Background: Pentatricopeptide repeat (PPR) proteins that are required for RNA editing frequently include a C-terminal DYW deaminase domain.
Results: Mutagenesis of a glutamate residue in the conserved deaminase HXE motif results in loss of editing activity.
Conclusion: The glutamate residue is required for editing.
Significance: The DYW deaminase domain of PPR proteins has the molecular characteristics of a deaminase.
Keywords: Cytidine Deaminase, Metalloenzyme, RNA-binding Protein, RNA Editing, RNA Metabolism, Chloroplasts, Zinc, DYW Deaminase Domain
Abstract
Many transcripts expressed from plant organelle genomes are modified by C-to-U RNA editing. Nuclear encoded pentatricopeptide repeat (PPR) proteins include an RNA binding domain that provides site specificity. In addition, many PPR proteins include a C-terminal DYW deaminase domain with characteristic zinc binding motifs (CXXC, HXE) and has recently been shown to bind zinc ions. The glutamate residue of the HXE motif is catalytically required in the reaction catalyzed by cytidine deaminase. In this work, we examine the activity of the DYW deaminase domain through truncation or mutagenesis of the HXE motif. OTP84 is required for editing three chloroplast sites, and transgenes expressing OTP84 with C-terminal truncations were capable of editing only one of the three cognate sites at high efficiency. These results suggest that the deaminase domain of OTP84 is required for editing two of the sites, but another deaminase is able to supply the deamination activity for the third site. OTP84 and CREF7 transgenes were mutagenized to replace the glutamate residue of the HXE motif, and transgenic plants expressing OTP84-E824A and CREF7-E554A were unable to efficiently edit the cognate editing sites for these genes. In addition, plants expressing CREF7-E554A exhibited substantially reduced capacity to edit a non-cognate site, rpoA C200. These results indicate that the DYW deaminase domains of PPR proteins are involved in editing their cognate editing sites, and in some cases may participate in editing additional sites in the chloroplast.
Introduction
RNA editing takes place in most land plant chloroplasts and mitochondria (1, 2). In flowering plants, the transcripts of chloroplasts and mitochondria are modified post-transcriptionally by C-to-U editing with about 35 C-to-U editing events in chloroplasts and hundreds of editing sites in the mitochondria (3). Editing in higher plants and in Physcomitrella patens is known to require nuclear proteins (4–8).
Pentatricopeptide repeat (PPR)2 genes have been shown to be required for RNA editing (9), and form a large family of protein-coding genes in higher plants with over 400 members in Arabidopsis (10). The known editing factors are members of the PLS subfamily of PPR proteins, which are composed of characteristic P, L (long), and S (short) repeats (11). Amino acid residues located in specific locations within the repeats have been shown to specify the base recognized in the cis-element (12–14), and the PLS repeat domain interacts with specific nucleotides within the cis-element to provide site specificity for RNA editing (12, 15).
The PLS subfamily of PPR proteins also includes characteristic C-terminal domains known as the E, E+, and DYW domains (10). Bioinformatics analysis identified characteristic structural motifs present in part of the E domain, the entire E+ domain, and most of the DYW domain that place the protein in the deaminase superfamily (16). This region has been identified as the “DYW family of nucleic acid deaminases” (Pfam 14432) (17) and is referred to as the “DYW deaminase domain” in this work.
There is mounting evidence that supports the role of the DYW deaminase domain as the catalytic component of the editing reaction. This region has canonical zinc binding motifs (HXE, CXXC) (18–20), which are conserved in deaminases that act on nucleotides, RNA, and DNA (16, 21–26). The DYW deaminase domain has recently been shown to bind zinc ions (19, 20). In addition, mutagenesis of the zinc binding motifs has been shown to interfere with editing in transgenic plants (20) and through transient expression in protoplasts (27). The glutamate residue of the HXE motif has been shown to be directly involved in the E. coli cytidine deaminase mechanism through deprotonation of the substrate water molecule and transfer of the proton to the product ammonia (21). Thus, the requirement of this conserved glutamate residue is a key characteristic expected for an editing deaminase.
The editing of the ndhD C2 site in Arabidopsis chloroplasts requires two PPR proteins, CRR4 and DYW1 (28). CRR4 lacks the entire DYW deaminase domain but has a canonical PLS repeat region and is apparently required for editing as a site specificity factor (29). DYW1 has an intact DYW deaminase domain; however, the N-terminal PLS-like region is small and composed of degenerate repeats. Furthermore, DYW1 has canonical zinc binding motifs and has been shown to bind zinc ions (19, 20). Mutagenesis of the zinc binding motifs and the catalytic glutamate residue of DYW1 ablated that ability to edit the ndhD site (20). Thus, the editing of the ndhD site requires both CRR4 and DYW1, and the functions of site recognition and deamination appear to be separated into two proteins in this case.
Many PPR proteins that are required for editing lack a DYW deaminase domain. In addition, there are several examples in which the DYW deaminase domain is present in a PPR, but may be eliminated by truncation and still support editing of the cognate sites (19, 30, 31). Finally, PPR genes have been shown to undergo truncation in evolution (19), indicating that the DYW deaminase domain is dispensable in an evolutionary context. Thus, the role of the DYW deaminase domain in these editing reactions has remained elusive.
In this work, we have investigated the role of the DYW deaminase domain of two PPR proteins, OTP84 and CREF7. In contrast to DYW1 and CRR4, both of these PPR proteins have canonical PLS repeat regions and an intact DYW deaminase domain. In this work, we examine the function of the DYW deaminase domain by gene truncation and mutagenesis of the catalytic glutamate residue and show that the DYW deaminase domain is required for editing the cognate editing sites.
EXPERIMENTAL PROCEDURES
Plant Materials
Arabidopsis T-DNA lines SALK_078415C (cref7-1), SALK_120902C (otp84-2), and SALK_142061C (otp84-3) were obtained from the Arabidopsis Biological Resource Center. Seeds for Arabidopsis ecotype Columbia (Col-0) were purchased from Lehle Seeds (Round Rock, TX) and were used for the wild type line. Transgenic plant lines were produced by introducing the following genes: OTP84 (At3g57430); OTP84-trcDYW (OTP84 truncated after residue Phe-770), OTP84trcPG (OTP84 truncated before the PG box after residue Lys-754); OTP84-E824A (Glu-to-Ala substitution at residue 824); and CREF7 (At5g66520) and CREF7-E554A (Glu to Ala substitution at residue 554).
Gene Cloning and Plant Transformation
Gene sequences from OTP84, OTP84trcPG (Met-1–Lys-754), and OTP84trcDYW (Met-1–Phe-770) were amplified by PCR to introduce 5′ BamHI and 3′ SalI restriction sites. Sequences for CREF7 were amplified with flanking BglII and SalI restriction sites. Mutations were introduced by amplification with primers that altered codon Glu-824 of OTP84 and Glu-554 of CREF7 to alanine codons. All gene fragments were cloned into pCHF1 using BamHI and SalI restriction sites (32). Binary vectors were electroporated into Agrobacterium strain ASE (33), and plants were transformed by floral dip (34). Seedlings were selected using 100 mg/liter gentamicin.
RNA Editing Analysis through Bulk Sequencing
Total RNA was isolated from green leaves using RiboZol from AMRESCO (Solon, OH). The GoScript reverse transcription system (Madison, WI) was used with random hexamers from Thermo Fisher to create a cDNA pool. Gene-specific primers were used to amplify sequences including editing sites from the cDNA templates with DreamTaq Green DNA polymerase from Thermo Fisher. Bulk sequencing of RT-PCR products was carried out at the University of California, Berkeley DNA Sequencing Facility. Peak heights were measured from electropherograms using BioEdit V7.0.9.0 to estimate the extent of RNA editing in RNA templates isolated from leaves.
Poisoned Primer Extension Editing Analysis
Poisoned primer extension assays were performed as described previously (35) with one modification. Purified PCR products were treated with FastAP from Thermo Scientific and incubated for 10 min at 37 °C followed by 5 min at 75 °C. This is similar to the ExoSAP-IT step of the poisoned primer extension assay from the Hanson laboratory (36). The addition of the FastAP step consistently reduced read-through to less than 3%. Oligonucleotide primers contained a 5′-hexachlorofluorescein tag from Eurofins (Ebersberg, Germany). Vent DNA polymerase and acyNTPs from New England Biolabs (Ipswich, MA) were utilized for chain termination reactions. Primer extension products were separated by electrophoresis on 12% polyacrylamide gels with 6 m urea. Gels were covered in plastic wrap and immediately scanned using a Typhoon Trio imager from GE Healthcare (Little Chalfont, UK). The percentage of conversion was calculated from the intensity of bands measured from a gel image using GELQUANT.NET V1.8.2.
Mass Spectrometry
The DYW deaminase domain from ELI1 (At4g37380: Asp-478–Trp-632) was amplified by PCR to add flanking BamHI and SalI restriction sites. A single nucleotide mutation (GAG to GCG) was introduced using a PCR primer to create the E566A mutation. The ELI1-E566A gene was cloned into pET28a using BamHI and SalI restriction sites and introduced into E. coli strain Rosetta 2 (DE3) pLysS from Novozymes (Bagsvaerd, Denmark). Recombinant ELI1-E566A was expressed as described previously (19) and purified. Native protein samples were dialyzed in 20 mm ammonium acetate prior to mass spectrometry. Denatured protein samples were treated with 50% acetonitrile and 1% formic acid prior to mass spectrometry. Protein samples were ionized with an electrospray ionization voltage of 3.6 kV, a cone voltage of 40 V, and a desolvation temperature of 120 °C. Mass determinations were made with a Waters QTOF2 mass spectrometer.
RESULTS
Plants Expressing Truncated OTP84 Edit One of Three Cognate Sites
Previous studies have examined the role of the DYW deaminase domain by expression of truncated variants, and in several examples, these experiments have demonstrated that the DYW deaminase domain was not required for editing of the cognate sites (19, 30, 37, 38). Because OTP84 has three editing site targets, the role of the DYW deaminase domain was examined on these editing sites.
The OTP84 gene has an intact DYW deaminase domain (Fig. 1) and is required for editing of ndhF C290, psbZ C50, and ndhB C1481 (39). Two truncated variants of OTP84 were each prepared and tested for editing activity in three independent transgenic plants (Fig. 1). Truncation immediately C-terminal to the PG box eliminated most of the DYW deaminase domain (OTP84trcDYW), and truncation immediately N-terminal of the PG box eliminated all of the PG box and downstream DYW deaminase domain (OTP84trcPG). The truncated variants were introduced into an OTP84 knock-out background (39). The effects of OTP84 truncation are shown in Fig. 2. Expression of the OTP84trcDYW transgene did not restore editing of ndhF and psbZ transcripts to wild type levels (Fig. 2, A and B, respectively), suggesting that the DYW deaminase domain is required for editing these two cognate editing sites. In contrast, the editing of ndhB C1481 remained at wild type levels in transgenic plants expressing OTP84trcDYW (Fig. 2C), which suggests that editing of the ndhB site does not require the OTP84 DYW deaminase domain. Truncations that eliminate the PG box (OTP84trcPG) resulted in highly reduced editing for all three sites. This result is consistent with earlier studies performed with ELI1 that indicated that the PG box is critical for editing a site in ndhB transcripts (19). Although OTP84 is required for editing three sites, transgenes lacking a large portion of the DYW deaminase domain were capable of restoring editing to wild type levels in ndhB transcripts, suggesting the participation of an unknown DYW deaminase(s) for that site.
FIGURE 1.
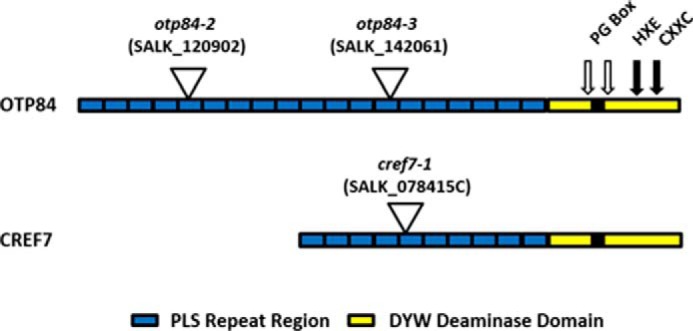
PPR domain architecture for OTP84 and CREF7. Repeats in the PLS region are indicated by rectangles. Features in the DYW deaminase domain include the PG box, which is indicated as the solid black rectangle, and the open arrows indicate the position of truncations in OTP84. The positions of the zinc binding and catalytic glutamate residues (HXE, CXXC) are indicated with solid arrows. The location of T-DNA insertions is shown at the point of the triangle.
FIGURE 2.
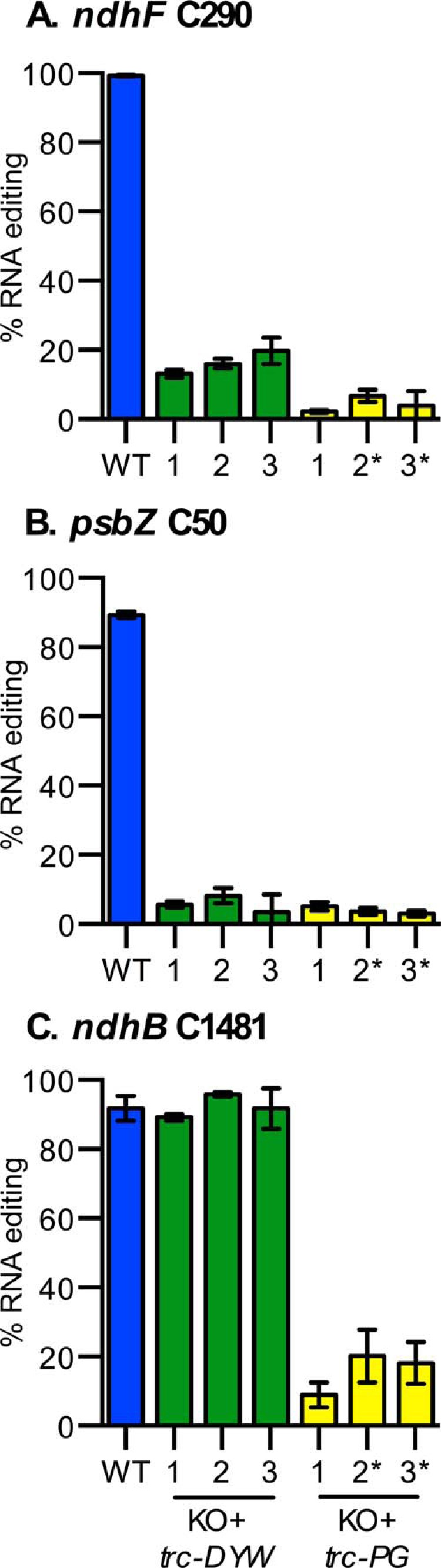
OTP84 truncation shows differential effects on the editing of three cognate sites. A–C show the response of editing ndhF C290, psbZ C50, and ndhB C1481, respectively. Truncation of the DYW domain (OTP84trcDYW) resulted in a large decrease in editing of the ndhF and psbZ sites, but the ndhB site was not significantly reduced. Three leaf samples were analyzed from three wild-type plants (WT). Three leaf samples were analyzed from each of three independent integration events for the OTP84trcDYW in the otp84-2 mutant background (trcDYW), and these samples are labeled 1, 2, and 3. Three leaf samples were analyzed from each of three independent integration events were analyzed for the OTP84trcPG in the otp84-2 mutant background (trcPG plant 1) or the otp84-3 background (trcPG plants 2* and 3*). Error bars show the S.D. of the three leaf samples from three independent plants in the case of WT and three leaf samples from a single plant in all other plants.
Mutagenesis of the HXE Motif of OTP84 Decreases Editing of the Three Cognate Sites
The HXE motif of OTP84 was mutagenized to HXA to produce OTP84-E824A, which was introduced into OTP84 knock-out line (otp84-3) and into the wild type Col-0 background. The conversion of the ndhF, psbZ, and ndhB editing sites is represented in Fig. 3, A, B, and C, respectively. Each editing site was converted at 90% or greater in the wild type plants and remained unedited in otp84-3, the OTP84 knock-out line. Complementation of otp84-3 with a wild type transgene restored editing to ∼90% or greater. In contrast, the OTP84-E824A transgene was unable to restore editing of the three editing sites to wild type levels (Fig. 3). Plants expressing OTP84-E824A did not edit the ndhF and psbZ sites. In contrast, 23% of transcripts were edited at ndhB C1481 in plants expressing OTP84-E824A.
FIGURE 3.
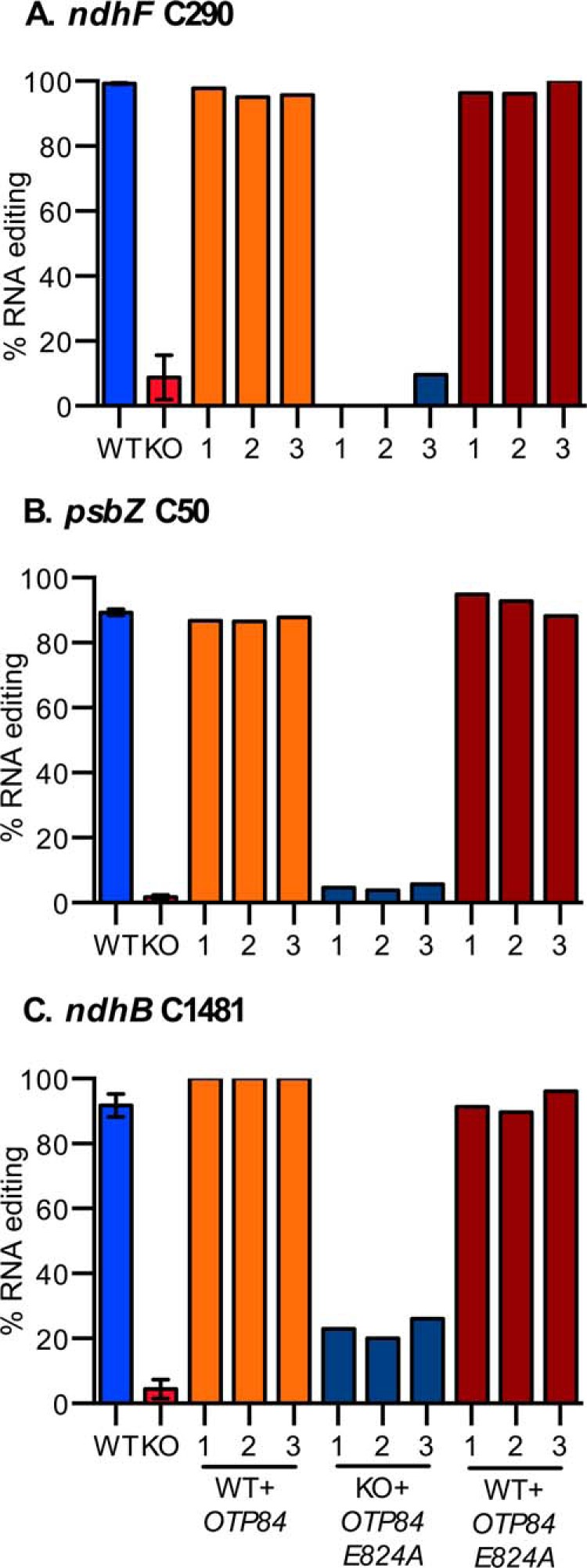
OTP84-E824A does not restore editing to wild type levels in OTP84 knock-out plants. A–C show the response of editing ndhF C290, psbZ C50, and ndhB C1481, respectively. Wild type OTP84 complemented the knock-out phenotype in the OTP84 knock-out line; however, OTP84-E824A did not increase editing of the ndhF or psbZ editing sites. Editing of the ndhB editing site was significantly greater (23%) over the level observed in the knock-out line (5%). A single leaf sample was analyzed from three independent wild type (WT) and otp84-3 knock-out (KO) plants. A single leaf sample was analyzed from each of three independent integration events for these backgrounds and transgenes: wild type plants with OTP84 transgene (WT + OTP84); otp84-3 with OTP84E824A (KO + OTP84 E824A); and wild type expressing OTP84E824A (WT + OTP84 E824A). Error bars show the S.D. of the three leaf samples from three independent plants in the case of WT and KO.
Because the OTP84-E824A transgene was unable to restore editing to the cognate sites, the mutant transgene might act in a dominant negative manner to suppress editing in wild type plants by formation of inactive editing complexes. Therefore, we tested whether expression of the OTP84-E824A transgene could disrupt editing in wild type plants. In three independent transgenic lines, the mutant transgene did not reduce editing levels in the wild type background and therefore did not act in a dominant negative manner over the native gene (Fig. 3).
Mutagenesis of the HXE Motif of CREF7 Decreases Editing of the Cognate Site
The CREF7 gene has an intact DYW deaminase domain (Fig. 1) and a single cognate editing site, ndhB C1255 (40). Transgenes for wild type CREF7 and a mutant variant with the HXE motif changed to HXA (CREF7-E554A) were introduced into a CREF7 knock-out line (cref7-1); in addition, the CREF7-E554A transgene was introduced into wild type plants. Transgenic plants were analyzed for editing of the cognate site (Fig. 4). The ndhB C1255 site was 100% edited in wild type plants, but remained unedited in the CREF7 knock-out plants. Editing of ndhB C1255 in CREF7 knock-out plants expressing the CREF7-E554A transgene was ∼10% based on Sanger sequencing. In contrast, plants with wild type background expressing CREF7-E554A edited ndhB transcripts at wild type levels, and the transgene did not behave in a dominant negative manner. These results demonstrate that the Glu-554 plays a crucial role in the activity of CREF7 in editing ndhB transcripts.
FIGURE 4.
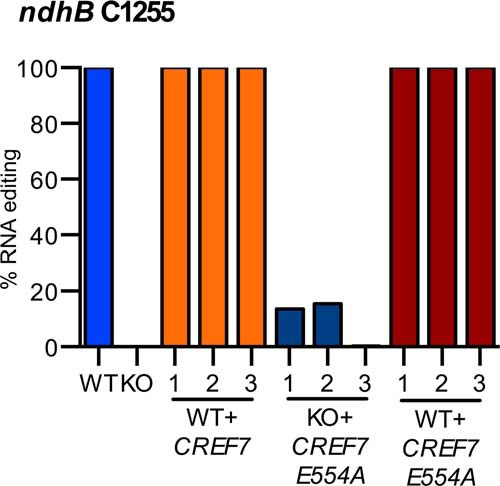
CREF7-E554A does not restore editing in CREF7 knock-out plants. Wild type CREF7 complemented the knock-out phenotype when expressed in the CREF7 knock-out line; however, CREF7-E554A only increased editing of ndhB C1255 to about 10%. A single leaf sample was analyzed from three independent wild type (WT) and cref7-1 knock-out (KO) plants. A single leaf sample was analyzed from each of three independent integration events for these backgrounds and transgenes: wild type plants with CREF7 transgene (WT + CREF7); cref7 with CREF7-E554A (KO + CREF7-E554A); and wild type expressing CREF7-E554A (WT + CREF7-E554A).
Expression of CREF7-E554A Reduces Editing of rpoA C200, a Non-cognate Site
Editing site conversion was determined for the 34 major chloroplast editing sites in plants expressing OPT84-E824A and CREF7-E554A in their respective gene knock-out backgrounds (data not shown). In several instances, small changes in editing site conversion were detected at non-cognate editing sites.
The strongest effect of the mutant transgene was observed on rpoA C200 editing in plants expressing CREF7-E554A (Fig. 5). The rpoA editing site is about 70% converted in wild type Col-0 plants, in cref7-1 knock-out plants, and in cref7-1 plants complemented with the wild type CREF7 gene. The CREF7-E554A transgene had no detectable effect when expressed in wild type plants; however, in cref7-1 knock-out plants, the E554A substitution caused a 25% reduction of rpoA editing to about 45%. Thus, expression of CREF7-E554A caused a significant reduction of rpoA editing, suggesting that CREF7 may participate in editing rpoA transcripts.
FIGURE 5.
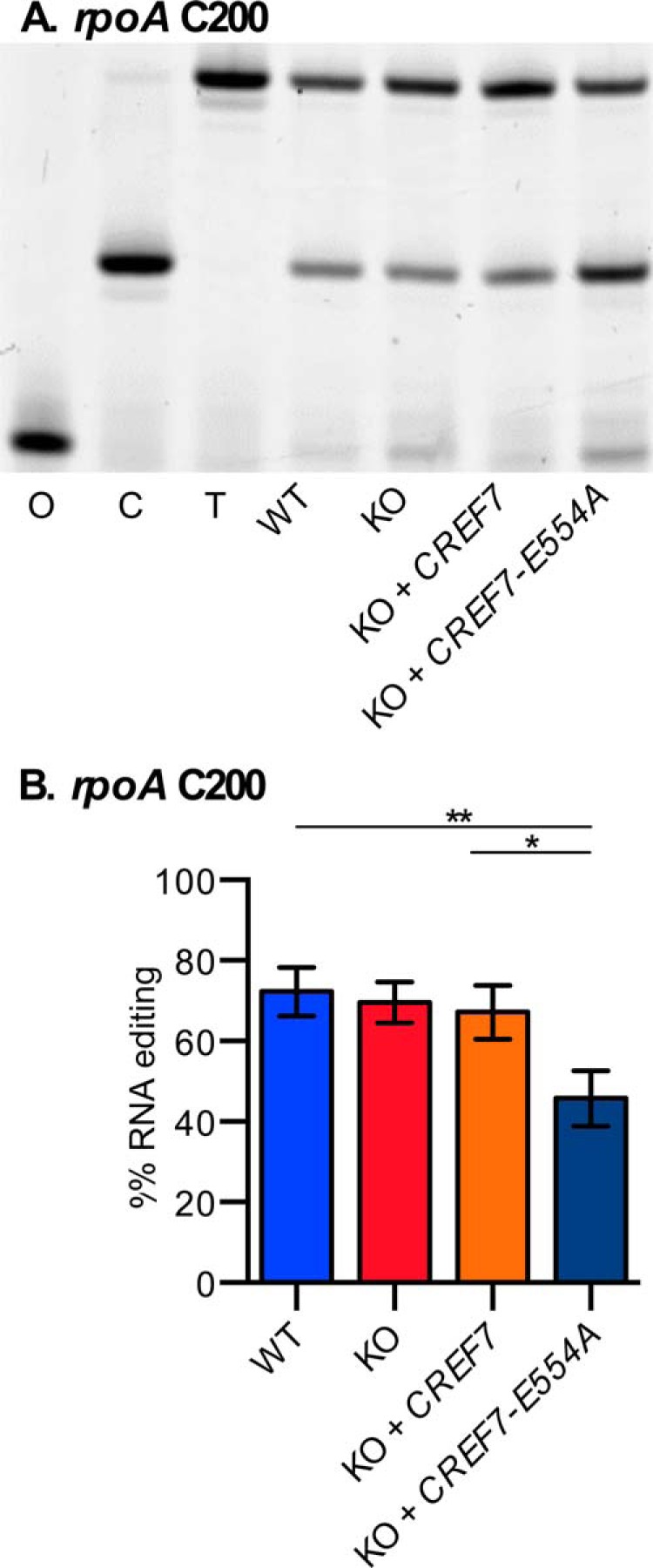
The CREF7-E554A mutation decreases editing of rpoA, a non-cognate editing site. A, poisoned primer extension assay was used to determine rpoA editing site conversion. Control lanes show oligonucleotide (O), unedited template (C), and edited template (T). B, editing of rpoA was about 70% in wild type and knock-out plants. Expression of CREF7-E554A in the CREF7 knock-out line resulted in a decrease in rpoA editing to about 45%. Three leaf samples were analyzed from wild type plants (WT) and cref7-1 knock-out (KO) plants. A single leaf sample was analyzed from each of three independent integration events for these backgrounds and transgenes: wild type plants with CREF7 transgene (WT + CREF7); and cref7 with CREF7-E554A (KO + CREF7-E554A). For statistical analysis, the Student's t test was performed with significance indicated as * and ** for >0.05 and >0.005 at the 0.05 level, respectively. Error bars show the S.D. of the three leaf samples from three independent plants.
The HXA Mutation of the DYW Deaminase Domain Maintains Zinc Binding
Mutagenesis may cause a protein to lose function because of improper folding, or through a direct effect on catalysis. Previous investigations of the structure and function of E. coli cytidine deaminase used circular dichroism and zinc stoichiometry to demonstrate that the polypeptide with the Glu-to-Ala substitution retained native structure (41). The ELI1 DYW deaminase domain was selected for zinc analysis to determine whether the DYW deaminase domain was able to fold into a native structure. A Glu-to-Ala mutation was introduced at position 566 in the ELI1 DYW deaminase domain (19). The recombinant protein was expressed in E. coli, purified, and analyzed by mass spectrometry as described previously (19). The purified recombinant ELI1-E566A protein had a native mass of 19,519 atomic mass units, and the denatured protein had a mass of 19,395 atomic mass units. The mass difference of 124 atomic mass unit is consistent with the native DYW deaminase domain binding two zinc molecules of 65.4 atomic mass unit and the loss of several protons from the cysteine ligands that are predicted to coordinate the zinc atoms (19). This result demonstrates that the ELI1-E566A mutant DYW deaminase domain binds two zinc ions and retains native structure.
DISCUSSION
The enzyme responsible for RNA editing in plants has been controversial. Early biochemical analyses indicated that the enzymatic reaction was probably a deamination reaction (42, 43), and in vitro editing assays established that the reaction occurred through a cytidine deamination mechanism (44–46). After discovery of the role of PPR proteins in RNA processing reactions in plant organelles (10), deaminase-like zinc binding motifs were recognized in the DYW domain (18). More recently, the DYW deaminase domain was shown to bind zinc as a prosthetic group (19, 20), further supporting the hypothesis that the DYW deaminase functions as the deaminase in plant editing. Mutagenesis of amino acid residues in the zinc binding motifs and of the catalytic glutamate (HXE, CXXC) of DYW1 resulted in the loss of the ability to edit ndhD C2 in transgenic plants (20). Thus, the DYW deaminase domain possesses several features that would be expected for an editing deaminase.
In this work, we explored the role of the glutamate residue of the HXE motif in full-length PPR proteins that have a canonical PLS repeat region and an intact DYW deaminase domain. Mutation of the putative catalytic glutamate residue caused a dramatic decrease in the editing of the cognate sites for OTP84 and CREF7. The requirement of the glutamate residue of the HXE domain and zinc binding by the DYW deaminase domain are two key features that are expected for an editing deaminase. They strongly support the hypothesis that the DYW deaminase domain provides the catalytic activity for the editing reaction.
There are several examples in which the DYW deaminase is dispensable for complementation of a knock-out mutant phenotype (19, 30, 31, 47). The truncation of OTP84 had markedly different effects on the three editing site targets. The OTP84trcDYW transgene restored editing of ndhB C1481 to wild type levels, and this site responded similarly to previously characterized editing sites for ELI1, CRR22, CRR28, OTP82, and MEF11 (19, 30, 31, 47). Editing of the psbZ and ndhF sites was highly reduced in the presence of the OTP84trcDYW transgene, although the ndhF site may have been somewhat higher than gene knock-out levels. The ndhF C290 editing site is also affected by disruption of the PPR gene, VAC1, which results in a partial loss of editing of the ndhF site (48). The DYW deaminase domain of VAC1 might participate with OTP84trcDYW to incompletely edit the ndhF C290 site. These results suggest that a set of editing sites can share a single specificity factor, but might rely on different deaminases in trans to complete the editing reaction.
Editing was highly reduced at the three cognate sites of OTP84 in plants expressing the OTP84-E824A transgene. The editing of the psbZ and ndhF sites was near gene knock-out levels, and the OTP84-E554A protein may form non-functional editing complexes for these sites. The editing of the ndhB C1481 site responded somewhat differently, and editing of the ndhB site increased well above knock-out levels to about 23%. The ndhB site also responded differently from the other two cognate sites in plants expressing OTP84trcDYW, and the increase in ndhB editing might result from the participation of other deaminases in the editing of this site.
Expression of CREF7-E554A substantially decreased editing of rpoA C200, a cognate editing site for CLB19 (49). Because CLB19 is truncated at the end of the E domain and lacks an intact DYW deaminase domain (49), it could recruit a deaminase domain from another PPR protein. The reduction in editing of a non-cognate site suggests that CREF7 may participate as a deaminase in rpoA C200 editing. Because editing of rpoA C200 is not reduced in the CREF7 knock-out line (Fig. 5), there may be other DYW deaminases that participate in rpoA editing.
Nineteen PPR proteins have been shown to be required for editing in Arabidopsis chloroplasts. Four of these PPRs (CLB19, CRR4, CRR21, and OTP80) have truncated DYW deaminase domains (37, 39, 49) and could not function in C deamination. A complex network of PPR proteins could be involved in RNA editing. The PLS repeat domain may be sufficient for specifying an editing site; however, one or more deaminases could be recruited in trans as enzymatic components. Table 1 summarizes the relationships between several chloroplast editing sites, site specificity factors, and possible PPR proteins involved in the deamination reaction. PPR proteins that lack complete DYW deaminase domains may acquire the activity from other proteins as in the case of CRR4 and DYW1. Based on these results, OTP84 acts as a site specificity factor for three cognate sites and participates as a deaminase along with other PPR proteins in the editing of these sites. CREF7 appears to function as both a specificity factor and a deaminase in the editing of its cognate site, but also contributes to rpoA editing with the CLB19 as a specificity factor. VAC1 is a PPR protein with a DYW domain, and the gene knock-out results in a strong lethal phenotype and partial editing of ndhF C290 (a cognate site of OTP84) and accD C794 (a cognate site of RARE1) (48). Because the VAC1 knock-out exhibits partial editing, VAC1 is not essential for each site. If other PPR proteins participate in these editing reactions, these proteins have partially redundancy. The partial reduction in editing of rpoA C200 by the CREF7-E554A mutant suggests that CREF7 is partially redundant with other deaminases in rpoA editing.
TABLE 1.
Possible interactions of PPR proteins in editing site recognition and deamination reactions
| Editing site | Site specificity factor | Putative deaminase |
|---|---|---|
| ndhD C2 | CRR4a (29) | DYW1a (28), DYW1b (20) |
| ndhB C1481 | OTP84a (39) | OTP84b (unknown) |
| psbZ C50 | OTP84a (39) | OTP84b,c |
| ndhF C290 | OTP84a (39) | OTP84b,c, VAC1a (48) |
| accD C794 | RARE1a (50) | VAC1a (48) |
| ndhB C1255 | CREF7a (40) | CREF7b |
| rpoA C200 | CLB19a (49) | CREF7b (unknown) |
a Gene knockout exhibits reduced editing.
b Glu-to-Ala mutant transgene incapable of restoring editing in knockout line.
c Transgene with truncation removing DYW deaminase incapable of restoring editing in knockout line.
Expression of catalytically deficient PPR transgenes by mutation of the HXE motif has proven to be a powerful way to distinguish the DYW deaminase function for a PPR protein from the site specificity role. Our results provide direct support for the model that PPR proteins have dual and separable functions as site specificity factors and as deaminases (13, 19, 47).
Acknowledgments
We thank Chelsea Pham for cloning OTP84 truncation constructs. Dr. Beniam Berhane performed mass spectroscopy of the recombinant ELI1-E566A protein. We thank Prof. Tom Poulos for thoughtful discussion of these results.
This work was supported by National Science Foundation Grant MCB-0929423 (to R. M. M.). In addition, this material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant DGE-1321846 (to M. F. D.).
- PPR
- pentatricopeptide repeat.
REFERENCES
- 1. Covello P. S., Gray M. W. (1989) RNA Editing in plant mitochondria. Nature 341, 662–666 [DOI] [PubMed] [Google Scholar]
- 2. Gualberto J. M., Lamattina L., Bonnard G., Weil J. H., Grienenberger J. M. (1989) RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature 341, 660–662 [DOI] [PubMed] [Google Scholar]
- 3. Bentolila S., Elliott L. E., Hanson M. R. (2008) Genetic architecture of mitochondrial editing in Arabidopsis thaliana. Genetics 178, 1693–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bentolila S., Heller W. P., Sun T., Babina A. M., Friso G., van Wijk K. J., Hanson M. R. (2012) RIP1, a member of an Arabidopsis protein family, interacts with the protein RARE1 and broadly affects RNA editing. Proc. Natl. Acad. Sci. U.S.A. 109, E1453–E1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kotera E., Tasaka M., Shikanai T. (2005) A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 433, 326–330 [DOI] [PubMed] [Google Scholar]
- 6. Takenaka M., Zehrmann A., Verbitskiy D., Kugelmann M., Härtel B., Brennicke A. (2012) Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proc. Natl. Acad. Sci. U.S.A. 109, 5104–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rüdinger M., Szövényi P., Rensing S. A., Knoop V. (2011) Assigning DYW-type PPR proteins to RNA editing sites in the funariid mosses Physcomitrella patens and Funaria hygrometrica. Plant J. 67, 370–380 [DOI] [PubMed] [Google Scholar]
- 8. Uchida M., Ohtani S., Ichinose M., Sugita C., Sugita M. (2011) The PPR-DYW proteins are required for RNA editing of rps14, cox1 and nad5 transcripts in Physcomitrella patens mitochondria. FEBS Lett. 585, 2367–2371 [DOI] [PubMed] [Google Scholar]
- 9. Barkan A., Small I. (2014) Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 65, 415–442 [DOI] [PubMed] [Google Scholar]
- 10. Lurin C., Andrés C., Aubourg S., Bellaoui M., Bitton F., Bruyère C., Caboche M., Debast C., Gualberto J., Hoffmann B., Lecharny A., Le Ret M., Martin-Magniette M. L., Mireau H., Peeters N., Renou J. P., Szurek B., Taconnat L., Small I. (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16, 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andres C., Lurin C., Small I. D. (2007) The multifarious roles of PPR proteins in plant mitochondrial gene expression. Physiol. Plantarum 129, 14–22 [Google Scholar]
- 12. Barkan A., Rojas M., Fujii S., Yap A., Chong Y. S., Bond C. S., Small I. (2012) A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS. Genet. 8, e1002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yagi Y., Hayashi S., Kobayashi K., Hirayama T., Nakamura T. (2013) Elucidation of the RNA recognition code for pentatricopeptide repeat proteins involved in organelle RNA editing in plants. PLoS One 8, e57286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takenaka M., Zehrmann A., Brennicke A., Graichen K. (2013) Improved computational target site prediction for pentatricopeptide repeat RNA editing factors. PLoS One 8, e65343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okuda K., Shoki H., Arai M., Shikanai T., Small I., Nakamura T. (2014) Quantitative analysis of motifs contributing to the interaction between PLS-subfamily members and their target RNA sequences in plastid RNA editing. Plant J. 80, 870–882 [DOI] [PubMed] [Google Scholar]
- 16. Iyer L. M., Zhang D., Rogozin I. B., Aravind L. (2011) Evolution of the deaminase fold and multiple origins of eukaryotic editing and mutagenic nucleic acid deaminases from bacterial toxin systems. Nucleic Acids Res. 39, 9473–9497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finn R. D., Bateman A., Clements J., Coggill P., Eberhardt R. Y., Eddy S. R., Heger A., Hetherington K., Holm L., Mistry J., Sonnhammer E. L., Tate J., Punta M. (2014) Pfam: the protein families database. Nucleic Acids Res. 42, D222–D230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salone V., Rüdinger M., Polsakiewicz M., Hoffmann B., Groth-Malonek M., Szurek B., Small I., Knoop V., Lurin C. (2007) A hypothesis on the identification of the editing enzyme in plant organelles. FEBS Lett. 581, 4132–4138 [DOI] [PubMed] [Google Scholar]
- 19. Hayes M. L., Giang K., Berhane B., Mulligan R. M. (2013) Identification of two pentatricopeptide repeat genes required for RNA editing and zinc binding by C-terminal cytidine deaminase-like domains. J. Biol. Chem. 288, 36519–36529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boussardon C., Avon A., Kindgren P., Bond C. S., Challenor M., Lurin C., Small I. (2014) The cytidine deaminase signature HxE(x)n CxxC of DYW1 binds zinc and is necessary for RNA editing of ndhD-1. New Phytol. 203, 1090–1095 [DOI] [PubMed] [Google Scholar]
- 21. Betts L., Xiang S., Short S. A., Wolfenden R., Carter C. W., Jr. (1994) Cytidine deaminase: the 2.3-Ä crystal structure of an enzyme: transition-state analog complex. J. Mol. Biol. 235, 635–656 [DOI] [PubMed] [Google Scholar]
- 22. Teng B., Burant C. F., Davidson N. O. (1993) Molecular cloning of an apolipoprotein-B messenger RNA editing protein. Science 260, 1816–1819 [DOI] [PubMed] [Google Scholar]
- 23. Xie K. F., Sowden M. P., Dance G. S. C., Torelli A. T., Smith H. C., Wedekind J. E. (2004) The structure of a yeast RNA-editing deaminase provides insight into the fold and function of activation-induced deaminase and APOBEC-1. Proc. Natl. Acad. Sci. U.S.A. 101, 8114–8119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spears J. L., Rubio M. A., Gaston K. W., Wywial E., Strikoudis A., Bujnicki J. M., Papavasiliou F. N., Alfonzo J. D. (2011) A single zinc ion is sufficient for an active Trypanosoma brucei tRNA editing deaminase. J. Biol. Chem. 286, 20366–20374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goodman R. A., Macbeth M. R., Beal P. A. (2012) ADAR proteins: structure and catalytic mechanism. Curr. Top Microbiol Immunol. 353, 1–33 [DOI] [PubMed] [Google Scholar]
- 26. Bransteitter R., Prochnow C., Chen X. S. (2009) The current structural and functional understanding of APOBEC deaminases. Cell. Mol. Life Sci. 66, 3137–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wagoner J. A., Sun T., Lin L., Hanson M. R. (2015) Cytidine deaminase motifs within the DYW domain of two pentatricopeptide repeat-containing proteins are required for site-specific chloroplast RNA editing. J. Biol. Chem. 290, 2957–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boussardon C., Salone V., Avon A., Berthomé R., Hammani K., Okuda K., Shikanai T., Small I., Lurin C. (2012) Two interacting proteins are necessary for the editing of the NdhD-1 site in Arabidopsis plastids. Plant Cell 24, 3684–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okuda K., Nakamura T., Sugita M., Shimizu T., Shikanai T. (2006) A pentatricopeptide repeat protein is a site recognition factor in chloroplast RNA editing. J. Biol. Chem. 281, 37661–37667 [DOI] [PubMed] [Google Scholar]
- 30. Okuda K., Chateigner-Boutin A. L., Nakamura T., Delannoy E., Sugita M., Myouga F., Motohashi R., Shinozaki K., Small I., Shikanai T. (2009) Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell 21, 146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okuda K., Hammani K., Tanz S. K., Peng L., Fukao Y., Myouga F., Motohashi R., Shinozaki K., Small I., Shikanai T. (2010) The pentatricopeptide repeat protein OTP82 is required for RNA editing of plastid ndhB and ndhG transcripts. Plant J. 61, 339–349 [DOI] [PubMed] [Google Scholar]
- 32. Fankhauser C., Yeh K. C., Lagarias J. C., Zhang H., Elich T. D., Chory J. (1999) PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284, 1539–1541 [DOI] [PubMed] [Google Scholar]
- 33. Fraley R. T., Rogers S. G., Horsch R. B., Eichholtz D. A., Flick J. S., Fink C. L., Hoffmann N. L., Sanders P. R. (1985) The Sev system: a new disarmed Ti plasmid vector system for plant transformation. Nat. Biotechnol. 10.1038/nbt0785-62 [DOI] [Google Scholar]
- 34. Clough S. J., Bent A. F. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 [DOI] [PubMed] [Google Scholar]
- 35. Roberson L. M., Rosenthal J. J. (2006) An accurate fluorescent assay for quantifying the extent of RNA editing. RNA 12, 1907–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayes M. L., Hanson M. R. (2007) Assay of editing of exogenous RNAs in chloroplast extracts of Arabidopsis, maize, pea, and tobacco. Methods Enzymol. 424, 459–482 [DOI] [PubMed] [Google Scholar]
- 37. Okuda K., Myouga F., Motohashi R., Shinozaki K., Shikanai T. (2007) Conserved domain structure of pentatricopeptide repeat proteins involved in chloroplast RNA editing. Proc. Natl. Acad. Sci. U.S.A. 104, 8178–8183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verbitskiy D., Zehrmann A., Brennicke A., Takenaka M. (2010) A truncated MEF11 protein shows site-specific effects on mitochondrial RNA editing. Plant Signal Behav. 5, 558–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hammani K., Okuda K., Tanz S. K., Chateigner-Boutin A. L., Shikanai T., Small I. (2009) A study of new Arabidopsis chloroplast RNA editing mutants reveals general features of editing factors and their target sites. Plant Cell 21, 3686–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yagi Y., Tachikawa M., Noguchi H., Satoh S., Obokata J., Nakamura T. (2013) Pentatricopeptide repeat proteins involved in plant organellar RNA editing. RNA Biol. 10, 1419–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carlow D. C., Short S. A., Wolfenden R. (1998) Complementary truncations of a hydrogen bond to ribose involved in transition-state stabilization by cytidine deaminase. Biochemistry 37, 1199–1203 [DOI] [PubMed] [Google Scholar]
- 42. Rajasekhar V. K., Mulligan R. M. (1993) RNA Editing in plant mitochondria: α-phosphate is retained during C-to-U conversion in mRNAs. Plant Cell 5, 1843–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu W., Schuster W. (1995) Evidence for a site-specific cytidine deamination reaction involved in C to U RNA editing of plant mitochondria. J. Biol. Chem. 270, 18227–18233 [DOI] [PubMed] [Google Scholar]
- 44. Hirose T., Sugiura M. (2001) Involvement of a site-specific trans-acting factor and a common RNA-binding protein in the editing of chloroplast mRNAs: development of a chloroplast in vitro RNA editing system. EMBO J. 20, 1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakajima Y., Mulligan R. M. (2005) Nucleotide specificity of the RNA editing reaction in pea chloroplasts. J. Plant Physiol. 162, 1347–1354 [DOI] [PubMed] [Google Scholar]
- 46. Hegeman C. E., Hayes M. L., Hanson M. R. (2005) Substrate and cofactor requirements for RNA editing of chloroplast transcripts in Arabidopsis in vitro. Plant J. 42, 124–132 [DOI] [PubMed] [Google Scholar]
- 47. Zehrmann A., Verbitskiy D., Härtel B., Brennicke A., Takenaka M. (2011) PPR proteins network as site-specific RNA editing factors in plant organelles. RNA Biol. 8, 67–70 [DOI] [PubMed] [Google Scholar]
- 48. Tseng C. C., Sung T. Y., Li Y. C., Hsu S. J., Lin C. L., Hsieh M. H. (2010) Editing of accD and ndhF chloroplast transcripts is partially affected in the Arabidopsis vanilla cream1 mutant. Plant Mol. Biol. 73, 309–323 [DOI] [PubMed] [Google Scholar]
- 49. Chateigner-Boutin A. L., Ramos-Vega M., Guevara-García A., Andrés C., de la Luz Gutiérrez-Nava M., Cantero A., Delannoy E., Jiménez L. F., Lurin C., Small I., León P. (2008) CLB19, a pentatricopeptide repeat protein required for editing of rpoA and clpP chloroplast transcripts. Plant J. 56, 590–602 [DOI] [PubMed] [Google Scholar]
- 50. Robbins J. C., Heller W. P., Hanson M. R. (2009) A comparative genomics approach identifies a PPR-DYW protein that is essential for C-to-U editing of the Arabidopsis chloroplast accD transcript. RNA 15, 1142–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]


