be explained by the recent finding that lactic acid promotes arginase-1 expression by macrophages in a HIF-1α-dependent manner (Colegio et al., 2014).
Finally, the use of immune competent murine models has firmly established that macrophage polarization and function within tumors is strongly influenced by lymphocytes through the production of multiple factors including interleukin (IL)-4, IL-10, IL-13, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and immunoglobulins (Andreu et al., 2010; DeNardo et al., 2009; Gocheva et al., 2010; Guiducci et al., 2005; Kang et al., 2011). These activities include mediating responses to therapy, as B cells coated in anti-CD20 antibody can suppress their phagocytic removal from circulation by Kupffer cells through the secretion of IL-10 (Horikawa et al., 2011), while B cell production of immunoglobulins (Affara et al., 2014) and CD4+ T cell expression of IL-4 (Shiao et al., 2015) suppress responses to cytotoxic therapy by altering macrophage polarization. Even the efficacy of CSF-1R inhibition depends upon altered macrophage polarization, rather than depletion, in certain models (Pyonteck et al., 2013).
Macrophage Function and Therapeutic Resistance
Regulation of tumor cell survival pathways by macrophages
The general concept that neoplastic cell extrinsic factors mediate resistance to cytotoxic therapy owes to three dimensional (3D) cell culture models evaluating microenvironmental-derived factors (Correia and Bissell, 2012), but in vivo studies have revealed that macrophages also mediate chemotherapy resistance by providing survival factors and/or activating anti-apoptotic programs in malignant cells. While macrophage-secreted soluble factors have usually been implicated, it is also possible that extracellular matrix deposition and/or remodeling, or direct cell-cell interactions are involved (Castells et al., 2012; Correia and Bissell, 2012; Meads et al., 2009).
Using a CSF-1 neutralizing antibody in combination with chemotherapy, Paulus and colleagues reported increased chemosensitivity of subcutaneous MCF-7 breast cancer xenografts (Paulus et al., 2006). Co-culture studies utilizing mammary carcinoma cell lines and bone marrow-derived macrophages revealed macrophage-mediated resistance to paclitaxel, doxorubicin and etoposide (Shree et al., 2011), and to gemcitabine in murine PDAC cells (Mitchem et al., 2013). At least with PDAC cells, this resistance is dependent on activation of signal transducer and activator of transcription 3 (STAT3), implicating macrophage IL-6 or other macrophage-derived factors such as milk-fat globule-epidermal growth factor-VIII, found to promote resistance to carboplatin in vivo and synergize with IL-6 to enhance tumor cell growth (Jinushi et al., 2011). STAT3 activation promotes neoplastic cell proliferation and survival, and multiple tumor cell lines exhibit IL-6 or STAT3-dependent chemoresistance in vitro (Taniguchi and Karin, 2014; Yu et al., 2014). Though autocrine production of IL-6 is common, tumor-associated macrophages produce IL-6 in vivo (DeNardo et al., 2009; Movahedi et al., 2010; Song et al., 2009), with expression induced in bone marrow-derived macrophages by co-culture with neoplastic cells (Mitchem et al., 2013). However, as the only in vivo evidence of IL-6 being chemoprotective derives from a murine lymphoma model where IL-6 is expressed by thymic endothelial cells (Gilbert and Hemann, 2010), the source and relevance of IL-6 during chemotherapy for solid tumors remains incompletely described.
Surprisingly, macrophage production of soluble chemoprotective factors is in part dependent on cathepsin protease activity, specifically cathepsin B and S, where inhibition of cathepsin activity in vivo enhances response of mammary carcinomas to paclitaxel (Shree et al., 2011). A possible underlying mechanism may derive from cathepsin B-dependent activation of inflammasomes in myeloid cells following treatment with gemcitabine or 5-FU, leading to IL-1β release and enhancement of a TH17 immune response (Bruchard et al., 2013). Tumor-associated macrophages isolated from ovarian cancer patients also direct IL-17 production in memory T cells through IL-1β and IL-23 production (Kryczek et al., 2009). As IL-17 induces IL-6 expression in multiple cell types, including melanoma, mesenchymal, endothelial, and immune cells (Wang et al., 2009), cathepsin B could therefore be linked indirectly to STAT3 activation. However, IL-17 has also been found to direct anti-tumor responses to subcutaneously implanted tumor cell lines following treatment with anthracycline chemotherapy (Ma et al., 2011), and a role for IL-17 fails to explain macrophage chemoprotection in the absence of T cells. A more direct pathway may be through IL-1β-induced IL-6 expression that occurs in multiple cell types, including monocytes and osteoblasts (Mori et al., 2011; Tosato and Jones, 1990).
Alternatively, as cathepsin B activity is important in trafficking of TNF-α-containing vesicles to the surface of macrophages (Ha et al., 2008), TNF-α may be one of the critical factors mediating chemoprotection, either directly through NF-κβ activation (Li and Sethi, 2010), or indirectly through induced IL-6 expression and subsequent STAT3 activation (Mori et al., 2011). Macrophages can be a critical source of TNF-α in vivo, as it has recently been demonstrated that macrophage-derived TNF-α imparts resistance to MAPK inhibitors in melanoma through NF-κβ-dependent expression of microphthalmia transcription factor (Smith et al., 2014). Further research is warranted to determine whether macrophages indeed mediate resistance to cytotoxic therapies via these pathways, and to extend these finding to other targeted therapeutics, as with the complexities of TNF-α and NF-κβ in cancer development/growth, the efficacy of targeting these pathways may be context specific (Balkwill, 2009).
Macrophages and tumor angiogenesis
Macrophages are well-described regulators of tumor angiogenesis, with supporting evidence derived from both clinical and experimental studies (Murdoch et al., 2008; Ruffell et al., 2012a), wherein much of their capability is associated with vascular endothelial growth factor (VEGF) signaling. This includes macrophage production of VEGF-A (Lin et al., 2007; Stockmann et al., 2008), production of VEGF homologues such as placental growth factor (Fischer et al., 2007; Rolny et al., 2011), enhancement of VEGF-A bioavailability through matrix metalloproteinase (MMP)-9 activity (Bergers et al., 2000; Du et al., 2008; Giraudo et al., 2004; Nakasone et al., 2012), and induction of VEGF-A production by endothelial cells via WNT7B expression (Yeo et al., 2014). VEGF-A drives formation of abnormal vasculature in tumors, consisting of excessive branching, dead-end vessels, and vessel leakiness, that together impact tumor hemodynamics and drug delivery (Heldin et al., 2004; Tredan et al., 2007). VEGF antagonists induce vascular normalization (Greenberg et al., 2008; Jain, 2005), and several studies have reported increased uptake of chemotherapeutics associated with this process, likely due to reduced vessel leakiness and interstitial fluid pressure (Chauhan et al., 2012; Tong et al., 2004; Turley et al., 2012). Although macrophages are not necessarily a dominant source of VEGF-A in all tumor tissue, specific deletion of VEGF-A in macrophages via lysozyme M promoter-driven Cre recombinase revealed their role in driving abnormal vascular phenotypes in tumors (Stockmann et al., 2008). Importantly, similar to the use of VEGF antagonists, tumors in these mice were more sensitive to chemotherapy; although unexpectedly, they also grew at a faster rate due to improved tissue perfusion and reduced hypoxia in the absence of therapeutic intervention (Stockmann et al., 2008).
While CSF-1 neutralization enhances response to chemotherapy in mammary carcinomas (DeNardo et al., 2011), this is not due to increased delivery of chemotherapeutic agents, at least for small molecules such as paclitaxel and doxorubicin (Ruffell et al., 2014). Why then does macrophage depletion not phenocopy specific VEGF-A inhibition? One possible explanation is that blockade of the CSF-1/CSF-1R pathway only partially depletes macrophages in tumors, with macrophages surrounding vasculature remaining (DeNardo et al., 2011; Pyonteck et al., 2012; Ruffell et al., 2014). This residual subset has not been analyzed in detail, but at least a portion of the remaining cells are composed of Tie2+ macrophages (Mitchem et al., 2013) associated with vascular programming and important mediators of tumor angiogenesis (De Palma et al., 2005; Mazzieri et al., 2011). Although CSF-1 neutralization could functionally impair the angiogenic potential of Tie2+ monocytes (Forget et al., 2014), neutralizing angiopoietin-2, the ligand for Tie2, inhibits growth of mammary carcinomas (Mazzieri et al., 2011) – a phenotype not observed following therapeutic inhibition of CSF-1 or CSF-1R – and exhibits efficacy in xenograft models when used in combination with chemotherapy or VEGF antagonists (Brown et al., 2010). Interfering with Tie2+ macrophage recruitment via CXCR4-blockade also enhances the effects of the vascular-disrupting agent CA-4-P (Welford et al., 2011), and macrophage depletion further suppresses tumor growth in the context of VEGF/VEGFR inhibition (Priceman et al., 2010; Zeisberger et al., 2006). Although Tie2 is also expressed by endothelial cells and pericytes (De Palma et al., 2005), and thus the results with angiopoietin-2 neutralization cannot be entirely ascribed to the role of Tie2 expression by macrophages in regulating vascular architecture (Mazzieri et al., 2011), it would be interesting to evaluate combination angiopoietin-2 and CSF-1R-blockade for synergistic efficacy.
Macrophages as mediators of immune suppression
In murine tumor models, macrophages contain immunosuppressive transcriptional profiles (Biswas et al., 2006; Ojalvo et al., 2009), and accordingly, can directly suppress CD8+ T cell proliferation in vitro (DeNardo et al., 2011; Doedens et al., 2010; Movahedi et al., 2010; Ruffell et al., 2014). Based on macrophage expression of CD163, CD204, and CD206 in human tumors, it is presumed that macrophages will exhibit similar profiles, although this has yet to be evaluated in cells isolated directly from tumors. That said, CD14+ myeloid cells from hepatocellular and ovarian carcinomas suppress autologous T cell proliferation and IFN-γ expression in vitro, and nullify anti-tumor T cell activity during in vivo adoptive transfer experiments (Kryczek et al., 2006; Kuang et al., 2009).
In mouse models, T cell suppression by immature myeloid cells is typically linked to nutrient depletion via metabolism of L-arginine or production of free radicals (Gabrilovich and Nagaraj, 2009). However, while hypoxia promotes macrophage suppressive activity via expression of arginase-1 (Doedens et al., 2010), and thyioglycollate-induced peritoneal macrophages suppress T cell proliferation through L-arginine depletion (Rodriguez et al., 2003), inhibition of arginase activity does not blunt in vitro suppressive functions of tumor macrophages (Movahedi et al., 2010). This seems to be the case even for MHCIILO macrophages associated with hypoxic areas of tumors. To date, only inhibition of inducible nitric oxide synthase (NOS) has been reported to reduce suppression by tumor macrophages isolated from subcutaneously implanted lung carcinomas (Movahedi et al., 2010). Whether macrophages directly suppress T cell activity in vivo remains compelling, albeit speculative, but one role may simply be to overwhelm T cells with non-productive interactions (Broz et al., 2014).
In humans, there is no evidence for a role of nutrient depletion in mediating immune suppression by macrophages, since macrophages conditioned by ovarian carcinoma ascites suppress T cell proliferation independent of arginase and NOS activity (Kryczek et al., 2006). Instead, macrophages directly suppress T cell responses through programmed death-ligand 1 (PD-L1) in hepatocellular carcinoma (Kuang et al., 2009) and B7-H4 in ovarian carcinoma (Kryczek et al., 2006). This is perhaps fortuitous as immune checkpoint blockade is therapeutically more attractive (Pardoll, 2012), with monoclonal antibodies against PD-1, PD-L1, and PD-L2 all in clinical trials. Notably, response rates in the PD-1/PD-L1 trials relate, at least partially, to PD-L1 expression in tumor stroma (Herbst et al., 2014; Tumeh et al., 2014), consistent with a role for macrophages, and/or other stromal cells, in blocking anti-tumor T cell responses. Could macrophage targeting enhance checkpoint blockade therapy? At least one study to date has reported this using an orthotopic implant model of PDAC, with CSF-1R inhibition providing additive efficacy to either PD-1 or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade in combination with gemcitabine (Zhu et al., 2014). Importantly, CSF-1R inhibition also enhanced response to combined PD-1/CTLA-4 blockade in the absence of chemotherapy (Zhu et al., 2014); thus, it would not be surprising for CSF-1R antagonists to be combined with checkpoint blockade antibodies in future clinical trials (NCT02323191).
Rather than directly suppressing anti-tumor T cell responses, macrophage may also regulate the immune microenvironment, such that T cell responses are controlled indirectly through an intermediate cell type, as was first suggested in human ovarian carcinoma with regulatory T (TReg) cell recruitment via CCL22 (Curiel et al., 2004). In vitro, TReg cells induce IL-6 and IL-10 expression by macrophages, leading to autocrine upregulation of B7-H4 and a suppressive phenotype (Kryczek et al., 2007; Kryczek et al., 2006). Macrophages are also a key source of IL-10 in murine mammary carcinomas, but in this system, macrophages do not express detectable levels of B7-H4 (Ruffell and Coussens; unpublished observations), and IL-10 was not a significant mediator of macrophage polarization or suppressive function (Ruffell et al., 2014). Instead, in mammary carcinomas exposed to paclitaxel, macrophage IL-10 suppresses the capacity of dendritic cells to express IL-12, thereby blocking productive cytotoxic CD8+ T cell responses (Ruffell et al., 2014). Increased understanding of interactions between macrophages and other immune cells in tumor microenvironments, and deconstruction of the molecular pathways underlying these interactions, will undoubtedly provide additional therapeutic targets to fine tune an immune response during therapy.
Macrophages and metastasis
From local invasion, intravasation into vessels, and extravasation at peripheral sites, macrophages (or their monocyte precursors) have been implicated as regulators of all stages of the metastatic process, often through positive feedback pathways involving CCL2 and/or CSF-1 (Joyce and Pollard, 2009). Studies with human tissues have also demonstrated a relationship between epithelial-mesenchymal transition and macrophage expression of CCL18, for which there is no murine homolog (Meng et al., 2015; Su et al., 2014). Preclinical mouse models of tumor development (mammary, pancreas, glioblastoma, etc) wherein macrophages have either been depleted (albeit not completely) or reprogrammed, exhibit diminished metastatic burden in end-stage mice (DeNardo et al., 2009; DeNardo et al., 2011; Gocheva et al., 2010; Lin et al., 2001; Qian et al., 2009; Rolny et al., 2011; Shree et al., 2011; Welm et al., 2007; Zabuawala et al., 2010). Mechanistically however, direct evidence for a pro-metastatic role is largely derived from directed migration of neoplastic cells in response to molecules secreted by macrophages (DeNardo et al., 2009; Mizutani et al., 2009; Qian et al., 2009). The combined impact of these studies has been interpreted to indicate that therapies targeting macrophage presence and/or polarization would ameliorate metastasis in late-stage cancer patients. The fact that malignant cells likely already reside in secondary metastatic niches long before clinical presentation of malignant primary disease (Valastyan and Weinberg, 2011), mandates that this therapeutic approach be evaluated carefully. A recent evaluation of preclinical mammary carcinoma metastasis models with CCL2 neutralizing antibodies revealed enhanced metastatic burden; experimental neutralization of CCL2, while limiting early metastatic processes, promoted metastasis following cessation of therapy by enhancing recruitment of monocytes to micrometastatic lesions (Bonapace et al., 2014). Therapies targeting the stromal compartment of primary tumors may also prove ineffective at treating metastasis if the pathways regulating the targeted process differ between the tissue of origin and the metastatic site. As a possible example of this, IL-34 mediates development of Langerhans cells and microglia through CSF-1R (Wang et al., 2012), whereas CSF-1R signaling is mediated in most tissues by CSF-1 (Pollard, 2009). Development of macrophage-directed therapeutics aiming to minimize or eradicate metastasis will therefore first require identification of pathways that drive neoplastic cell survival, proliferation, angiogenesis, and immune suppression in ectopic sites. Some progress has been made in the lung, where both VEGF-A and angiopoietin-2 appear important for angiogenesis in metastatic tumors derived from mammary carcinomas (Bonapace et al., 2014; Mazzieri et al., 2011); however, as mentioned, this may not be directly linked to macrophage function. Interestingly, CSF-1R inhibition reverses the effects observed following cessation of CCL2 neutralization (Bonapace et al., 2014), even though CSF-1R inhibition does not alter the number of macrophages in lungs (Strachan et al., 2013). This could hint at a possible role for CSF-1R signaling in mediating macrophage polarization in metastatic lungs, similar to the observations in glioblastoma multiforme (Pyonteck et al., 2013). Nevertheless, it remains uncertain whether macrophages are important in mediating therapeutic resistance at metastatic sites, or even the degree to which they are involved in mediating metastatic outgrowth. As the majority of patients succumb to metastatic disease, this is an urgent area of research that has been largely unexplored, in part due to experimental obstacles and maintaining mice with spontaneous tumors prone to metastasize where primary tumor burden limits duration of study.
Macrophages as Therapeutic Targets
Based on compelling preclinical data from numerous laboratories indicating that macrophage presence and/or activity are malleable in vivo (Figure 3), clinical studies are now ongoing in several solid tumor types wherein macrophages are being targeted via CSF-1R inhibitors or blocking monoclonal antibodies (Table 1). While a goal of these clinical studies is to reduce the presence of tumor-associated macrophages, based on preclinical and clinical studies, we anticipate that not all macrophages will be eradicated. The hope is however, that those remaining will be reprogramed towards an anti-tumor phenotypic state wherein they would support T cell responses, and together with cytotoxic therapy, limit ongoing tumor growth. CSF-1R antagonists appear well tolerated as single agents in both pre-clinical and clinical studies (Radi et al., 2011; Ries et al., 2014; Ruffell et al., 2014), but with that significant macrophage depletion in the colon and liver is observed in nonhuman primates, toxicity is a significant concern for combinatorial studies moving forward. It should also be noted that while increased CSF-1 serum concentrations resulting from the use of CSF-1R antagonists (Ries et al., 2014) provides an excellent biomarker to evaluate on target efficacy, recent findings with CCL2 inhibition (Bonapace et al., 2014) indicate that recurrence or exacerbation of disease is a possibility after therapy cessation. These potential issues will need to be incorporated into the design of clinical studies for appropriate drug combinations and patient monitoring.
Figure 3. Macrophage function in the tumor microenvironment.
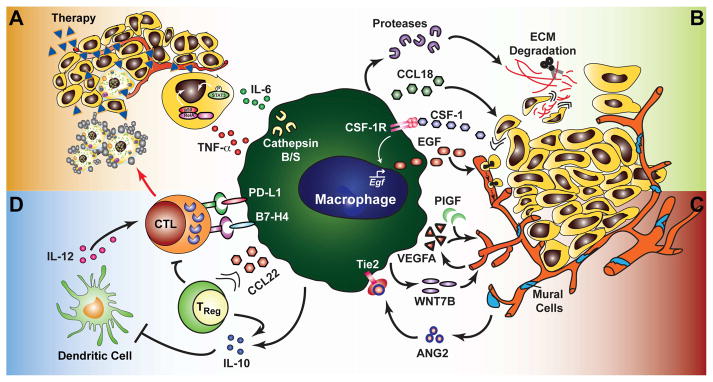
(a) Macrophage expression of IL-6 and TNF-α promotes survival signaling in neoplastic cells and resistance to chemotherapy and targeted agents. Expression of survival factors is dependent upon the protease activity of cathepsin B and/or S. (b) Neoplastic cell invasion of ectopic tissue can be promoted through directed release of cytokines/chemokines such as EGF and CCL18, or through protease-dependent ECM remodeling that may directly affect neoplastic migration or increase chemoattractant bioavailability. EGF expression is driven by signaling through the CSF-1R via neoplastic cell production of CSF-1, as well as T cell-derived IL-4 (not shown). (c) Macrophages directly promote angiogenesis via production of VEGFA and other angiogenic factors, and can enhance VEGFA expression by endothelial cells through WNT7B. A subset of macrophages expressing the Tie2 receptor are recruited to the vasculature by mural cell/pericyte expression of ANG2 and are important in regulating vascular structure. (d) Direct suppression of a cytotoxic T cell (CTL) response can occur via expression of B7 family ligands (PD-L1, B7-H4). Indirect suppression may occur through release of IL-10 or recruitment of IL-10-expressing regulatory T cells (TReg) via CCL22, whereby IL-10 suppresses the capacity of dendritic cells to produce IL-12 and promote a TH1/CTL anti-tumor immune response.
Blocking macrophage recruitment into tumors (DeNardo et al., 2011; Shiao et al., 2015), pro-tumor polarization (Affara et al., 2014; Pyonteck et al., 2013; Shiao et al., 2015) effector function (Ruffell et al., 2014; Shree et al., 2011), or directly promoting macrophage activation (Beatty et al., 2011), have all been used successfully in preclinical models to enhance response to cytotoxic therapy. The question remains, which of these approaches (Table 1) will be most efficacious when combined with cytotoxic, targeted, or immune checkpoint blockade therapy. At least in a murine model of squamous cell carcinogenesis, repolarizing macrophages was more effective than blocking recruitment, and in fact, repolarized macrophages were necessary for recruitment of CD8+ T cells via CCR5 during paclitaxel chemotherapy (Affara et al., 2014). For this reason, going forward it will be critical to understand if depletion, or instead repolarization is the “best” therapeutic approach to accompany combination therapy, for which tumor types, and at which stage of tumor progression (primary or metastatic disease). Multiple agents targeting TH2 cytokines and their receptors have gone beyond Phase II clinical trials with demonstrated efficacy in autoimmune disorders and acceptable safety profiles (Beck et al., 2014; Corren et al., 2011; Danese et al., 2015). Although none of these compounds have been re-directed towards therapy in solid tumors, we have recently found that targeting this pathway (IL-4, IL-13, IL-4Rα) improves response to cytotoxic therapy (Shiao et al., 2015).
One lesson learned from results comparing efficacy of immunotherapy in mice bearing orthotopic versus subcutaneously implanted tumors is that the later exhibit enhanced sensitivity (Devaud et al., 2014), indicating that context matters. In light of these findings, and given the dearth of late-stage metastatic studies, it will be important to be mindful of the fact that a one size-fits-all approach, while attractive, may not be realistic as we strive to translate preclinical findings to the clinic. A major question to address from these preclinical and clinical studies with either macrophage depletion or reprogramming approaches will be to evaluate the durability of resultant anti-tumor immune responses formed against the tumor. Along these lines, evaluating patients longitudinally for indicators of T cell function could reveal an important diagnostic opportunity whereby monitoring circulating T cell receptor diversity becomes a routine diagnostic strategy to indicate when addition of combination therapy, e.g., immune checkpoint or anti-tumor vaccine, might be beneficial to the patient.
Acknowledgments
The authors thank members of the Coussens laboratory for critical discussions, Nesrine Affara for graphical assistance, and all contributors to the field whose data was not acknowledged herein due to space limitations. The authors acknowledge support from the NIH/NCI (BR and LMC), and grants from the DOD BCRP Era of Hope Scholar Expansion Award, Susan B Komen Foundation, Breast Cancer Research Foundation, and AACR-SU2C to LMC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O’Dwyer PJ. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, Ming JE, Ren H, Kao R, Simpson E, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- Brown JL, Cao ZA, Pinzon-Ortiz M, Kendrew J, Reimer C, Wen S, Zhou JQ, Tabrizi M, Emery S, McDermott B, et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol Cancer Ther. 2010;9:145–156. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- Buddingh EP, Kuijjer ML, Duim RA, Burger H, Agelopoulos K, Myklebost O, Serra M, Mertens F, Hogendoorn PC, Lankester AC, Cleton-Jansen AM. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res. 2011;17:2110–2119. doi: 10.1158/1078-0432.CCR-10-2047. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, Au A, Baehner F, Chen Y, Malaka DO, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011;128:703–711. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Castells M, Thibault B, Delord JP, Couderc B. Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. Int J Mol Sci. 2012;13:9545–9571. doi: 10.3390/ijms13089545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung FT, Lee KY, Wang CW, Heh CC, Chan YF, Chen HW, Kuo CH, Feng PH, Lin TY, Wang CH, et al. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer. 2012;131:E227–235. doi: 10.1002/ijc.27403. [DOI] [PubMed] [Google Scholar]
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Danese S, Rudzinski J, Brandt W, Dupas JL, Peyrin-Biroulet L, Bouhnik Y, Kleczkowski D, Uebel P, Lukas M, Knutsson M, et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut. 2015;64:243–249. doi: 10.1136/gutjnl-2014-308004. [DOI] [PubMed] [Google Scholar]
- De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud C, Westwood JA, John LB, Flynn JK, Paquet-Fifield S, Duong CP, Yong CS, Pegram HJ, Stacker SA, Achen MG, et al. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol Ther. 2014;22:18–27. doi: 10.1038/mt.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, Denardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage Expression of Hypoxia-Inducible Factor-1{alpha} Suppresses T-Cell Function and Promotes Tumor Progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Forget MA, Voorhees JL, Cole SL, Dakhlallah D, Patterson IL, Gross AC, Moldovan L, Mo X, Evans R, Marsh CB, Eubank TD. Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer. PLoS One. 2014;9:e98623. doi: 10.1371/journal.pone.0098623. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, Oda Y, Yamada H, Okada S, Watari K, Ono M, et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol. 2011;179:1157–1170. doi: 10.1016/j.ajpath.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried E, Kunz-Schughart LA, Weber A, Rehli M, Peuker A, Muller A, Kastenberger M, Brockhoff G, Andreesen R, Kreutz M. Expression of CD68 in non-myeloid cell types. Scand J Immunol. 2008;67:453–463. doi: 10.1111/j.1365-3083.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- Ha SD, Martins A, Khazaie K, Han J, Chan BM, Kim SO. Cathepsin B is involved in the trafficking of TNF-alpha-containing vesicles to the plasma membrane in macrophages. J Immunol. 2008;181:690–697. doi: 10.4049/jimmunol.181.1.690. [DOI] [PubMed] [Google Scholar]
- Hameed A, Hruban RH, Gage W, Pettis G, Fox WM., 3rd Immunohistochemical expression of CD68 antigen in human peripheral blood T cells. Hum Pathol. 1994;25:872–876. doi: 10.1016/0046-8177(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama S, Ishii G, Nagai K, Ono S, Kojima M, Yamauchi C, Aokage K, Hishida T, Yoshida J, Suzuki K, Ochiai A. Prognostic impact of CD204-positive macrophages in lung squamous cell carcinoma: possible contribution of Cd204-positive macrophages to the tumor-promoting microenvironment. J Thorac Oncol. 2012;7:1790–1797. doi: 10.1097/JTO.0b013e3182745968. [DOI] [PubMed] [Google Scholar]
- Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121:4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe E, Elkrim Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805:167–180. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Lin EY, Li JF, Bricard G, Wang W, Deng Y, Sellers R, Porcelli SA, Pollard JW. VEGF Restores Delayed Tumor Progression in Tumors Depleted of Macrophages. Mol Oncol. 2007;1:288–302. doi: 10.1016/j.molonc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E, Green K, Dickinson R, Wang XN, Low D, et al. Human dermal CD14(+) cells are a transient population of monocyte-derived macrophages. Immunity. 2014;41:465–477. doi: 10.1016/j.immuni.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- Meng F, Li W, Li C, Gao Z, Guo K, Song S. CCL18 promotes epithelial-mesenchymal transition, invasion and migration of pancreatic cancer cells in pancreatic ductal adenocarcinoma. Int J Oncol. 2015;46:1109–1120. doi: 10.3892/ijo.2014.2794. [DOI] [PubMed] [Google Scholar]
- Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H, Pienta KJ. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11:1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L, Graeber TG, West BL, Bollag G, Ribas A. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 2014;74:153–161. doi: 10.1158/0008-5472.CAN-13-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, Pinto AR, Klapproth K, Henri S, Malissen B, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211:2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Miyamoto T, Yoshida H, Asakawa M, Kawasumi M, Kobayashi T, Morioka H, Chiba K, Toyama Y, Yoshimura A. IL-1beta and TNFalpha-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol. 2011;23:701–712. doi: 10.1093/intimm/dxr077. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, Fein M, Rasch MG, Tan YX, Qiu J, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. The tissue microlocalisation and cellular expression of CD163, VEGF, HLA-DR, iNOS, and MRP 8/14 is correlated to clinical outcome in NSCLC. PLoS One. 2011;6:e21874. doi: 10.1371/journal.pone.0021874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SM, Tan YC, Beretta O, Jiang D, Yeap WH, Tai JJ, Wong WC, Yang H, Schwarz H, Lim KH, et al. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur J Immunol. 2012;42:89–100. doi: 10.1002/eji.201141825. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus P, Stanley ER, Schafer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006;66:4349–4356. doi: 10.1158/0008-5472.CAN-05-3523. [DOI] [PubMed] [Google Scholar]
- Perdiguero EG, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2014 doi: 10.1038/nature13989. Epub 2014 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML, Wu L. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyonteck SM, Gadea BB, Wang HW, Gocheva V, Hunter KE, Tang LH, Joyce JA. Deficiency of the macrophage growth factor CSF-1 disrupts pancreatic neuroendocrine tumor development. Oncogene. 2012;31:1459–1467. doi: 10.1038/onc.2011.337. [DOI] [PubMed] [Google Scholar]
- Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376–389. [PMC free article] [PubMed] [Google Scholar]
- Radi ZA, Koza-Taylor PH, Bell RR, Obert LA, Runnels HA, Beebe JS, Lawton MP, Sadis S. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am J Pathol. 2011;179:240–247. doi: 10.1016/j.ajpath.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et al. HRG Inhibits Tumor Growth and Metastasis by Inducing Macrophage Polarization and Vessel Normalization through Downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev. 2012;38:451–466. doi: 10.1016/j.ctrv.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012a;33:119–125. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012b;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CMT, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 Blocks CD8(+) T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-polarized CD4+ T cells and macrophages limit efficacy of radiation therapy. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–2479. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MP, Sanchez-Laorden B, O’Brien K, Brunton H, Ferguson J, Young H, Dhomen N, Flaherty KT, Frederick DT, Cooper ZA, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFalpha. Cancer Discov. 2014;4:1214–1229. doi: 10.1158/2159-8290.CD-13-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, Ara T, Silverman AM, DeClerck YA, Seeger RC, Metelitsa LS. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, Daniel D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology. 2013;2:e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- Tosato G, Jones KD. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 1990;75:1305–1310. [PubMed] [Google Scholar]
- Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley RS, Fontanella AN, Padussis JC, Toshimitsu H, Tokuhisa Y, Cho EH, Hanna G, Beasley GM, Augustine CK, Dewhirst MW, Tyler DS. Bevacizumab-induced alterations in vascular permeability and drug delivery: a novel approach to augment regional chemotherapy for in-transit melanoma. Clin Cancer Res. 2012;18:3328–3339. doi: 10.1158/1078-0432.CCR-11-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide RH, Bajor DL, Winograd R, Evans RA, Bayne LJ, Beatty GL. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother. 2013;62:949–954. doi: 10.1007/s00262-013-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM, Lewis CE. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969–1973. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welm AL, Sneddon JB, Taylor C, Nuyten DS, van de Vijver MJ, Hasegawa BH, Bishop JM. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci U S A. 2007;104:7570–7575. doi: 10.1073/pnas.0702095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo EJ, Cassetta L, Qian BZ, Lewkowich I, Li JF, Stefater JA, 3rd, Smith AN, Wiechmann LS, Wang Y, Pollard JW, Lang RA. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74:2962–2973. doi: 10.1158/0008-5472.CAN-13-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- Zabuawala T, Taffany DA, Sharma SM, Merchant A, Adair B, Srinivasan R, Rosol TJ, Fernandez S, Huang K, Leone G, Ostrowski MC. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res. 2010;70:1323–1333. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenay S, Reis e Sousa C. Adaptive immunity after cell death. Trends Immunol. 2013;34:329–335. doi: 10.1016/j.it.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2014 doi: 10.1007/s10120-014-0422-7. Epub 2014 Sep 18. [DOI] [PubMed] [Google Scholar]
- Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]