Abstract
Smaller hippocampal volume has been reported in individuals with post‐traumatic stress disorder (PTSD) and dissociative identity disorder (DID), but the regional specificity of hippocampal volume reductions and the association with severity of dissociative symptoms and/or childhood traumatization are still unclear. Brain structural magnetic resonance imaging scans were analyzed for 33 outpatients (17 with DID and 16 with PTSD only) and 28 healthy controls (HC), all matched for age, sex, and education. DID patients met criteria for PTSD (PTSD–DID). Hippocampal global and subfield volumes and shape measurements were extracted. We found that global hippocampal volume was significantly smaller in all 33 patients (left: 6.75%; right: 8.33%) compared with HC. PTSD–DID (left: 10.19%; right: 11.37%) and PTSD‐only with a history of childhood traumatization (left: 7.11%; right: 7.31%) had significantly smaller global hippocampal volume relative to HC. PTSD–DID had abnormal shape and significantly smaller volume in the CA2‐3, CA4‐DG and (pre)subiculum compared with HC. In the patient groups, smaller global and subfield hippocampal volumes significantly correlated with higher severity of childhood traumatization and dissociative symptoms. These findings support a childhood trauma‐related etiology for abnormal hippocampal morphology in both PTSD and DID and can further the understanding of neurobiological mechanisms involved in these disorders. Hum Brain Mapp 36:1692–1704, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: dissociative disorders, stress, neuroimaging, hippocampal volume, childhood abuse, gray matter
INTRODUCTION
Recent epidemiological and neurobiological research in trauma‐related disorders has focused on the relationship between childhood and chronic trauma and dissociation. This has led to the recent nosological inclusion of a dissociative subtype of Post‐traumatic Stress Disorder (PTSD) [Lanius et al., 2010] in the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) [American Psychiatric Association, 2013]. The dissociative disorders category has also recently been placed next to the trauma‐ and stress‐related disorders category “to indicate the close relationship between them” [Spiegel et al., 2013]. However, neurobiological evidence for a close relationship between PTSD and dissociative disorders is sparse. Furthermore, to date, the link between neuroanatomical abnormalities and childhood traumatization and/or dissociative symptoms in PTSD and dissociative disorders remains unclear.
A smaller hippocampal volume is the most consistently reported neuroanatomical finding in individuals with a history of childhood adversity, with or without psychiatric disorders [Andersen et al., 2008; Bremner et al., 2003; Dannlowski et al., 2012; Samplin et al., 2013; Stein et al., 1997; Thomaes et al., 2010]. Relevant to our study, a meta‐analysis of structural brain imaging studies in childhood‐related PTSD has revealed significantly smaller hippocampal volume in adults, but not children, with PTSD compared to healthy controls (HC) [Woon and Hedges, 2008]. However, none of these PTSD studies has investigated hippocampal shape or the regional specificity of hippocampal volume reductions in this disorder. The relation between hippocampal morphology and childhood adversity also remains unknown. Furthermore, evidence relating hippocampal structural abnormality to the severity of dissociative symptoms in PTSD is mixed. While some PTSD studies have reported a negative correlation between global hippocampal volume and severity of dissociative symptoms [Bremner et al., 2003; Stein et al., 1997], others reported no significant association [Bremner et al., 1995; Nardo et al., 2013]. Therefore, the relationship between hippocampal morphology, childhood maltreatment, and dissociative symptoms in a sufficiently large sample of PTSD patients is still open to test.
So far, only few structural imaging studies have investigated hippocampal volume in dissociative disorders [Ehling et al., 2008; Irle et al., 2009; Stein et al., 1997; Tsai et al., 1999; Vermetten et al., 2006; Weniger et al., 2008]. Studies in patients with dissociative disorders and comorbid PTSD [Ehling et al., 2008; Irle et al., 2009; Stein et al., 1997; Tsai et al., 1999; Vermetten et al., 2006] have found smaller hippocampal volume in these individuals relative to HC, and one study [Ehling et al., 2008] reported a negative correlation between hippocampal volume and severity of life‐time traumatizing experience and dissociative symptoms in individuals with dissociative identity disorder (DID). DID is the most severe dissociative disorder, and has been conceptualized as a severe childhood‐onset PTSD [Van der Hart et al., 2006]. Interestingly, hippocampal volume has been reported as preserved in patients with dissociative disorders without comorbid PTSD [Weniger et al., 2008]. Unfortunately, these studies suffered from several limitations: small sample sizes; inclusion of patients with mixed diagnoses of dissociative disorders without differentiating these groups within the analyses (DID, dissociative amnesia, dissociative disorder‐not otherwise specified); and age differences between patients and controls [Ehling et al., 2008; Irle et al., 2009; Stein et al., 1997; Tsai et al., 1999; Vermetten et al., 2006; Weniger et al., 2008].
Most studies on the effects of early stress on the hippocampus, including those on PTSD or DID, have only examined differences in global hippocampal volume. However, the hippocampus consists of several histologically distinct subfields, each with distinct structural and functional connections with the cortex, specialized functional properties, and different developmental trajectories [Wang et al., 2010]. In individuals from the general community, childhood traumatization (assessed using the Childhood Trauma Questionnaire [CTQ; Bernstein et al., 1994]) is specifically associated with relatively small volume within the CA2‐3 (CA: cornu ammonis), CA4‐DG (DG: dentate gyrus), subiculum, and to a lesser extent with smaller volume of the CA1 hippocampal subfields, revealing a relationship between childhood adversity maltreatment and small hippocampal subfield volumes [Teicher et al., 2012]. So far, only two studies evaluated hippocampal subfield volumes in PTSD patients [Bonne et al., 2008; Wang et al., 2010]. These studies found smaller volume of the CA3/DG and posterior regions in PTSD patients compared with controls. However, these studies did not investigate the association with childhood traumatization or dissociative symptoms. Indeed, regional hippocampal volume, and shape abnormalities have never been investigated in dissociative disorders.
This study is the first to investigate hippocampal morphological correlates of childhood traumatization and dissociative symptoms in both DID and PTSD patients. To this end, we obtained structural magnetic resonance imaging (MRI) scans from HC and patients with DID and/or PTSD. All participants were carefully matched for age, sex, and education. We investigated global hippocampal volume, subfield volumes, as well as hippocampal regional shape deformations. In the patient samples, we examined the association between hippocampal volume and severity of self‐reported early childhood traumatization, that is physical maltreatment, sexual and emotional abuse, and emotional neglect, and severity of dissociative symptoms. We tested three a priori hypotheses: (1) both DID and PTSD patients, as compared with HC, would have smaller global hippocampal volume, regional volumetric abnormalities, and shape deformations in various hippocampal subfields; (2) global hippocampal volume, as well as, regional volume in the CA4‐DG, CA2‐3, and subiculum subfields would be negatively associated with the severity of childhood traumatization; and (3) global hippocampal and regional volumes would be negatively associated with dissociative symptoms.
MATERIALS AND METHODS
Subjects
Sixty‐five women (only female patients with DID volunteered to participate in this study) underwent MRI: 17 with a diagnosis of DID, 16 with a diagnosis of PTSD, and 32 HC. Four HC were excluded from the demographic and morphological analyses due to the presence of artifacts in the MRI scans. Participants were matched for sex, age, years of education (Table 1), and Western European ancestry. PTSD patients with a history of interpersonal traumatizing events and DID patients were recruited via mental healthcare institutions and internet advertisements.
Table 1.
Demographic and clinical characteristics of the participants
| All‐PTSD to HC comparison | Different group comparisons | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | t‐Test: P value | Mean (SD) | t‐Test: P value | |||||
| All‐PTSD (n = 33) | HC (n = 28) | All‐PTSD vs. HC | PTSD–DID (n = 17) | PTSD‐only (n = 16) | PTSD–DID vs. HC | PTSD–DID vs. PTSD‐only | PTSD‐only vs. HC | |
| Demographics | ||||||||
| Age, years | 42.33 (10.91) | 41.75 (12.29) | 0.85 | 43.82 (9.85) | 40.75 (12.05) | 0.56 | 0.45 | 0.78 |
| Education, years | 14.91 (0.91) | 15.04 (1.20) | 0.64 | 14.88 (0.99) | 14.94 (0.85) | 0.64 | 0.88 | 0.77 |
| Handedness, n (%right) | 29 (90.63%) | 27 (96.43%) | 0.37 | 14 (87.50%) | 15 (93.75%) | 0.54 | 0.99 | 0.99 |
| Medication history | ||||||||
| Antipsychotics: n (typical,atypical) | Past:2(1,1) current:8(2,6)a | Past: 0 current: 0 | — | Past:2(1,1) current:8(2,6)a | Past: 0 current: 0 | — | — | — |
| Antiepileptics: n | Past:1 current:3 | Past: 0 current: 0 | — | Past:1 current:3 | Past: 0 current: 0 | — | — | — |
| Antidepressant: n | Past: 2 current:12 | Past: 0 current: 0 | — | Past: 2 current:10 | Past: 0 current: 2 | — | — | — |
| Clinical measures | ||||||||
| Dissociative symptoms | ||||||||
| Psychoform (DES) | 38.79 (22.09) | 5.02 (3.10) | <0.001* | 54.41 (16.18) | 22.18 (13.83) | <0.001* | <0.001* | <0.001* |
| Somatoform (SDQ‐20) | 45.24 (19.65) | 22.04 (2.21) | <0.001* | 57.06 (17.26) | 32.69 (13.43) | <0.001* | <0.001* | <0.001* |
| Traumatic experience checklist (TEC) | ||||||||
| Total lifetime trauma | 14.40 (5.16) | 1.96 (1.93) | <0.001* | 17.53 (4.08) | 11.06 (4.01) | <0.001* | <0.001* | <0.001* |
| Childhood Trauma Questionnaire (CTQ)b | ||||||||
| emotional neglect | 19.90 (5.69) | 10.36(4.14) | <0.001* | 23.40 (2.26) | 16.63 (6.02) | <0.001* | <0.001* | <0.001* |
| physical neglect | 13.87 (5.22) | 7.42 (2.28) | <0.001* | 17.47 (3.87) | 10.50 (3.93) | <0.001* | <0.001* | 0.020* |
| emotional abuse | 18.48 (6.56) | 7.50 (2.56) | <0.001* | 22.80 (3.30) | 14.44 (6.31) | <0.001* | <0.001* | <0.001* |
| physical abuse | 12.35 (5.94) | 5.43 (1.34) | <0.001* | 15.60 (5.37) | 9.31 (4.84) | <0.001* | <0.001* | 0.018* |
| sexual abuse | 13.84 (7.69) | 5.29 (0.73) | <0.001* | 17.87 (7.32) | 10.06 (6.06) | <0.001* | <0.001* | 0.024* |
| Total trauma | 78.45 (26.92) | 36.00 (8.00) | <0.001* | 97.13 (16.63) | 60.94 (22.70) | <0.001* | <0.001* | <0.001* |
PTSD‐only = patients with only post‐traumatic stress disorder; PTSD–DID = patients with PTSD and dissociative identity disorder; All‐PTSD = includes both PTSD‐only and PTSD‐DID patient groups; HC = healthy controls.
*P‐value ≤ 0.05
One PTSD–DID patient used typical antipsychotics in the past but stopped and was using atypical antipsychotics at the time of the MRI scan. Another PTSD–DID patient was using atypical antipsychotics in the past but was not using any antipsychotics at the time of the MRI scan.
CTQ data was available for 15 PTSD–DID, 16 PTSD‐only, and 14 HC
The diagnosis of DID was confirmed by one of two DID experts (E.N. or N.D.) using the Structural Clinical Interview for DSM‐IV Dissociative Disorders (SCID‐D) [Boon and Draijer, 1993a; Steinberg, 1993], during which PTSD comorbidity was assessed as well. The evaluation revealed that all DID patients met criteria for either current comorbid PTSD (82.35%) or PTSD in remission (17.65%). Therefore, we refer to this sample as “PTSD–DID.” The personal therapists of the patients with PTSD–DID reported the following comorbid disorders based on DSM‐IV classification [American Psychiatric Association, 1994]: somatoform disorder (N = 2), recurrent major depression (N = 4), dysthymic disorder (N = 1), trauma‐related specific phobias (N = 2), personality disorder‐not otherwise specified (N = 2), mixed personality disorders (N = 2), borderline personality disorder symptoms (N = 3), dependent personality disorder symptoms (N = 1), histrionic personality disorder symptoms (N = 1), eating disorder (N = 2), sleeping disorder (N = 2), and catalepsy (N = 1).
Severity of psychoform and somatoform dissociative symptoms were evaluated using the Dissociative Experiences Scale (DES) [Bernstein and Putnam, 1986] and Somatoform Dissociation Questionnaire (SDQ‐20) [Nijenhuis et al., 1996], respectively. The five‐item SDQ‐5 was derived from the SDQ‐20. These five items as a group discriminate best between patients with and without a dissociative disorder [Nijenhuis et al., 1997, 1998]. The cutoff scores that we used for the DES and SDQ‐5 were 25 and 7, respectively [Boon and Draijer, 1993b; Nijenhuis et al., 1997]. Severity of lifetime traumatizing events were assessed with the Traumatic Experiences Checklist (TEC) [Nijenhuis et al., 2002]. PTSD–DID patients completed these questionnaires in their predominant identity state and all of them reported experiencing severe traumatizing events starting from their childhood and extended into their adult life including severe emotional neglect and abuse, physical maltreatment or extreme physical punishments, and sexual abuse. Childhood maltreatment was retrospectively assessed using the CTQ [Bernstein et al., 1994]. The CTQ is a retrospective 28‐item self‐report inventory that measures the severity of five different types of childhood traumatization (i.e., emotional abuse and neglect, physical abuse and neglect, and sexual abuse) with scores ranging from 5 to 25 for each trauma type. Total childhood traumatization is calculated as the sum of all the five subscores. In PTSD–DID, CTQ scores were obtained from a trauma‐conscious state.
In the 16 PTSD patients, symptom severity was assessed using the Clinician Administered PTSD Scale (CAPS) interview [Blake et al., 1995] conducted by researchers E.V. and M.G. Eleven of the PTSD patients reported multiple types of interpersonal traumatizing events during childhood (N = 6) or starting from childhood and continuing into adult life (N = 5). The remaining five PTSD patients reported traumatizing events only during adult life. Two PTSD patients scored high on the DES/SDQ‐5 (both reported DES>25 and one of them also reported SDQ‐5>7), and therefore, underwent a SCID‐D interview and DID was excluded. Hence, we refer to this patient group as “PTSD‐only.” As both PTSD–DID and PTSD‐only groups shared the diagnosis of PTSD, we further merged them into one larger group, referred to as “All‐PTSD,” to investigate the common morphological features of PTSD.
HC were recruited through advertisements in local newspapers. Additional exclusion criteria for HC were: a high score of (psychoform/somatoform) dissociative symptoms (evaluated with the DES and SDQ‐20, respectively), psychiatric disorder in the past or at present, or a high score on the TEC. All HC were free of present and past psychiatric medication. Exclusion criteria for all participants were: age outside the range of 18–65, pregnancy, systemic or neurological illness, claustrophobia, presence of metal implants in the body, and alcohol/drug abuse. Details of psychotropic medication usage are provided in Table 1.
After complete description of the study to the subjects, written informed consent was obtained according to procedures approved by the Medical Ethical Committee (METc) of the University Medical Center Groningen (UMCG) and of the Amsterdam Medical Center (AMC).
Image Acquisition
T1‐weighted anatomical MR scans (MPRAGE, repetition time (TR) = 9.95 ms, echo time (TE) = 5.6 ms, flip‐angle = 8°, 1 × 1 × 1 mm voxels, number of slices = 160, total scan‐time = 10 min 14 s) were acquired on two (UMCG and AMC) 3T MR scanners (Philips Medical Systems, Best, NL) in The Netherlands after a reproducibility study determined one optimized structural MRI protocol for the two centers [Chalavi et al., 2012]. All‐PTSD patients and their matched HC were scanned interleaved within a short time interval and the samples were balanced over the two centers: 20 All‐PTSD patients (10 PTSD–DID, 10 PTSD‐only) and 19 HC were scanned at UMCG (See Supporting Information 1 for more details).
Image Analysis
Manual measures of global volume and shape analysis of the hippocampus
After preprocessing the MR images, the hippocampi were manually traced using MultiTracer by a single rater (S.C.), who was blind to all demographical variables and was trained by an expert (J.H.C.) according to an established protocol [Thompson et al., 2004] (details in Supporting Information 1). Hippocampal global volumes obtained from these tracings were statistically analyzed. To assess the shape deformations of different hippocampal subfields, an anatomical surface mesh modeling method was applied according to standard procedures [Thompson et al., 2004]. In brief, localized gray matter (GM) contractions and expansions of the hippocampal surface were established corresponding to the CA1, CA2‐3, and subiculum. In each individual, the medial core, a central 3D curve threading down the long axis of the structure, was computed and from each point on the hippocampal surface, a radial distance measure was derived to the medial core. Statistical comparisons were made at each hippocampal surface point between the groups to index contrasts on a local scale. Probability values from these statistical comparisons were mapped onto an average hippocampal shape for the entire sample to generate a 3D representation of the structural differences between the groups. The approximate overlay of the hippocampal subfields was defined based on the Duvernoy atlas ([Duvernoy, 1988], Fig. 1a).
Figure 1.
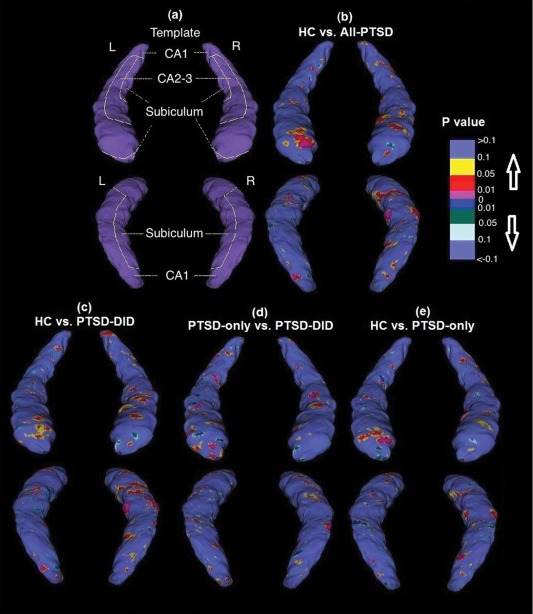
(a) A schematic representation of the hippocampal subfields mapped onto a representative hippocampal surface obtained by averaging the surface from all the participants. In addition, 3D maps of regional hippocampal shape differences (uncorrected) are shown comparing (b) All‐PTSD vs. HC, (c) PTSD–DID vs. HC, (d) PTSD‐DID vs. PTSD‐only, and (e) PTSD‐only vs. HC. Upper rows represent anterior view and lower rows represent posterior view. Abbreviations: PTSD‐only = patients with only post‐traumatic stress disorder; PTSD–DID= patients with PTSD and dissociative identity disorder; All‐PTSD= includes both PTSD‐only and PTSD‐DID patient groups; HC= healthy controls. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Automated extraction of hippocampal subfield volumes
We used FreeSurfer v5.1 (http://surfer.nmr.mgh.harvard.edu) to segment all images into tissue classes and to extract estimates of hippocampal subfield volumes (CA1, CA2‐CA3, CA4‐DG, subiculum, presubiculum, and fimbria) [Van Leemput et al., 2009]. An estimate of parenchymal volume (total GM + total white matter) was also obtained using FreeSurfer and was used in subsequent statistical analyses to correct for whole‐brain size.
Statistical Analysis
The effect of PTSD diagnosis was first investigated on the global hippocampal and subfield volumes by comparing volumetric measurements between All‐PTSD and HC. To this end, a repeated‐measures analysis of covariance (ANCOVA) was used with hemisphere (left or right) as the repeated measure and age and parenchymal volume as covariates. The analysis was then followed by pairwise t‐tests to compare left and right hippocampal volumes separately between: (1) PTSD–DID versus HC, (2) PTSD–DID versus PTSD‐only, and (3) PTSD‐only versus HC. Furthermore, to ascertain that our findings are not due to the differences in the medication usage between the groups, these analyses were repeated after excluding the patients with a history of using different types of psychiatric medications (see Supporting Information 2: Tables S1–S3).
To assess regional hippocampal shape deformations, statistical regression analyses, with age, and parenchymal volume as covariates, were conducted at each hippocampal surface point to map the associations between group and radial distance, a measure of local hippocampal shape. The resulting statistical maps (P‐map) of group differences (uncorrected) were displayed on the hippocampal surface template, which was created by averaging hippocampal shapes from the entire sample. Furthermore, permutation tests with 10,000 iterations and a threshold of P < 0.05 were run to obtain an omnibus‐corrected P‐value for each P‐map.
In the All‐PTSD group, we tested the association of severity of childhood traumatizing events with global hippocampal and subfield volumes using partial correlations while controlling for age and parenchymal volume. Furthermore, a possible link between global and subfield hippocampal volumes and severity of dissociative symptoms was tested using partial correlations while adjusting for age and parenchymal volume.
RESULTS
Hippocampal Global and Subfield Volumes
We found a significant main effect of PTSD on global hippocampal volumes (F(2,54) = 6.65, P = 0.003) which was independent of hemisphere (group × side; Wilks' Lambda = 0.97, F(1,57) = 1.69, P = 0.20). We also found a significant main effect of PTSD diagnosis on hippocampal subfield volumes (F(12,44) = 3.19, P = 0.002), also independent of hemisphere (group × side: Wilks' Lambda = 0.99, F(1,55) = 0.052, P = 0.82).
Bilateral global hippocampal volumes were significantly smaller (left: 6.75%; right 8.33%) in All‐PTSD compared to HC (Table 2). Further pairwise t‐tests (Table 2) showed that PTSD–DID had significantly smaller bilateral hippocampal volumes as compared to PTSD‐only (left: 7.25%; right: 6.58%) and to HC groups (left: 10.19%; right: 11.37%). We also found a trend for a smaller right hippocampal volume in PTSD‐only as compared to HC (right: 5.13%; P = 0.067). Post hoc analyses (see Supporting Information 3) revealed that bilateral hippocampal volumes were only significantly smaller in those PTSD‐only patients with childhood onset traumatizing events (left: 7.11%; right: 7.31%; see Fig. 2).
Table 2.
Statistical analyses of parenchymal (cm3), hippocampal global (mm3), and subfields (0.5 mm3) volumes
| Measurement | All‐PTSD to HC comparison | Different group comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | t‐Test: P value (change%) | Mean (SD) | t‐Test: P value (change%) | |||||
| All‐PTSD (n = 33) | HC (n = 28) | All‐PTSD vs. HC | PTSD‐DID (n = 17) | PTSD‐only (n = 16) | PTSD‐DID vs. HC | PTSD‐DID vs. PTSD‐only | PTSD‐only vs. HC | |
| Parenchymal | 1072 (779) | 1084 (599) | 0.69 (−1.11) | 1068 (819) | 1075 (759) | 0.48 (−1.48) | 0.78 (−0.65) | 0.70 (−0.83) |
| Global volume | ||||||||
| Left hippocampus | 2086 (229) | 2237 (196) | 0.012* (−6.75) | 2009 (208) | 2166 (228) | 0.001* (−10.19) | 0.046* (−7.25) | 0.30 (−3.17) |
| Right hippocampus | 2145 (204) | 2340 (205) | 0.001* (−8.33) | 2074 (185) | 2220 (202) | <0.001* (−11.37) | 0.047* (−6.58) | 0.067^ (−5.13) |
| Subfield volumes | ||||||||
| Left CA1 | 2436 (321) | 2421 (304) | 0.56 (0.62) | 2366 (330) | 2511 (302) | 0.78 (−2.28) | 0.16 (−5.77) | 0.20 (3.70) |
| Right CA1 | 2425 (300) | 2551 (265) | 0.090^ (−4.94) | 2382 (258) | 2471 (341) | 0.024* (−6.62) | 0.12 (−3.61) | 0.60 (−3.12) |
| Left CA2‐3 | 6789 (857) | 7284 (832) | 0.031* (−6.80) | 6578 (995) | 7012 (640) | 0.009* (−9.70) | 0.12 (−6.19) | 0.38 (−3.73) |
| Right CA2‐3 | 7303 (930) | 7778 (851) | 0.043* (−6.11) | 7074 (838) | 7547 (986) | 0.006* (−9.06) | 0.052^ (−6.26) | 0.57 (−2.98) |
| Left CA4‐DG | 3836 (506) | 4094 (462) | 0.053^ (−6.30) | 3680 (580) | 4003 (361) | 0.007* (−10.12) | 0.046* (−8.07) | 0.66 (−2.22) |
| Right CA4‐DG | 4032 (464) | 4361 (468) | 0.009* (−7.54) | 3942 (455) | 4128 (470) | 0.003* (−9.61) | 0.13 (−4.50) | 0.19 (−5.34) |
| Left presubiculum | 3521 (361) | 3721 (364) | 0.027* (−5.37) | 3413 (361) | 3635 (336) | 0.007* (−8.28) | 0.11 (−6.09) | 0.35 (−2.32) |
| Right presubiculum | 3413 (350) | 3591 (377) | 0.12 (−4.96) | 3430 (344) | 3396 (368) | 0.30 (−4.48) | 0.64 (1.00) | 0.13 (−5.43) |
| Left subiculum | 4680 (561) | 4919 (439) | 0.082^ (−4.86) | 4476 (567) | 4896 (482) | 0.007* (−9.00) | 0.022* (−8.57) | 0.89 (−0.46) |
| Right subiculum | 4694 (435) | 4905 (442) | 0.085^ (−4.30) | 4613 (412) | 4780 (455) | 0.020* (−5.95) | 0.10^ (−3.49) | 0.63 (−2.54) |
| Left fimbria | 498 (104) | 498 (164) | 0.90 (0.00) | 491 (115) | 507 (95) | 0.83 (−1.58) | 0.85 (−3.20) | 0.99 (1.67) |
| Right fimbria | 503 (90) | 471(158) | 0.25 (6.79) | 521 (92) | 485 (87) | 0.11 (10.59) | 0.24 (7.48) | 0.80 (2.89) |
PTSD‐only = patients with only post‐traumatic stress disorder; PTSD‐DID = patients with PTSD and dissociative identity disorder; All‐PTSD = includes both PTSD‐only and PTSD‐DID patient groups; HC = healthy controls.
*P‐value ≤ 0.05
^ 0.05 < P‐value ≤ 0.1 (a trend)
Figure 2.
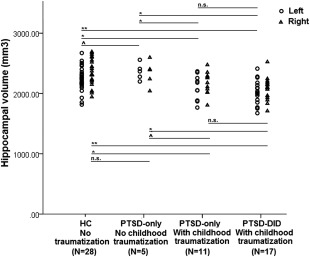
Left and right hippocampal volumes in different diagnosis groups (** P‐value ≤ 0.001; * P‐value ≤ 0.05; ^0.05 < P‐value ≤ 0.1; n.s.: not significant). Abbreviations: PTSD‐only = patients with only post‐traumatic stress disorder; PTSD–DID= patients with PTSD and dissociative identity disorder; HC= healthy controls.
Compared to HC, the All‐PTSD group had significantly smaller volume in the bilateral CA2‐3, right CA4‐DG, and left presubiculum, and, at trend level, also in the right CA1, left CA4‐DG, and bilateral subiculum. Pairwise t‐tests revealed that PTSD–DID patients had significantly smaller right CA1, bilateral CA2‐3, CA4‐DG and subiculum, and left presubiculum volumes than HC. Furthermore, the PTSD–DID group showed significantly smaller left CA4‐DG and subiculum volumes than the PTSD‐only group. In contrast, hippocampal subfield volumes of the PTSD‐only group were not different from those of HC.
Hippocampal Shape Analysis
As compared to HC, All‐PTSD (Fig. 1b) as well as both PTSD‐DID (Fig. 1c) and PTSD‐only (Fig. 1e) showed deformations in the CA1, CA2‐3, and subiculum. Direct comparison of shape measures between PTSD–DID and PTSD‐only showed relative contractions in the CA1, CA2‐3, and subiculum in PTSD–DID (Fig. 1d). The results of these shape analyses did not survive multiple comparison correction with permutations. Uncorrected shape deformation results are presented for exploratory purposes because they support and inform on the significant volumetric results.
Hippocampal Volume and Severity of Childhood Traumatization
Bilateral global hippocampal volumes were significantly, or at a trend level, negatively correlated with severity of childhood emotional neglect, physical neglect, emotional abuse, sexual abuse, and total traumatization (Table 3 and Fig. 3). Subfield volumes of the left CA1, CA2‐3, CA4‐DG, and (pre)subiculum, also showed correlations with severity of childhood traumatizing events. The strongest correlations were observed between left presubiculum volume and total childhood trauma (r = −0.64, P < 0.001).
Table 3.
Correlations between hippocampal global and subfield volumes and severity of dissociative symptoms or childhood traumatizing events in the patients
| Partial correlation r (P‐value)a | ||||||||
|---|---|---|---|---|---|---|---|---|
| DES | SDQ‐20 | Childhood Trauma Questionnaire (CTQ) | ||||||
| Emotional neglect | Physical neglect | Emotional abuse | Physical abuse | Sexual abuse | TOTAL trauma | |||
| Global volume | ||||||||
| Left hippocampus | −0.11 (0.57) | −0.20 (0.29) | −0.49 (0.006*) | −0.38 (0.039*) | −0.32 (0.094^) | −0.24 (0.22) | −0.43 (0.020*) | −0.33 (0.020*) |
| Right hippocampus | −0.20 (0.29) | −0.22 (0.23) | −0.39 (0.038*) | −0.37 (0.051^) | −0. 32 (0.093^) | −0.27 (0.16) | −0.44 (0.018*) | −0.41 (0.026*) |
| Subfield volumes | ||||||||
| Left CA1 | −0.26 (0.15) | −0.15 (0.42) | −0.30 (0.11) | −0.13 (0.49) | −0.25 (0.19) | −0.22 (0.24) | −0.41 (0.029*) | −0.32 (0.094^) |
| Right CA1 | −0.15 (0.43) | 0.001 (0.99) | −0.25 (0.18) | −0.25 (0.19) | −0.12 (0.54) | −0.05 (0.79) | −0.25 (0.19) | −0.21 (0.27) |
| Left CA2‐3 | −0.21 (0.25) | −0.20 (0.28) | −0.35 (0.064^) | −0.19 (0.32) | −0.36 (0.057^) | −0.24 (0.20) | −0.44 (0.018*) | −0.38 (0.044*) |
| Right CA2‐3 | −0.14 (0.44) | 0.05 (0.79) | −0.34 (0.069^) | −0.30 (0.11) | −0.27 (0.15) | −0.15 (0.45) | −0.35 (0.059^) | −0.33 (0.081^) |
| Left CA4‐DG | −0.25 (0.17) | −0.25 (0.17) | −0.37 (0.051^) | −0.26 (0.16) | −0.38 (0.044*) | −0.31 (0.10^) | −0.42 (0.022*) | −0.41 (0.027*) |
| Right CA4‐DG | −0.09 (0.63) | −0.05 (0.77) | −0.29 (0.12) | −0.27 (0.15) | −0.25 (0.18) | −0.13 (0.50) | −0.30 (0.12) | −0.29 (0.13) |
| Left presubiculum | −0.39 (0.031*) | −0.49 (0.005*) | −0.46 (0.012*) | −0.60 (0.001*) | −0.56 (0.002*) | −0.59 (0.001*) | −0.54 (0.003*) | −0.64 (<0.001*) |
| Right presubiculum | 0.02 (0.90) | −0.21 (0.25) | −0.13 (0.51) | −0.17 (0.37) | −0.27 (0.15) | −0.26 (0.18) | −0.14 (0.45) | −0.22 (0.24) |
| Left subiculum | −0.37 (0.040*) | −0.31 (0.086^) | −0.46 (0.013*) | −0.38 (0.041*) | −0.45 (0.013*) | −0.46 (0.013*) | −0.56 (0.001*) | −0.54 (0.002*) |
| Right subiculum | −0.17 (0.35) | −0.19 (0.31) | −0.33 (0.081^) | −0.31 (0.11) | −0.21 (0.26) | −0.12 (0.52) | −0.35 (0.060^) | −0.31 (0.10^) |
| Left fimbria | −0.03 (0.87) | 0.20 (0.28) | −0.06 (0.72) | 0.006 (0.98) | 0.02 (0.90) | 0.01 (0.95) | 0.004 (0.98) | −0.002 (0.99) |
| Right fimbria | 0.20 (0.27) | 0.19 (0.31) | 0.10 (0.96) | 0.17 (0.37) | 0.08 (0.69) | 0.09 (0.63) | 0.14 (0.45) | 0.12 (0.54) |
CTQ = childhood trauma questionnaire; DES = dissociative experience scale; SDQ = somatoform dissociative questionnaire.
*P‐value ≤ 0.05
^0.05 < P‐value ≤ 0.1 (a trend)
Controlled for age and parenchymal volume
Figure 3.
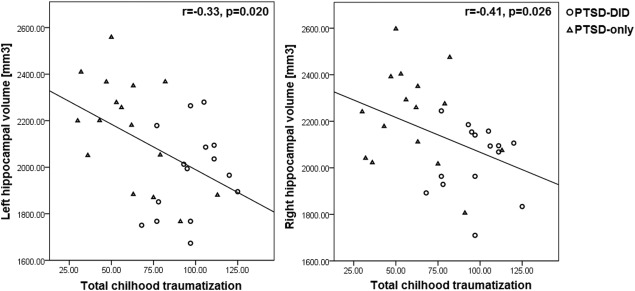
Scatter plots of the bilateral global hippocampal volumes from PTSD–DID and PTSD‐only groups in relation to total childhood traumatization (as assessed using CTQ). Abbreviations: PTSD‐only = patients with only post‐traumatic stress disorder; PTSD–DID= patients with PTSD and dissociative identity disorder; CTQ: Childhood Trauma Questionnaire.
Hippocampal Volume and Dissociative Symptoms
Significant negative correlations were found between severity of dissociative symptoms and the volumes of the left subiculum and presubiculum (Table 3). That is, the higher the severity of the psychoform and somatoform dissociative symptoms, the smaller the volume of the left subiculum and presubiculum.
DISCUSSION
This study is the first to investigate clinical correlates of global and regional morphological abnormalities of the hippocampus in DID and PTSD patients. As hypothesized, we found that in all patients with PTSD and patients with DID, relative to HC, global hippocampal volume is smaller and regional volumetric abnormalities are localized in the subfields CA2‐3, CA4‐DG, and subiculum. Furthermore, these findings are supported by evidence of hippocampal surface contractions in the subfields CA1, CA2‐3, and subiculum. Another important finding is that within our patient sample, the severity of childhood traumatizing events was negatively correlated with global and subfield hippocampal volumes. However, severity of dissociative symptoms (psychoform or somatoform) was negatively associated with the volumes of the left presubiculum and subiculum only. These findings support a link between hippocampal morphological abnormalities and childhood traumatization in both DID and PTSD patients.
The subgroup with DID, who all had comorbid PTSD (PTSD‐DID), had significantly smaller global volumes as compared to HC (left: 10.19%; right: 11.37%) as well as compared to PTSD patients without DID (PTSD‐only; left: 7.25%; right: 6.58%). The PTSD‐only group showed a trend level difference in the right global hippocampal volume when compared to HC (right: 5.13%; P = 0.067), and post hoc analyses (see Supporting Information 3) revealed that bilateral hippocampi were significantly smaller in those PTSD‐only patients with a history of childhood onset traumatizing events (left: 7.11%; right: 7.31%), suggesting again that childhood traumatization is an important factor in the hippocampal abnormalities. These findings concur with prior neuroimaging studies in adult victims of childhood adversity, with or without PTSD [Andersen et al., 2008; Bremner et al., 2003] and in DID patients [Ehling et al., 2008; Irle et al., 2009; Stein et al., 1997; Tsai et al., 1999; Vermetten et al., 2006]. Also, our findings support and advance a previous report of smaller hippocampal volume in DID patients with comorbid PTSD [Vermetten et al., 2006] which was limited by the unmatched characteristics of their control group [Vermetten, 2006], an issue we did not have in this study.
Smaller hippocampal volumes were particularly located in the subfields CA1, CA2‐3, CA4‐DG, and (pre)subiculum, a finding also supported by shape analysis results. The subiculum has specifically been associated with memory retrieval, whereas regions corresponding to CA2‐3 are involved in the encoding of episodic information [Eldridge et al., 2005]. The role of CA1 in encoding and retrieval of contextual memory has been reported in animal studies [Daumas et al., 2005]. Therefore, abnormalities of any of these subfields could result in memory abnormalities and the localized deformations and GM loss in different hippocampal subfields observed in PTSD–DID and PTSD‐only patients could underlie memory alterations reported in DID patients [Dorahy, 2001] and the impaired (non)declarative memory often reported in PTSD patients [for review see Samuelson, 2011]. Our study opens avenues to investigate the cognitive correlates of hippocampal abnormalities, and we suggest that future research investigate the relationship between memory performance and hippocampal abnormalities in DID and PTSD with childhood traumatizing events.
Our findings of smaller volume and deformed shape of the hippocampal subfields in PTSD patients with childhood adversity and in DID patients are consistent with evidence of a relationship between stress and/or elevated level of glucocorticoids (the main stress‐related hormones) and morphological alteration of the hippocampal CA1, CA2‐3, CA4‐DG, and (pre)subiculum subfields [Teicher et al., 2012; Wang et al., 2010]. Elevated levels of stress hormones can result in reduced branching of dendrites, reduced synaptic plasticity, neuronal loss, or suppression of neurogenesis [Sapolsky, 1993]. The CA2‐3 and subiculum subfields have the highest density of glucocorticoid receptors [Sarrieau et al., 1986] and hence are the most susceptible subfields to the adverse effect of stress. The CA1 neurons in the anterior hippocampus in humans project to the medial prefrontal cortex [Small et al., 2011]. The morphological abnormalities of this subfield could perhaps indicate a potential disturbance in the prefrontal–limbic system, including in the regulation of the hypothalamic–pituitary–adrenal (HPA) axis during stress [Herman et al., 2005]. Therefore, they could possibly be, at least in part, related to the HPA axis dysfunction reported in the patients with PTSD [for a review see De Kloet et al. 2006] or with a dissociative disorder [Simeon et al., 2007]. The dentate gyrus is involved in neurogenesis and it has been suggested that childhood traumatization can suppress neurogenesis and hence result in smaller CA4‐dentate gyrus subfield [Teicher et al., 2012]. This is of course conjecture and the direct relationship between hippocampal morphometric abnormalities and these stress hormone pathways and neuronal properties would need to be confirmed experimentally.
Our finding of a relationship between abnormalities of global and subfield hippocampal volume and severity of childhood traumatizing events in DID, provides a first neuroanatomical support for the hypothesis that the pathophysiology of DID is related to childhood traumatization [Van der Hart et al., 2006; Reinders et al., 2003, 2006, 2012]. Although, the present findings need to be confirmed by other neuroanatomical studies, they are in line with the negative correlations previously reported between severity of childhood traumatizing events and hippocampal global [Andersen et al., 2008; Dannlowski et al., 2012; Samplin et al., 2013] and subfield [Teicher et al., 2012] volumes in adults from the general community with a history of early‐life adversity. However, as this study is a cross‐sectional study, we could not examine direct or indirect links between these measures and hence longitudinal studies are needed to further explore these.
In the two patient groups, severity of dissociative symptom was negatively correlated with the volume of presubiculum and the subiculum, but not with global hippocampal volumes. So far, limited studies have investigated this relationship in traumatized individuals and some studies reported a negative relationship consistent with our results [Bremner et al., 2003; Ehling et al., 2008; Stein et al., 1997], but other studies did not find any significant association [Bremner et al., 1995; Nardo et al., 2013]. While it is possible that the hippocampal morphological abnormalities in our patient samples were at least, in part, involved in the dissociative symptoms, it can be speculated that the associations between morphological measures and dissociative symptoms are actually mediated by childhood traumatization. Future studies can explore this relationship by including individuals with dissociative symptoms but without childhood traumatization.
It has been reported that some psychiatric medications including typical antipsychotics [Chakos et al., 2005], antiepileptics [Watanabe et al., 1992], and antidepressants [Vermetten et al., 2003] can change the hippocampal morphology. Therefore, it might be argued that our findings of smaller hippocampal global and subfield volumes in PTSD–DID, and to a lesser extent in PTSD‐only, are due to the higher level of medications in these patients. However, when patients with a history of using typical antipsychotics (Supporting Information Table S1) or antiepileptics were excluded (Supporting Information Table S2), the majority of our results remained preserved. Nevertheless, when patients with a history of using antidepressants (i.e., 10 PTSD–DID and 2 PTSD‐only) were excluded the group differences in the hippocampal global volume remained significant but the group differences in the subfield volumes became less or nonsignificant (see Supporting Information Tables S3). The latter finding can be as a result of the insufficient statistical power to detect the subtle changes due to the exclusion of a large portion of the patients. Altogether, the results of these post hoc analyses may indicate that the smaller hippocampal global and subfield volume in PTSD–DID and PTSD‐only as compared to HC are robust findings and are not due to the history of medication usage.
Some strengths and limitations: although our sample size of 17 PTSD–DID and 16 PTSD‐only patients can be considered as modest, it is in fact the largest sample of individuals with DID in which hippocampal morphology has been studied. PTSD severity was evaluated differently across the two patient groups limiting us to investigate the morphological correlates of PTSD severity. Our findings of more pronounced morphological abnormalities in DID might be due to an interaction between (comorbid) PTSD severity and childhood traumatization [Van Voorhees et al., 2012]. Medication effects were tested (see Supporting Information 2) and showed that the smaller hippocampal volume in PTSD–DID as compared to HC is a robust finding. However, the shape analyses did not survive multiple comparison correction, which is likely due to insufficient statistical power for conducting multiple tests across the hippocampal surface. Nevertheless, permutation testing can be considered too conservative in this context, as all the tests are treated independently, when in fact many of the surface points are highly related.
In conclusion, we provide novel evidence that smaller hippocampal global and subfield volumes and contractions of hippocampal surface in PTSD patients, with or without DID, are related to the severity of childhood traumatizing events and dissociative symptoms. Our findings are in line with the clinical observation that DID is related to chronic childhood abuse and neglect. These findings can help to understand the neurobiological mechanisms involved in PTSD and DID.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. .The authors would like to thank all the participants and their therapists. The authors also thank J.A. den Boer, R. Renken, A. Nederveen, A.J. Sibeijn‐Kuiper, J. Streurman and R. van Luijk‐Snoeks, S. van den Berg‐Faay for their assistance with data acquisition, M. Jongsma and J. Reisel for their assistance with patient inclusion and H. Hofstetter for her help with the initial phases of the project.
REFERENCES
- American Psychiatric Association (1994): Diagnostic and statistical manual of mental disorders: DSM‐IV. Washington, DC: American Psychiatric Association. Book, Whole. [Google Scholar]
- American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders: DSM 5. Arlington, VA: American Psychiatric Publishing. Book, Whole. [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH (2008): Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 20:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein EM, Putnam FW (1986): Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 174:727–735. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J (1994): Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151:1132–1136. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM (1995): The development of a Clinician‐Administered PTSD Scale. J Trauma Stress 8:75–90. [DOI] [PubMed] [Google Scholar]
- Bonne O, Vythilingam M, Inagaki M, Wood S, Neumeister A, Nugent AC, Snow J, Luckenbaugh DA, Bain EE, Drevets WC, Charney DS (2008): Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry 69:1087–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon S, Draijer N (1993a): Multiple personality disorder in The Netherlands: A clinical investigation of 71 patients. Am J Psychiatry 150:489–494. [DOI] [PubMed] [Google Scholar]
- Boon S, Draijer N (1993b): Multiple personality disorder in The Netherlands. A study on reliability and validity of the diagnosis. Lisse: Swets & Zeitlinger. Book, Whole. [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB (1995): MRI‐based measurement of hippocampal volume in patients with combat‐related posttraumatic stress disorder. Am J Psychiatry 152:973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS (2003): MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 160:924–932. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Schobel SA, Gu H, Gerig G, Bradford D, Charles C, Lieberman JA (2005): Duration of illness and treatment effects on hippocampal volume in male patients with schizophrenia. Br J Psychiatry J Ment Sci 186:26–31. [DOI] [PubMed] [Google Scholar]
- Chalavi S, Simmons A, Dijkstra H, Barker GJ, Reinders AATS (2012): Quantitative and qualitative assessment of structural magnetic resonance imaging data in a two‐center study. BMC Med Imaging 12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H (2012): Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71:286–293. [DOI] [PubMed] [Google Scholar]
- Daumas S, Halley H, Frances B, Lassalle JM (2005): Encoding, consolidation, and retrieval of contextual memory: Differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn Mem Cold Spring Harb N 12:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG (2006): Assessment of HPA‐axis function in posttraumatic stress disorder: Pharmacological and non‐pharmacological challenge tests, a review. J Psychiatr Res 40:550–567. [DOI] [PubMed] [Google Scholar]
- Dorahy MJ (2001): Dissociative identity disorder and memory dysfunction: The current state of experimental research and its future directions. Clin Psychol Rev 21:771–795. [DOI] [PubMed] [Google Scholar]
- Duvernoy H (1988): The Human Hippocampus: An Atlas of Applied Anatomy. Munich, Germany: JF Bergman Verlag. Book, Whole. [Google Scholar]
- Ehling T, Nijenhuis ERS, Krikke AP (2008): Volume of discrete brain structures in complex dissociative disorders: Preliminary findings. Prog Brain Res 167:307–310. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ (2005): A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci Off J Soc Neurosci 25:3280–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H (2005): Limbic system mechanisms of stress regulation: hypothalamo‐pituitary‐adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29:1201–1213. [DOI] [PubMed] [Google Scholar]
- Irle E, Lange C, Sachsse U, Weniger G (2009): Further evidence that post‐traumatic stress disorder but not dissociative disorders are related to amygdala and hippocampal size reduction in trauma‐exposed individuals. Acta Psychiatr Scand 119:330–1; discussion 331. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D (2010): Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry 167:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardo D, Högberg G, Lanius RA, Jacobsson H, Jonsson C, Hällström T, Pagani M (2013): Gray matter volume alterations related to trait dissociation in PTSD and traumatized controls. Acta Psychiatr Scand 128:222–233. [DOI] [PubMed] [Google Scholar]
- Nijenhuis ERS, Spinhoven P, Van Dyck R, Van der Hart O, Vanderlinden J (1996): The development and psychometric characteristics of the Somatoform Dissociation Questionnaire (SDQ‐20). J Nerv Ment Dis 184:688–694. [DOI] [PubMed] [Google Scholar]
- Nijenhuis ERS, Spinhoven P, van Dyck R, van der Hart O, Vanderlinden J (1997): The development of the somatoform dissociation questionnaire (SDQ‐5) as a screening instrument for dissociative disorders. Acta Psychiatr Scand 96:311–318. [DOI] [PubMed] [Google Scholar]
- Nijenhuis ERS, Spinhoven P, van Dyck R, van der Hart O, Vanderlinden J (1998): Psychometric characteristics of the somatoform dissociation questionnaire: A replication study. Psychother Psychosom 67:17–23. [DOI] [PubMed] [Google Scholar]
- Nijenhuis ERS, Van der Hart O, Kruger K (2002): The psychometric characteristics of the Traumatic Experiences Checklist (TEC): First findings among psychiatric outpatients. Clin Psychol Psychother 9:200–210. [Google Scholar]
- Reinders AATS, Nijenhuis ERS, Paans AM, Korf J, Willemsen ATM, den Boer JA (2003): One brain, two selves. NeuroImage 20:2119–2125. [DOI] [PubMed] [Google Scholar]
- Reinders AATS, Nijenhuis ERS, Quak J, Korf J, Haaksma J, Paans AM, Willemsen ATM, den Boer JA (2006): Psychobiological characteristics of dissociative identity disorder: A symptom provocation study. Biol Psychiatry 60:730–740. [DOI] [PubMed] [Google Scholar]
- Reinders AATS, Willemsen ATM, Vos HP, den Boer JA, Nijenhuis ERS (2012): Fact or factitious? A psychobiological study of authentic and simulated dissociative identity states. PloS One 7:e39279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samplin E, Ikuta T, Malhotra AK, Szeszko PR, Derosse P (2013): Sex differences in resilience to childhood maltreatment: Effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J Psychiatr Res 47:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson KW (2011): Post‐traumatic stress disorder and declarative memory functioning: A review. Dialogues Clin Neurosci 13:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM (1993): Potential behavioral modification of glucocorticoid damage to the hippocampus. Behav Brain Res 57:175–182. [DOI] [PubMed] [Google Scholar]
- Sarrieau A, Dussaillant M, Agid F, Philibert D, Agid Y, Rostene W (1986): Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post‐mortem brain. J Steroid Biochem 25:717–721. [DOI] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM (2007): Hypothalamic‐pituitary‐adrenal axis function in dissociative disorders, post‐traumatic stress disorder, and healthy volunteers. Biol Psychiatry 61:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011): A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev 12:585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D, Lewis‐Fernandez R, Lanius R, Vermetten E, Simeon D, Friedman M (2013): Dissociative disorders in DSM‐5. Annu Rev Clin Psychol 9:299–326. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B (1997): Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 27:951–959. [DOI] [PubMed] [Google Scholar]
- Steinberg M (1993): Structured Clinical Interview for DSM‐IV Dissociative Disorders (SCID‐D). Washington DC: American Psychiatric Press. Book, Whole. [Google Scholar]
- Teicher MH, Anderson CM, Polcari A (2012): Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 109:E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH, Veltman DJ (2010): Reduced anterior cingulate and orbitofrontal volumes in child abuse‐related complex PTSD. J Clin Psychiatry 71:1636–1644. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TG, Cannon TD, Toga AW (2004): Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. NeuroImage 23 Suppl 1:S2–S18. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Condie D, Wu MT, Chang IW (1999): Functional magnetic resonance imaging of personality switches in a woman with dissociative identity disorder. Harv Rev Psychiatry 7:119–122. [PubMed] [Google Scholar]
- Vermetten E (2006): Dr. Vermetten replies (letter). Am J Psychiatry 163:1643. 16946196 [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD (2003): Long‐term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry 54:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD (2006): Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry 163:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Hart O, Nijenhuis ERS, Steele K (2006): The Haunted Self: Structural Dissociation and the Treatment of Chronic Traumatization. New York, London: W. W. Norton & Company. Book, Whole. [Google Scholar]
- Van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J, Dickerson BC, Golland P, Fischl B (2009): Automated segmentation of hippocampal subfields from ultra‐high resolution in vivo MRI. Hippocampus 19:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhees EE, Dedert EA, Calhoun PS, Brancu M, Runnals J, Beckham JC, VA Mid‐atlantic MIRECC Workgroup (2012): Childhood trauma exposure in Iraq and Afghanistan war era veterans: Implications for posttraumatic stress disorder symptoms and adult functional social support. Child Abuse Negl 36:423–432. [DOI] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N (2010): Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry 67:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS (1992): Phenytoin prevents stress‐ and corticosterone‐induced atrophy of CA3 pyramidal neurons. Hippocampus 2:431–435. [DOI] [PubMed] [Google Scholar]
- Weniger G, Lange C, Sachsse U, Irle E (2008): Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatr Scand 118:281–290. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW (2008): Hippocampal and amygdala volumes in children and adults with childhood maltreatment‐related posttraumatic stress disorder: A meta‐analysis. Hippocampus 18:729–736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


