Abstract
Background
Ethanol causes neurotoxicity by several mechanisms including excitotoxicity and neuroinflammation, but little is known about the interaction between these mechanisms. Because neuroinflammation is known to enhance excitotoxicity, we hypothesized that neuroinflammation contributes to the enhanced excitotoxicity which is associated with ethanol withdrawal (EWD). The aim of this study was to evaluate the lipopolysaccharide (LPS)-induced inflammatory response of cultured hippocampal tissue during EWD and its effects on the enhanced N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity which occurs at this time.
Methods
Using a neonatal organotypic hippocampal slice culture (OHSC) model, we assessed the effects of NMDA and LPS (separately or combined) during EWD after 10 days of ethanol exposure. Neurotoxicity was assessed using propidium iodide uptake and the inflammatory response was evaluated by measuring the release of TNF-alpha (quantified by ELISA) and nitric oxide (quantified by the Griess reaction) into culture media. Furthermore, we explored the potential role of the microglial cell type using immortalized BV2 microglia treated with ethanol for 10 days and challenged with LPS during EWD.
Results
As predicted, NMDA-induced toxicity was potentiated by LPS under control conditions. However, during EWD the reverse was observed and LPS inhibited peak NMDA-induced toxicity. Additionally, LPS-induced release of TNF-alpha and nitric oxide during EWD was reduced compared to control conditions. In BV2 microglia, following ethanol exposure, LPS-induced release of nitric oxide was reduced whereas TNF-alpha release was potentiated.
Conclusions
During EWD following chronic ethanol exposure, OHSC exhibited a desensitized inflammatory response to LPS and the effects of LPS on NMDA toxicity were reversed. This might be explained by a change in microglia to an anti-inflammatory and neuroprotective phenotype. In support, studies on BV2 microglia indicate that ethanol exposure and EWD does alter the response of these cells to LPS, but this cannot fully explain the changes observed in the OHSC. The data suggest that neuroinflammation and excitotoxicity do interact during EWD. However, the interaction is not as simple as we originally proposed. This in turn illustrates the need to assess the extent, importance and relation of these mechanisms in models of ethanol exposure producing neurotoxicity.
Keywords: ethanol induced neurotoxicity, neuroinflammation, excitotoxicity, ethanol withdrawal, rat hippocampal slice culture
1. Introduction
Ethanol causes neurotoxicity via several mechanisms (Crews et al., 2004) at different points in the cycle of dependence, including ethanol exposure and ethanol withdrawal (EWD). Ethanol exposure is generally associated more with oxidative stress (Haorah et al., 2008) and neuroinflammation (Crews and Nixon, 2009) whereas EWD is associated with excitotoxicity (Lovinger, 1993). These mechanisms have mostly been studied as if they were separate, but the possibility that there are important interactions between them has rarely been considered. For example, it is unclear whether neuroimmune signaling changes induced by ethanol exposure extend into EWD and potentiate excitotoxicity observed at that time. In this study we investigate the potential interaction between neuroinflammatory signaling and excitotoxicity during EWD in organotypic hippocampal slice cultures (OHSC).
The precise mechanisms that cause excitotoxicity during EWD are uncertain, but increased activity of the glutamate system is fundamental (Holmes et al., 2013). Most evidence supports two possible mechanisms that are not exclusionary. One is that ethanol exposure upregulates the number of N-methyl-D-aspartate receptors (NMDAR) (Follesa and Ticku, 1996) and/or changes their subunit composition (Nagy et al., 2003), resulting in hypersensitive conformations of the receptor (Nagy et al., 2005). The second is that release of glutamate (Rossetti et al., 1999) and polyamines (Gibson et al., 2003) is greater during EWD, resulting in the overactivation of NMDARs. These mechanisms of EWD-induced excitotoxicity are closely interrelated in vivo, but they can be evaluated separately ex vivo in OHSC (Noraberg et al., 2005) where exogenous activation of NMDARs can be used to cause neurotoxicity. Studies on OHSC (e.g. (Gibson et al., 2003, Harris et al., 2003)) suggest that both the functional upregulation of NMDARs and increased release of glutamate and polyamines contribute to EWD-induced excitotoxicity in this model. In the current study we focused on NMDAR hypersensitivity by activating these receptors with exogenous NMDA during EWD. Many previous studies (e.g. (Mayer et al., 2002, Self et al., 2004)) have shown that NMDA-induced toxicity is enhanced under EWD conditions.
None of the above studies exclude other mechanisms, such as neuroinflammation, as contributing to excitotoxicity during EWD. In fact, it has previously been reported that neuroinflammation induced by exogenous application of TNF-alpha enhances glutamate excitotoxicity in OHSC (Zou and Crews, 2005). Therefore, the current study tested the primary hypothesis that ethanol exposure causes neuroinflammation, which then potentiates excitotoxicity during EWD.
To date, research on ethanol exposure and neuroinflammation has focused on changes that occur in the presence of ethanol rather than during EWD. Increased release of proinflammatory mediators from neuroimmune cells as a consequence of ethanol exposure is probably fundamental, but as with excitotoxicity, two major mechanisms have been proposed for this. One is that ethanol exposure results in increased influx of lipopolysaccharide (LPS), a bacterial endotoxin, via the gut (Leclercq et al., 2012) and blood brain barrier (Haorah et al., 2005), thereby activating neuroinflammatory processes. The second is that ethanol exposure directly affects neuroimmune cells, such as microglia (Fernandez-Lizarbe et al., 2009), and sensitizes their response to LPS (Qin et al., 2008). The possible contribution of these mechanisms is difficult to separate in vivo but, as with excitotoxicity, OHSC can be used to dissect these possibilities because exogenous LPS can be applied to the culture (Johansson et al., 2005).
If ethanol exposure sensitizes neuroimmune cells, OHSC should release greater amounts of proinflammatory mediators in response to LPS during EWD. In turn, the enhanced proinflammatory response should lead to a further enhancement of excitotoxicity induced by an NMDA challenge. Previous studies have used OHSC to study the effects of ethanol on excitotoxicity (Thomas et al., 1998) and neuroinflammation (Moon et al., 2014) but there are no published studies on the interaction between these.
Microglia have a fundamental role in the hypotheses that connect ethanol-induced neurotoxicity with neuroinflammation (Yang et al., 2014). However, all brain preparations, including OHSCs, contain other types of glia, including astrocytes (Benediktsson et al., 2005) and oligodendrocytes (Haber et al., 2009) as well as microglia (Dailey and Waite, 1999). All these cell types may contribute to the LPS-induced inflammatory response (Salmina, 2009) and modify excitotoxicity. In an attempt to address this issue the current study included experiments on the effects of chronic ethanol exposure and response to LPS during EWD on cultures in which only immortalized microglia of the BV2 cell line are present, a suitable alternative to primary microglia (Henn et al., 2009), thereby mirroring the studies on OHSC. The acute effects of ethanol on BV2 microglia in response to a variety of inflammatory stimuli have been previously investigated and compared to primary microglia (Lee et al., 2004). Both primary and immortalized cultures behaved similarly making this cell line a suitable system to investigate the effects of chronic ethanol on microglia.
These studies aim to increase our understanding of the mechanisms underlying excitotoxicity associated with EWD. It should be emphasized that OHSC and immortalized BV2 microglia are being used to dissect mechanisms of ethanol-induced neurotoxicity rather than to “model” all aspects of ethanol-induced neurodegeneration. Subsequent studies in vivo will be necessary to confirm the observations in vitro, but the culture systems provide a means of identifying specific cell types and molecular mechanisms that are important to this aspect of alcoholism.
2. Materials and methods
2.1. Organotypic hippocampal slice culture (OHSC) preparation
OHSC were prepared essentially as described by Stoppini et al. (Stoppini et al., 1991). Briefly, hippocampi were aseptically removed from 8-day-old Sprague-Dawley male and female rat pups and sliced at a transverse thickness of 200 μm using a McIlwain tissue chopper (Campden Instruments Ltd., Lafayette, ID). Slices were transferred to sterile culture inserts (4 slices per insert) and placed in 6-well-plates containing culture medium (Minimum Essential Medium (Life Technologies Corporation, Grand Island, NY), 200mM glutamine (Invitrogen, Carlsbad, CA), 25mM HEPES (ATCC, Manassas, VA), 50uM penicillin/streptomycin (ATCC, Manassas, VA), 36mM glucose, 25% (v/v) Hank’s buffered salt solution (Gibco BRL, Gaithersburg, MD), 25% heat-inactivated horse serum (Sigma, St. Louis, MO)). Cultures were maintained at 37°C in an atmosphere of 5% CO2/95% air in 95% humidity for 5 days in vitro (DIV) to allow slices to adhere to the insert membrane. The care of animals was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996), as well as the University of Kentucky’s Institutional Animal Care and Use Committee.
2.2. Ethanol exposure and withdrawal (EWD)
At 6 DIV, slices were placed in culture media with or without the addition of 100mM ethanol, incubated for 10 DIV and then subjected to EWD for 24h, 48h or 72h. During exposure, the plates were placed in topless polypropylene containers containing 50mL of 100mM ethanol or dH2O accordingly, and placed inside sealable plastic bags filled with 5% CO2, 21% oxygen, and a balance of nitrogen. The ethanol solution was used as an evaporating source of ethanol to counter the evaporation of ethanol from the culture wells resulting in an average ethanol concentration of 65mM during the 5 DIV between media changes. At the onset of EWD, slices were further separated into groups and treated accordingly: control media, 5uM NMDA (Sigma Aldrich Co. LCC., St. Louis, MO), 10ug/mL lipopolysaccharide from Escherichia coli 026:B6 (LPS, Lot #: 021M4072V; Sigma Aldrich Co. LCC., St. Louis, MO) or both NMDA and LPS combined.
2.3. Assessment of toxicity by propidium iodide uptake
Propidium iodide (PI) is a membrane impermeable, DNA intercalating fluorescent molecule that is commonly used in OSHC as a semi-quantitative stain for cellular toxicity and has been significantly correlated to other reliable markers of cell death (Zimmer et al., 2000). Although there has been extensive work on NMDA and EWD toxicity in OHSC using a variety of cell-type specific markers (Wilkins et al., 2006), it is uncertain how these markers are affected by LPS in this model. The current study focused on the interaction between NMDA and LPS and since PI uptake has been used in OHSC to assess both NMDA (Wilkins et al., 2006) and LPS (Johansson et al., 2005) induced toxicity separately, it was therefore chosen for the current study to assess overall toxicity.
For 24h EWD, slices were directly challenged in culture media containing 3.74uM propidium iodide (PI; Sigma Aldrich Co. LCC., St. Louis, MO). For 48h and 72h EWD, slices were originally challenged in culture media without PI and each well was supplemented with 10uL of concentrated PI (374uM) to obtain a final concentration of 3.74uM PI 24h prior to imaging. Slice images were captured using SPOT Advanced software (Version 4.0.9; W. Nuhsbaum Inc., McHenry, IL) connected to an inverted Leica DMIRB microscope (W. Nuhsbaum Inc.) fitted for fluorescence detection (mercury-arc lamp) and connected to a computer via a SPOT 7.2 color mosaic camera (W. Nuhsbaum Inc). PI uptake in the CA1, CA3, and DG cell layers was measured using ImageJ software (Version 1.46; National Institute of Health, Bethesda, MD). Background signal was subtracted from intensities obtained for each cell layer resulting in specific intensities which were used for statistical analysis. These values were then converted to % control (no EWD, no NMDA, and no LPS) within each preparation for graphical representation and clarity across time points.
2.4. Assessment of inflammatory mediator release
Once slices were imaged, inserts were discarded and the resulting media was collected for assessment of inflammatory mediator release. Nitric oxide (NO) release was assessed by the Griess Reagent System (Promega Corporation, Madison, WI) according to the manufacturer’s instructions. All samples were assayed in duplicate and nitrite content was estimated using a reference NaNO2 standard curve performed with each assay. TNF-alpha content was assessed by enzyme linked immunosorbent assay kit (ELISA; Ready-Set-Go!® ELISA, eBioscience Inc., San Diego, CA) according to the manufacturer’s instructions. All samples were assayed in duplicate and TNF-alpha content was estimated from a reference TNF-alpha standard curve performed with each assay.
2.5. BV2 microglia culture
BV2 microglia are derived raf/myc-immortalized murine neonatal microglia. They were cultured in Dulbecco’s Modified Eagle Medium/Ham’s F12 nutrient mix supplemented with 10% fetal bovine serum and antibiotics (penicillin 100U/ml, streptomycin 100ug/ml; all from Life Technologies Corp., Grand Island, NY). Cells were kept at 37°C in a humidified atmosphere of air and 5% CO2 and propagated in T25 flasks (Techno Plastic Products AG, Trasadingen, Switzerland) in media with or without 100mM EtOH in sealable plastic bags as described above to avoid EtOH evaporation. They were split every 2–3 days and subjected to EWD after 10 days. During EWD, cells were seeded in 24 well plates at densities of 5×105 cells/well, allowed to adhere overnight and then challenged with 1μg/mL LPS for 24h. Culture media was collected and assessed for inflammatory mediators as described above. Cell viability was measured using resazurin salt fluorescence (7-hydroxy-3H-phenoxazin-3-one-10-oxide sodium salt; Sigma Aldrich Co. LCC., St. Louis, MO) and normalized to percentage control (no LPS group).
2.6. Statistical analysis
Data were analyzed using IBM Statistical Package for the Social Sciences (SPSS) Version 21 (IBM Corporation, Armonk, NY) and graphed using Prism (Graphpad Software Inc., La Jolla, CA). PI uptake was measured in three different regions (DG, CA3 and CA1). Thus, PI uptake was analyzed by multi-factorial, repeated measures analysis of variance (ANOVA) with region as within-subjects variables and sex, EWD, NMDA, and LPS as between-subjects factors. Data were obtained from separate preparations for each length of treatment and EWD (24h, 48h, and 72h). Thus, preparation and time point were used as covariates to control for differences across litters/culture preparations and time of exposure. Significant interactions were further investigated at each time point using post hoc pair-wise comparisons using Fisher’s LSD test. TNF-alpha and NO release from OHSC were also analyzed by multi-factorial ANOVA with the following factors: sex, EWD, NMDA and LPS as well as preparation and time point as covariates. Significant interactions were further investigated at each time point using post hoc pair-wise comparisons with Tukey’s LSD correction. TNF-alpha and NO release from BV2 microglia were analyzed by non-repeated measures two-way ANOVA with EWD and LPS as factors followed by Bonferroni post-hoc analyses.
3. Results
3.1. Effects of NMDA, LPS and EWD on cellular damage in OHSC as measured by PI uptake
An overall repeated-measures multi-factorial ANOVA was first performed on PI uptake across the treatment groups to assess whether there were any differences in the separate regions of the hippocampus and whether there were any sex effects. This analysis revealed that sex did not interact with any factors but that there was an effect of region and the highest order significant interaction included all factors except for sex (region x EWD x NMDA x LPS [F(1.09,1765.03) = 15.02, p < 0.0001] corrected using Greenhouse-Geisser). Therefore, subsequent analyses were performed within each region at each time point collapsed across sex. Analysis within each region revealed minor effects of drug treatment on PI uptake in the DG and CA3 in comparison to those observed in the CA1. Therefore, assessment of cellular damage by PI uptake was focused on the CA1 region of the hippocampus.
3.1.1. LPS treatment potentiates NMDA-induced cellular damage under control conditions
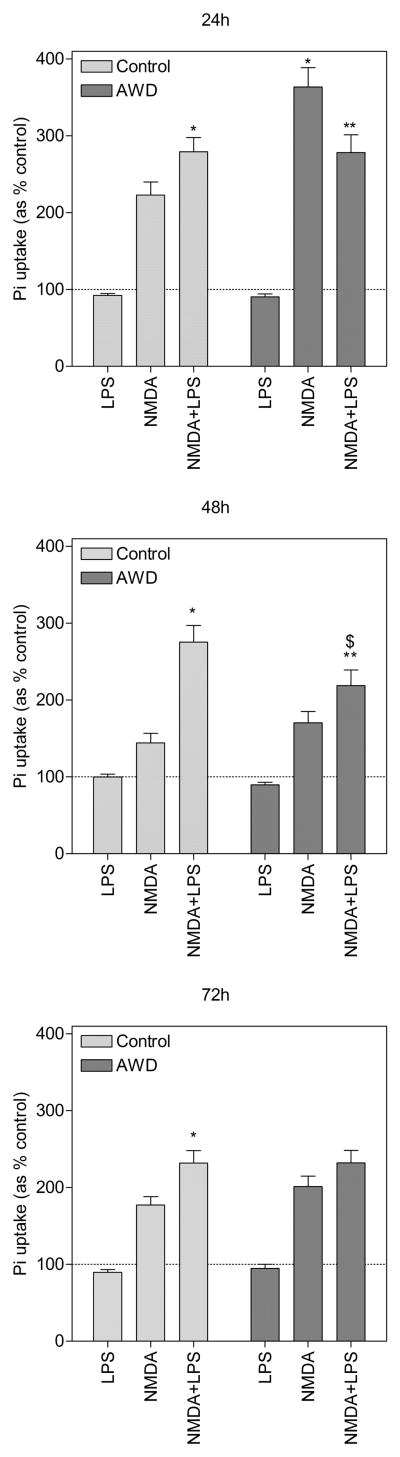
Before we could evaluate the combined effects of NMDA and LPS on hippocampal damage, we examined the effects of LPS alone under control conditions and during EWD. Slices treated with LPS alone under both conditions exhibited a speckled pattern of PI uptake throughout the slice that was not observed in untreated slices. However, when quantified in the CA1 region of the hippocampus, it did not differ from untreated control or EWD slices (fig. 1). Despite no quantifiable effect alone, LPS significantly potentiated NMDA-induced damage under control conditions. Slices co-exposed to LPS and NMDA exhibited greater PI uptake at all time points in comparison to slices treated with NMDA alone (24h = 279%, post hoc [p < 0.01], 48h = 275%, post hoc [p < 0.0001] and 72h = 201%, post hoc [p < 0.0001], all compared to NMDA alone).
Figure 1.
Propidium iodide uptake in the CA1 region of the hippocampus in slices treated with lipopolysaccharide (LPS), N-methyl-D-aspartate (NMDA) or a combination of both for 24h, 48h and 72h under control conditions (light bars) and under ethanol withdrawal (EWD) conditions (dark bars). *p < 0.01 compared to NMDA alone under control conditions. **p < 0.05 compared to NMDA alone during EWD. $p < 0.01 compared to NMDA+LPS under control conditions. Data are expressed as percent of untreated control (means ± SEM). Dotted line represents untreated control. n = 64–72 slices for each treatment group (control, NMDA, LPS, NMDA+LPS), under both control and EWD conditions, at all time points.
3.1.2. EWD potentiates peak NMDA-induced cellular damage
OHSC treated with NMDA under control conditions exhibited significant cellular damage in the CA1 region of the hippocampus as measured by PI uptake (fig. 1 - 222%, post hoc [p < 0.0001] compared to control). When coupled with EWD, PI uptake was significantly potentiated at the 24h time point (363%, post hoc [p < 0.0001] compared to NMDA). At later time points (48h and 72h), PI uptake for these groups remained significantly higher than control (post hoc [p < 0.05] compared to control at 48h and 72h) but they were not statistically different from each other.
3.1.3. LPS treatment reduces peak NMDA-induced cellular damage during EWD
Despite potentiation of NMDA-induced cellular damage by LPS under control conditions, the reverse was observed during peak toxicity under EWD conditions (fig. 1). Slices co-exposed to NMDA and LPS during EWD exhibited lower PI uptake (278%) than slices treated with NMDA alone during EWD (363%, post hoc [p < 0.0001]). This relationship was however not observed at later time points. At the 48h and 72h time points, slices co-exposed to NMDA and LPS during EWD exhibited higher PI uptake (48h = 218% and 72h = 231%) than slices treated with NMDA alone during EWD (48h = 170%, post hoc [p < 0.05] and 72h = 201%, post hoc [p < 0.05]). Interestingly, at the 48h time point, slices co-exposed to NMDA and LPS during EWD (218%) exhibited significantly lower PI uptake than slices co-exposed to NMDA and LPS under control conditions (275%, post hoc [p < 0.01]).
3.2. Effects of NMDA, LPS and EWD on inflammatory mediator release from OHSCs
An overall statistical analysis on TNF-alpha and NO levels measured in culture media was first performed across the treatment groups to assess whether there were effects of sex. This analysis revealed that there was no main effect of sex on TNF-alpha release [F(1,162) = 3.492, p = 0.063] or NO release [F(1,358) = 0.200, p = 0.655]. Therefore, further analyses were performed collapsed across this factor.
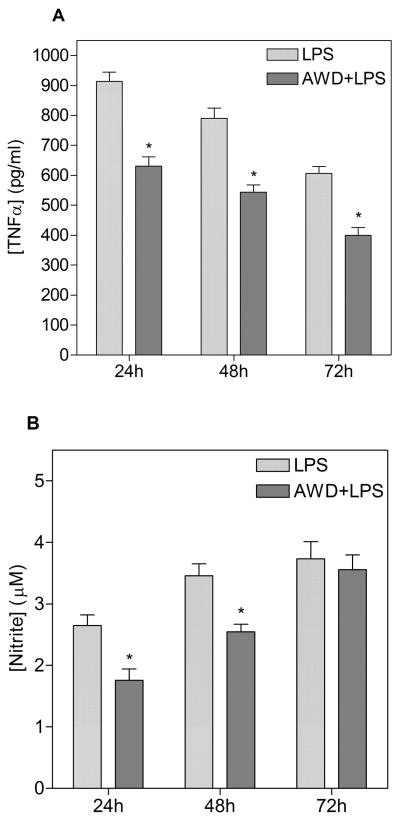
3.2.1. EWD reduces LPS-induced proinflammatory mediator release
LPS treatment induced TNF-alpha release both under control conditions and during EWD (fig. 2A). However, in comparison to control conditions, the response was reduced during EWD (main effect of EWD [F(1,170) = 238.2, p < 0.0001]) at all time points (post hoc at 24h, 48h and 72h [p < 0.0001]). Similarly, LPS treatment induced NO release under control conditions and during EWD (fig. 2B - main effect of LPS [F(1,374) = 1508.1, p < 0.0001]). However, in comparison to control conditions, this effect was also reduced by EWD (EWD x LPS interaction [F(1,374) = 42.7, p < 0.0001]) at least at earlier time points (post hoc at 24h and 48h [p < 0.0001]). At the 72h time point, NO release from EWD slices normalized to control condition levels and there was no significant difference at that time (post hoc at 72h [p = 0.689]).
Figure 2.
Release of TNF-alpha (A) and nitric oxide (NO) (B) from OHSCs after 24h, 48h and 72h of LPS treatment under control conditions (light bars) and under ethanol withdrawal (EWD) conditions (dark bars). *p < 0.0001 compared to LPS alone; n.d. not detected. n = 16–18 samples for each treatment group (control, LPS), under both control and EWD conditions at all time points.
3.2.2. NMDA treatment does not induce proinflammatory mediator release and does not interact with the response to LPS
Under control conditions, NMDA alone did not induce TNF-alpha release (TNF-alpha not detected) and had no effect on LPS-induced TNF-alpha release. There were no statistical differences between TNF-alpha levels measured in media from slices co-exposed to NMDA and LPS and slices treated with LPS alone (post hoc at 24h [p = 0.133], at 48h [p = 0.647] and at 72h [p = 0.167]). The same pattern was observed in slices undergoing EWD. There were no statistical differences between TNF-alpha levels measured in media from slices co-exposed to NMDA and LPS and slices treated with LPS alone during EWD (post hoc at 24h [p = 0.142], 48h [p = 0.471] and 72h [p = 0.315]). Similar results were observed for NO release under control conditions (no main effect of NMDA [F(1,374) = 0.487, p = 0.486]; no NMDA x LPS interaction [F(1,374) = 0.331, p = 0.566]) and during EWD (no EWD x NMDA interaction [F(1,374) = 0.196, p = 0.658]; no EWD x NMDA x LPS interaction [F(1, 374) = 0.844, p = 0.359]).
3.3. Effects of LPS and EWD on inflammatory mediator release from BV2 microglia
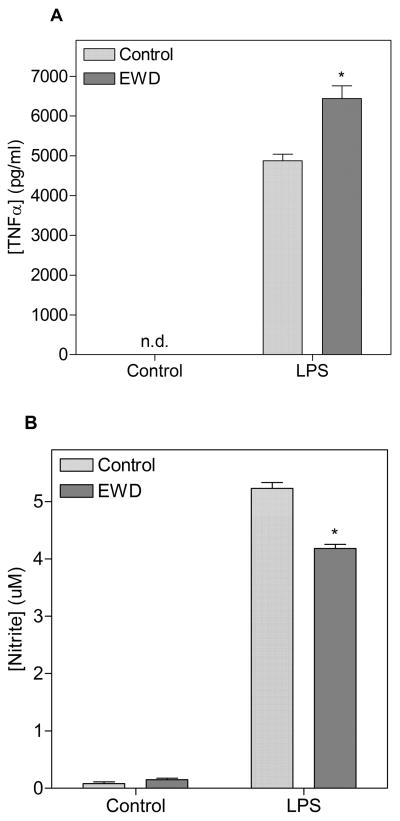
BV2 microglia exposed to ethanol for 10 days did not exhibit morphological changes compared to control cultures. Additionally, cell viability, as measured by resazurin fluorescence did not differ between ethanol exposed and control cultures during these experiments. LPS treatment induced release of TNF-alpha and NO under both control and EWD conditions (fig. 3). BV2 microglia undergoing EWD exhibited a reduction in LPS-induced release of NO compared to controls (main effect of EWD [F(1, 36) = 214.1, p < 0.0001]). On the other hand, BV2 microglia undergoing EWD exhibited a potentiated release of TNF-alpha induced by LPS compared to control conditions (main effect of EWD [F(1, 22) = 19.46, p < 0.001).
Figure 3.
Release of TNF-alpha (A) and nitric oxide (NO) (B) from BV2 microglia after 24h of LPS treatment under control conditions (light bars) and under ethanol withdrawal (EWD) conditions (dark bars). *p < 0.05 compared to LPS under control conditions; n.d. not detected. Treatment groups were run in quadruplicates.
4. Discussion
The present studies were undertaken to test the hypothesis that ethanol exposure causes neuroinflammation which in turn potentiates excitotoxicity associated with EWD. Specifically, following ethanol exposure, OHSC were treated with NMDA, LPS, or the combination of both and evaluated for toxicity (PI uptake) and release of proinflammatory mediators (TNF-alpha and NO).
In the absence of chronic ethanol treatment (no EWD), LPS treatment alone did not produce quantifiable toxicity but OHSC co-exposed to NMDA and LPS exhibited significantly more toxicity than cultures treated with NMDA alone (fig. 1). Therefore, LPS enhances NMDA toxicity, suggesting that neuroinflammation enhances excitotoxicity. This supports previous findings by Zou and Crews who used hippocampal-entorhinal cortical slices (similar to OHSC) to show that TNF-alpha potentiates glutamate toxicity (Zou and Crews, 2005).
Numerous mechanisms have been proposed for the potentiation of excitotoxicity by neuroinflammation (Tilleux and Hermans, 2007). Of particular interest to this study, NO and TNF-alpha released from immune cells have been found to contribute to excitotoxic injury by increasing extra-synaptic glutamate. In co-culture experiments, NO released from astrocytes and microglia has been shown to induce glutamate release from neurons by inhibiting neuronal respiration (Bal-Price and Brown, 2001). In parallel, TNF-alpha has been shown to down-regulate glutamate reuptake by decreasing EAAT2/GLT1 expression on glia (Carmen et al., 2009). In our study, release of TNF-alpha and NO was induced by LPS and their presence in culture media could explain LPS-enhanced NMDA toxicity. As an alternative explanation, activated microglia have been shown to release quinolinic acid (Espey et al., 1997) and glutamate (Barger et al., 2007), both of which activate NMDARs. Therefore, LPS activation of microglia may have increased the overall concentration of NMDAR agonists in the culture media thereby potentiating NMDA-induced toxicity. The precise mechanisms by which LPS enhances NMDA toxicity in the current study remain uncertain at this stage. However, these data support the fact that neuroimmune signaling is capable of potentiating NMDAR mediated excitotoxicity and this might have important implications for neurotoxicity associated with EWD. For example, if release of NO and TNF-alpha elicited by LPS is enhanced during EWD, this may further potentiate excitotoxicity observed at that time.
Many previous studies (Mayer et al., 2002, Self et al., 2004) using OHSC have shown that NMDA-induced toxicity is enhanced during EWD and we were able to replicate these findings in the current study. NMDA treatment alone produced robust toxicity and this was significantly potentiated during EWD.
Since NMDA toxicity is enhanced by LPS under control conditions and NMDA toxicity is also enhanced by EWD, we predicted that NMDA toxicity would be further enhanced by LPS under EWD conditions. Furthermore, this was predicted to be accompanied by a greater release of proinflammatory mediators induced by LPS because ethanol exposure has been shown to sensitize the inflammatory response to LPS (Qin et al., 2008). The results do not support these predictions. During peak EWD, co-exposure to LPS reduced NMDA toxicity (fig. 1) and LPS-induced release of proinflammatory mediators was reduced (fig. 2) at that time. Therefore, in OHSC, ethanol exposure resulted in a desensitized response to LPS and the effects of LPS on NMDA toxicity are reversed during EWD.
A potential explanation for the desensitization to LPS following ethanol exposure is by a mechanism similar to endotoxin tolerance. Endotoxin tolerance occurs when immune tissues or cells are chronically exposed to an inflammatory stimulus resulting in a desensitized response to subsequent challenges with the same stimulus (Morris and Li, 2012). For example, Antonietta Ajmone-Cat et al. (Antonietta Ajmone-Cat et al., 2013) show in an elegant study that when OHSC are treated repeatedly with LPS, the cultures exhibit a reduced inflammatory response to a subsequent challenge. This is presumably the result of persistent activation of the primary molecular target for LPS, toll-like receptor 4 (TLR4). Ethanol has been shown to directly activate TLR4 on isolated astrocytes (Blanco et al., 2004) and microglia (Fernandez-Lizarbe et al., 2009). In our study, it is therefore possible that ethanol persistently activated TLR4 resulting in tolerance to a subsequent challenge with LPS during EWD. In support, ethanol pretreatment has been found to produce tolerance to a subsequent LPS challenge in human monocytes and immortalized macrophages (Bala et al., 2012).
The desensitized inflammatory response to LPS following ethanol exposure does not however fully explain the reversed effects of LPS on NMDA toxicity during EWD. Proinflammatory mediators, TNF-alpha and NO (known to enhance excitotoxicity, see above), are still present in culture media and should still be capable of enhancing excitotoxicity. Therefore, other changes to OHSC induced by ethanol must have had to occur for LPS to reduce NMDA toxicity during EWD. An elegant study by Marshall et al recently reported that in rats exposed to a modified Marjchowicz 4-day binge ethanol paradigm, microglia are partially activated and exhibit an anti-inflammatory phenotype (Marshall et al., 2013). This phenotype has been extensively demonstrated to be neuroprotective against a variety of neurotoxic stimuli (Choi et al., 2012, Stirling et al., 2014). In our study, ethanol exposure may have produced preparations containing anti-inflammatory and neuroprotective microglia which could explain the apparent neuroprotection by LPS against NMDA toxicity during EWD. This interpretation has important implications for the role microglia may play in neurotoxicity associated with EWD. Therefore, we investigated how ethanol exposure affects the response to LPS on microglia specifically.
In OHSC, both TNF-alpha and NO release elicited by LPS were reduced following ethanol exposure. In BV2 microglia, LPS-induced NO release was also reduced following ethanol exposure. However, in contrast, LPS-induced TNF-alpha release was increased following ethanol exposure (fig. 3). Accordingly, following ethanol exposure, BV2 microglia exhibit an unusual inflammatory phenotype that is not characteristic of traditional classical and/or alternative microglial activation states (Boche et al., 2013, Colton and Wilcock, 2010) but instead could suggest another activation state such as an immunoregulatory phenotype (Chhor et al., 2013). In comparison to the results obtained from OHSC, in which the overall phenotype of the slices is anti-inflammatory, the changes observed in BV2 microglia may suggest that other cell types are involved in the changes observed in the slice cultures. However, the use of an immortalized cell line in these studies is a limitation and may not reflect the effects of chronic ethanol in vivo. Therefore, additional studies are necessary to fully characterize neuroimmune changes to all cell types in OHSC, including microglia, astrocytes, oligodendrocytes and neurons, following ethanol exposure.
OHSC used in these studies are taken originally from neonatal rats at postnatal day 8 and, although they are not analyzed until 16, 17 or 18 DIV, the effects of ethanol on these cultures is almost certainly relevant to effects on the developing brain. Indeed, we have previously used these preparations to study the sensitivity of different developmental stages to the effects of excitotoxicity during EWD (Barron et al., 2008). Additionally, others (Moon et al., 2014) have used similar cultures to study the contribution of neuroinflammation on ethanol-induced neurotoxicity specifically during brain development. Their data focused on the role of increased phospholipase A2 activation in neuroinflammation in adolescent cultures, a change that we have previously reported in adult brain (John et al., 1985). The current studies therefore provide further support that changes to the glutamate and neuroimmune systems may contribute to ethanol related toxicity in the developing brain. Furthermore, they suggest that the pathological mechanisms and their interactions examined in this research may be relevant to ethanol-induced neurotoxicity at different developmental ages.
In summary, in OHSC, LPS enhances NMDA toxicity under control conditions but the reverse is observed during EWD. In addition, ethanol exposure results in a desensitized inflammatory response to LPS. The primary hypothesis is therefore not supported by the data. Furthermore, the specific role of microglia in neuroimmune changes induced by ethanol in this model remains uncertain but data obtained from BV2 microglia suggest that other cell types are important for these changes in OHSC. This has important implications for the treatment of ethanol-induced neurotoxicity because, following ethanol exposure, the neuroimmune system may be initially protective against excitotoxicity associated with EWD. In contrast, repeated EWDs may in turn dysregulate neuroimmune processes and contribute to excitotoxic injury observed during EWD. These studies do not invalidate neuroinflammation or excitotoxicity as potential pathological mechanisms to target for the treatment of ethanol induced neurodegeneration but illustrate the necessity to clearly assess the extent, importance and interaction between these mechanisms when using models of ethanol induced neurotoxicity.
Acknowledgments
This work was supported in part by NIAAA (National Institute on Alcohol Abuse and Alcoholism) grants (R21-AA020188, R42-AA014555, and R42-AA015475) awarded to Dr. Littleton as Principal Investigator. The authors would like to thank Dr. Mark Prendergast for allowing use of his microscope and Dr. Linda Van Eldik from the Sanders Brown Center on Ageing at the University of Kentucky for providing BV2 microglia.
References
- Antonietta Ajmone-Cat M, Mancini M, De Simone R, Cilli P, Minghetti L. Microglial polarization and plasticity: Evidence from organotypic hippocampal slice cultures. Glia. 2013;61:1698–1711. doi: 10.1002/glia.22550. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–91. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala S, Tang A, Catalano D, Petrasek J, Taha O, Kodys K, Szabo G. Induction of Bcl-3 by acute binge alcohol results in Toll-like receptor 4/LPS tolerance. Journal of Leukocyte Biology. 2012;92:611–620. doi: 10.1189/jlb.0112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. Journal of Neurochemistry. 2007;101:1205–1213. doi: 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron S, Mulholland PJ, Littleton JM, Prendergast MA. Age and Gender Differences in Response to Neonatal Ethanol Withdrawal and Polyamine Challenge in Organotypic Hippocampal Cultures. Alcoholism: Clinical and Experimental Research. 2008;32:929–936. doi: 10.1111/j.1530-0277.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141:41–53. doi: 10.1016/j.jneumeth.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Pascual M, Valles SL, Guerri C. Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-κB. NeuroReport. 2004;15:681–685. doi: 10.1097/00001756-200403220-00021. [DOI] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Carmen J, Rothstein JD, Kerr DA. Tumor necrosis factor-α modulates glutamate transport in the CNS and is a critical determinant of outcome from viral encephalomyelitis. Brain Research. 2009;1263:143–154. doi: 10.1016/j.brainres.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhor V, Le Charpentier T, Lebon S, Ore MV, Celador IL, Josserand J, Degos V, Jacotot E, Hagberg H, Savman K, Mallard C, Gressens P, Fleiss B. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, Kim HW, Jackson SH, Bosetti F. Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. J Neurochem. 2012;120:292–301. doi: 10.1111/j.1471-4159.2011.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–91. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, Pentney R, Snell LD, Tabakoff B, Zou J, Noronha A. Alcohol-induced neurodegeneration: when, where and why? Alcohol Clin Exp Res. 2004;28:350–64. doi: 10.1097/01.alc.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–27. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey ME, Waite M. Confocal imaging of microglial cell dynamics in hippocampal slice cultures. Methods. 1999;18:222–30. 177. doi: 10.1006/meth.1999.0775. [DOI] [PubMed] [Google Scholar]
- Espey MG, Chernyshev ON, Reinhard JF, Jr, Namboodiri MA, Colton CA. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport. 1997;8:431–4. doi: 10.1097/00001756-199701200-00011. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical Role of TLR4 Response in the Activation of Microglia Induced by Ethanol. The Journal of Immunology. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Follesa P, Ticku MK. Chronic ethanol-mediated up-regulation of the N-methyl-D-aspartate receptor polypeptide subunits in mouse cortical neurons in culture. J Biol Chem. 1996;271:13297–9. doi: 10.1074/jbc.271.23.13297. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Harris BR, Prendergast MA, Hart SR, Blanchard JA, 2nd, Holley RC, Pedigo NW, Littleton JM. Polyamines contribute to ethanol withdrawal-induced neurotoxicity in rat hippocampal slice cultures through interactions with the NMDA receptor. Alcohol Clin Exp Res. 2003;27:1099–106. doi: 10.1097/01.ALC.0000075824.10502.DD. [DOI] [PubMed] [Google Scholar]
- Haber M, Vautrin S, Fry EJ, Murai KK. Subtype-specific oligodendrocyte dynamics in organotypic culture. Glia. 2009;57:1000–13. doi: 10.1002/glia.20824. [DOI] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–32. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45:1542–50. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart SR, Scotland RL, Foster TC, Pedigo NW, Littleton JM. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2003;27:1724–35. doi: 10.1097/01.ALC.0000093601.33119.E3. [DOI] [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjarn M, Schrattenholz A, Porzgen P, Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. Altex. 2009;26:83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 2013;229:539–54. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Bohman S, Radesater AC, Oberg C, Luthman J. Salmonella lipopolysaccharide (LPS) mediated neurodegeneration in hippocampal slice cultures. Neurotox Res. 2005;8:207–20. doi: 10.1007/BF03033974. [DOI] [PubMed] [Google Scholar]
- John GR, Littleton JM, Nhamburo PT. Increased activity of Ca2+-dependent enzymes of membrane lipid metabolism in synaptosomal preparations from ethanol-dependent rats. J Neurochem. 1985;44:1235–41. doi: 10.1111/j.1471-4159.1985.tb08749.x. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, Delzenne NM, De Timary P. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 2012;26:911–8. doi: 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Lee H, Jeong J, Son E, Mosa A, Cho GJ, Choi WS, Ha JH, Kim IK, Lee MG, Kim CY, Suk K. Ethanol selectively modulates inflammatory activation signaling of brain microglia. Journal of Neuroimmunology. 2004;156:88–95. doi: 10.1016/j.jneuroim.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Excitotoxicity and alcohol-related brain damage. Alcohol Clin Exp Res. 1993;17:19–27. doi: 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Marshall SA, Mcclain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis. 2013;54:239–51. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S, Harris BR, Gibson DA, Blanchard JA, Prendergast MA, Holley RC, Littleton J. Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcohol Clin Exp Res. 2002;26:1468–78. doi: 10.1097/01.ALC.0000033261.14548.D2. [DOI] [PubMed] [Google Scholar]
- Moon KH, Tajuddin N, Brown J, 3rd, Neafsey EJ, Kim HY, Collins MA. Phospholipase A2, oxidative stress, and neurodegeneration in binge ethanol-treated organotypic slice cultures of developing rat brain. Alcohol Clin Exp Res. 2014;38:161–9. doi: 10.1111/acer.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Li L. Molecular mechanisms and pathological consequences of endotoxin tolerance and priming. Arch Immunol Ther Exp (Warsz) 2012;60:13–8. doi: 10.1007/s00005-011-0155-9. [DOI] [PubMed] [Google Scholar]
- Nagy J, Kolok S, Boros A, Dezso P. Role of altered structure and function of NMDA receptors in development of alcohol dependence. Curr Neuropharmacol. 2005;3:281–97. doi: 10.2174/157015905774322499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J, Kolok S, Dezso P, Boros A, Szombathelyi Z. Differential alterations in the expression of NMDA receptor subunits following chronic ethanol treatment in primary cultures of rat cortical and hippocampal neurones. Neurochem Int. 2003;42:35–43. doi: 10.1016/s0197-0186(02)00062-1. [DOI] [PubMed] [Google Scholar]
- Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–52. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93:1135–40. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- Salmina AB. Neuron-glia interactions as therapeutic targets in neurodegeneration. J Alzheimers Dis. 2009;16:485–502. doi: 10.3233/JAD-2009-0988. [DOI] [PubMed] [Google Scholar]
- Self RL, Mulholland PJ, Harris BR, Nath A, Prendergast MA. Cytotoxic effects of exposure to the human immunodeficiency virus type 1 protein Tat in the hippocampus are enhanced by prior ethanol treatment. Alcohol Clin Exp Res. 2004;28:1916–24. doi: 10.1097/01.alc.0000148108.93782.05. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P. Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain. 2014;137:707–23. doi: 10.1093/brain/awt341. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Thomas MP, Davis MI, Monaghan DT, Morrisett RA. Organotypic brain slice cultures for functional analysis of alcohol-related disorders: novel versus conventional preparations. Alcohol Clin Exp Res. 1998;22:51–9. [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–70. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Wilkins LH, Jr, Prendergast MA, Blanchard J, Holley RC, Chambers ER, Littleton JM. Potential value of changes in cell markers in organotypic hippocampal cultures associated with chronic EtOH exposure and withdrawal: comparison with NMDA-induced changes. Alcohol Clin Exp Res. 2006;30:1768–80. doi: 10.1111/j.1530-0277.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Yang JY, Xue X, Tian H, Wang XX, Dong YX, Wang F, Zhao YN, Yao XC, Cui W, Wu CF. Role of microglia in ethanol-induced neurodegenerative disease: Pathological and behavioral dysfunction at different developmental stages. Pharmacol Ther. 2014;144:321–337. doi: 10.1016/j.pharmthera.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Kristensen BW, Jakobsen B, Noraberg J. Excitatory amino acid neurotoxicity and modulation of glutamate receptor expression in organotypic brain slice cultures. Amino Acids. 2000;19:7–21. doi: 10.1007/s007260070029. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]