Abstract
The expression of the leukotoxin of Aggregatibacter actinomycetemcomitans is regulated by the leukotoxin promoter. A 530-bp deletion or an 886-bp insertion sequence (IS) element in this region has earlier been described in highly leukotoxic isolates. Here, we report on highly leukotoxic isolate with a 640-bp deletion, which was detected in an adolescent of Ethiopian origin.
Keywords: leukotoxicity, deletion, JP2, aggressive periodontitis, geographic spreading, Africa
The Gram-negative oral bacterial species, Aggregatibacter actinomycetemcomitans, expresses a leukotoxin that links the bacterium to aggressive periodontitis (1–6). The toxin expression, which is associated with four structural genes (ltxA, ltxB, ltxC, and ltxD), is regulated by the leukotoxin promoter (Fig. 1). Although the genes of the leukotoxin operon appear to be present in all strains of A. actinomycetemcomitans, the toxin expression varies substantially between different strains (7–9). High expression of leukotoxin has been detected in A. actinomycetemcomitans isolates with a 530-bp deletion in the promoter region and in isolates carrying an 886-bp IS element in the same region (7–10) (Fig. 1).
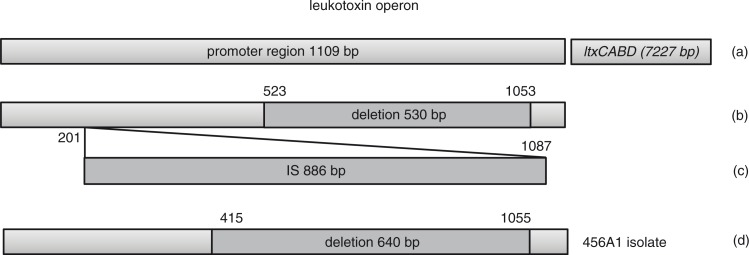
Fig. 1.
Schematic illustration of the leukotoxin promoter from A. actinomycetemcomitans genotype JP2, non-JP2, IS, and the most recent isolate 456A1. (a) The leukotoxin promoter region of A. actinomycetemcomitans (non-JP2): 1,109 bp (the sequence from the first nucleotide after the stop codon of glyA to the stop codon of promoter region is incorporated) (11). (b) The position of the 530-bp deletion (JP2) within the promoter region (9). (c) The position of the 886-bp insertion sequence (IS) within the promoter region (10). (d) The position of the 640-bp deletion (456 A1 isolate) within the promoter region (this study).
Isolates characterized by the 530-bp deletion comprise the so-called JP2 genotype, and isolates with a complete leukotoxin promoter, which consequently are distributed within the group often called the non-JP2 genotype, are described as low or moderately leukotoxic (11). Reports about other leukotoxin promoter types than the JP2, the non-JP2 genotype, and the genotype with the IS element are absent.
The JP2 genotype of A. actinomycetemcomitans, which has been studied for more than 20 years from an experimental perspective, is almost exclusively isolated from individuals of African origin living in North and West Africa (12, 13). However, a Sudanese subject, positive for the JP2 genotype, was reported recently (14). Although much rarer, findings of the JP2 genotype among non-Africans also have been described, indicating that this genotype is more widespread than was initially believed (15–17). Based on longitudinal studies, individuals carrying the JP2 genotype have an enhanced risk of developing aggressive periodontitis (3, 5, 6). However, an association with aggressive periodontitis among carriers of the non-JP2 genotype cannot be neglected (6, 18, 19).
Recently, we identified a leukotoxin promoter type of A. actinomycetemcomitans, which has not previously been reported. It was detected in two periodontal plaque samples collected from a 16-yr old female periodontitis patient of Ethiopian descent (Fig. 2). The samples were sent in vials containing VMGA III medium (20) to our clinical laboratory at the Dental School in Umeå, Sweden. After a procedure as described (17), it was found that the plaque samples contained high proportions of A. actinomycetemcomitans (Table 1). According to the routine procedure, two isolates (456A1, 456A2) were serotyped according to Suzuki and co-workers (21). Since the two isolates belonged to serotype b, they were typed according to the leukotoxin promoter as described (22). Surprisingly, both isolates were characterized by a leukotoxin promoter region that was approximately 100 bp smaller than the JP2 promoter region, i.e., they harboured a previously undescribed promoter type (Figs. 1 and 2). This finding was confirmed when the bacterial DNA, taken directly from the samples (455V and 456V) in the transport vials (VMGA), was amplified with leukotoxin promoter-specific primers (Fig. 2). The identical characteristics of the analyzed isolates and samples indicate that the patient most likely is colonized by a single clone of A. actinomycetemcomitans.
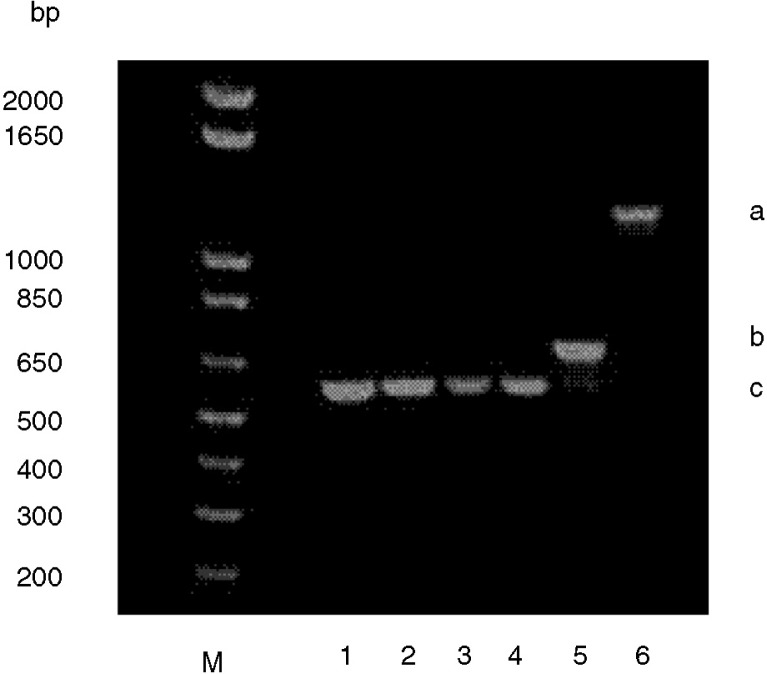
Fig. 2.
A. actinomycetemcomitans isolates 456A1 [1], 456A2 [2], sample 455 [3], sample 456 [4], JP2 [5], and D7s [6]. (a) Strain with a complete leukotoxin promoter region, (b) strain with a 530-bp deletion in the leukotoxin promoter region, (c) strain with a 640-bp deletion in the leukotoxin promoter region. Molecular weight marker (M).
Table 1.
Aggregatibacter actinomycetemcomitans (A.a.) in subgingival plaque samples quantified by cultivation on blood agar plates and selective plates (TBV)
| Sample number | Total viable count per sample, millions | A.a. per sample, millions | % |
|---|---|---|---|
| 455-13 | 1.2 | 0.82 | 68 |
| 456-13 | 2.4 | 1.1 | 47 |
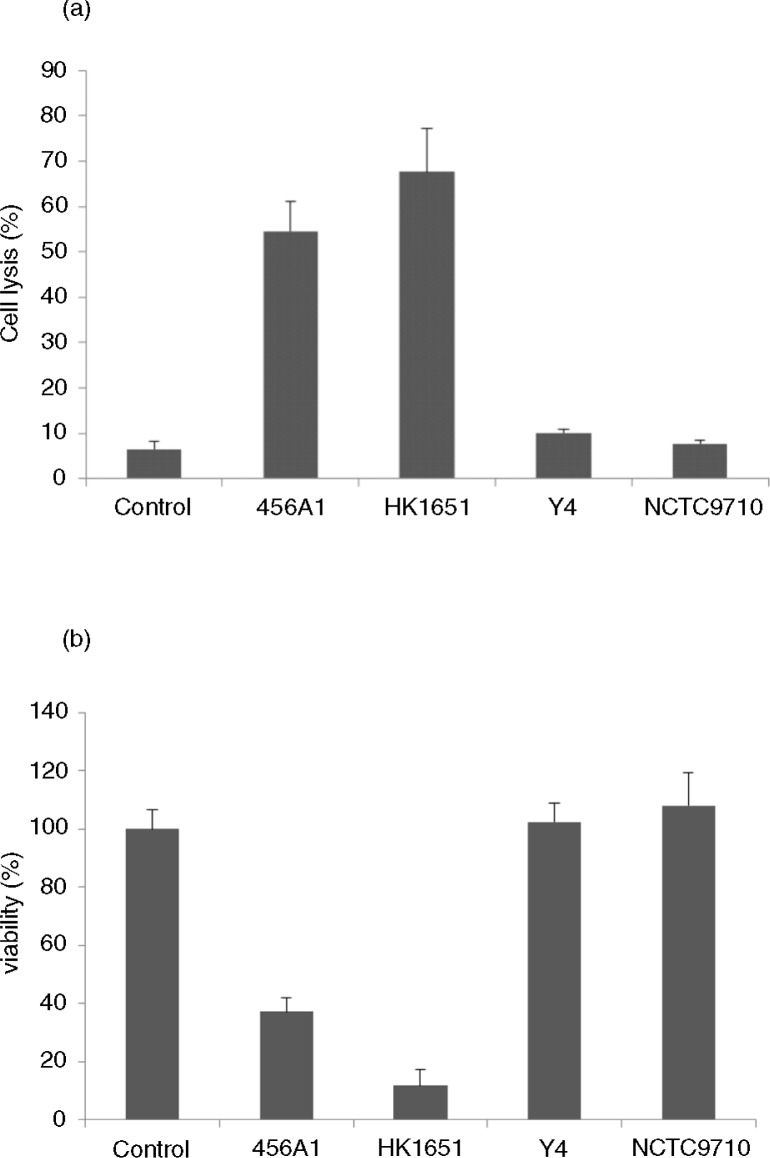
DNA was purified from one of the two isolates (456A1) with a DNA-purifying kit (Ready-To-Go Kit; GE Healthcare, Little Chalfont, UK) for further characterization. First, when the promoter region was sequenced as described (6), it was found to be associated with a 640-bp deletion, i.e., it lacked 640 bp as compared with strains with the full-length leukotoxin promoter (Fig. 1). In comparison with the 530-bp deletion in the leukotoxin promoter region in JP2 genotype strains, the additional 110 bp missing in the sequenced isolate (456A1) were located 108 bp upstream and 2 bp downstream from the site of the JP2 genotype–associated deletion. Although isolate 456A1 carried a different leukotoxin promoter than JP2 genotype strains, it was found to have leukotoxicity similar to that of the JP2 genotype of A. actinomycetemcomitans (strain HK1615), when leukotoxic activity was determined as described (6) (Fig. 3).
Fig. 3.
Leukotoxicity of A. actinomycetemcomitans strains with different leukotoxin promoter types on cultures of PMA-differentiated THP-1 cells. Strains: 456A1 (novel), HK1651 (JP2), Y4 (non-JP2), and NCTC9710 (non-JP2). The THP-1 cells were exposed for 2 hours with 10% of a NaCl surface extract based on a bacterial density of OD 10 at 600 nm. Mean and standard deviations from two different experiments with four replicates in each are shown. (a) Cell lysis as a percentage of LDH release in relation to that of a triton-lysed control sample. (b) Viability as a percentage of neutral red uptake in relation to a plane control sample.
By sequencing the hemoglobin binding gene (hbp2), JP2 genotype isolates from North Africa, which have an ‘A’ nucleotide in position 525285 (213 within the 329-bp gene), are distinguished from isolates of West African origin, which have a ‘G’ in the same position. Sequencing of the hbpA gene in the 456A1 isolate according to Eriksen and co-workers (23) revealed a North Africa–associated point mutation.
Multilocus sequence analysis (MLSA) of JP2 genotype isolates based on the housekeeping genes adk, atpG, frB, recA, and the iron acquisition–associated genes hbpA and tbpA revealed 11 sequence types among 66 isolates (24). However, none of the 11 sequence types was identical with the sequence type revealed when the 456A1 isolate was subjected to MLSA as earlier described (6). Compared with the JP2 genotype reference strain, named HK1651, a polymorphism was detected in four of the genes (Table 2). The deletion in tbpA in the 456A1 isolate has been detected only in the non-JP2 genotype of A. actinomycetemcomitans (24). This deletion may be a valuable marker for future evaluation of the dissemination routes of isolates with this mutation.
Table 2.
Polymorphic sites in housekeeping genes of the 456A1 isolate in comparison with the Aggregatibacter actinomycetemcomitans reference strain HK1651
| atpG | hbpA-1 | hbpA-2 | tbpA | ||
|---|---|---|---|---|---|
| 231/500 | 44/439 | 6/329 | 213/329 | 340/344 | |
| HK 1651 | G | T | T | A | A |
| 456A1 | C | G | G | G | – |
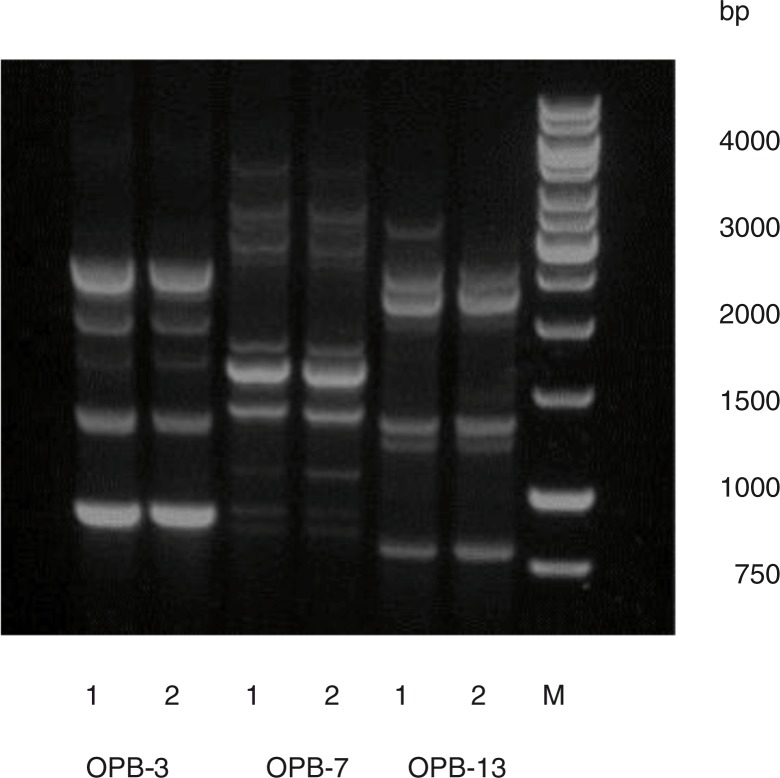
Genetic similarities between the 456A1 isolate and a JP2 genotype strain were indicated by AP-PCR (arbitrarily primed PCR) analyzes (6) (Fig. 4). This AP-PCR pattern has been detected in other JP2 genotype isolates, as well as among some of the serotype b non-JP2 genotype isolates (6).
Fig. 4.
AP-PCR banding pattern of HK 1651 (JP2) [1] and isolate 456A1 [2] obtained by use of primers OPB-3, OPB-7, and OPB-13. Molecular weight marker (M).
Three different leukotoxin promoter types of A. actinomycetemcomitans have been described. In this paper, we present a promoter type which has not previously been reported. It is characterized by a missing 640-bp sequence in the leukotoxin promoter region. Like the JP2 genotype, it is highly leukotoxic according to the results obtained in leukotoxicity assays (6). In addition, it might follow a dissemination route not identical to the one for the JP2 genotype. For confirmation and further characterization of this putative novel leukotoxin promoter type, identification of additional carriers and access to additional isolates is a prerequisite.
Acknowledgements
The authors thank laboratory technicians Ewa Engbo-Strömqvist and Chrissie Roth for their valuable contributions to the detection and characterization of the novel leukotoxin promoter type.
Conflict of interest and funding
The authors declare that they have no conflict of interest. This study was supported by the Research Fund (TUA) of Västerbotten County, Sweden; the Swedish Dental Society; the Danish Dental Association; and the Ingeborg and Leo Dannins Foundation.
References
- 1.Haubek D, Ennibi O-K, Poulsen K, Poulsen S, Benzarti N, Kilian M. Early-onset periodontitis in Morocco is associated with the highly leukotoxic clone of Actinobacillus actinomycetemcomitans . J Dent Res. 2001;80:1580–3. doi: 10.1177/00220345010800062001. [DOI] [PubMed] [Google Scholar]
- 2.Haubek D, Ennibi O-K, Poulsen K, Benzarti N, Baelum V. The highly leukotoxic JP2 clone of Actinobacillus atinomycetemcomitans and progression of periodontal attachment loss. J Dent Res. 2004;83:767–70. doi: 10.1177/154405910408301006. [DOI] [PubMed] [Google Scholar]
- 3.Haubek D, Ennibi O-K, Poulsen K, Vaeth M, Poulsen S, Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a perspective longitudinal cohort study. Lancet. 2008;371:237–42. doi: 10.1016/S0140-6736(08)60135-X. [DOI] [PubMed] [Google Scholar]
- 4.Höglund Åberg C, Antonoglou G, Haubek D, Kwamin F, Claesson R, Johansson A. Cytolethal distending toxin in isolates of Aggregatibacter actinomycetemcomitans from Ghanaian adolescents and association with serotype and disease progression. PLoS One. 2013;8:e65781. doi: 10.1371/journal.pone.0065781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Höglund Åberg C, Kwamin F, Claesson R, Dahlén G, Johansson A, Haubek D. Progression of attachment loss is strongly associated with presence of the JP2 genotype of Aggregatibacter actinomycetemcomitans: a prospective cohort study of a young adolescent population. J Clin Periodontol. 2014;41:232–41. doi: 10.1111/jcpe.12209. [DOI] [PubMed] [Google Scholar]
- 6.Höglund Åberg C, Haubek D, Kwamin F, Johansson A, Claesson R. Leukotoxic activity of Aggregatibacter actinomycetemcomitans and periodontal attachment loss. PLoS One. 2014;9:e104095. doi: 10.1371/journal.pone.0104095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zambon J, Slots J, Genco RJ. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983;41:19–27. doi: 10.1128/iai.41.1.19-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spitznagel J, Jr., Kraig E, Kolodrubetz D. Regulation of leukotoxin in leukotoxic and nonleukotoxic strains of Actinobacillus actinomycetemcomitans . Infect Immun. 1991;59:1394–401. doi: 10.1128/iai.59.4.1394-1401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brogan JM, Lally ET, Poulsen K, Kilian M, Demuth DR. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect Immun. 1994;62:501–8. doi: 10.1128/iai.62.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He T, Nishihara T, Demuth DR, Ishikawa I. A novel insertion sequence increases the expression of leukotoxicity in Actinobacillus actinomycetemcomitans clinical isolates. J Periodontol. 1999;70:1261–8. doi: 10.1902/jop.1999.70.11.1261. [DOI] [PubMed] [Google Scholar]
- 11.Kolodrubetz D, Spitznagel J, Jr., Wang B, Phillips LH, Jacobs C, Kraig E. cis Elements and trans factors are both important in strain-specific regulation of the leukotoxin gene in Actinobacillus actinomycetemcomitans . Infect Immun. 1996;64:3451–60. doi: 10.1128/iai.64.9.3451-3460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haubek D, Johansson A. Pathogenicity of the highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans and its geographic dissemination and role in aggressive periodontitis. J Oral Microbiol. 2014;6:23980. doi: 10.3402/jom.v6.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine DH, Kaplan JB, Kachlany SC, Schreiner HC. How we got attached to Actinobacillus actinomycetemcomitans: a model for infectious diseases. Periodontol 2000. 2006;42:114–57. doi: 10.1111/j.1600-0757.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- 14.Elabdeen HRZ, Mustafa M, Hasturk H, Klepac-Ceraj V, Ali RW, Paster BJ, et al. Subgingival microbial profiles of Sudanese patients with aggressive periodontitis. J Periodontal Res. 2014 doi: 10.1111/jre.12250. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guthmiller ET, Lally ET, Korostoff J. Beyond the specific plaque hypothesis: are highly leukotoxic strains of Actinobacillus actinomycetemcomitans a paradigm for periodontal pathogenesis? Crit Rev Oral Biol Med. 2001;12:116–24. doi: 10.1177/10454411010120020201. [DOI] [PubMed] [Google Scholar]
- 16.Orru G, Marini MF, Ciusa Ml, Isola D, Cotti M, Baldoni M, et al. Usefulness of real time PCR for the differentiation and quantification of 652 and JP2 Actinobacillus actinomycetemcomitans genotypes in dental plaque and saliva. BMC Infect Dis. 2006;6:98. doi: 10.1186/1471-2334-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claesson R, Lagervall M, Höglund Åberg C, Johansson A, Haubek D. Detection of the highly leucotoxic JP2 clone of Aggregatibacter actinomycetemcomitans in members of a Caucasian family living in Sweden. J Clin Periodontol. 2011;38:115–21. doi: 10.1111/j.1600-051X.2010.01643.x. [DOI] [PubMed] [Google Scholar]
- 18.Van der Velden U, Abbas F, Armand S, Loos BG, Timmerman MF, van der Weijden GA, et al. Java project on periodontal diseases. The natural development of periodontitis: risk factors, risk predictors and risk determinants. J Clin Periodontol. 2006;33:540–8. doi: 10.1111/j.1600-051X.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- 19.Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, et al. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol. 2007;45:3859–69. doi: 10.1128/JCM.00653-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Höglund Åberg C, Sjödin B, Laiko L, Pussinen PJ, Johansson A, Claesson R. Presence of Aggregatibacter actinomycetemcomitans in young individuals: a 16 year clinical and microbiological follow-up study. J Clin Periodontol. 2009;36:815–22. doi: 10.1111/j.1600-051X.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki N, Nakano Y, Yoshida Y, Ikeda D, Koga T. Identification of Actinobacillus actinomycetemcomitans serotypes by multiplex PCR. J Clin Microbiol. 2001;39:2002–5. doi: 10.1128/JCM.39.5.2002-2005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulsen K, Ennibi O-K, Haubek D. Improved PCR for detection of the highly leukotoxic JP2 clone of Actinobacillus actinomycetemcomitans in subgingival plaque samples. J Clin Microbiol. 2003;41:4829–32. doi: 10.1128/JCM.41.10.4829-4832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksen KT, Haubek D, Poulsen K. Intragenomic recombination in the highly leukotoxic JP2 clone of Actinobacillus actinomycetemcomitans . Microbiology. 2005;151:3371–9. doi: 10.1099/mic.0.28193-0. [DOI] [PubMed] [Google Scholar]
- 24.Haubek D, Poulsen K, Kilian M. Microevolution and patterns of dissemination of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans . Infect Immun. 2007;75:3080–8. doi: 10.1128/IAI.01734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]