Abstract
This scoping review synthesizes existing research on two major transitions in females’ lives: puberty and perimenopause. These two periods of vast physiological change demarcate the beginning and the end of the reproductive life cycle and are associated with major neuroendocrine reorganization across two key systems, the hypothalamic-pituitary-gonadal (HPG) axis the hypothalamus-pituitary-adrenal (HPA) axis. Despite growing evidence suggesting that the timing and experience of puberty and perimenopause are related to various physical and mental health outcomes (e.g., mood disorders, metabolism, cardiovascular health, autoimmune conditions and cancer), these two processes are rarely examined together. In this paper, we bridge these disparate literatures to highlight similarities, isolate inconsistencies, and identify important areas for future research in women’s health.
Keywords: puberty, perimenopause, chronic disease, women’s health, life course health, HPG axis, HPA axis
Puberty and perimenopause demarcate the beginning and the end of the female reproductive life cycle and are two major transitions in a woman’s life. Despite underlying biological parallels, previous research suggests a weak or non-existent relationship between these reproductive life events (Forman, Mangini, Thelus-Jean, & Hayward, 2013). Growing evidence, however, suggests that the experience and timing of puberty and perimenopause are independently associated with the many of the same health outcomes. For example, prevalence of mental health conditions, autoimmune diseases, and cardiometabolic risk all appear to increase during both puberty (Patton & Viner, 2007) and perimenopause (Greendale, Lee, & Arriola, 1999). Similarly, the timing in which these reproductive events occur (i.e., early or late as compared to average) in women’s lives is also associated with various disease outcomes and mortality (Forman et al., 2013).
A life course approach to women’s health recognizes that biological and social factors act interactively and cumulatively throughout the entire lifespan to shape health outcomes in later life (Kuh & Hardy, 2002). However, distant developmental periods (such as adolescence and middle age) are rarely examined together in an integrative and cohesive way to understand the epidemiology of common disease processes. The aim of this paper was to map a wide range of literature on the association of chronic conditions with puberty and perimenopause in order to identify the similarities and differences across these two key reproductive transitions. We propose that exploring these chronologically distant, yet physiologically connected, reproductive events together will help us understand important issues in women’s health.
First, we summarized the neuroendocrine processes that define these two periods of change and development. Next, we conducted a scoping review (Arksey & O'Malley, 2005; Armstrong, Hall, Doyle, & Waters, 2011; Levac, Colquhoun, & O’Brien, 2010) of these fields to examine the relationships between progression and timing of puberty and perimenopause with long-term health and disease risk. We concluded with the implications of these findings and identified important areas for future research.
Neuroendocrine changes during puberty and perimenopause
Puberty is initiated in late childhood through a cascade of neuroendocrine changes that results in extensive physical growth, sexual maturation, and reproductive capability. Pubertal maturation consists of two associated but independent processes: adrenarche, the reappearance of adrenal androgen production (around age 6–8); and gonadarche, the pubertal reactivation of the hypothalamic-pituitary-gonadal (HPG) axis a few years later (Grumbach, 2004). Menarche, the initiation of the menstrual cycle, occurs toward the end of puberty, around the ages of 12–13 in most developed countries (Patton & Viner, 2007).
Perimenopause is defined as the period immediately preceding menopause when endocrinological, biological, and clinical features of approaching menopause commence (Hale & Burger, 2009; J. Prior, 1998). Women typically begin the shift from a reproductive state to non-reproductive state during their mid-to late 40s, and they remain in this transitory state for approximately 4–5 years before reaching menopause (Burger, Woods, et al., 2007; J. Prior & Hitchcock, 2011). Perimenopause culminates with menopause, when menses have ceased for a period of at least 12 consecutive months (Burger, 2008).
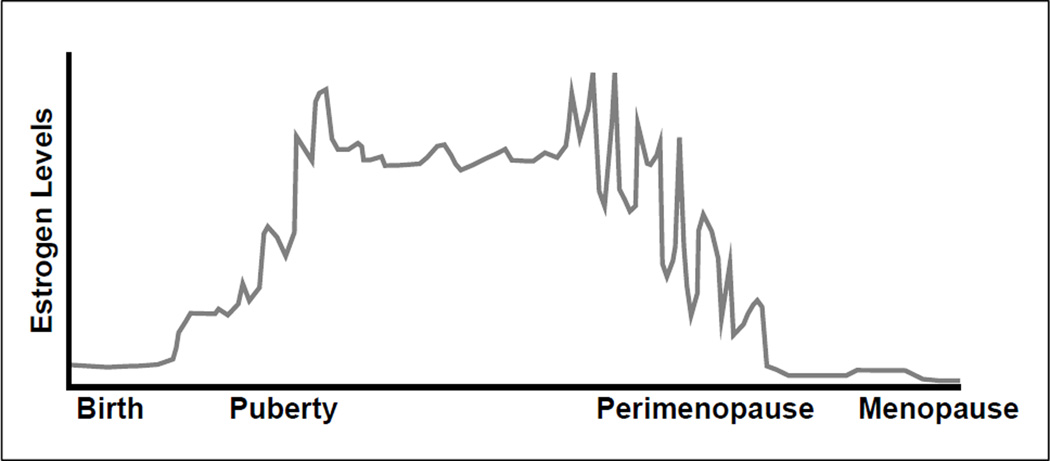
The neuroendocrine system presides over the significant hormonal changes occurring in the HPG axis during puberty and perimenopause (see Table 1 for a summary). In particular, both of these periods are characterized by major changes in the production of luteinizing hormone (LH) and follicle stimulating hormone (FSH), which together regulate ovarian follicle growth and ovulation, and estradiol, the most abundant form of endogenous estrogen (Albin, Niklasson, Westgren, & Norjavaara, 2012; Archibald, Graber, & Brooks-Gunn, 2003; Burger, 2008; Burger, Hale, Robertson, & Dennerstein, 2007; Burger, Woods, et al., 2007; Nelson, 2008; J. Prior & Hitchcock, 2011; J. C. Prior, 2006). Mean levels of estradiol increase across pubertal development until menarche, when estradiol levels stabilize and then cycle regularly through the menstrual cycle each month. During late perimenopause, concentration of estradiol falls markedly from its elevated levels present during early perimenopause and eventually begins to stabilize (see Figure 1).
Table 1.
Summary of changes in the hypothalamic pituitary gonadal (HPG) axis during puberty and perimenopause
| Puberty | Perimenopause | |
|---|---|---|
|
Gonadotropin-releasing hormone (GnRH) is produced by the hypothalamus and controls the synthesis and secretion of LH and FSH. In the brain, steroids influence GnRH secretion via neuroendocrine feedback loops to determine reproductive status during development. |
The onset of puberty is characterized by a gradual increase in the frequency and amplitude of intermittent episodes of GnRH secretion. |
GnRH pulse frequency decreases, in particular, during early perimenopause. |
|
Luteinizing hormone (LH), produced by the pituitary gland, stimulates the ovaries to form androgenic precursors of estradiol. During the reproductive lifespan, a mid-cycle surge of LH triggers ovulation. |
There are sleep-related increases in the pulsatile release of LH at the beginning of puberty, which eventually persist into the daytime and begin to cycle regularly by menarche. |
Few observable changes in LH transpire until late perimenopause, when intermittent elevations in LH concentration and pulse amplitude occur. |
|
Follicle-stimulating hormone (FSH), produced by the pituitary gland, stimulates gonadal growth and the production of gonadal hormones, such as estradiol and progesterone. |
Starting at the beginning of puberty, FSH is secreted in parallel with LH, but increases relatively less. LH-to- FSH ratios are typically less than 1 during childhood and greater than 1 during puberty. |
Intermittent elevations in FSH concentration occur at the beginning of perimenopause. The rise in FSH accelerates during late perimenopause. |
|
Estradiol is the primary form of estrogen produced in women during her reproductive years. It is mostly released from the ovaries and adrenal glands, which subsequently downregulates secretion of LH and FSH. |
Estradiol increases many-fold across pubertal development, and then levels off and begins to cycle regularly approximately one year after menarche. |
Estradiol is erratic and elevated throughout early perimenopause. Estradiol decreases and is less erratic towards the end of perimenopause, accompanied by large increases in FSH and LH. |
|
Progesterone is a hormone produced mainly by the ovaries and plays an important role in regulating the menstrual cycle. Production ceases if the egg is not fertilized, upon which menstruation occurs. |
Production begins with menarche. Youth tend to have low or variable progesterone levels during the first few years after menarche. |
Progesterone decreases gradually but continuously during perimenopause. |
Figure 1.
Life cycle of estrogen.
Note. This depiction of the estrogen changes across women’s life cycles provides a representation of the chaotic and higher estrogen levels in pubertal and perimenopausal women. (Fluctuations do not correlate to precise changes in estrogen levels). Adapted from Prior (2006) and reprinted with permission.
In addition to the HPG axis changes related to puberty and perimenopause, another set of endocrine changes occur in the hypothalamus-pituitary-adrenal (HPA) axis. Levels of cortisol, the major hormonal output of the HPA system, vary throughout the day based on: (1) a strong circadian rhythm (i.e., basal pattern with high morning levels, low evening levels, and a strong negative slope), and (2) experiences of stress or challenge (i.e., cortisol reactivity) (McEwen et al., 1997). A growing body of research suggests that the overall basal activity of the HPA axis increases with sexual maturation in girls (i.e., higher average levels of cortisol across the day) (Adam, 2006; Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Legro, Lin, Demers, & Lloyd, 2003; Netherton, Goodyer, Tamplin, & Herbert, 2004; Schiefelbein & Susman, 2006; Shirtcliff, Granger, Booth, & Johnson, 2005; Stroud et al., 2009). Initial research also suggests that girls experience increased cortisol reactivity (i.e., hypercortisolism) to stressful tasks across puberty (Gunnar et al., 2009; Stroud et al., 2009; Stroud, Papandonatos, Williamson, & Dahl, 2004).
Although comparatively less is known about the HPA axis changes during perimenopause, some studies have found an increase in cortisol levels as women transition from an early to late menopausal transition stage (Woods, Carr, Tao, Taylor, & ES, 2006; Woods, Mitchell, & Smith-DiJulio, 2009). Other research suggests that estrogen regulates corticotropin-releasing hormone gene expression, resulting in elevated cortisol levels (Vamvakopoulos & Chrousos, 1993). Therefore, as women approach menopause, increasing levels of FSH stimulate ovarian follicles to produce excess estrogen, which may influence cortisol levels (Santoro, Brown, Adel, & Skurnick, 1996). There is also some evidence for greater HPA reactivity among postmenopausal women, with older women showing higher cortisol in response to challenge compared to both younger women and similarly-aged male (Seeman, Singer, Wilkinson, & McEwen, 2001). Importantly, HPA activity is involved in regulating many physiological processes relevant to health including cardiovascular activity, blood pressure, and immune and inflammatory functioning (Chrousos & Gold, 1998).
The years surrounding both the initiation and completion of the female reproductive cycle are associated with major neuroendocrine reorganization that is distinct from other periods of the life course. In particular, dramatic changes occur across two key systems: the HPG axis— including fluctuations of LH, FSH, and estradiol— and hyperactivation of the HPA axis. By exploring the shared biological processes and associated health outcomes related to both puberty and perimenopause, we sought to answer: (1) What chronic diseases show a discontinuous increase in prevalence across both transitions? (2) Independent of the experience of these transitions, what is the association of pubertal and perimenopausal timing on health and disease?
Methods
To examine the extent, range, and nature of these literatures, we conducted a comprehensive scoping review to summarize a breadth of evidence on chronic diseases and conditions related to puberty and perimenopause. Following methodological guidelines for this type of literature review (Arksey & O'Malley, 2005; Armstrong et al., 2011; Levac et al., 2010) we defined our research focus as links between reproductive transitions and health outcomes. We included all literature presenting theoretical or empirical approaches to this broad area and that directly pertained to a health condition and puberty or menopause or whose study participants were pubertal or menopausal females. We narrowed our focus to include evidence from industrialized countries only and selected papers published between 1990 and 2014. Exceptions included significant theoretical works published earlier or older papers related to particular diseases when more recent papers could not be identified.
All studies and reviews from peer-reviewed journals and books were identified using electronic databases (Pub Med, Google Scholar, Psych Info), key health journals relevant in the areas of puberty, menopause, and women’s health, and topic-related expert networks and websites (e.g., Global Library of Women’s Medicine, NIH Office of Research on Women’s Health.) We also reviewed the reference lists of relevant articles to identify additional studies our search strategy may have missed.
Keywords for the literature search were selected from two broad areas: female reproductive transition (e.g., puberty, menarche, pubertal timing; perimenopause, menopause, menopause timing) and health outcome (e.g., cancer, cardiovascular disease, life expectancy, mortality, diabetes, mental health, depression, anxiety, obesity, insulin resistance, autoimmune disease). We also searched specific types of cancer (e.g., breast, endometrial, ovarian), and specific types of autoimmune diseases (e.g., lupus, rheumatoid arthritis). Data from the literature review were charted to identify specific disease outcomes relevant to both reproductive transitions. For instances when evidence for a health outcome was found for one transition, but not the other, another round of selection and review was conducted.
Five major health outcomes were identified: mental health, cardiometabolic health, autoimmune conditions, cancer, and mortality. Over 5,000 articles were assessed in the initial screening process, although only 300 representative studies, reviews, and chapters were ultimately included in the final stage of review and systematized using a bibliographic-managing software (EndNote®). These studies were charted according to key issues and themes, and discrepancies between literatures from the two reproductive periods were mapped. Finally, the reviewed literature was systematically reported, with results structured thematically along each dimension of health.
Results
1. Association of pubertal and perimenopausal transitions with health outcomes
Biological, behavioral, environmental, and social influences across the life span continuously interact to affect health of both individuals and populations (Ben-Shlomo & Kuh, 2002; Halfon, Larson, Lu, Tullis, & Russ, 2014). Central to the life course framework is the idea that health trajectories may be altered more readily during certain windows of rapid development or biological reorganization, also termed “critical” or “sensitive periods.” Whereas exposures acting in critical periods affect health in such a way that cannot be modified in any dramatic way by later experience, sensitive periods are windows when an exposure has a stronger effect on development and subsequent disease risk than it would at other times (Kuh, Ben-Shlomo, Lynch, Hallqvist, & Power, 2003). Although most of the literature on critical and sensitive periods has focused on prenatal development or the first few years of life, there is considerable evidence that discrete periods of hormonal activity during puberty and perimenopause also hold important implications for mental and physical health outcomes. Hormones mediate the interaction between an individual the environment (McEwen & Wingfield, 2003); therefore, hormonal changes occurring in the HPG and HPA systems during these transitional periods may cause females to become particularly sensitive to their environment.
Mental health
Puberty is marked by a substantial increase, and emerging sex difference, in many psychological and physiological disorders. In particular, a large body of work identifies a rise in clinical depression and depressive symptoms in girls around the middle of puberty (Angold, Costello, & Worthman, 1998; Joinson et al., 2012; Saluja et al., 2004), leading to a gender gap in depression prevalence between the ages of 11–15 that persists into adulthood (Cyranowski, Frank, Young, & Shear, 2000; Kessler, 2003). Many studies also indicate that the risk of depression is greater for perimenopausal women compared with pre-menopausal or post-menopausal women, even after adjusting for variables such as history of depression, premenstrual syndrome, hot flashes, health behaviors, BMI, stressful life events, age, race, and employment status (Freeman, Sammel, Lin, & Nelson, 2006; Freeman et al., 2004; Halbreich & Kahn, 2001; Schmidt, 2005; Schmidt, Haq, & Rubinow, 2004). Women may also be more susceptible to high anxiety during perimenopause: at least one study has found that odds of high anxiety peak during late perimenopause—although not among women with high anxiety upon entering the menopausal transition (Bromberger et al., 2013).
Higher prevalence of mood disorders and symptoms during puberty and perimenopause is usually tied to fluctuations in sex hormones (Angold, Costello, Erkanli, & Worthman, 1999; Freeman et al., 2006; Freeman et al., 2004; Goodyer, Park, Netherton, & Herbert, 2001; Goodyer, Tamplin, Herbert, & Altham, 2000; Halbreich & Kahn, 2001; Nolen-Hoeksema, 2001; Schmidt, 2005; Steiner, Dunn, & Born, 2003). Most theories propose one of two types of models to explain the developmental rise of mood disorders during reproductive transitions: an indirect model whereby hormones affect some general physiological system such as psychological arousal, neurotransmitters, or reactivity to stressors that increases risk for mood disorders; or an interaction model in which social context or environments, in addition to hormones, contribute to risk (Hyde, Mezulis, & Abramson, 2008). For instance, gonadal steroids estrogen and progesterone have been shown to affect brain regions known to be involved in the modulation of mood and behavior, including the prefrontal cortex, hippocampus, thalamus, and brain stem (Soares & Zitek, 2008). Gonadal steroids also modulate regulation of the HPA axis. Therefore, hormonal changes may trigger dysregulation of the biological stress response during puberty and perimenopause, making these women more vulnerable to depression, particularly when they are confronted with stress (Nolen-Hoeksema, 2001).
Metabolism and cardiovascular health
Increases in ovarian steroids affect metabolism and promote fat deposition, contributing to the large sex difference in body composition initiated at puberty (Worthman, 2002). Rapid changes in body size and shape, combined with the formation of dietary and physical activity patterns during puberty, implicate this window as a sensitive period for the development of excessive weight gain and obesity (Alberga, Sigal, Goldfield, Prud'Homme, & Kenny, 2012; Daniels et al., 2005; Dietz, 1994; Lawlor & Chaturvedi, 2006). Studies similarly find increased risk for unhealthy weight gain and excessive adiposity during perimenopause. Research indicates that, independent of aging, abdominal adiposity increases during the menopausal transition, coinciding with a decrease in lean mass (Sternfeld, Aradhana, Wang, Sharp, & Quesenberry, 2005; Toth, Tchernof, Sites, & Poehlman, 2000).
Independent, but related, changes in insulin sensitivity also occur during puberty and perimenopause, with studies showing that females are more insulin resistant than males throughout both reproductive transitions (Goran & Gower, 2001; Moran et al., 1999; Schianca, Castello, Rapettie, LImoncini, & Bartoli, 2006; Wildman et al., 2008). While insulin resistance appears to play an integral role in healthy somatic growth at the beginning of puberty (likely driven by both growth hormones and sex steroids), continued insulin resistance later in puberty could lead to unhealthy weight gain and put youth at increased risk for type 2 diabetes during adolescence and for cardiovascular disease (CVD) later in life (Goran & Gower, 2001). Likewise, changes in body fat distribution and insulin resistance during perimenopause are associated with increased risk for CVD (Matthews et al., 1989; Schianca et al., 2006).
Other evidence, however, suggests that women’s increased vulnerability to developing CVD during the menopausal transition may be due to factors independent of weight gain or changes in insulin homeostasis (Schianca et al., 2006). For example, one study has shown that the thickness and diameter of the carotid artery changes during perimenopause, which increase women’s vulnerability to developing CVD (El Khoudary et al., 2013). Another study indicates that low-density lipoprotein (LDL) cholesterol (i.e., “bad” cholesterol) and total cholesterol rises during late perimenopause increase women’s risk of developing CVD (Matthews et al., 2009). Still other studies have found associations between vasomotor menopausal symptoms and risk of future coronary heart disease (Gast et al., 2011; Thurston, Sutton-Tyrell, Everson-Rose, Hess, & Matthews, 2008). Although the precise biological mechanisms are yet to be specified, it appears that risk for CVD increases during both reproductive transitions.
Autoimmune conditions
Other health outcomes associated with endocrine changes occurring during puberty and perimenopause include a number of autoimmune conditions, such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes, and autoimmune thyroid conditions (Bove, 2013; Patton & Viner, 2007; Wilder, 1995). Changes in risk, onset, and progression of such conditions occur during puberty and perimenopause, and these conditions are significantly more prevalent among females. The sexual dimorphism in autoimmune disorders may be tied to the different effects of estradiol and testosterone on the initiation or course of underlying autoimmune processes (Lamason et al., 2006; Verthelyi, 2001).
Cancer
The pubertal transition is a sensitive period specifically for the developing mammary gland with respect to future cancer risk. Pubertal girls who are exposed to carcinogens in the presence of a changing hormonal profile may be at higher risk for cancer as a result of susceptibility of the mammary epithelial cells to early insults and mutations that make them vulnerable to carcinogenesis (Golub et al., 2008; Hiatt, Haslam, & Osuch, 2009), although the underlying mechanisms are still unknown (Diamanti-Kandarakis et al., 2009). Even less is known about the possibility of perimenopause as a sensitive period for hormonal carcinogenesis. Although some research in the 1980s hypothesized an increased risk of breast cancer for perimenopausal women (Alexander & Roberts, 1987; Korenman, 1980), there is no conclusive evidence at this time. Associations between various cancers and puberty and perimenopause are more related to the timing of these events rather than their experience.
2. The effect of pubertal and perimenopausal timing on health and disease
The sensitive period hypothesis described in the previous section focused on the discrete set of years during which females transition into and out of the reproductive lifecycle. However, much of the research relating either puberty or perimenopause with long-term health outcomes centers on the relative timing of these transitions (i.e., pubertal or perimenopausal onset). Overall, there is no consistent evidence for an association between pubertal onset and age at menopause (Forman et al., 2013). However, the timing of puberty and natural age at menopause are each independently associated with a number of chronic diseases as well as all-cause mortality. In most cases, the proposed mechanism is related to hormonal exposure over the life course. However, we also highlight a number of behavioral, social, and psychological mechanisms and modifiers that may help explain inconsistencies in a direct-hormonal hypothesis.
Mental health
A large body of work has found that early pubertal timing is associated with heightened prevalence and intensity of depressive symptoms, adjustment problems, anxiety, and psychopathology among adolescent females (Ge, Elder Jr, Regnerus, & Cox, 2001; Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1997; Harlow, Cohen, Otto, Spiegelman, & Cramer, 1999; Mendle, Turkheimer, & Emery, 2007; Natsuaki, Biehl, & Ge, 2009). Biological explanations for these associations stem from evidence that early maturing girls secrete higher levels of estradiol from adolescence across the transition to adulthood, which may cause heightened sensitivity to negative life events (Apter, Reinilä, & Vihko, 1989). Individual differences in the age of pubertal onset also create variation in the point at which the brain is exposed to sex hormones, influencing the developmental trajectory of neural maturation and risk for sex-biased psychopathologies (Sisk & Foster, 2004; Sisk & Zehr, 2005). Some scholars have found opposing evidence, however, arguing that estrogen has stimulating effects on the serotonergic system in the brain. Girls who experience early puberty, therefore, may have better mental health outcomes in the long run than later maturing girls (Herva et al., 2004).
Other theories suggest that the psychological and behavioral implications associated with either early or late pubertal timing may be implicated in the increasing incidence of and gender disparity in mental health problems starting in adolescence (Caspi & Moffitt, 1991). The most common psychosocial theory, the early timing hypothesis, holds that early pubertal girls must confront new norms and expectations before they are psychologically and cognitively prepared for such challenges, and may experience distress related to poor body image or harassment from peers tied to exhibiting the physical manifestations of puberty (e.g., secondary sex characteristics) before their peers (Ge, Conger, & Elder, 1996; Mendle et al., 2007). However, others argue that both early or late pubertal timing (off-time hypothesis) may be associated with heightened stress related to social comparisons (Weichold, Silbereisen, & Schmitt-Rodermund, 2003). An interaction hypothesis, which alternatively suggests that only pubertal timing combined with stressful life events increases the risk for depression (Ge, Conger, & Elder Jr, 2001; Hyde et al., 2008), could help to explain inconsistencies in the literature.
Evidence is less conclusive about the impact of menopausal timing on mental health outcomes. One study found that psychological distress was unrelated to timing of menopausal transition (Busch, Zonderman, & Costa Jr, 1994), while other studies have found an inverse relationship between menopausal age and late-life depression among women (Harlow, Wise, Otto, Soares, & Cohen, 2003; Ryan, Carrière, Scali, Ritchie, & Ancelin, 2008). Women with relatively lower levels of education appear to be at particular risk of depression from a lower age at menopause (Ryan et al., 2008). Like the early puberty hypothesis, it is plausible that menopause timing occurring significantly earlier than the average age in the population may be a source of psychological distress for some women (Lennon, 1982). The relationship between depression and earlier age of menopause may also stem from prolonged exposure to a hypoestrogenic state (Ryan et al., 2008).
Cardiovascular disease
Emerging evidence from epidemiological studies indicates that early pubertal timing is associated with increased risk of CVD and CVD-precursors and comorbid conditions, such as hypertension and diabetes (He et al., 2010; Jacobsen, Oda, Knutsen, & Fraser, 2009; Lakshman et al., 2009; Remsberg et al., 2005). In a recent meta-analysis, early menarche was associated with a 15% increase in CVD risk (Prentice & Viner, 2012). The association between pubertal timing and poor cardiovascular health may also reflect pre-existing biological risk, related to childhood or adult body size (Bleil et al., 2013; Bleil et al., 2012; Kivimäki et al., 2008; Patton & Viner, 2007), or differences in health-risk behavior associated with early timing, such as substance use and low physical activity (Baker, Birch, Trost, & Davison, 2007; Davison, Werder, Trost, Baker, & Birch, 2007; Kaltiala-Heino, Marttunen, Rantanen, & Rimpela, 2003).
One major criticism of previous studies examining the relationship between pubertal timing and adult cardiovascular health outcomes is the lack of measurement of child or adolescent body weight. Many scholars contend that early menarche is only a risk marker, and not an independent predictor of cardiovascular disease. In other words, elevated childhood BMI contributes to an earlier age of menarche, which in turn leads to a higher adult BMI and associated cardiovascular risk (Kivimäki et al., 2008). Critics question, therefore, whether menarcheal age is an independent predictor of cardiovascular risk factors in adulthood.
Research has generally found a similar association between an early age at menopause (i.e., before age 40) and an increased risk for coronary heart disease (Atsma, Bartelink, Grobbee, & van der Schouw, 2006; Mondul, Rodriguez, Jacobs, & Calle, 2005; Shuster, Rhodes, Gostout, Grossardt, & Rocca, 2010), although a number of studies have produced inconsistent results (Atsma et al., 2006). The main challenges associated with determining the relationship between timing of menopause and cardiovascular disease include distinguishing the effect of menopause from age-related effects (Lawlor, Ebrahim, & Smith, 2002) and health-related behaviors (van der Schouw, van der Graaf, Steyerberg, Eijkemans, & Banga, 1996). Although several studies have reported adverse relationships between menopause and lipid profiles, blood pressure, and weight gain, these factors also vary with age (Lawlor et al., 2002). Smoking is also a well-known risk factor for CVD and is associated with decreased age of menopause. A meta-analysis of early menopause and risk factors for cardiovascular disease found, however, that the effects of smoking are unclear. Although the relationship between postmenopausal status and CVD disappears upon controlling for smoking, the effect of early menopause on CVD was also more pronounced after controlling for smoking (Atsma et al., 2006).
Biological mechanisms proposed to explain the association between age at menopause and CVD include the role of estrogen in the maintenance of immune function (Mondul et al., 2005) and a cardioprotective effect of estrogen (Ossewaarde, Bots, Verbeek, & Peeters, 2005; Roeters van Lennep, Westerveld, Erkelens, & van der Wall, 2002). Decreasing estrogen levels during the menopausal transition have been linked to adverse vascular changes. In particular, low-density lipoprotein cholesterol (LDL-C) levels increase, while high-density lipoprotein cholesterol (HDL-C) levels remain stable or slightly increase. These changes have consistently been found associated with increased risk of CVD (Woodard et al., 2011).
Autoimmune conditions
Almost all autoimmune diseases are more prevalent in women than men, and it has been hypothesized that hormonal or reproductive factors, such as estrogen exposure, may play an important role in disease pathogenesis (Simard & Costenbader, 2007). While a few studies suggest that early menarche is associated with systemic lupus erythematosus (SLE) (Costenbader, Feskanich, Stampfer, & Karlson, 2007) and rheumatic arthritis (Avila et al., 1990; Karlson, Mandl, Hankinson, & Grodstein, 2004), findings are not consistent across studies. In other research, no clear association was found between age of menarche and SLE (Cooper, Dooley, Treadwell, St Clair, & Gilkeson, 2002), and still others have found that earlier age of menarche was inversely related to rheumatic arthritis (Pedersen et al., 2006; Pikwer, Bergstrom, Nilsson, Jacobsson, & Turesson, 2012).
More consistent observations have been found between an earlier age at menopause (i.e., prior to age 45) and autoimmune diseases, including disorders involving the thyroid and adrenal glands, pernicious anemia, alopecia, Chron’s disease, SLE, and rheumatoid arthritis (Bove, 2013; Costenbader et al., 2007; Leidy, 1994; Pikwer et al., 2012; Sammaritano, 2012). The pathophysiological mechanisms explaining these relationships are likely complex, although hypothesized explanations for this relationship include an insufficient HPA axis or immune changes regulated by estrogen, androgens, progesterone, or estrogen/androgen ratios.
Cancer
Numerous studies have shown that earlier pubertal timing is a key risk factor for breast cancer (Ahlgren, Melbye, Wohlfahrt, & Sorensen, 2004; D. Apter & Vihko, 1983; Hsieh, Trichopoulos, Katsouyanni, & Yuasa, 1990; Kelsey & Bernstein, 1996), endometrial cancer (Kaaks, Lukanova, & Kurzer, 2002; Purdie & Green, 2001) and ovarian cancer (Jordan, Webb, & Green, 2005). A later age at menopause, on the other hand, is also associated with an increased risk of breast and endometrial cancer (DeLellis Henderson, Bernstein, Henderson, Kolonel, & Pike, 2008; Ossewaarde et al., 2005; Purdie & Green, 2001). Additionally, some studies find an association between later age of menopause and colon cancer, although this link is less well established relative to the associations found with reproductive tract cancers (Franceschi et al., 2000).
For both early puberty and late menopause, the hypothesized biological pathway to the occurrence of various cancers is increased lifetime exposure to estrogen and progesterone (DeLellis Henderson et al., 2008; Key et al., 2011). Although early menarche and late menopause increase cancer risk, the effects are not necessary equivalent. For example, excess risk for breast cancer is greater if women’s reproductive years are extended by one year at puberty compared with excess risk associated with lengthening one year at menopause (Collaborative Group on Hormonal Factors in Breast Cancer, 2012).
The relation between reproductive timing and cancer risk is potentially confounded or modified by BMI and cigarette smoking. Not only is higher BMI associated with both earlier puberty and later menopause, but obesity may also heighten oxidative stresses, an independent risk factor for a wide range of cancers (Dai et al., 2009; Vincent & Taylor, 2006). BMI also affects the concentration of circulating sex hormones (Akahoshi et al., 2002; Gold et al., 2013) and research indicates that a higher BMI modifies the risk of breast, endometrial, and colon cancer depending upon menopausal status (Reeves et al., 2007). Cigarette smoking is one of the strongest and most consistently associated factors for an earlier age of menopause (Hardy, Kuh, & Wadsworth, 2000; Harlow & Signorello, 2000). The association found between late menopause and endometrial cancer, for example, may be due to the anti-estrogenic effects of cigarette smoking (Terry & Rohan, 2002; Zhou et al., 2008).
Mortality
Some evidence from epidemiologic research suggests that early pubertal timing is associated with a shorter lifespan. For example, one study found a 4% reduced risk of all-cause mortality per year delay in onset of menarche in a population-based cohort study in the United Kingdom (Lakshman et al., 2009). This finding is consistent with previous research in Norway (Jacobsen, Heuch, & Kvale, 2007) and California (Jacobsen et al., 2009) that found a 2.4 and 4.5% (respectively) reduced risk of overall mortality associated with a one-year delay in onset of menarche. Many studies have also linked all-cause mortality to an early age at menopause (some studies indicate earlier than age 40, while others suggest the relationship occurs prior to age 45) (Gold, 2011; Jacobsen, Heuch, & Kvale, 2003; Li et al., 2013; Mondul et al., 2005; Ossewaarde et al., 2005; Shuster et al., 2010). Studies also have found that women who experienced menopause prior to age 40 experience mortality ranging from 35–95% higher compared with women who reported menopause occurring at age 50 years or older (Gold et al., 2001).
Many complex mechanisms may underlie the relationship between timing of reproductive events and mortality, including genetic, behavioral, environmental, and hormonal factors (Gold et al., 2001; Mishra, Cooper, Tom, & Kuh, 2009). While there is growing evidence that reproductive timing is associated with a number of specific diseases (as discussed in the sections above), some argue that early reproductive timing is a biological marker of cumulative stress (Barkow, 1984; Belsky, Steinberg, & Draper, 1991; Chisholm, Quinlivan, Petersen, & Coall, 2005) or accelerated somatic aging (Li et al., 2013; Svejme, Ahlborg, Nilsson, & Karlsson, 2012).
Discussion
The aim of this article was to review the dramatic physiological changes occurring during the beginning and end of women’s reproductive lives and how they are linked to later life health outcomes and mortality. We presented evidence using the sensitive periods model to suggest that events occurring during puberty and perimenopause—as well as their timing—are correlated with or influence risk for some of the leading causes of morbidity and mortality in women. We do not deny that other life course models (e.g., accumulation/chains of risk) may also influence women’s health; rather, we chose to highlight the importance of exposure-time interactions with respect to puberty and perimenopause.
Hormonal fluctuations that are common across both reproductive life events may help explain why puberty and perimenopause are sensitive periods for the development of depression and anxiety, metabolic and cardiovascular risks, and autoimmune conditions. Overall, it is hypothesized that hormones increase vulnerability that, in interaction with stressful life events, chronic stress, risky behaviors, or poor environments cause initiation or progression of disease. Although less studied, some research suggests that these hormonal shifts provoke high arousal, excitability, or excessive emotionality more generally, which may make individuals more sensitive to all environmental conditions (Steiner et al., 2003). We urge future researchers to look beyond risks, and examine these reproductive transitions as periods of opportunity to improve positive health and psychological well-being.
Despite dramatic changes across both gonadal and adrenal systems during the pubertal and perimenopausal transitions, most of the literature is focused on sex steroids, namely estradiol, with relatively little research on the integrative functioning of the HPG and HPA axes (Nolen-Hoeksema, 2001; Woods, Mitchell, & Smith-DiJulio, 2009). There is evidence showing that the adrenal axis regulates gonadal function through inhibitory effects of stress on reproductive behavior and the release of sex steroids (Viau, 2002), but the relation is not unidirectional. The HPA axis is also subject to gonadal influences, as evidenced by gender differences in basal cortisol levels, stress reactivity of the HPA system, and prevalence rates of stress-related diseases (Dallman et al., 2002; Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999). However, how sex steroids operate within the central nervous system to regulate the HPA axis remains unresolved. Future studies should examine the interactive and bidirectional effects of stress steroids and glucocorticoids during these important reproductive transitions to help identify underlying causes of health and disease.
While this review has highlighted many similarities between the beginning and end of the female reproductive lifespan that may underlay common disease pathways, we have also found inconsistencies between puberty and perimenopause timing and their associated health outcomes. In particular, earlier ages of both puberty and menopause have been associated with higher risk of CVD and decreased life expectancy. There is considerable evidence for the protective effect of estrogen against atherosclerosis in older women, however estrogen exposure earlier in life may be harmful. It is possible that the timing of estrogen exposures may play at least as important a role in affecting health as lifetime exposure. For example, there are robust estrogen benefits for cardiovascular health during the perimenopausal and early postmenopausal years, with no or possibly deleterious estrogen effects during the late postmenopausal years (Clarkson, 2007). Such inconsistencies underlie the complexity of the relationship between hormonal exposure and disease and highlight the importance of developmental timing, genetic, environment, and behavioral influences.
There is also still much to learn about the initiation of puberty and perimenopause, which together determine the length of a female’s reproductive lifespan. Although some research suggests that reproductive characteristics in puberty and perimenopause may simply represent markers of the same underlying process (Kuh & Hardy, 2002), there is conflicting evidence concerning the direct relationship between timing of puberty (usually measured by menarche) and the timing of menopause. A recent review of the literature identified 36 studies that examine the association between these two sentinel events and found that ten reported a significant direct association (i.e., early menarche was associated with early menopause), two an inverse association (i.e., early menarche was associated with later menopause), and the majority had null findings (Forman et al., 2013).
The association between age at menarche and menopause and health conditions is complicated by the many social and environmental exposures that interact with a woman’s physiology across her lifespan. Beyond a strong genetic component that influences timing of puberty and perimenopause, variation in timing is linked to socio-demographic characteristics, body size, and exposure to endocrine-disrupting chemicals (Gold, 2011; Patton & Viner, 2007). In particular, results from several large-scale studies suggest that non-Hispanic Black girls, and girls who are overweight or obese, experience puberty earlier than their peers (Chumlea et al., 2003; Kaplowitz, Slora, Wasserman, Pedlow, & Herman-Giddens, 2001). There is also a substantial body of work suggesting that stress-inducing family processes (e.g., harsh parenting, marital conflict) may accelerate onset of puberty (Belsky et al., 2007), and some evidence linking low socioeconomic status (SES) to early pubertal development, although results are not consistent across all measures (Braithwaite et al., 2009; Parent et al., 2003). Similarly, timing of menopause also varies by race/ethnicity (i.e. many studies report that African American and Latina women experience menopause earlier than White and Asian women). Lower SES, smoking, and exposure to endocrine disruptors have all been associated with earlier menopausal age, while higher parity and BMI have been associated with later menopausal age (Gold, 2011). Although all of the empirical studies in this scoping review included some set of basic controls, covariate selection varied widely based on data availability and disciplinary priorities. In order to understand the constellation of factors related to pubertal onset and age at menopause, it is important to examine these dynamic and interrelated variables, which have important implications for reproductive timing and health trajectories.
Additionally, some life course researchers examine pregnancy as another sensitive period for health (Rich-Edwards, 2002). Although we have focused this review on the beginning and end of the reproductive life cycle for women, pregnancy represents another window of vast hormonal and physiological changes and may be similarly linked with long-term health outcomes. Research indicates that pregnancy produces significant changes in estrogen levels as well as suppression of the HPA axis during this time (Mastorakos & Ilias, 2000; Steiner et al., 2003), and changes in neuroendocrine activity may imply increased vulnerability or sensitivity to psychosocial, environmental, and physiological factors (Steiner et al., 2003).
The unique contribution this review provides to existing literature is the linkage of the physiological similarities between puberty and perimenopause and their relation to various health outcomes. Although the more narrow foci of the studies we reviewed presents critical information about pubertal and perimenopausal females’ health, this review offers a broad perspective of how the changes occurring at the beginning and culmination of women’s reproductive lives influence long-term health trajectories. This review has illustrated the complexity of the relationship between reproductive transitions, their timing, and health outcomes, revealing that exposure to various hormone changes during puberty and perimenopause do not always produce parallel responses. Such information may be useful in the study of how health capacities develop across different phases or periods of development (Halfon et al., 2014), as well as to policymakers or clinicians in deciding the most efficient way to organize health programs or services. Greater understanding of women’s health across the life course and how different periods relate to each other may also inform disease risk and provide insight on how to modify health trajectories.
Highlights.
Puberty and perimenopause involve reorganization across HPG and HPA systems.
Many chronic health risks increase during both puberty and perimenopause.
Environmental influences on health may intensify when hormone levels are changing.
This review reveals inconsistent health consequences of lifetime estrogen exposure.
Acknowledgements
The authors received financial support from the Robert Wood Johnson Foundation Health and Society Scholars Program (Hoyt) and the National Institute of Child Health & Human Development, grant # T32-HD007275 (Falconi). The authors wish to thank Dr. Jerilynn Prior, for permission to include a figure from her previously published work, and Dr. Nancy Adler, for helpful comments on a previous draft of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam E. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TIA. Growth patterns and the risk of breast cancer in women. New England Journal of Medicine. 2004;351(16):1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- Akahoshi M, Soda M, Nakashima E, Tominaga T, Ichimaru S, Seto S, Yano K. The effects of body mass index on age at menopause. International Journal of Obesity. 2002;26(7):961–968. doi: 10.1038/sj.ijo.0802039. [DOI] [PubMed] [Google Scholar]
- Alberga A, Sigal R, Goldfield G, Prud'Homme D, Kenny G. Overweight and obese teenagers: Why is adolescence a critical period? Pediatric Obesity. 2012;7(4):261–273. doi: 10.1111/j.2047-6310.2011.00046.x. [DOI] [PubMed] [Google Scholar]
- Albin A, Niklasson A, Westgren U, Norjavaara E. Estradiol and pubertal growth in girls. Hormone Research in Paediatrics. 2012;78:218–225. doi: 10.1159/000343076. [DOI] [PubMed] [Google Scholar]
- Alexander FE, Roberts MM. The menopause and breast cancer. Journal of Epidemiology and Community Health. 1987;41(2):94–100. doi: 10.1136/jech.41.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological medicine. 1999;29(5):1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological medicine. 1998;28(1):51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. International journal of cancer. 1989;44(5):783–787. doi: 10.1002/ijc.2910440506. [DOI] [PubMed] [Google Scholar]
- Apter D, Vihko R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. The Journal of Clinical Endocrinology & Metabolism. 1983;57(1):82–86. doi: 10.1210/jcem-57-1-82. [DOI] [PubMed] [Google Scholar]
- Archibald AB, Graber JA, Brooks-Gunn J. Pubertal processes and physiological growth in adolescence. In: Adams G, Berzonsky B, editors. Blackwell handbook of adolescence. Malden, MA: Blackwell Publishing Ltd; 2003. pp. 24–47. [Google Scholar]
- Arksey H, O'Malley L. Scoping studies: towards a methodological framework. International journal of social research methodology. 2005;8(1):19–32. [Google Scholar]
- Armstrong R, Hall BJ, Doyle J, Waters E. ‘Scoping the scope’ of a cochrane review. Journal of Public Health. 2011;33(1):147–150. doi: 10.1093/pubmed/fdr015. [DOI] [PubMed] [Google Scholar]
- Atsma F, Bartelink M-L, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause. 2006;13(2):265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- Avila MH, Liang MH, Willett WC, Stampfer MJ, Colditz GA, Rosner B, Speizer FE. Reproductive factors, smoking, and the risk for rheumatoid arthritis. Epidemiology. 1990;1(4):285–291. doi: 10.1097/00001648-199007000-00005. [DOI] [PubMed] [Google Scholar]
- Baker BL, Birch LL, Trost SG, Davison KK. Advanced pubertal status at age 11 and lower physical activity in adolescent girls. The Journal of Pediatrics. 2007;151(5):488. doi: 10.1016/j.jpeds.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkow JH. The distance between genes and culture. Journal of Anthropological Research. 1984:367–379. [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62(4):647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg LD, Houts RM, Friedman SL, DeHart G, Cauffman E, Susman E. Family rearing antecedents of pubertal timing. Child Development. 2007;78(4):1302–1321. doi: 10.1111/j.1467-8624.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International journal of epidemiology. 2002;31(2):285–293. [PubMed] [Google Scholar]
- Bleil ME, Adler NE, Appelhans BM, Gregorich SE, Sternfeld B, Cedars MI. Childhood adversity and pubertal timing: Understanding the origins of adulthood cardiovascular risk. Biological psychology. 2013;93(1):213–219. doi: 10.1016/j.biopsycho.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil ME, Appelhans BM, Adler NE, Gregorich SE, Sternfeld B, Cedars MI. Pubertal timing, androgens, and obesity phenotypes in women at midlife. The Journal of Clinical Endocrinology & Metabolism. 2012;97(10):E1948–E1952. doi: 10.1210/jc.2012-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove R. Autoimmune diseases and reproductive aging. Clinical Immunology. 2013;149:251–264. doi: 10.1016/j.clim.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite D, Moore DH, Lustig RH, Epel ES, Ong KK, Rehkopf DH, Hiatt RA. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes & Control. 2009;20(5):713–720. doi: 10.1007/s10552-008-9284-9. [DOI] [PubMed] [Google Scholar]
- Bromberger J, Kravitz H, Chang Y, Randolph JJ, Avis N, Gold E, Matthews K. Does risk for anxiety increase during the menopausal transition? Study of women's health across the nation. Menopause. 2013;20(5):488–495. doi: 10.1097/GME.0b013e3182730599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H. The menopausal transition - endocrinology. Journal of Sexual Medicine. 2008;5:2266–2273. doi: 10.1111/j.1743-6109.2008.00921.x. [DOI] [PubMed] [Google Scholar]
- Burger H, Hale G, Robertson D, Dennerstein L. A review of hormonal chnages during the menopausal transition: Focus on findings from the Melbourne Women's Midlife Health Project. Human Reproduction Update. 2007;13(6):559–565. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- Burger H, Woods N, Dennerstein L, Alexander J, Kotz K, Richardson G. Nomenclature and endocrinology of menopause and perimenopause. Expert Review of Neurotherapeutics. 2007;7(11 Suppl):S35–S43. doi: 10.1586/14737175.7.11s.S35. [DOI] [PubMed] [Google Scholar]
- Busch C, Zonderman A, Costa P., Jr. Menopausal transition and psychological distress in a nationall representative sample: Is menopause associated with psychological distress? Journal of Aging and Health. 1994;6:209–228. [Google Scholar]
- Caspi A, Moffitt T. Individual differences are accentuated during periods of social change: The sample case of girls at puberty. Journal of Personality and Social Psychology. 1991;61(1):157. doi: 10.1037//0022-3514.61.1.157. [DOI] [PubMed] [Google Scholar]
- Chisholm JS, Quinlivan JA, Petersen RW, Coall DA. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Human Nature. 2005;16(3):233–265. doi: 10.1007/s12110-005-1009-0. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. A healthy body in a healthy mind—and vice versa—the damaging power of “uncontrollable” stress. Journal of Clinical Endocrinology & Metabolism. 1998;83(6):1842. doi: 10.1210/jcem.83.6.4908. [DOI] [PubMed] [Google Scholar]
- Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, Sun SS. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111(1):110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14(3):373–384. doi: 10.1097/GME.0b013e31803c764d. [DOI] [PubMed] [Google Scholar]
- Cohen L, Soares C, Vitonis A, Otto M, Harlow B. Risk for new onset of depression during the menopausal transition: The Harvard Study of Moods and Cycles. Archives of General Psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Indivdiual participant meta-analysis, including 118,964 women with breast cancer from 117 epidemiological studies. Lancet Oncology. 2012;13(11):1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. Hormonal and reproductive risk factors for development of systemic lupus erythematosus: Results of a population based, case-control study. Arthritis & Rheumatism. 2002;46(7):1830–1839. doi: 10.1002/art.10365. [DOI] [PubMed] [Google Scholar]
- Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis & Rheumatism. 2007;56(4):1251–1262. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: A theoretical model. Archives of General Psychiatry. 2000;57(1):21. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Dai Q, Gao Y-T, Shu X-O, Yang G, Milne G, Cai Q, Li H. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women's Health Study. Journal of Clinical Oncology. 2009;27(15):2482–2488. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Viau VG, Bhatnagar S, Gomez F, Laugero K, Bell M. Corticotropin-releasing factor, corticosteroids, stress, and sugar: Energy balance, the brain, and behavior. Hormones, brain and behavior. 2002;1:571–631. [Google Scholar]
- Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, Williams CL. Overweight in children and adolescents pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111(15):1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- Davison KK, Werder JL, Trost SG, Baker BL, Birch LL. Why are early maturing girls less active? Links between pubertal development, psychological well-being, and physical activity among girls at ages 11 and 13. Social science & medicine (1982) 2007;64(12):2391. doi: 10.1016/j.socscimed.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLellis Henderson K, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the multiethnic cohort study. American Journal of Epidemiology. 2008;167(11):1287–1294. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, Gore AC. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocrine Reviews. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. Critical periods in childhood for the development of obesity. The American journal of clinical nutrition. 1994;59(5):955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- El Khoudary S, Wildman R, Matthews K, Thurston R, Bromberger J, Sutton-Tyrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20(1):8–14. doi: 10.1097/gme.0b013e3182611787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolescent Health, Medicine and Therapeutics. 2013;4:1–21. doi: 10.2147/AHMT.S15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi S, Gallus S, Talamini R, Tavani A, Negri E, La Vecchia C. Menopause and colorectal cancer. British journal of cancer. 2000;82(11):1860–1862. doi: 10.1054/bjoc.1999.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of General Psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Archives of General Psychiatry. 2004;61(1):62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- Gast G, Pop V, Samsioe G, Grobbee D, Nilsson P, Keyzer J, van der Schouw Y. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause. 2011;18(2):146–151. doi: 10.1097/gme.0b013e3181f464fb. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH. Coming of age too early: Pubertal influences on girls' vulnerability to psychological distress. Child Development. 1996;67(6):3386–3400. [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental psychology. 2001;37(3):404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Elder GH, Jr., Regnerus M, Cox C. Pubertal transitions, perceptions of being overweight, and adolescents' psychological maladjustment: Gender and ethnic differences. Social Psychology Quarterly. 2001:363–375. [Google Scholar]
- Gold E. The timing of the age at which natural menopause occurs. Obstetrics and Gynecology Clinics of North America. 2011;38(3):425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E, Bromberger J, Crawford S, Samuels S, Greendale G, Harlow S, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. American Journal of Epidemiology. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- Gold E, Crawford S, Avis N, Crandall C, Matthews K, Waetjen L, Harlow S. Factors related to age at natural menopause: Longitudinal analyses from SWAN. American Journal of Epidemiology. 2013;178(1):70–83. doi: 10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Collman GW, Foster PMD, Kimmel CA, Rajpert-De Meyts E, Reiter EO, Toppari J. Public health implications of altered puberty timing. Pediatrics. 2008;121(Supplement 3):S218–S230. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- Goodyer I, Park R, Netherton C, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. The British Journal of Psychiatry. 2001;179(3):243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Tamplin A, Herbert J, Altham P. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. The British Journal of Psychiatry. 2000;177(6):499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- Graber J, Lewinsohn P, Seeley J, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(12):1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Greendale G, Lee N, Arriola E. The menopause. The Lancet. 1999;353(9152):571–580. doi: 10.1016/S0140-6736(98)05352-5. [DOI] [PubMed] [Google Scholar]
- Grumbach MM. The neuroendocrinology of human puberty revisited. Hormone Research in Paediatrics. 2004;57(Suppl. 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: normative changes and associations with puberty. Development and Psychopathology. 2009;21(01):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Kahn L. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- Hale G, Burger H. Hormonal changes and biomarkers in late reproductive age, menopausal transition, and menopause. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23:7–23. doi: 10.1016/j.bpobgyn.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Halfon N, Larson K, Lu M, Tullis E, Russ S. Lifecourse health development: Past, present, and future. Journal of Maternal and Child Health. 2014;18:344–365. doi: 10.1007/s10995-013-1346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R, Kuh D, Wadsworth M. Smoking, body mass index, socioeconomic status, and the menopausal transition in a British national cohort. International journal of epidemiology. 2000;29(5):845–851. doi: 10.1093/ije/29.5.845. [DOI] [PubMed] [Google Scholar]
- Harlow B, Cohen L, Otto M, Spiegelman D, Cramer D. Prevalence and predictors of depressive symptoms in older premenopausal women: the Harvard Study of Moods and Cycles. Archives of General Psychiatry. 1999;56(5):418–424. doi: 10.1001/archpsyc.56.5.418. [DOI] [PubMed] [Google Scholar]
- Harlow B, Signorello L. Factors associated with early menopause. Maturitas. 2000;35(1):3–9. doi: 10.1016/s0378-5122(00)00092-x. [DOI] [PubMed] [Google Scholar]
- Harlow B, Wise L, Otto M, Soares C, Cohen L. Depression and its influence on reproductive endocrine and menstrual cycle markers associated with perimenopause. Archives of General Psychiatry. 2003;60:29–36. doi: 10.1001/archpsyc.60.1.29. [DOI] [PubMed] [Google Scholar]
- He C, Zhang C, Hunter DJ, Hankinson SE, Louis GMB, Hediger ML, Hu FB. Age at menarche and risk of type 2 diabetes: Results from 2 large prospective cohort studies. American Journal of Epidemiology. 2010;171(3):334–344. doi: 10.1093/aje/kwp372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herva A, Jokelainen J, Pouta A, Veijola J, Timonen M, Karvonen JT, Joukamaa M. Age at menarche and depression at the age of 31 years: Findings from the Northern Finland 1966 Birth Cohort Study. Journal of psychosomatic research. 2004;57(4):359–362. doi: 10.1016/j.jpsychores.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Hiatt RA, Haslam SZ, Osuch J. The breast cancer and the environment research centers: Transdisciplinary research on the role of the environment in breast cancer etiology. Environmental health perspectives. 2009;117(12):1814. doi: 10.1289/ehp.0800120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: Associations and interactions in an international case control study. International journal of cancer. 1990;46(5):796–800. doi: 10.1002/ijc.2910460508. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review. 2008;115(2):291. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Jacobsen B, Heuch I, Kvale G. Age at natural menopause and all-cause mortality: A 37-year follow-up of 19,731 Norwegian women. American Journal of Epidemiology. 2003;157(10):923–929. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- Jacobsen B, Heuch I, Kvale G. Association of low age at menarche with increased all-cause mortality: A 37-year follow-up of 61,319 Norwegian women. American Journal of Epidemiology. 2007;166(12):1431–1437. doi: 10.1093/aje/kwm237. [DOI] [PubMed] [Google Scholar]
- Jacobsen B, Oda K, Knutsen S, Fraser G. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: The Adventist Health Study, 1976-88. International journal of epidemiology. 2009;38(1):245–252. doi: 10.1093/ije/dyn251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joinson C, Heron J, Araya R, Paus T, Croudace T, Rubin C, Lewis G. Association between pubertal development and depressive symptoms in girls from a UK cohort. Psychological medicine. 2012;42(12):2579–2589. doi: 10.1017/S003329171200061X. [DOI] [PubMed] [Google Scholar]
- Jordan SJ, Webb PM, Green AC. Height, age at menarche, and risk of epithelial ovarian cancer. Cancer Epidemiology Biomarkers & Prevention. 2005;14(8):2045–2048. doi: 10.1158/1055-9965.EPI-05-0085. [DOI] [PubMed] [Google Scholar]
- Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk A synthetic review. Cancer Epidemiology Biomarkers & Prevention. 2002;11(12):1531–1543. [PubMed] [Google Scholar]
- Kaltiala-Heino R, Marttunen M, Rantanen P, Rimpela M. Early puberty is associated with mental health problems in middle adolescence. Social Science & Medicine. 2003;57(6):1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108(2):347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast feeding and other reproductive factors influence future risk of rheumatoid arthritis?: Results from the Nurses' Health Study. Arthritis & Rheumatism. 2004;50(11):3458–3467. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annual review of public health. 1996;17(1):47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. Journal of affective disorders. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Key T, Appleby P, Reeves G, Roddam A, Helzlsouer K, Alberg A, Overvad K. Circulating sex hormones and breast cancer risk factors in postmenopausal women: Reanalysis of 13 studies. British journal of cancer. 2011;105(5):709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic medicine. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kivimäki M, Lawlor D, Smith G, Elovainio M, Jokela M, Keltikangas-Jarvinen L, Viikari J. Association of age at menarche with cardiovascular risk factors, vascular structure, and function in adulthood: the Cardiovascular Risk in Young Finns study. The American journal of clinical nutrition. 2008;87(6):1876–1882. doi: 10.1093/ajcn/87.6.1876. [DOI] [PubMed] [Google Scholar]
- Korenman S. Oestrogen window hypothesis of the aetiology of breast cancer. The Lancet. 1980;315(8170):700–701. [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. Journal of Epidemiology and Community Health. 2003;57:778–783. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Hardy R, editors. A life course approach to women's health. Oxford: Oxford University Press; 2002. [Google Scholar]
- Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. Journal of Clinical Endocrinology & Metabolism. 2009;94(12):4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- Lamason R, Zhao P, Rawat R, Davis A, Hall J, Chae J, Kanneboyina N. Sexual dimorphism in immune response genes as a function of puberty. BMC Immunology. 2006;7(2) doi: 10.1186/1471-2172-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D, Chaturvedi N. Treatment and prevention of obesity—are there critical periods for intervention? International journal of epidemiology. 2006;35(1):3–9. doi: 10.1093/ije/dyi309. [DOI] [PubMed] [Google Scholar]
- Lawlor D, Ebrahim S, Smith G. A Life Course Approach to Coronary Heart Disease and Stroke. In: Kuh D, Hardy R, editors. A Life Course Approach to Women's Health. New York: Oxford University Press; 2002. [Google Scholar]
- Legro RS, Lin HM, Demers LM, Lloyd T. Urinary free cortisol increases in adolescent Caucasian females during perimenarche. Journal of Clinical Endocrinology & Metabolism. 2003;88(1):215–219. doi: 10.1210/jc.2002-020256. [DOI] [PubMed] [Google Scholar]
- Leidy L. Biological aspects of menopause: Across the lifespan. Annual Review of Anthropology. 1994;23:231–253. doi: 10.1146/annurev.an.23.100194.001311. [DOI] [PubMed] [Google Scholar]
- Lennon M. The psychological consequences of menopause: The importance of timing of a life stage event. Journal of Health and Social Behavior. 1982;23(4):353–366. [PubMed] [Google Scholar]
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: Advancing the methodology. Implement Sci. 2010;5(1):1–9. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Rosenberg L, Wise L, Boggs D, LaValley M, Palmer J. Age at natural menopause in relation to all-cause and cause-specific mortality in a follow-up study of U.S. Black women. Maturitas. 2013;75:246–252. doi: 10.1016/j.maturitas.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal hypothalamic-pituitary-adrenal axis in pregnancy and the postpartume period. Annals of the New York Academy of Sciences. 2000;900:95–106. doi: 10.1111/j.1749-6632.2000.tb06220.x. [DOI] [PubMed] [Google Scholar]
- Matthews K, Crawford S, Chae C, Everson-Rose S, Sowers M, Sternfeld B, Sutton-Tyrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition. Journal of the American College of Cardiology. 2009;54(25):2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Meilahn E, Kuller L, Kelsey S, Caggiula A, Wing R. Menopause and risk factors for coronary heart disease. New England Journal of Medicine. 1989;321(10):641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Spencer RL. The role of adrenocorticoids as modulators of immune function in health and disease: Neural, endocrine and immune interactions. Brain Research Reviews. 1997;23(1–2):79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Mendle J, Turkheimer E, Emery RE. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Developmental Review. 2007;27(2):151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra GD, Cooper R, Tom SE, Kuh D. Early life circumstances and their impact on menarche and menopause. Womens Health. 2009;5(2):175–190. doi: 10.2217/17455057.5.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondul A, Rodriguez C, Jacobs E, Calle E. Age at natural menopause and cause-specific mortality. American Journal of Epidemiology. 2005;162(11):1089–1097. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- Moran A, Jacobs DR, Steinberger J, Hong C-P, Prineas R, Luepker R, Sinaiko AR. Insulin resistance during puberty: Results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- Natsuaki MN, Biehl MC, Ge X. Trajectories of depressed mood from early adolescence to young adulthood: The effects of pubertal timing and adolescent dating. Journal of Research on Adolescence. 2009;19(1):47–74. [Google Scholar]
- Nelson H. Menopause. The Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29(2):125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in depression. Current directions in psychological science. 2001;10(5):173–176. [Google Scholar]
- Ossewaarde M, Bots M, Verbeek A, Peeters P. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- Parent A-S, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon J-P. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocrine Reviews. 2003;24(5):668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. The Lancet. 2007;369(9567):1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, Frisch M. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis research & therapy. 2006;8(4):R133. doi: 10.1186/ar2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikwer M, Bergstrom U, Nilsson J-A, Jacobsson L, Turesson C. Early menopause is an independent predictor of rheumatoid arthritis. Annals of the Rheumatic Diseases. 2012;71:378–381. doi: 10.1136/ard.2011.200059. [DOI] [PubMed] [Google Scholar]
- Prentice P, Viner R. Pubertal timing and adult obesity and cardiometabolic risk in women and men: A systematic review and meta-analysis. International Journal of Obesity. 2012:1–8. doi: 10.1038/ijo.2012.177. [DOI] [PubMed] [Google Scholar]
- Prior J. Perimenopause: The Complex Endocrinology of the Menopausal Transition. Endocrine Reviews. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- Prior J, Hitchcock C. The endocrinology of perimenopause: Need for paradigm shift. Frontiers in Bioscience. 2011;3:474–486. doi: 10.2741/s166. [DOI] [PubMed] [Google Scholar]
- Prior JC. Perimenopause lost—reframing the end of menstruation. Journal of reproductive and infant psychology. 2006;24(4):323–335. [Google Scholar]
- Purdie DM, Green AC. Epidemiology of endometrial cancer. Best Practice & Research Clinical Obstetrics & Gynaecology. 2001;15(3):341–354. doi: 10.1053/beog.2000.0180. [DOI] [PubMed] [Google Scholar]
- Reeves G, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ. 2007;335(7630):1134–1145. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: The Fels Longitudinal Study. Journal of Clinical Endocrinology & Metabolism. 2005;90(5):2718–2724. doi: 10.1210/jc.2004-1991. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards J. A life course approach to women's reproductive health. In: Kuh D, Hardy R, editors. A Life Course Approach to Women's Health. Oxford, UK: Oxford University Press; 2002. pp. 23–37. [Google Scholar]
- Roeters van Lennep J, Westerveld T, Erkelens D, van der Wall E. Risk factors for coronary heart disease: Implications of gender. Cardiovascular Research. 2002;53:538–549. doi: 10.1016/s0008-6363(01)00388-1. [DOI] [PubMed] [Google Scholar]
- Ryan J, Carrière I, Scali J, Ritchie K, Ancelin M-L. Lifetime hormonal factors may predict late-life depression in women. International Psychogeriatrics. 2008;20(06):1203–1218. doi: 10.1017/S1041610208007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja G, Iachan R, Scheidt PC, Overpeck MD, Sun W, Giedd JN. Prevalence of and risk factors for depressive symptoms among young adolescents. Archives of pediatrics & adolescent medicine. 2004;158(8):760–765. doi: 10.1001/archpedi.158.8.760. [DOI] [PubMed] [Google Scholar]
- Sammaritano L. Menopause in patients with autoimmune diseases. Autoimmunity Review. 2012;11:A430–A436. doi: 10.1016/j.autrev.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Santoro N, Brown J, Adel T, Skurnick J. Characterization of reproductive hormonal dynamics in the perimenopause. The Journal of Clinical Endocrinology & Metabolism. 1996;81(4):1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- Schianca G, Castello L, Rapettie R, LImoncini A, Bartoli E. Insulin sensitivity: Gender-related differences in subjects with normal glucose tolerance. Nutrition, Metabolism, & Cardiovascular Disease. 2006;16:339–344. doi: 10.1016/j.numecd.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Schiefelbein VL, Susman EJ. Cortisol levels and longitudinal cortisol change as predictors of anxiety in adolescents. The Journal of Early Adolescence. 2006;26(4):397–413. [Google Scholar]
- Schmidt PJ. Mood, depression, and reproductive hormones in the menopausal transition. The American journal of medicine. 2005;118(12):54–58. doi: 10.1016/j.amjmed.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. American Journal of Psychiatry. 2004;161(12):2238–2244. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- Seeman T, Singer B, Wilkinson C, McEwen B. Gender differences in age-related changes in HPA-axis reactivity. Psychoneuroendocrinology. 2001;26:225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17(1):167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shuster L, Rhodes D, Gostout B, Grossardt B, Rocca W. Premature menopause or early menopause: Long-term health consequences. Maturitas. 2010;65(2):161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard J, Costenbader K. What can epidemiology tell us about systemic lupus erythematosus? International journal of clinical practice. 2007;61(7):1170–1180. doi: 10.1111/j.1742-1241.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster D. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in neuroendocrinology. 2005;26(3):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: A continuum of vulnerability? Journal of Psychiatry & Neuroscience. 2008;33(4):331–343. [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. Journal of affective disorders. 2003;74(1):67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Aradhana B, Wang H, Sharp T, Quesenberry CJ. Menopause, physical activity, and body composition/fat distribution in midlife women. Medicine & Social Science in Sports & Exercise. 2005;37(7):1195–1202. doi: 10.1249/01.mss.0000170083.41186.b1. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21(1):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in the effects of pubertal development on responses to a corticotropin releasing hormone challenge: The Pittsburgh psychobiologic studies. Annals of the New York Academy of Sciences. 2004;1021(1):348–351. doi: 10.1196/annals.1308.043. [DOI] [PubMed] [Google Scholar]
- Svejme O, Ahlborg H, Nilsson J-A, Karlsson M. Early menopause and risk of osteoporosis, fracture and mortality: A 34-year prospective observational study in 390 women. BJOG: An International Journal of Obstetrics and Gynaecology. 2012;119:810–816. doi: 10.1111/j.1471-0528.2012.03324.x. [DOI] [PubMed] [Google Scholar]
- Terry P, Rohan T. Cigarette smoking and the risk of breast cancer in women: A review of the literature. Cancer Epidemiology Biomarkers & Prevention. 2002;11(10 Pt 1):953–971. [PubMed] [Google Scholar]
- Thurston R, Sutton-Tyrell K, Everson-Rose S, Hess R, Matthews K. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women's Health Across the Nation. Circulation. 2008;118(12):1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M, Tchernof A, Sites C, Poehlman E. Menopause-related changes in body fat distribution. Annals of the New York Academy of Sciences. 2000;904:502–506. doi: 10.1111/j.1749-6632.2000.tb06506.x. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression: Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. Journal of Clinical Investigation. 1993;92(4):1896. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schouw YT, van der Graaf Y, Steyerberg E, Eijkemans M, Banga J. Age at Menopause as a Risk Factor for Cardiovascular Mortality. The Lancet. 1996;347:714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- Verthelyi D. Sex hormones as immunomodulators in health and disease. International Immunopharmacology. 2001;1:983–993. doi: 10.1016/s1567-5769(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. Journal of neuroendocrinology. 2002;14(6):506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. International Journal of Obesity. 2006;30(3):400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- Weichold K, Silbereisen RK, Schmitt-Rodermund E. Short-term and long-term consequences of early versus late physical maturation in adolescents. In: Hayward C, editor. Gender differences at puberty. New York, NY: Cambridge Univerisity Press; 2003. pp. 241–276. [Google Scholar]
- Wilder R. Neuroendocrine-immune system interactions and autoimmunity. Annual Review of Immunology. 1995;13:307–338. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- Wildman R, Colvin A, Powell L, Matthews K, Everson-Rose S, Hollenberg S, Sutton-Tyrell K. Associations of endogenous sex hormones with the vasculature in menopausal women: The study of women's health across the nation. Menopause. 2008;15(3):414–421. doi: 10.1097/gme.0b013e318154b6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard G, Brooks M, Barinas-Mitchell E, Mackey R, Matthews K, Sutton-Tyrell K. Lipids, menopause, and early atherosclerosis in Study of Women's Health Across the Nation Heart women. Menopause. 2011;18(4):376–384. doi: 10.1097/gme.0b013e3181f6480e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES. Increased urinary cortisol levels during the menopausal transition. Menopause. 2006;13(2):212–221. doi: 10.1097/01.gme.0000198490.57242.2e. [DOI] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES, Smith-DiJulio K. Cortisol levels during the menopausal transition and early postmenopause: Observatiosn from the Seattle Midlife Women's Health Study. Menopause. 2009;16(4):708–718. doi: 10.1097/gme.0b013e318198d6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthman CM. Endocrine pathways in differential well-being across the life course. In: Kuh DH, Rebecca, editors. A Life Course Approach to Women's Health. Oxford: Oxford University Press; 2002. [Google Scholar]
- Zhou B, Yang L, Sun Q, Cong R, Gu H, Tang N, Wang B. Cigarette smoking and the risk of endometrial cancer: A meta-analysis. American Journal of Medicine. 2008;121(6):501–508. doi: 10.1016/j.amjmed.2008.01.044. [DOI] [PubMed] [Google Scholar]