Abstract
The Dietary Supplement Ingredient Database (DSID) is a federally funded, publicly accessible dietary supplement database that currently contains analytically-derived information on micronutrients in selected adult and children’s multivitamin and mineral (MVM) supplements. Other constituents in dietary supplement products such as botanicals are also of interest and thus are being considered for inclusion in the DSID. Thirty-eight constituents, mainly botanicals were identified and prioritized by a federal interagency committee. Green tea was selected from this list as the botanical for expansion of the DSID. This paper describes the process for prioritizing dietary ingredients in the DSID. It also discusses the criteria for inclusion of these ingredients, and the approach for selecting and testing products for the green tea pilot study.
Keywords: Green tea, dietary supplements, databases, analytical methods, reference materials, botanicals
Introduction
Dietary supplement databases are needed to calculate total dietary intakes of nutrients and other constituents in foods and supplements. Analytically verified databases are helpful in health research and public policy decision-making for verifying or modifying estimates of intakes from product labels. The purpose of the Dietary Supplement Ingredient Database (DSID) is to provide publicly accessible, analytically derived data on levels of dietary ingredients in dietary supplement products (Dwyer and others 2006, 2007, 2008). Members of an ad hoc cross-agency federal working group (Working Group) that include scientists from the US Departments of Health and Human Services, Agriculture and Defense act as the steering committee for the DSID. The initial focus in populating the DSID was on commonly consumed dietary supplements containing micronutrients of public health interest that are also found in foods. The Institute of Medicine (IOM)’s Food and Nutrition Board has established Dietary Reference Intakes for these micronutrients and the U.S. Food and Drug Administration (FDA) has established reference Daily Values for labeling purposes. Only products that are labeled as a “Dietary Supplement” and carry a “Supplement Facts” panel are currently eligible for inclusion in the DSID1. Thus, the DSID currently contains estimates of vitamins and minerals in adult and child multi-vitamin and mineral (MVM) supplements (USDA 2014). Sales of vitamin and mineral products were estimated at 41 percent of all dietary supplement sale in 2012 (NBJ 2013). Estimates of calcium, copper, folic acid, iodine, iron, magnesium, manganese, niacin, phosphorus, potassium, riboflavin, selenium, thiamin, vitamin A, vitamin B-6, vitamin B-12, vitamin C, vitamin D, vitamin E, and zinc are reported in DSID. Studies designed to measure these nutrients in prenatal MVM dietary supplements sold behind and over-the-counter are now in progress. However, other ingredients such as botanicals are also of health concern and thus are being considered for inclusion in the DSID.
There is no uniform approach for labeling dietary ingredients without Daily Values. Often, the constituent(s) listed on labels depends on the claimed product benefit and which constituent(s) is purported to provide that benefit. In contrast, the constituent(s) of interest to researchers may not be those listed on labels. For example, it would be valuable to report an estimate of total flavonoid content as well as specific flavonoids such as catechins for flavonoid-containing supplements, if researchers wish to estimate total and specific flavonoid intake from foods and supplements.
The Working Group next evaluated the feasibility of adding botanicals to the database. Botanicals, are the third most popularly consumed dietary supplement category after vitamin and mineral and omega-3 fatty acid products. Omega-3 fatty acids in representative fish, plant and fish/plant blend dietary supplements have been analyzed and the results will be reported in an upcoming release of the DSID. According to data from NHANES, 7.5 percent of the adult US population reported using a botanical in the last 30 days (Bailey and others 2013). Herbal and botanical dietary supplement products were estimated at 17 percent of all dietary supplement sales in 2012 (NBJ 2013).
The FDA has not established Daily Values for botanicals. FDA gives manufacturers considerable flexibility in how they can declare these dietary ingredients within the Supplement Facts panel, but this flexibility makes determining the amount of a dietary ingredient, based on label information, in a botanical product difficult. Manufacturers have three options: 1) list the amount of each dried botanical, or extract, e.g., Chinese green tea, 100 mg, 2) list the botanical as a component of a blend, without providing the amount of each component in the blend, e.g., proprietary blend (green tea extract (leaf), cocoa bean extract (seed), tyrosine, trimethylglycine HCl, taurine, coenzyme Q10 (CoQ10)), 750 mg, or 3) list it with “other ingredients”, if it is a source of a dietary ingredient, e.g., rose hips which are added as a source of vitamin C (FDA 2005).
The Dietary Supplement Label Database (DSLD), another publicly-available database, contains label information on the composition on dietary supplement products offered for sale in the U.S. Of the over 30,000 products currently in the database, around 1000 contain green tea as a dietary ingredient. There were 90 different synonyms or ways that green tea was listed on the label. Examples include whole leaf, various extracts, powders and decaffeinated green tea. In about 600 products the amount of green tea was listed on the label. In the remaining products the amount of green tea was not provided or it was listed as a component of a blend.
Populating the DSID is a three-step process. The first step is prioritizing products and ingredients. The second step is sampling and analyzing products. The third step is evaluating the analytical data and reporting the results. This paper focuses on the first step and describes the process the Working Group used for prioritizing ingredients for the first botanical study for the DSID. It also includes a discussion of available analytical methods, reference materials, and the program to qualify laboratories for analyzing the active constituents in green tea dietary supplement products, since these are key requirements in the decision making for the inclusion of products reported in the database.
Materials and Methods
The input from members of the Working Group and a review of studies funded by the National Institutes of Health (NIH) were used to arrive at the initial list of botanical and a few non-vitamin and mineral dietary ingredients of interest to the Working Group. The 38 ingredients were acai, aloe, beta-carotene, black cohosh, cascara, chondroitin, cocoa flavonols, CoQ10, cranberry, creatine, echinacea, elderberry, flaxseed, garlic, Ginkgo biloba, ginseng, glucosamine, goldenseal, green tea catechins, guarana, lutein, lycopene, milk thistle, methylsulfonylmethane (MSM), natural herbal sources of vitamin C, phytosterols, prebiotics, probiotics, psyllium, red clover, S-adenosylmethionine (SAMe), saw palmetto, soy products, stevia, synephrine/bitter orange, turmeric, valerian, and yohimbe. The criteria used to rank these 38 dietary ingredients were similar to those previously used for prioritizing vitamins and minerals: 1) exposure (intake), 2) sales, 3) availability of validated analytical methods, and 4) availability of analytical reference materials, and 5) public health significance (Dwyer and others 2006). The fifth criterion was changed to “scientific, economic, and safety factors” since adequate information to rank “public health significance”, the criterion used for nutrients, was not available for botanicals. The four parameters used in scoring the “scientific, economic, and safety factors” criterion were the existence of adequate clinical trials, the existence of adequate epidemiologic studies, a variety of safety concerns, and the prevalence of economic adulteration.
Each of the five criteria described above were then scored on a scale of 1 to 5 where 5 represented the highest (most exposure and available data) score, and the scores were tallied to determine the overall highest scoring ingredients. Table 1 shows the sources of data used to score these five criteria. The availability of analytical methods and reference materials made up 40 percent of the total score.
Table 1.
Sources of data used to score the five criteria to rank botanicals.
| Criteria | Data Source (5 criteria were weighted equally) |
|---|---|
| 1. Public exposure intake |
National Health and Nutrition Examination Survey (NHANES)* and consumer responses to commonly consumed natural products in the National Health Interview Survey (NHIS)§. |
| 2. Public exposure sales |
Sales and five-year market trend data of individual botanicals and other dietary ingredients as reported by the Nutrition Business Journal (NBJ) |
| 3. Availability of validated analytical methods |
The availability of a validated analytical method(s), such as an AOAC Official Method of Analysis (OMA) which received the highest rank. |
| 4. Availability of analytical reference materials |
The availability of certified reference materials (CRMs) such as a NIST Standard Reference Material® (SRM) which received the highest rank. |
| 5. Scientific, economic, and safety factors |
Scored based on four parameters:
|
NHANES and HNIS are two programs administered by the Centers for Disease Control and Prevention (CDC). NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the United States.
HNIS is a tool used to monitor the health of Americans. Data are collected through personal household interviews.
Results
Eleven dietary ingredients that received the highest scores from the original list of 38 were identified for further evaluation. Table 2 shows the scores for these 11 dietary ingredients (green tea catechins, garlic, CoQ10, saw palmetto, Ginkgo biloba. glucosamine, ginseng, milk thistle, echinacea, flaxseed, and turmeric (curcumin)). Green tea products were selected by the Working Group because green tea scored high as a dietary ingredient of interest, there was other federal and scientific research interest in green tea, green tea also occurred in food products, and the USDA has released an expanded database for the flavonoid content of selected foods (Bhagwat and others 2014; Dwyer and Peterson 2013; Harnly and others 2006). There are also indicators that some green tea extracts may pose safety concerns, such as acute liver injury (Navarro and others 2013; NLM 2014). The decision was also made to include in the pilot study products where green tea is the only or a primary ingredient. Green tea dietary supplements will be analyzed for the chemical constituents listed in Table 3.
Table 2.
Scores for the eleven highest ranking dietary ingredients selected from the original list of 38 identified by the Working Group.
| Component | Scores for the Five criteria1 | |||||
|---|---|---|---|---|---|---|
| 1. Public exposure/ intake (NHANES/ NHIS)2 |
2. Public exposure/ sales (NBJ) |
3. Availability of validated methods for analysis |
4.Availability of reference materials for analysis |
5. Scientific, economic, and safety factors3 |
Total | |
| Botanicals | ||||||
| 1. Green Tea Catechins (EGCG) |
3/4 | 5 | 4 | 5 | 3.5 | 21.5 |
| 2. Garlic | 3/5 | 4 | 5 | 5 | 2 | 21 |
| 3. Saw palmetto | /4 | 4 | 5 | 5 | 2.5 | 20.5 |
| 4. Ginkgo biloba | /5 | 4 | 4 | 5 | 2 | 20 |
| 5. Ginseng | 3/5 | 4 | 5 | - | 3.5 | 17.5 |
| 6. Flaxseed | /5 | - | 3 | 5 | 2 | 15 |
| 7. Milk Thistle | /4 | 4 | 3 | - | 1 | 12 |
| 8. Echinacea | 3/5 | 4 | - | - | 2.5 | 11.5 |
| 9. Turmeric (curcumin) |
- | 4 | - | - | 2 | 6 |
| Non-Botanicals | ||||||
| 10. CoQ10 | /5 | 5 | 5 | 5 | 1 | 21 |
| 11. Glucosamine | 5/5 | 5 | 5 | - | 3.5 | 18.5 |
Each criteria were scored on a scale of 5-1, where 5 represents the highest score
Higher of two scores is included in the total
Evaluated based on availability of adequate clinical trials, the existence of adequate epidemiologic studies, a variety of safety concerns, and the prevalence of economic adulteration.
Table 3.
Testing procedures to be utilized in the pilot study for the analysis of green tea botanical dietary supplements.
| Ingredient Analysis/Test | Method | Units reported |
Reason for Analysis |
|---|---|---|---|
| Individual catechins, includes: (+)-catechin (−)-epicatechin (−)-epicatechin gallate (−)-epigallocatechin (−)-epigallocatechin gallate (EGCG) (−)-gallocatechin (−)-gallocatechin gallate |
HPLC | mg/g | Catechins, especially EGCG, are believed to be one of the main primary bioactives components in green tea. |
| Gallic acid | HPLC | mg/g | Organic acid with antioxidant properties |
| Methylxanthines, include: Caffeine Theobromine Theophylline |
HPLC | mg/g | Methylxanthines are alkaloids found in many plants. |
| L-theanine | HPLC | mg/g | Dried tea extract contains 4 to 6 percent theanine, an amino acid found only in tea. Theanine is sometimes added to dietary supplements as a purified extract from green tea. |
| Total Polyphenols | Folin C | Gallic Acid Equivalents (GAE) |
This is a non-specific test, but polyphenol content is the most commonly reported value on green tea dietary supplement labels |
| Botanical Identity testing | DNA | NA | Products with green tea leaf listed on the label will be considered for botanical identity testing |
Discussion
Green tea ingredients
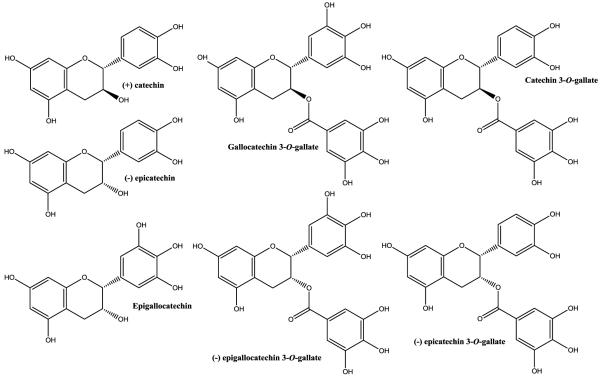
When deciding what to measure it is important to note the form and range of constituents in tea. Tea (Camellia sinensis) is available in several forms, including unfermented (green), semi-fermented (oolong), or fermented (black). Green tea is a popular ingredient in dietary supplements, and health benefits are often attributed to the catechins. Green tea is particularly rich in catechins, a subclass of flavonoids. Fermentation of tea reduces the catechins in it. Flavonoids are categorized into subclasses that encompass monomers (flavonols, flavones, flavanones, catechins (flavanols or flavan-3-ols), anthocyanidins, and isoflavones) and polymers (Dwyer and Peterson 2013). There are seven “signature” monomer catechins in green tea that provide its characteristic chemical profile, including epigallocatechin-3-gallate (EGCG) and epicatechin-3-gallate (Figure 1). However, there are over 200 other active constituents in green tea, including polyphenols as well as xanthines (caffeine, theophylline, and theobromine), the amino acid theanine, inorganic salts, and elements (Dwyer and Peterson 2013). The presence and amount of methylxanthines in green tea extracts is dependent upon the specific commercial extraction and processing procedures used (Roman and others 2013).
Figure 1.
Major catechins in green tea.
Availability of analytical methods
The technology now exists for the identification and quantification of catechins in tea. For the flavonoid monomers, the method of choice is high-performance or ultra-high performance liquid chromatography (HPLC or UHPLC) using a reversed phase column with either ultraviolet molecular absorbance (UV) or mass spectrometric (MS) detection (Machonis and others 2012, 2014). The separation of flavan-3-ol oligomers and polymers), can be achieved by normal-phase HPLC for proanthocyanidins up to ten subunits (decamers) in length. Lower molecular weight proanthocyanidins, dimers through hexamers in length, can be separated by reverse phase HPLC (Lin and Harnly 2014). All of the separation methods can be enhanced with the use of high-resolution MS (HRMS).
Commercial standards for the common catechins are readily available, and catechins can be determined using HPLC-UV (Roman and others 2013). A survey of standards showed them to be generally free of contamination (fairly easily isolated) and having low levels of water of hydration, hence drying of the standards is usually not necessary for accurate results (Lin and Harnly 2012).
Availability of reference materials
The National Institute of Standards and Technology (NIST) now offers a suite of green tea reference materials and a catechin calibration solution developed to evaluate the chemical composition of green tea dietary supplement products. These Standard Reference Materials® (SRMs), SRM 3254, SRM 3255, SRM 3256, and SRM 3257 are available on the NIST web site (http://www.nist.gov/srm/index.cfm) (Bedner and others 2010, 2011; Sander and others 2012). The Certificates of Analysis that accompany the SRMs provide assigned values for concentrations of seven catechins and gallic acid, three xanthine alkaloids (including caffeine), theanine, and toxic elements (arsenic, cadmium, lead, and mercury). Table 4 provides the concentrations of flavonoids, caffeine, and theobromine in SRM 3254 Camellia sinensis (Green Tea) Leaves, SRM 3255 Camellia sinensis (Green Tea) Extract, SRM 3256 Green Tea-Containing Solid Oral Dosage Form, and catechins in SRM 3257 Catechin Calibration Solutions. These materials were developed for use in the validation of analytical methods for the determination of catechins and xanthines as well as for quality control, either as a test material or for assigning values to in-house quality control materials. The suite of green tea-containing materials was initially designed only for quantitative use. However, due to community interests, the inclusion of DNA data for the authentication of SRM 3254 is being investigated. Since natural-matrix materials should not be used as calibrants, which may introduce bias during the extraction of the analytes of interest, NIST offers SRM 3257 Catechin Calibration Solutions, which has certified values for seven catechins.
Table 4.
| SRM 3254 Camellia sinensis (Green Tea) Leaves mg/g |
SRM 3255 Camellia sinensis (Green Tea) Extract mg/g |
SRM 3256 Green Tea- Containing Solid Oral Dosage Form mg/g |
SRM 3257 Catechin Calibration Materials μg/g |
||
|---|---|---|---|---|---|
| Catechins | |||||
| (+)-Catechin | 1.01 ± 0.41 | 9.17 ± 0.93 | 2.63 ± 0.18 |
23.54 ±
0.53 |
|
| (−)-Epicatechin | 9.0 ± 1.6 | 47.3 ± 6.7 | 12.0 ± 2.6 | 93.9 ± 2.1 | |
| (−)-Epicatechin gallate |
12.7 ± 1.2 | 100.3 ± 7.8 | 17.0 ± 2.6 | 203.9 ± 4.6 | |
| (−)-Epigallocatechin | 25.2 ± 4.5 | 81.8 ± 6.5 | 30.7 ± 5.7 | 232.9 ± 6.6 | |
| (−)-Epigallocatechin gallate |
52.0 ± 2.2 |
442.0 ±
19.0 |
71.1 ± 6.6 | 549 ± 12 | |
| (−)-Epigallocatechin methylgallate |
- | 6.87 ± 0.44 | - | - | |
| (−)- Gallocatechin | 2.4 ± 1.1 | 22.0 ± 1.7 |
7.55 ±
0.28 |
62.7 ± 1.8 | |
| (−)- Gallocatechin gallate |
0.99 ± 0.21 | 39.0 ± 2.0 | 4.6 ± 1.8 | 76.6 ± 1.7 | |
| Other compounds | |||||
| Gallic acid | 1.12 ± 0.61 | 3.231 ± 0.086 |
13.10 ±
0.49 |
-- | |
| Caffeine | 23.5 ± 1.8 | 36.9 ± 2.7 | 70.0 ± 2.6 | - | |
| Theobromine |
0.463 ±
0.052 |
0.867 ±
0.076 |
1.04 ± 0.15 | - | |
| Theophylline | - | 0.087 ± 0.002 |
0.060 ± 0.002 |
- | |
| L-theanine | 2.130 ± 0.054 |
0.340 ± 0.008 |
3.7 ± 1.2 | - |
Certified values are provided in bold and reference values are provided in normal typeface ± the uncertainty.
Each certified and reference value, expressed as a mass fraction, is an equally weighted mean of results provided by using LC/UV, LC/MS, and the individual means of collaborating laboratories’ data where available. In cases where NIST data were provided using UV and MS detectors arranged in series, the average was treated as a single method mean when it was combined with other data. The uncertainty provided with each value is an expanded uncertainty about the mean to cover the measurand with approximately 95 percent confidence; it incorporates Type B uncertainty components related to the analyses, as well as a component related to moisture correction, and expresses both the observed difference between the results from the methods and their respective uncertainties.
Availability of qualified analytical laboratories
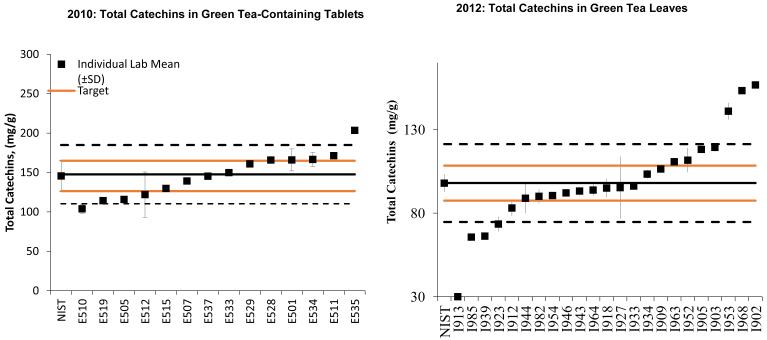
Under the NIST Dietary Supplement Laboratory Quality Assurance Program (DSQAP), participating laboratories measure concentrations of active and/or marker compounds and nutritional and toxic elements in samples distributed by NIST. Many laboratories are capable of making accurate measurements of catechins in green tea-containing supplements in DSQAP (Phillips and other 2013). The proficiency of laboratories to measure catechins in green tea has been tested two times in 2010 and 2012 (Figure 2). Participating laboratories used in-house analytical methods to determine the mass fractions of seven catechins (catechin, epicatechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, gallocatechin, and gallocatechin gallate) as well as the total amount of catechins in the green tea supplements. Figure 2 demonstrates the performance of the laboratories in 2010 (Green Tea-Containing Solid Oral Dosage Form sample) and 2012 (Green Tea Leaves sample) for measuring total catechins in a Camellia sinensis extract. In 2010, many of the laboratory results fell along a diagonal line (laboratories that reported low results on the extract typically reported low results on the pulverized tablets as well) indicating that many laboratories had a measurement bias due to calibration issues. In 2012, the results were more scattered, indicating fewer sources of systematic bias, and the consensus values (blue dotted line) surround the target values (red solid line) closely, indicating good method performance. While NIST does not disclose the identity of laboratories participating in the DSQAP to others, the laboratories are welcome to share their individual data as evidence of measurement capabilities and the results of the exercises demonstrate that many laboratories are capable of making acceptable determinations of individual catechins in green tea matrices. Reports from the DSQAP studies can be viewed and downloaded from the NIST Web site (NIST 2014).
Figure 2.
Results of the 2010 and 2012 DSQAP studies for measurement of total catechins in green tea products
Plans for green tea pilot study
The pilot study is designed to obtain estimates of the content and variability for catechins, caffeine, and other ingredients in representative green tea dietary supplements; evaluate the methods of analysis for these ingredients by using multiple laboratories; and identify methods for reporting and comparing the analyzed values with the information declared on the label.
A market survey of products and available information on product composition will be used to select products for the pilot study. Based on a limited survey of green tea dietary supplement products offered for sale in the greater Washington DC area in 2013, weight loss products containing green tea, typically listed green tea in proprietary blends and so neither the amount of green tea nor its active constituents were provided. Other green tea products sold listed the amount of green tea on the labels and declared the percent of polyphenols in green tea extracts. The amount or concentration of catechins, particularly EGCG, was provided on a few labels.
For the pilot study, candidate dietary supplement products will be identified from reported usage in the NHANES and from information in the Dietary Supplement Label Database (DSLD) (NHANES 2014). Products in which green tea is the only or primary ingredient, will be analyzed, as flavonoids are found in many botanical materials and are not unique to green tea. About half of the top 100 dietary supplements in the US ranked by sales contain some flavonoids, and high levels of flavonoids are also found in Ginkgo biloba, echinacea, red clover, berries, grape extracts, cocoa, citrus, and soy ingredients (NBJ 2013).
When selecting products for the study, multiple forms will be included (caplets, capsules, liquids, powders, softgels, and teabags) and products will be purchased from a variety of market channels. These market channels include the brick-and-motar or mass market and the natural health market stores, multi-level or network marketing, and direct channels, which includes products sold exclusively on the internet.
Conclusions
The challenges associated with adding botanical dietary supplements to analytically based databases are many. First it is necessary to determine the analytes to measure and the materials to analyze. Botanicals are complex mixtures, and their effects cannot be attributed to a single botanical or ingredient. A second problem is determining how to report the information to be provided in the database, since there is no standardized approach to reporting these dietary ingredients within the Supplement Facts panel.
The Working Group will evaluate the findings from the green tea pilot study to determine the feasibility of including green tea containing and other botanicals in the DSID. Comments from researchers and other interested parties are welcome.
Practical Application.
This paper describes the available reference materials, analytical methods, and the program to qualify laboratories for analyzing the active constituents in green tea dietary supplement products, and how these criteria have been used to expand a dietary supplement composition database for green tea products. This information may be useful for product developers, chemists, and researchers involved in the analysis and formulation of products, and those making public policy decisions.
Acknowledgments
This study was funded by the Office of Dietary Supplements at the National Institutes of Health. We thank Julia Peterson, PhD, Adjunct Assistant Professor at Tufts University’s Friedman School of Nutrition Science and Policy for her professional review of this paper.
Footnotes
The Dietary Supplement Health and Education Act of 1994 (DSHEA) defined a dietary supplement and permitted the addition of dietary ingredients if they meet the Act's requirements (1). A dietary ingredient is a vitamin; a mineral; an herb or other botanical; an amino acid; a dietary substance for use by man to supplement the diet by increasing total dietary intake; or a concentrate, metabolite, constituent, extract, or combination of any of the above dietary ingredients. In contrast, a dietary supplement contains dietary ingredients and is limited to products that are intended for ingestion in tablet, capsule, powder, softgel, gelcap, and liquid form, that are not represented as a conventional food or as the sole item of a meal or of the diet, and are labeled as dietary supplements.
Author Contributions.
All authors are members of an ad hoc federal dietary supplement database working group that guides the development of dietary supplement databases.
Johanna Dwyer leads the ad hoc federal dietary supplement database working group and oversees the development of the DSID.
Karen Andrews manages the DSID at the Nutrient Data Laboratory, USDA and is overseeing the green tea pilot study.
Joseph Betz is the Director of the Analytical Methods and Reference Materials Program at the NIH that funds the development and validation of analytical methods and reference materials for dietary supplements.
James Harnly is the Research Leader of the Food Composition and Methods Development Laboratory, USDA, which is involved in the development and validation of food and supplement methods, and wrote the analytical methods section.
Pamela Pehrsson is the Research Leader of the USDA’s Nutrient Data Laboratory, USDA, responsible for the development of authoritative nutrient databases.
Catherine Rimmer is one of three coordinators of the Dietary Supplement Laboratory Quality Assurance Program at NIST.
Sushma Savarala is a researcher at the Nutrient Data Laboratory, USDA and assists with the coordination and management of the green tea pilot study.
References
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;11173(5):355–61. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- Bedner M, Duewer DL. Dynamic calibration approach for determining catechins and gallic acid in green tea using LC-ESI/MS. Anal Chem. 2011;1583(16):6169–76. doi: 10.1021/ac200372d. [DOI] [PubMed] [Google Scholar]
- Bedner M, Sander LC, Sharpless KE. An LC-ESI/MS method for determining theanine in green tea dietary supplements. Anal Bioanal Chem. 2010;397(5):1773–77. doi: 10.1007/s00216-010-3713-9. [DOI] [PubMed] [Google Scholar]
- Bhagwat S, Haytowitz DB, Holden JM. USDA database for the flavonoid content of selected foods, Release 3.1. 2014 Available at: http://www.ars.usda.gov/Services/docs.htm?docid=6231. Accessed 2014 October 20.
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey Available at: http://www.cdc.gov/nchs/nhanes.htm. Accessed 2014 October 20.
- Centers for Disease Control and Prevention (CDC) National Health Interview Survey. Available at: http://www.cdc.gov/nchs/nhis.htm. Accessed 2014 October 20.
- Dwyer JT, Holden J, Andrews K, Roseland J, Zhao C, Schweitzer A, Perry CR, Harnly J, Wolf WR, Picciano MF, Fisher KD, Saldanha LG, Yetley EA, Betz JM, Coates PM, Milner JA, Whitted J, Burt V, Radimer K, Wilger J, Sharpless KE, Hardy CJ. Measuring vitamins and minerals in dietary supplements for nutrition studies in the USA. Anal Bioanal Chem. 2007;389(1):37–46. doi: 10.1007/s00216-007-1456-z. [DOI] [PubMed] [Google Scholar]
- Dwyer JT, Peterson J. Tea and flavonoids: where we are, where to go next. Am J Clin Nutr. 2013;98(6 Suppl):1611S–18S. doi: 10.3945/ajcn.113.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JT, Picciano MF, Betz JM, Fisher KD, Saldanha LG, Yetley EA, Coates PM, Radimer K, Bindewald B, Sharpless KE, Holden J, Andrews K, Zhao C, Harnly J, Wolf WR, Perry CR. Progress in development of an integrated dietary supplement ingredient database at the NIH Office of Dietary Supplements. Journal of Food Composition and Analysis. 2006;19:S108–S14. doi: 10.1016/j.jfca.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JT, Picciano MF, Betz JM, Fisher KD, Saldanha LG, Yetley EA, Coates PM, Milner JA, Whitted J, Burt V, Radimer K, Wilger J, Sharpless KE, Holden JM, Andrews K, Roseland J, Zhao C, Schweitzer A, Harnly J, Wolf WR, Perry CR. Progress in developing analytical and label-based dietary supplement databases at the NIH Office of Dietary Supplements. J Food Comp and Analysis. 2008;21:S83–S93. doi: 10.1016/j.jfca.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006;54(26):9966–77. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- Lin L, Sun J, Chen P, Monagas M, Harnly JM. UHPLC-PDA-ESI/HRMSn profiling method to identify and quantify oligomeric proanthocyanidins in plant products. J Agric Food Chem. 2014;62(39):9387–400. doi: 10.1021/jf501011y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-Z, Harnly JM. Quantitation of flavanols, proanthocyanidins, isoflavones, flavanones, dihydrochalcones, stilbenes, benzoic acid derivatives using ultraviolet absorbance after identification by liquid chromatography-mass spectrometry. J Agric Food Chem. 2012;60(23):5832–40. doi: 10.1021/jf3006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machonis PR, Jones MA, Schaneberg BT, Kwik-Uribe CL, Dowell D. Method for the determination of catechin and epicatechin enantiomers in cocoa-based ingredients and products by high-performance liquid chromatography: First Action 2013.04. J AOAC Int. 2014;97(2):506–9. doi: 10.5740/jaoacint.13-351. [DOI] [PubMed] [Google Scholar]
- Machonis PR, Jones MA, Schaneberg BT, Kwik-Uribe CL. Method for the determination of catechin and epicatechin enantiomers in cocoa-based ingredients and products by high-performance liquid chromatography: Single-laboratory validation. J AOAC Int. 2012;95(2):500–7. doi: 10.5740/jaoacint.11-324. [DOI] [PubMed] [Google Scholar]
- National Institute of Standards and Technology (NIST) Dietary Supplement Laboratory Quality Assurance Program Information. Available at: http://www.nist.gov/mml/csd/dsqaprogram.cfm. Accessed 2014 October 20.
- National Library of Medicine (NLM) Dietary Supplements Label Database (DSLD) Available: http://dsld.nlm.nih.gov. Accessed 2014 October 20.
- National Library of Medicine (NLM) LiverTox. Clinical and research information on drug-induced liver injury. Available: http://livertox.nih.gov/GreenTea.htm. Accessed 2014 October 20. [PubMed]
- Navarro VJ, Bonkovsky HL, Hwang SI, Vega M, Barnhart H, Serrano J. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci. 2013;58(9):2682–90. doi: 10.1007/s10620-013-2687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutrition Business Journal (NBJ) Supplement Business Report. Penton Media, Inc.; 2012. 2013. Available: http://newhope360.com/2012-supplement-business-report. Accessed 2014 October 20. [Google Scholar]
- Phillips MM, Rimmer CA, Wood LJ, Murphy KE, Sharpless KE, Vetter TW. Dietary Supplement Laboratory Quality Assurance Program: Exercise I Final Report. NIST Interagency/Internal Report (NISTIR) 20137955 Available at: http://www.nist.gov/customcf/get_pdf.cfm?pub_id=914292. Accessed 2014 October 20.
- Roman M, Hildreth J, Bannister S. Determination of catechins and caffeine in Cameilla sinensis raw materials, extracts, and dietary supplements by HPLC-UV: Single-laboratory validation. J AOAC Int. 2013;96(5):933–41. doi: 10.5740/jaoacint.10-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander LC, Bedner M, Tims MC, Yen JH, Duewer DL, Porter B, Christopher SJ, Day RD, Long SE, Molloy JL, Murphy KE, Lang BE, Lieberman R, Wood LJ, Payne MJ, Roman MC, Betz JM, NguyenPho A, Sharpless KE, Wise SA. Development and certification of green tea-containing standard reference materials. Anal Bioanal Chem. 2012;402(1):473–87. doi: 10.1007/s00216-011-5472-7. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, Agriculture Research Service (USDA) and National Institutes of Health, Office of Dietary Supplements (NIH) Dietary Supplement Ingredient Database (DSID 2.0) Available at: http://dietarysupplementdatabase.usda.nih.gov/. Accessed 2014 October 20.
- U.S. Food and Drug Administration (FDA) Dietary Supplement Labeling Guide. 2005 Apr; Available at: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/DietarySupplements/ucm2006823.htm. Accessed 2014 October 20.