Abstract
Piperlongumine, a natural alkaloid isolated from the long pepper, selectively increases reactive oxygen species production and apoptotic cell death in cancer cells but not in normal cells. However, the molecular mechanism underlying piperlongumine-induced selective killing of cancer cells remains unclear. In the present study, we observed that human breast cancer MCF-7 cells are sensitive to piperlongumine-induced apoptosis relative to human MCF-10A breast epithelial cells. Interestingly, this opposing effect of piperlongumine appears to be mediated by heme oxygenase-1 (HO-1). Piperlongumine upregulated HO-1 expression through the activation of nuclear factor-erythroid-2-related factor-2 (Nrf2) signaling in both MCF-7 and MCF-10A cells. However, knockdown of HO-1 expression and pharmacological inhibition of its activity abolished the ability of piperlongumine to induce apoptosis in MCF-7 cells, whereas those promoted apoptosis in MCF-10A cells, indicating that HO-1 has anti-tumor functions in cancer cells but cytoprotective functions in normal cells. Moreover, it was found that piperlongumine-induced Nrf2 activation, HO-1 expression and cancer cell apoptosis are not dependent on the generation of reactive oxygen species. Instead, piperlongumine, which bears electrophilic α,β-unsaturated carbonyl groups, appears to inactivate Kelch-like ECH-associated protein-1 (Keap1) through thiol modification, thereby activating the Nrf2/HO-1 pathway and subsequently upregulating HO-1 expression, which accounts for piperlongumine-induced apoptosis in cancer cells. Taken together, these findings suggest that direct interaction of piperlongumine with Keap1 leads to the upregulation of Nrf2-mediated HO-1 expression, and HO-1 determines the differential response of breast normal cells and cancer cells to piperlongumine.
Keywords: apoptosis, breast cancer, HO-1, Nrf2, piperlongumine
INTRODUCTION
Phytochemicals have been emerging as the most dependable resource of therapeutic agents for inflammatory disorders and cancers. Piperlongumine, a natural alkaloid isolated from the long pepper (Piper longum L.), is known to have insecticidal, bactericidal (Yang et al., 2002), anti-diabetic effects (Rao et al., 2012), and anti-atherosclerotic effects (Son et al., 2012) as well as cytotoxic and anti-tumor effects (Raj et al., 2011). It has been reported that piperlongumine can selectively kill various cancer cells and transformed cells overexpressing oncogenes (e.g., ERBB2 and/or HRAS), but not normal cells (Raj et al., 2011). Moreover, this molecule dramatically suppressed tumor growth, angiogenesis and metastasis in bladder, breast and lung tumor tissues without affecting normal tissues in tumor xenograft mouse models (Raj et al., 2011). The differential response of cancer cells and normal cells to piperlongumine appears to be mediated by targeting the higher dependency of cancer cells on the oxidative stress-response pathway relative to normal cells. Piperlongumine enhances the accumulation of reactive oxygen species (ROS) in cancer cells by interfering with redox and ROS homeostatic regulators such as glutathione S-transferase pi 1 (GSTP1) and carbonyl reductase 1 (CBR1) (Raj et al., 2011). It has also been demonstrated that piperlongumine-induced ROS generation is dependent on the activation of mitogen activated protein kinases (MAPKs) including c-Jun-N-terminal kinase (JNK) and p38 (Liu et al., 2013).
Heme oxygenase-1 (HO-1), also known as heat shock protein 32 (HSP32), is a representative redox-regulated protein involved in cellular antioxidant and cytoprotective mechanisms (Bauer and Bauer, 2002). Under normal physiological conditions, induction of HO-1 expression is considered as an important adaptive survival response in stressed cells challenged with oxidative stress, UV irradiation, pro-inflammatory cytokines, cisplatin and 6-hydroxydopamine (Bae et al., 2013; Ewing et al., 2005; Kim et al., 2006; Lin et al., 2005; 2007). In addition to its cytoprotective effect, HO-1 has been reported to induce apoptosis and suppress proliferation and invasion in breast cancer cells (Hill et al., 2005; Lee et al., 2014; Lin et al., 2008). In hepatocellular carcinomas, cell migration and xenograft tumor growth were significantly inhibited by HO-1 overexpression (Zou et al., 2011). Furthermore, several studies have revealed that chemopreventive agents, including sulforaphane, suppress tumorigenesis through induction of HO-1 expression (Cornblatt et al., 2007; Keum et al., 2006), indicative of the antitumor activity of HO-1.
HO-1 is well-known to be regulated by nuclear factor-erythroid-2-related factor 2 (Nrf2), a stress-responsive transcription factor. Nrf2 binds to antioxidant response element (ARE), a cis-regulatory DNA sequence located in the promoter of target genes encoding phase 2 detoxifying enzymes and cytoprotective proteins (Itoh et al., 1997). Under physiological conditions, Nrf2 is bound to the Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and undergoes proteasomal degradation by the Keap1-Cul3 E3 ubiquitin ligase complex (Alam et al., 1999). In response to oxidative or electrophilic stress, however, Nrf2 dissociates from Keap1, thereby escaping from proteasomal degradation and translocating into the nucleus where it heterodimerizes with other transcription factors such as small Maf and binds to ARE (Kensler et al., 2007). Several studies have demonstrated that the dissociation of Nrf2 from Keap1 is most likely regulated by alterations in Keap1 structure. Oxidative stress or electrophiles can cause oxidation or covalent modification of cysteine residues within Keap1, thereby activating Nrf2 signaling (Kim et al., 2014; Kobayashi et al., 2009; Yamamoto et al., 2008).
Numerous chemopreventive phytochemicals which contain an α,β-unsaturated carbonyl moiety activate Nrf2 signaling and subsequently induce the expression of cytoprotective enzymes including HO-1 (Zhang et al., 2004). Piperlongumine also bears two electrophilic α,β-unsaturated carbonyl groups, and hence is expected to upregulate the expression of HO-1 through activation of Nrf2 signaling. In this study, we explored the piperlongumine-induced selective killing of cancer cells with special focus on HO-1. Furthermore, we demonstrated the possible mechanism involved in piperlongumine-mediated Nrf2 activation and HO-1 induction.
MATERIALS AND METHODS
Materials
Piperlongumine was purchased from Biovision (USA). Dulbecco’s Modified Eagle Medium (DMEM) was obtained from Welgene (Korea). Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s nutrient mixture F-12, horse serum and fetal bovine serum (FBS) were produced from Gibco BRL (USA). The primary antibody against HO-1 was purchased from Stressgen (Canada), and an antibody against cleaved poly (ADP-ribose) polymerase (PARP) was obtained from Cell Signaling (USA). Primary antibodies against Nrf2, lamin B and α-tubulin were purchased from Santa Cruz Biotechnology (USA). The anti-rabbit, anti-mouse and anti-goat horseradish peroxidase-conjugated secondary antibodies were obtained from Zymed Laboratories (USA). Zinc protoporphyrin (ZnPP) was the product of Alexis Corporation (Lausen, Switzerland). N-Acetyl-L-cysteine (NAC), (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (trolox) and dithiothreitol (DTT) were supplied from Sigma Chemical Co. (USA).
Cell culture
MCF-7 and MCF-10A cells were purchased from American Type Culture Collection (USA). MCF-7 cells were cultured in DMEM with 10% FBS, 100 μg/ml streptomycin and 100 U/ml penicillin, and MCF-10A cells were maintained in DMEM/F12 medium supplemented with 10 μg/ml insulin (bovine), 100 ng/ml cholera toxin, 0.5 μg/ml hydrocortisone, 20 ng/ml recombinant human epidermal growth factor, 2 mM L-glutamine, 100 μg/ml penicillin/streptomycin/fungi zone mixture and 5% heat-inactivated horse serum in humidified 5% CO2 at 37°C.
MTT assay
Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma). In brief, MCF-10A and MCF-7 cells were plated at a density of 3 × 104 cells in 48-well plates and cultured at 37°C for 24 h. The medium was then replaced with fresh medium containing piperlongumine at various concentrations (0, 1 and 5 μM). After incubation for 24 h, the supernatant was removed and cells were treated with MTT solution (0.25 mg/ml) for 2 h at 37°C. The formazan crystals that had formed in viable cells were measured at 570 nm using a microplate reader. The results are expressed as the percentage of MTT reduction, assuming that the absorbance of control cells was 100%.
Flow cytometry analysis
Apoptotic cell death was measured by annexin-V-FITC staining according to the manufacturer’s instructions. Cells were treated with piperlongumine for 36 h and collected. Washed cell pellets were resuspended in 100 μl of annexin V binding buffer (Invitrogen, USA) and incubated with 5 μl of FITC-conjugated annexin V (Invitrogen) and 1 μl of propidium iodide (Invitrogen) for 15 min in the dark. Annexin V binding buffer (400 μl) was then added, and the cells were analyzed by flow cytometry.
Cell cycle analysis
MCF-10A and MCF-7 cells were treated with 5 μM of piperlongumine for 24 h and harvested. After washing with ice-cold PBS, the cells were fixed using 500 μl of ice-cold 70% ethanol, and incubated overnight at 4°C. Cells were washed twice with 500 μl of PBS and incubated in 500 μl of PBS containing 50 μg/ml of propidium iodide and 50 μl of 1 mg/ml of RNase A in the dark for 30 min at room temperature. Cell cycle was analyzed by flow cytometry.
Preparation of cytosolic and nuclear extracts
MCF-10A and MCF-7 cells were collected and suspended in 100 μl of hypotonic buffer A [10 mM HEPES (pH 7.8), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.2 mM phenylmethanesulphonylfluoride (PMSF)] for 15 min on ice, and 1 μl of 10% Nonidet P-40 solution was added for 5 min. The mixture was centrifuged at 12,000 × g for 5 min. The supernatant containing cytosolic proteins was collected and stored at −70°C. The pellets were washed with hypotonic buffer and resuspended in hypertonic buffer C [20 mM HEPES (pH 7.8), 20% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF] for 1 h on ice and centrifuged at 12,000 × g for 7 min. The supernatant containing nuclear proteins was collected and stored at −70°C after determination of protein concentrations. The protein concentration of the cytosolic and nuclear extracts was determined using the BCA protein assay kit (Pierce, USA).
Protein extraction and Western blot analysis
Cell extracts were prepared by suspending cells directly in the radioimmunoprecipitation assay (RIPA) buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leuptin, 1 mM PMSF] for 1 h on ice, followed by centrifugation for 15 min at 12000 × g. Protein lysates (15 μg) were electrophoresed by sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis (PAGE), and the separated proteins were transferred to a polyvinyl difluoride (PVDF) membrane (0.22 μm thickness; Gelman Laboratory, USA). To block the non-specific binding of proteins with primary antibodies, the blots were incubated in a 5% non-fat dry milk-PBST buffer [PBS containing 0.1% Tween-20] for 1 h at room temperature. The membranes were then incubated with the primary antibody suspended in 3% non-fat milk PBST buffer overnight at 4°C. This was followed by washing with 1× PBST and incubation using appropriate secondary antibody coupled to horseradish peroxidase. Proteins tagged with specific primary antibodies were visualized with an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, UK).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from MCF10A and MCF7 cells using TRIzol reagent (Invitrogen). One microgram of total RNA was reverse transcribed with murine leukemia virus reverse transcriptase (Promega, USA) at 42°C for 50 min and at 72°C for 15 min. Reverse transcriptase-PCR was performed following standard procedures. PCR conditions for HO-1 and the house keeping gene, glyceraldehydes-3-phosphate dehydrogenase (GAPDH), were as follows: 30 cycles of 95°C for 30 s; 55°C for 30 s and 72°C for 1 min. The primers used for the reverse transcription-PCR are as follows (forward and reverse, respectively): HO-1; 5′-CAGGCAGAGAATGCTGAGTTC-3′ and 5′-GATGTTGAGCAGGAACGCAGT-3′ and GAPDH; 5′-TGAAGGTCGGTGTCAACGGATTTGGC-3′ and 5′-CATGTAGGCCATGAGGTCCACCAC-3′. Amplification products were analyzed on 2% agarose gel electrophoresis, stained with ethidium bromide and photographed under ultraviolet light.
Transfection
siRNA oligonucleotides targeting HO-1 and Nrf2 were purchased from Santa Cruz Biotechnology. Cells (3 × 105/60-mm dish) were transfected with 25 nM of specific or scrambled siRNA oligonucleotides using Lipofectamine RNAiMAX according to the manufacturer’s instructions (Invitrogen).
Measurement of glutathione levels
Cell pellets were resuspended in 5% metaphosphoric acid (Sigma-Aldrich), and supernatants were collected to determine total glutathione and oxidized glutathione (GSSG) concentrations using the EnzyChrom GSH/GSSG assay kit according to the manufacturer’s protocol (Bioassay systems, USA). 5,5′-Dithiobis (2-nitrobenzoic acid) (DNTB) reacts with reduced GSH and subsequently forms a yellow product. The change in absorbance was monitored at 410 nm for 10 min, and the reduced glutathione (GSH) concentration was obtained by subtracting the oxidized from the total concentration.
Measurement of intracellular accumulation of reactive oxygen species
The intracellular accumulation of hydrogen peroxide was assessed by flow cytometry using the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCF-DA) (Molecular Probe, USA). Cells were washed twice with Hanks balanced salt solution (HBSS; Cellgro, USA) and incubated with 10 μM of DCF-DA in humidified 5% CO2 at 37°C. After 30 min, cells were washed twice with HBSS solution, suspended in complete media and analyzed by flow cytometry.
In vitro pull-down assay
Cell lysates (500 mg) were incubated with either Sepharose 4B or piperlongumine-Sepharose 4B beads in reaction buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% NP-40, and 2 mg/ml bovine serum albumin). After incubation with gentle rocking overnight at 4°C, the beads were washed with buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT and 0.01% NP-40], and the interaction between piperlongumine and Keap1 was visualized by Western blot analysis.
Statistical analysis
Values were expressed as the mean ± S.D. of three independent experiments. Statistical significance was determined by Student’s t-test and the p-value of less than 0.05 was considered to be statistically significant.
RESULTS
Piperlongumine selectively kills human breast cancer MCF-7 cells but not human MCF-10A breast epithelial cells
To determine the effects of piperlongumine on the viability of normal and cancer cells, human MCF-10A breast epithelial cells and breast cancer MCF-7 cells were stimulated with varying concentrations of piperlongumine for 24 h. As shown in Fig. 1A, piperlongumine increased MCF-7 cell death in a concentration-dependent manner, but it barely affected the viability of MCF-10A cells. Maximum selective killing effects of piperlongumine on cancer cells were observed at 5 μM. Next, we evaluated apoptosis in MCF-10A and MCF-7 cells after piperlongumine (5 μM) treatment. Through annexin V and PI double staining, it was revealed that piperlongumine-treated MCF-7 cells underwent apoptosis whereas MCF-10A cells were less sensitive to piperlongumine-induced apoptosis. The apoptosis indices were 21.9% in MCF-7 cells and 6.7% in MCF-10A cells, respectively, after treatment of piperlongumine for 36 h (Fig. 1B). Moreover, piperlongumine significantly increased the proportion of MCF-7 cells in the sub-G1 stage, but not in MCF-10A cells (Fig. 1C). We then examined PARP cleavage, a hallmark of apoptosis. Following piperlongumine treatment, cleaved PARP levels increased in time- (Fig. 1D) and dose-dependent (Fig. 1E) manners in MCF-7 cells, but not in MCF-10A cells. These data clearly show that piperlongumine has a cancer cell-selective killing effect.
Fig. 1.
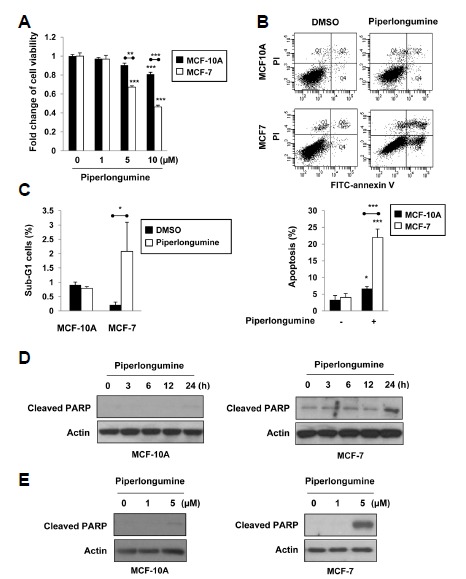
Piperlongumine induces apoptosis in human breast cancer MCF-7 cells relative to human MCF-10A breast epithelial cells. (A) MCF-10A and MCF-7 cells were treated with 0, 1, 5 or 10 μM of piperlongumine for 24 h. Cell viability was evaluated by the MTT assay. (B, C) Cells were treated with 5 μM of piperlongumine for 36 h. Quantification of apoptosis was determined by flow cytometry (B). The proportion of the cells at the sub-G1 phase was also assessed by flow cytometry analysis as described in the Materials and Methods section (C). Means ± S.D. (n=3), *p < 0.05, **p < 0.01, ***p < 0.001. (D, E) MCF-10A and MCF-7 cells were treated with 5 μM of piperlongumine for the indicated time periods (D) and 0, 1 or 5 μM of piperlongumine for 24 h (E). Total protein isolated from cell lysates was subjected to immunoblot analysis for the measurement of cleaved PARP. Actin was used as an equal loading control for normalization.
Piperlongumine-induced HO-1 expression plays a different role in MCF-10A and MCF-7 cells, thereby mediating the selective effect on cancer cell apoptosis
To elucidate the mechanism underlying the selective killing effect of piperlongumine on MCF-7 cells, we examined the level of cytoprotective proteins including HO-1 and Nrf2 after piperlongumine treatment. In contrast to our expectation, although piperlongumine selectively induced apoptosis in MCF-7 cells, HO-1 and Nrf2 expression levels were upregulated by piperlongumine in both MCF-10A and MCF-7 cells (Figs. 2A and 2B). Piperlongumine upregulated the expression of HO-1 and Nrf2 in MCF-10A cells at an earlier time compared to MCF-7 cells. In parallel with the elevated protein level of HO-1, the expression of its mRNA transcript was also increased in both MCF-10A and MCF-7 cells after piperlongumine treatment (Fig. 2C). To investigate whether HO-1 plays a role in determining the differential effects of piperlongumine, we utilized siRNA against HO-1. As shown in Fig. 2D, piperlongumine increased the level of cleaved PARP in MCF-10A cells transfected with HO-1 siRNA, indicating that HO-1 protects MCF-10A cells from piperlongumine-induced apoptosis. In contrast to MCF-10A cells, MCF-7 cells became less sensitive to piperlongumine-induced apoptosis when HO-1 was silenced (Figs. 2D and 2E), suggesting that piperlongumine triggers apoptosis of MCF-7 cells via HO-1 induction. The different role of HO-1 in piperlongumine-treated MCF-10A and MCF-7 cells was also confirmed by ZnPP (a pharmacological inhibitor of HO-1 activity) treatment. Similar to HO-1 siRNA, ZnPP abrogated piperlongumine-induced apoptosis in MCF-7 cells, but stimulated apoptosis of MCF-10A cells in response to piperlongumine (Fig. 2F). These findings suggest that HO-1 is the key protein involved in determining the selective effect of piperlongumine on cancer cell apoptosis. HO-1 protects MCF-10A cells from piperlongumine-induced apoptosis while amplifying the cytotoxicity of piperlongumine in MCF-7 cells.
Fig. 2.
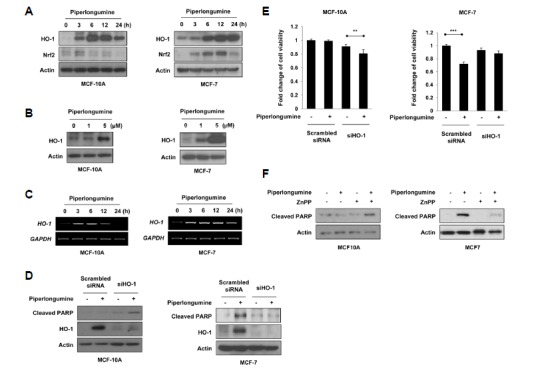
HO-1 mediates the selective effect of piperlongumine on cancer cell apoptosis. (A-C) MCF-10A and MCF-7 cells were treated with 5 μM of piperlongumine for the indicated time periods (A, C) and 0, 1 or 5 μM of piperlongumine for 24 h (B). (A, B) Total protein isolated from cell lysates was subjected to immunoblot analysis for the measurement of HO-1 and Nrf2. Actin was used as an equal loading control for normalization. (C) The expression of HO-1 mRNA was determined by semi-quantitative RT-PCR. The level of GAPDH mRNA was used as an internal control. (D, E) Cells were transfected with scrambled or HO-1 siRNA for 24 h, and then exposed to piperlongumine for additional 24 h. The protein levels of cleaved PAPR, HO-1 and actin were determined by Western blot analysis (D). Cell viability was evaluated by the MTT assay (E). Means ± S.D. (n = 3), **p < 0.01, ***p < 0.001. (F) MCF-10A and MCF-7 cells were treated with piperlongumine in the absence or presence of ZnPP for 24 h. The protein levels of cleaved PAPR, HO-1 and actin were determined by Western blot analysis.
Piperlongumine-induced Nrf2 activation and HO-1 expression do not rely on ROS generation
Because Nrf2 is one of major transcription factors involved in HO-1 regulation (Itoh et al., 1999), we examined whether piperlongumine could activate Nrf2 signaling. Following piperlongumine treatment, Nrf2 was translocated into the nucleus in both MCF-10A and MCF-7 cells (Fig. 3A). To determine whether HO-1 upregulation by piperlongumine was mediated via Nrf2 activation, cells were transiently transfected with siRNA against Nrf2. As shown in Fig 3B, piperlongumine failed to upregulate HO-1 expression in Nrf2 knockdown cells, indicating that activation of Nrf2 signaling is important for piperlongumine-induced HO-1 expression. It has been reported that piperlongumine modulates redox signaling, thereby increasing ROS production in cancer cells but not in normal cells (Raj et al., 2011). In the present study, we also observed that piperlongumine treatment lowered total and GSH levels, while increasing the level of GSSG in MCF-7 cells. However, there were no significant changes in the total GSH level and the GSH/GSSG ratio in piperlongumine-treated MCF-10A cells compared with those in DMSO-treated cells (Fig. 4A). Besides GSH depletion, piperlongumine treatment led to accumulation of intracellular ROS in MCF-7 cells, but this ROS generation was abrogated by pretreatment of NAC or trolox (Fig. 4B). Next, we examined the possible involvement of ROS in piperlongumine-induced Nrf2 activation and HO-1 expression. As shown in Figs. 4C and 4D, piperlongumine-induced nuclear translocation of Nrf2 and upregulation of HO-1 expression were markedly suppressed by NAC treatment in MCF-7 cells. Although piperlongumine did not increase ROS levels in MCF-10A cells, NAC also abolished piperlongumine-induced Nrf2 activation and HO-1 overexpression in MCF-10A cells, indicating that NAC, which can act as a thiol reducing agent, directly inhibited piperlongumine-mediated stimulation of the Nrf2/HO-1 pathway, rather than acting as a ROS scavenger. To confirm the role of ROS in piperlongumine-mediated effects, cells were treated with piperlongumine in the absence or presence of trolox, which also has ROS scavenging activity. Trolox failed to reverse the effect of piperlongumine on Nrf2 activation and HO-1 expression both in MCF-10A and MCF-7 cells (Figs. 4E and 4F). Moreover, the selective killing effect of piperlongumine on MCF-7 cells was not inhibited by trolox treatment (Fig. 4F). These results suggest that piperlongumine-induced Nrf2 activation, HO-1 expression and cancer cell death do not rely on ROS generation.
Fig. 3.
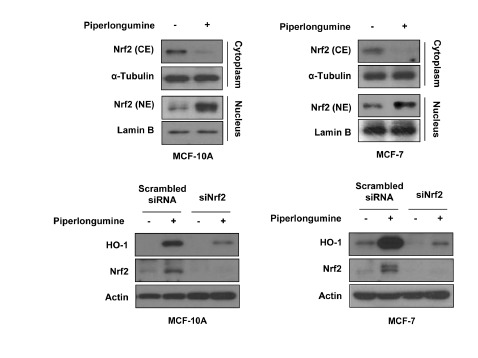
Piperlongumine upregulates HO-1 expression through Nrf2 activation. (A) Cytosolic and nuclear extracts were prepared from MCF-10A and MCF-7 cells treated with or without piperlongumine (5 μM) for 3 h (MCF-10A) or 12 h (MCF-7). The protein levels of Nrf2 were measured by Western blot analysis. α-Tubulin and Lamin B1 was used as an equal loading control for normalization. (B) Cells were transfected with scrambled or Nrf2 siRNA for 24 h, and then treated with piperlongumine for additional 3 h (MCF-10A) or 12 h (MCF-7). The protein levels of HO-1, Nrf2 and actin were determined by Western blot analysis.
Fig. 4.
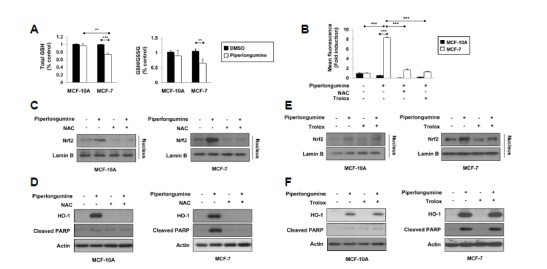
Piperlongumine-induced Nrf2 activation and HO-1 expression is not dependent on ROS generation. (A) Reduced GSH and GSSG levels were determined after cells were treated with piperlongumine for 3 h. (B) MCF-10A and MCF-7 cells were stimulated with piperlongumine in the absence or presence of either NAC or trolox for 3 h, and the intracellular ROS levels were measured by flow cytometry as described in the Materials and methods section. Means ± S.D. (n = 3), ** p < 0.01, *** p < 0.001. (C, E) Cells were pretreated with either NAC (C) or trolox (E) for 2 h, followed by piperlongumine treatment for 3 h (MCF-10A) or 12 h (MCF-7). Nuclear extracts were subjected to immunoblot analysis for the measurement of Nrf2. Lamin B was used as an equal loading control for normalization. (D, F) MCF-10A and MCF-7 cells were pretreated with either NAC (D) or trolox (F) for 2 h, followed by treatment of piperlongumine for 24 h. Levels of HO-1, cleaved PARP and actin were measured by Western blot analysis.
Piperlongumine induces Nrf2 activation and HO-1 expression through modification of Keap1 cysteine residues
It is well established that Keap1 is bound to Nrf2 under physiological conditions and inhibits the nuclear translocation of Nrf2. Oxidative stress or electrophiles can cause oxidation or covalent modification of cysteine residues present in Keap1, thereby activating Nrf2 signaling (Kim et al., 2014). Considering that piperlongumine contains the α,β-unsaturated carbonyl moiety (Fig. 5A), we expected that piperlongumine-induced Nrf2 activation and HO-1 expression is mediated by thiol modification of cysteine residues present in Keap1. In support with our hypothesis, the effects of piperlongumine on the nuclear translocation of Nrf2 and the expression of HO-1 were abrogated when cells were pre-treated with DTT, a thiol reducing agent (Figs. 5B and 5C). To investigate whether piperlongumine induces thiol modification through direct binding to Keap1, pull-down assays were performed using piperlongumine-conjugated Sepharose 4B beads with MCF-10A and MCF-7 cell lysates. As shown in Fig. 5D, piperlongumine directly bound to Keap1 both in MCF-10A and MCF-7 cells. These data imply that piperlongumine induces Nrf2 activation and HO-1 expression through modification of Keap1 cysteine residues.
Fig. 5.
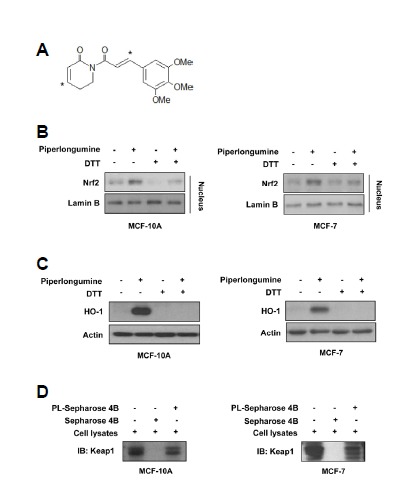
Interaction of piperlongumine with cysteine residues within Keap1 activates Nrf2/HO-1 pathway. (A) The structure of piperlongu-mine. Electrophilic carbons containing α,β-unsaturated carbonyl moiety are denoted by asterisks. (B) Cells were treated with 5 μM of piperlongumine in the absence or presence of DTT for 3 h (MCF-10A) or 12 h (MCF-7). Nuclear extracts were subjected to immunoblot analysis for the measurement of Nrf2. Lamin B was used as an equal loading control for normalization. (C) MCF-10A and MCF-7 cells were exposed to piperlongumine for 24 h. Levels of HO-1, cleaved PARP and actin were measured by Western blot analysis. (D) Cell lysates were incubated with piperlongumine-conjugated Sepharose 4B beads (PL-Sepharose 4B) or Sepharose 4B beads alone, and then the pulled-down Keap1 was detected by immunoblot analysis.
DISCUSSION
Since conventional chemotherapy for breast cancer treatment faces serious challenges such as damage to normal cells, drug resistance and toxic side effects, a dietary phytochemical has been increasingly considered as an alternative due to its safety as well as its efficient therapeutic effect. To date, various phytochemicals have been found to exert anti-oxidant, anti-inflammatory, anti-proliferative and pro-apoptotic effects, thereby being utilized as a source of anticancer drugs as well as chemopreventive agents (Russo et al., 2010). Piperlongumine, a biologically active alkaloid/amide derived from the long pepper (Piper longum L.), has been widely used in Indian Ayurvedic medicine for thousands of years to treat bronchitis, asthma, respiratory infections, cholera, malaria, diarrhea, stomachache and tumors (Bezerra et al., 2012). Interestingly, the most recent finding implies a cancer cell-selective killing effect of piperlongumine (Raj et al., 2011). In the present study, we also found that piperlongumine selectively kills human breast cancer MCF-7 cells but not human MCF-10A breast epithelial cells, supporting the potential of piperlongumine as a promising therapeutic for breast cancer treatment.
As a consequence of malignant transformation, cancer cells become to have high levels of ROS compared to normal cells, and show a strong reliance on the ROS stress-response pathway. Accumulating data indicate that ROS plays a key role in the cancer cell-selective cytotoxicity of piperlongumine (Liu et al., 2013; Raj et al., 2011). Through direct binding to GSTP1 and CBR1 involved in oxidative stress balance, piperlongumine can selectively decrease the level of reduced GSH and increase the level of oxidized GSSG, leading to ROS accumulation and subsequent apoptosis in cancer cells (Raj et al., 2011). In agreement with this report, we observed piperlongumine-mediated depletion of GSH, a reduction in the GSH/GSSG ratio and accumulation of intracellular ROS in MCF-7 cells but not in MCF-10A cells. However, piperlongumine-induced ROS generation is unlikely to be involved in the pro-apoptotic effect of piperlongumine in cancer cells. Although piperlongumine-mediated accumulation of ROS was completely blocked by pretreatment with NAC or trolox, both of which have the ability to quench ROS, piperlongumine-induced apoptosis was abrogated only in NAC-treated MCF7 cells but not in trolox-treated cells. Besides acting as a ROS scavenger, NAC is used as a precursor of GSH and a thiol reducing agent, thereby increasing the cell content of GSH and preventing oxidation of thiol groups (Dent et al., 1997). Based on this notion, we speculate that NAC-mediated suppression of apoptosis is mediated by inhibition of piperlongumine-induced thiol modification of proteins rather than preventing ROS generation.
Even though the role of HO-1 in cancer cells remains controversial, growing evidence supports the anti-tumor action of HO-1. Hill et al. (2005) showed that upregulation of HO-1 expression by cobalt protoporphyrin or transfection with adenoviral vectors containing the HO-1 gene led to decreased proliferation of breast cancer cell. Moreover, HO-1 induction lowered the lymph node metastasis in human colorectal and oral carcinomas (Becker et al., 2007; Tsuji et al., 1999) while downregulation of HO-1 expression stimulated malignant progression of hepatocellular carcinomas (Caballero et al., 2004). In agreement with these reports, we found that piperlongumine upregulated HO-1 expression, and suppression of HO-1 expression and activity abrogated the ability of piperlongumine to induce apoptosis in MCF-7 cells. To the best of our knowledge, this is the first report demonstrating that piperlongumine induces breast cancer cell apoptosis through the upregulation of HO-1 expression. Notably, HO-1 determines the differential response of MCF-10A and MCF-7 cells to piperlongumine. Piperlongumine increased the expression of HO-1 not only in MCF-7 cells, but MCF-10A cells. However, in contrast to MCF-7 cells, MCF-10A cells were sensitized to the cytotoxicity of piperlongumine when HO-1 expression was silenced or its activity was blocked, indicative of the cytoprotective action of HO-1 in normal cells. Taken together, piperlongumine-induced HO-1 appears to act differently depending on the cell type, mediating the killing effect of piperlongumine in cancer cells and the protective effect in normal cells.
The transcriptional activation of HO-1 is mainly regulated by the redox-sensitive transcription factor, Nrf2. Upon electrophilic or oxidative stress, Nrf2 dissociates from the Keap1-E3 ubiquitin ligase complex, translocates into the nucleus and initiates the transcription of HO-1 via ARE binding sites (Itoh et al., 1997). In the present study, we have found that piperlongumine upregulates HO-1 expression through Nrf2 activation in both MCF-10A and MCF-7 cells. The ability of piperlongumine to induce Nrf2-mediated HO-1 expression was attenuated by pretreatment with NAC. However, it was assumed that NAC reverses piperlongumine-mediated Nrf2 activation and HO-1 induction not by scavenging ROS because piperlongumine did not lead to an increasing ROS levels in MCF-10A, and trolox failed to suppress piperlongumine-induced Nrf2 activation and HO-1 expression in both MCF-10A and MCF-7 cells. These findings suggest that piperlongumine-induced Nrf2 activation and HO-1 expression do not rely on ROS generation.
It has been suggested that various chemopreventive agents activate Nrf2 signaling though cysteine modification-mediated Keap1 inactivation (Kobayashi et al., 2006). Through site-directed mutagenesis of cysteine residues within Keap1, it has been revealed that Cys151, Cys273 and Cys288 are essential for regulating Nrf2 activation. These cysteines function as cellular redox sensors and can be subjected to oxidative or covalent modification (Kobayashi et al., 2009; Yamamoto et al., 2008; Zhang et al., 2004). Since piperlongumine possesses two electrophilic α,β-unsaturated carbonyl groups capable of altering reactive cysteines, we expected that piperlongumine stimulates Nrf2 activation through direct cysteine modification. To examine the possibility of piperlongumine-mediated Nrf2 through direct Keap1 cysteine thiol modification, we utilized DTT, a strong reducing agent which can interfere with cysteine modification by electrophiles. In the presence of DTT, piperlongumine failed to upregulate Nrf2 activation and HO-1 expression in MCF-10A and MCF-7 cells, indicating that DTT keeps Keap1 thiol groups in a reduced form, thereby hampering interaction between piperlongumine and critical Keap1 cysteine residues. Moreover, our study clearly showed that piperlongumine directly bound to Keap1 as determined by the pull-down assays.
In conclusion, the results from our present study demonstrate a novel mechanism underlying a cancer cell-selective killing effect of piperlongumine (Fig. 6). We suggest that HO-1 is a key factor determining the differential response of breast normal cells and cancer cells to piperlongumine, and the thiol modification of Keap1 is responsible for piperlongumine-induced activation of Nrf2 signaling and HO-1 expression.
Fig. 6.
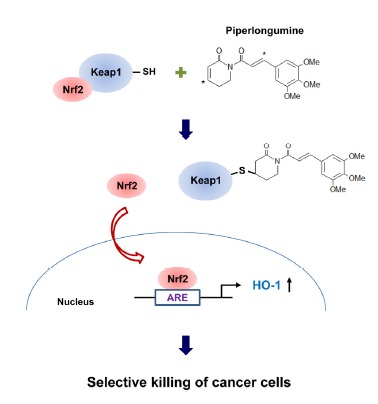
The proposed mechanism underlying a cancer cell-selective killing effect of piperlongumine. Through direct binding, piperlongumine induces thiol modification of Keap1, thereby allowing nuclear translocation of Nrf2 and subsequent upregulation of HO-1 expression in normal and cancer cells. HO-1 determines the differential response of breast normal cells and cancer cells to piperlongumine, resulting in selective killing of cancer cells.
Acknowledgments
This research has been supported by grants from the Radiation Bio-Resource Research Program of the Korea Institute of Radiological and Medical Sciences (No. 740802) funded by the Ministry of Education, Science and Technology in the Republic of Korea.
REFERENCES
- Alam J., Stewart D., Touchard C., Boinapally S., Choi A.M., Cook J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol.Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Bae J., Lee D., Kim Y.K., Gil M., Lee J.Y., Lee K.J. Berberine protects 6-hydroxydopamine-induced human dopaminergic neuronal cell death through the induction of heme oxygenase-1. Mol. Cells. 2013;35:151–157. doi: 10.1007/s10059-013-2298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M., Bauer I. Heme oxygenase-1: Redox regulation and role in the hepatic response to oxidative stress. Antioxid. Redox Signal. 2002;4:749–758. doi: 10.1089/152308602760598891. [DOI] [PubMed] [Google Scholar]
- Becker J.C., Fukui H., Imai Y., Sekikawa A., Kimura T., Yamagishi H., Yoshitake N., Pohle T., Domschke W., Fujimori T. Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand. J. Gastroenterol. 2007;42:852–858. doi: 10.1080/00365520701192383. [DOI] [PubMed] [Google Scholar]
- Bezerra D.P., Pessoa C., de Moraes M.O., Saker-Neto N., Silveira E.R., Costa-Lotufo L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2012;48:453–463. doi: 10.1016/j.ejps.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Caballero F., Meiss R., Gimenez A., Batlle A., Vazquez E. Immunohistochemical analysis of heme oxygenase-1 in preneoplastic and neoplastic lesions during chemical hepatocarcinogenesis. Int. J. Exp. Pathol. 2004;85:213–221. doi: 10.1111/j.0959-9673.2004.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt B.S., Ye L.X., Dinkova-Kostova A.T., Erb M., Fahey J.W., Singh N.K., Chen M.S.A., Stierer T., Garrett-Mayer E., Argani P., et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- Dent G., Rabe K.F., Magnussen H. Augmentation of human neutrophil and alveolar macrophage LTB4 production by N-acetylcysteine: role of hydrogen peroxide. Br. J. Pharmacol. 1997;122:758–764. doi: 10.1038/sj.bjp.0701428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing P., Wilke A., Eissner G., Holler E., Andreesen R., Gerbitz A. Expression of heme oxygenase-1 protects endothelial cells from irradiation-induced apoptosis. Endothelium. 2005;12:113–119. doi: 10.1080/10623320500189814. [DOI] [PubMed] [Google Scholar]
- Hill M., Pereira V., Chauveau C., Zagani R., Remy S., Tesson L., Mazal D., Ubillos L., Brion R., Ashgar K., et al. Heme oxygenase-1 inhibits rat and human breast cancer cell proliferation: mutual cross inhibition with indoleamine 2,3-dioxygenase. FASEB J. 2005;19:1957–1968. doi: 10.1096/fj.05-3875com. [DOI] [PubMed] [Google Scholar]
- Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Keum Y.S., Han Y.H., Liew C., Kim J.H., Xu C.J., Yuan X.L., Shakarjian M.P., Chong S.H., Kong A.N. Induction of heme oxygenase-1 (HO-1) and NAD[P]H: Quinone oxidoreductase 1 (NQO1) by a phenolic antioxidant, butylated hydroxyanisole (BHA) and its metabolite, tertbutylhydroquinone (tBHQ) in primary-cultured human and rat hepatocytes. Pharm. Res. 2006;23:2586–2594. doi: 10.1007/s11095-006-9094-2. [DOI] [PubMed] [Google Scholar]
- Kim H.J., So H.S., Lee J.H., Lee J.H., Park C., Park S.Y., Kim Y.H., Youn M.J., Kim S.J., Chung S.Y., et al. Heme oxygenase-1 attenuates the cisplatin-induced apoptosis of auditory cells via down-regulation of reactive oxygen species generation. Free Radic. Biol. Med. 2006;40:1810–1819. doi: 10.1016/j.freeradbiomed.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Lee I.C., Baek H.S., Shin I.S., Moon C., Bae C.S., Kim S.H., Kim J.C., Kim H.C. Mechanism for the protective effect of diallyl disulfide against cyclophosphamide acute urotoxicity in rats. Food Chem. Toxicol. 2014;64:110–118. doi: 10.1016/j.fct.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kang M.I., Watai Y., Tong K.I., Shibata T., Uchida K., Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The Antioxidant Defense System Keap1-Nrf2 Comprises a Multiple Sensing Mechanism for Responding to a Wide Range of Chemical Compounds. Mol. Cell. Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.Y., Chen Y.C., Shih C.M., Lin C.M., Cheng C.H., Chen K.C., Lin C.W. The induction of heme oxygenase-1 suppresses heat shock protein 90 and the proliferation of human breast cancer cells through its byproduct carbon monoxide. Toxicol. Appl. Pharmacol. 2014;274:55–62. doi: 10.1016/j.taap.2013.10.027. [DOI] [PubMed] [Google Scholar]
- Lin H.Y., Shen S.C., Chen Y.C. Anti-inflammatory effect of heme oxygenase 1: Glycosylation and nitric oxide inhibition in macrophages. J. Cell Physiol. 2005;202:579–590. doi: 10.1002/jcp.20160. [DOI] [PubMed] [Google Scholar]
- Lin H.Y., Shen S.C., Lin C.W., Yang L.Y., Chen Y.C. Baicalein inhibition of hydrogen peroxide-induced apoptosis via ROS-dependent heme oxygenase 1 gene expression. Biochim. Biophys. Acta. 2007;1773:1073–1086. doi: 10.1016/j.bbamcr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Lin C.W., Shen S.C., Hou W.C., Yang L.Y., Chen Y.C. Heme oxygenase-1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase-9. Mol. Cancer Ther. 2008;7:1195–1206. doi: 10.1158/1535-7163.MCT-07-2199. [DOI] [PubMed] [Google Scholar]
- Liu J.M., Pan F., Li L., Liu Q.R., Chen Y., Xiong X.X., Cheng K.J., Bin Yu. S., Shi Z., Yu A.C.H., et al. Piperlongumine selectively kills glioblastoma multiforme cells via reactive oxygen species accumulation dependent JNK and p38 activation. Biochem. Biophys. Res. Commun. 2013;437:87–93. doi: 10.1016/j.bbrc.2013.06.042. [DOI] [PubMed] [Google Scholar]
- Raj L., Ide T., Gurkar A.U., Foley M., Schenone M., Li X., Tolliday N.J., Golub T.R., Carr S.A., Shamji A.F., et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rao V.R., Muthenna P., Shankaraiah G., Akileshwari C., Babu K.H., Suresh G., Babu K.S., Chandra Kumar R.S., Prasad K.R., Yadav P.A., et al. Synthesis and biological evaluation of new piplartine analogues as potent aldose reductase inhibitors (ARIs). Eur. J. Med. Chem. 2012;57:344–361. doi: 10.1016/j.ejmech.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M., Spagnuolo C., Tedesco I., Russo G.L. Phytochemicals in Cancer Prevention and Therapy: Truth or Dare? Toxins. 2010;2:517–551. doi: 10.3390/toxins2040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son D.J., Kim S.Y., Han S.S., Kim C.W., Kumar S., Park B.S., Lee S.E., Yun Y.P., Jo H., Park Y.H. Piperlongumine inhibits atherosclerotic plaque formation and vascular smooth muscle cell proliferation by suppressing PDGF receptor signaling. Biochem. Biophys. Res. Commun. 2012;427:349–354. doi: 10.1016/j.bbrc.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M.H., Yanagawa T., Iwasa S., Tabuchi K., Onizawa K., Bannai S., Toyooka H., Yoshida H. Heme oxygenase-1 expression in oral squamous cell carcinoma as involved in lymph node metastasis. Cancer lett. 1999;138:53–59. doi: 10.1016/s0304-3835(98)00372-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Suzuki T., Kobayashi A., Wakabayashi J., Maher J., Motohashi H., Yamamoto M. Physiological significance of reactive cysteine residues of keap1 in determining Nrf2 activity. Mol. Cell. Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.C., Lee S.G., Lee H.K., Kim M.K., Lee S.H., Lee H.S. A piperidine amide extracted from Piper longum L. fruit shows activity against Aedes aegypti mosquito larvae. J. Agric. Food Chem. 2002;50:3765–3767. doi: 10.1021/jf011708f. [DOI] [PubMed] [Google Scholar]
- Zhang D.D., Lo S.C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C., Zhang H., Li Q., Xiao H., Yu L., Ke S., Zhou L., Liu W., Wang W., Huang H., et al. Heme oxygenase-1: a molecular brake on hepatocellular carcinoma cell migration. Carcinogenesis. 2011;32:1840–1848. doi: 10.1093/carcin/bgr225. [DOI] [PubMed] [Google Scholar]


