Background: SCF-Fbw7 participates in stability control of several Cdc4 phosphodegron-containing proteins phosphorylated by glycogen synthase kinase 3, an E3 ligase.
Results: Ubiquitin-dependent degradation of GATA-binding protein 2 is promoted by Fbw7, is cyclin B-CDK1-mediated Thr176 phosphorylation-dependent, and influences hematopoietic cell differentiation.
Conclusion: GATA-binding protein 2 is a novel target for Fbw7.
Significance: The molecular mechanism of post-transcriptional control of GATA-binding protein 2 is clarified.
Keywords: Cyclin-dependent Kinase (CDK), GATA Transcription Factor, Phosphorylation, Protein Degradation, Ubiquitin Ligase, Ubiquitylation (Ubiquitination), Fbw7
Abstract
A GATA family transcription factor, GATA-binding protein 2 (GATA2), participates in cell growth and differentiation of various cells, such as hematopoietic stem cells. Although its expression level is controlled by transcriptional induction and proteolytic degradation, the responsible E3 ligase has not been identified. Here, we demonstrate that F-box/WD repeat-containing protein 7 (Fbw7/Fbxw7), a component of Skp1, Cullin 1, F-box-containing complex (SCF)-type E3 ligase, is an E3 ligase for GATA2. GATA2 contains a cell division control protein 4 (Cdc4) phosphodegron (CPD), a consensus motif for ubiquitylation by Fbw7, which includes Thr176. Ectopic expression of Fbw7 destabilized GATA2 and promoted its proteasomal degradation. Substitution of threonine 176 to alanine in GATA2 inhibited binding with Fbw7, and the ubiquitylation and degradation of GATA2 by Fbw7 was suppressed. The CPD kinase, which mediates the phosphorylation of Thr176, was cyclin B-cyclin-dependent kinase 1 (CDK1). Moreover, depletion of endogenous Fbw7 stabilized endogenous GATA2 in K562 cells. Conditional Fbw7 depletion in mice increased GATA2 levels in hematopoietic stem cells and myeloid progenitors at the early stage. Increased GATA2 levels in Fbw7-conditional knock-out mice were correlated with a decrease in a c-Kit high expressing population of myeloid progenitor cells. Our results suggest that Fbw7 is a bona fide E3 ubiquitin ligase for GATA2 in vivo.
Introduction
Ubiquitin-proteasome systems control the stability of many cellular proteins and participate in regulation of various biological processes, such as cell proliferation, cell differentiation, signal transduction, and apoptosis (1, 2). Polyubiquitin chain conjugation to substrate proteins is mediated by E1, E2, and E3. E3 ubiquitin ligases are of the RING finger type (3), HECT type (4), and U-box type (5), and they bind to and polyubiquitylate their specific substrates. F-box/WD repeat-containing protein 7 (Fbw7; also called Fbxw7, Cdc4, and Sel10), an F-box protein, is a substrate recognition molecule of Skp1, Cullin, F-box-containing complex (SCF)3-type E3 ubiquitin ligase (6). It has been reported that Fbw7 targets cyclin E (7), c-Myc (8, 9), c-Jun (10, 11), Notch1 (12, 13), SREBP (14, 15), mTOR (16), c-Myb (17–19), MCL1 (20, 21), NFκB2 (22), and GATA3 (23) for ubiquitin-mediated proteasomal degradation (24, 25). Ablation of the Fbw7 gene was reported in human breast carcinomas, colon cancers, and T cell acute lymphoblastic leukemias (26, 27). Moreover, conditional Fbw7-knock-out mice developed thymus enlargement, thymic lymphomas, and defects of bone marrow (BM) hematopoietic stem cells (HSCs) (27). Because Fbw7 promotes destabilization of many oncogenic proteins and cell differentiation regulators, it is important to identify unknown substrates of Fbw7 to aid understanding of hematopoietic cell differentiation and Fbw7-associated cancer development.
Recently, we reported that hematopoietic transcription factor GATA-binding protein 3 (GATA3) is a novel target for Fbw7 (23). GATA3 is a member of the GATA family of transcription factors, which consists of GATA1, -2, -3, -4, -5, and -6 (28, 29). We found that conditional inactivation of Fbw7 in mouse T-cell development skewed thymic CD8 single positive lineage differentiation, which exhibited a higher incidence of apoptosis (23). Similar perturbations during development of CD8-positive cells were studied with transgenic mice, in which GATA3 expression was enforced throughout T-cell development. Excess GATA3 induced thymic lymphomas in the transgenic mice (30). Interestingly, thymic lymphomas also developed in mice when Fbw7 was conditionally ablated in the T-cell lineage alone (31). It is speculated that uncontrolled GATA3 protein levels result in the formation of lymphoblastoid tumors at a specific stage of thymic development. Fbw7 binds to a high affinity recognition motif termed the Cdc4 phosphodegron (CPD), with a consensus sequence of Ser(P)/Thr(P)-Pro-X-X-Ser(P)/Thr(P)/Glu/Asp and often promotes the turnover of substrates via phosphorylation of the CPD (18, 32). We found a CPD motif in GATA3 amino acid (aa) sequences and demonstrated that Fbw7-mediated ubiquitylation required phosphorylation of Thr156 in CPD in GATA3 (23). We also found the CPD motif in GATA-binding protein 1 (GATA1) and GATA-binding protein 2 (GATA2), suggesting that they might be targets for Fbw7. Among the GATA family, GATA1, -2, and -3 are classified as hematopoietic GATA factors, based on their ability to regulate distinct and overlapping aspects of hematopoiesis. Especially, aa sequences among GATA3 and GATA2 are highly conserved. GATA3 is expressed in HSCs in addition to T-lymphocytes (33). GATA2 is also expressed in HSCs and in hematopoietic progenitors, erythroid precursors, megakaryocytes, and eosinophils (28, 29). GATA2 participates in proliferation and differentiation of hematopoietic cell lineages. Although GATA1 is also expressed in erythroid precursors, megakaryocytes, and eosinophils (34), the identity of aa among GATA3 or GATA2 and GATA1 is high in zinc finger domains but low in other regions. It was reported that mutations of one allele of GATA2 participate in hematopoietic or immune system diseases (35, 36). Therefore, it is important to clarify the molecular mechanisms involved in the control of GATA2 levels. Although cellular GATA2 levels are regulated by transcriptional control and proteasome-mediated degradation (37, 38), ubiquitin E3 ligase, which ubiquitylates GATA2 to promote degradation via the ubiquitin-proteasome system, has not been identified. In the present study, we demonstrated that GATA2 is a novel CPD-dependent substrate for Fbw7. Furthermore, we identified the involvement of cyclin B-cyclin-dependent kinase 1 (CDK1), which is different from the CPD kinases identified for known substrates. We also demonstrated the physiological functions of Fbw7-dependent control of GATA2 using cultured cells and Fbw7-conditional knock-out mice.
EXPERIMENTAL PROCEDURES
Cell Lines, Cell Culture and Synchronization
HEK293 and HeLa cells were obtained from American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37 °C. Neuro2A cells were maintained in DMEM supplemented with 10% FBS, 1 mm sodium pyruvate, 2 mm l-glutamine, 10 ml/liter nonessential amino acids, and the above described antibiotics. K562 cells were obtained from the RIKEN Cell Bank and cultured in RPMI1640 supplemented with 10% FBS and the above described antibiotics. To obtain cell lysates synchronized at G1/S, S, and M phases, HeLa cells were treated with 1 μg/ml aphidicolin for 16 h and then released from arrest by washing with fresh medium for 10 h (G1/S and S phase cells). Then cells were treated with aphidicolin for 16 h (G1/S phase cells), then released from arrest by washing with fresh medium for 5 h, and harvested by treating trypsin (S phase cells). M phase cells were treated with 1 μg/ml aphidicolin for 16 h, released from arrest by washing with fresh medium for 4 h, treated with 100 ng/ml nocodazole for 16 h, and harvested by adding trypsin. To determine the contribution of cyclin B-CDK1, cells were treated with a CDK inhibitor, such as butyrolactone I (39, 40) or RO-3306 (Calbiochem).
Antibodies
The antibodies used in this study were anti-Myc 9E10 (Roche Applied Science), anti-FLAG M2 (Sigma), anti-HA 3F10 (MBL), anti-GATA2 PA5–17368 (Thermo), ab109241 (Abcam), RC1.1.1 (38), anti-Fbw7 A 301-720A-1 (Bethyl), anti-cyclin B v-152 (Santa Cruz Biotechnology, Inc.), and anti-β actin AC15 (Sigma). PE-Cy5-conjugated anti-c-Kit (2B8) was purchased from Biolegend. Biotinylated anti-CD127 (B12-1), mouse lineage depletion mixture, and streptavidin particles Plus (streptavidin-conjugated magnetic nanoparticles) were purchased from BD Bioscience. Alexa Fluor® 488-conjugated anti-GATA2 was prepared by Alexa Fluor® 488 monoclonal antibody labeling kit (Zenon). Anti-human GATA2 phosphorylated Thr176 polyclonal antibody (anti-p-T176-GATA2) was raised against keyhole limpet hemocyanin-conjugated chemically synthesized phosphorylated Thr176 peptide, corresponding to the CPD region of GATA2 (aa residues 172–181) (MBL). Antiserum obtained from an immunized guinea pig was bound to column chromatography conjugated with P-T176 peptide. The affinity-purified anti-p-T176-GATA2 was then passed through a column conjugated to nonphosphorylated Thr176 peptide (aa residues 172–181 of GATA2) to deplete antibodies against nonphosphorylated antigen. The specific binding ability of the purified antibody to P-T176 peptide was confirmed by ELISA.
Plasmids and Transfection
Human GATA2 cDNA was cloned into pcDNA3.1/myc-His (Invitrogen). Substitution of Thr176 to alanine in GATA2 was generated using PCR-based mutagenesis. cDNAs encoding mouse Fbw7 (Fbxw7α) or its ΔF mutant were subcloned into p3×FLAG-CMV 7.1 (Sigma). cDNAs encoding human Fbw7 were cloned into pCGN with an HA tag or pcDNA3 with a FLAG tag. The expression plasmid for ubiquitin (pCGN-HA-Ub) was described previously (41). Plasmids were transfected into HEK293 or HeLa cells using the calcium phosphate method or X-treme GENE 9 (Roche Applied Science), respectively. Cells were harvested by a scraper and lysed with lysis buffer (50 mm Tris-HCl, pH 7.5, 300 mm NaCl, 0.5% Triton X-100, 10 μg/ml each of antipain, pepstatin, E-64, leupeptin, and trypsin inhibitor, 2.5 μg/ml chymostatin, phosphatase inhibitor mix) (23).
RNA Interference
siRNA oligonucleotides for GATA2 or Fbw7 or control siRNAs were transfected into HeLa cells using RNAiMax (Life Technologies), according to the manufacturer's protocol. ON-TARGETplus human SMARTpool for GATA2 (L-009024-00-0005) was purchased from Thermoscientific Dharmacon. The nucleotide sequences of Fbw7 siRNAs were 5′-GUGUGGAAUGCAGAGACUGGAGA-3′ (Fbw7-A), 5′-AAUGAAAGCACAUAGAGUGCCAACU-3′ (Fbw7-B), 5′-ACAGGACAGUGUUUACAAA-3′ (Fbw7-C), and 5′-ACCUUCUCUGGAGAGAGAAAUGC-3′ (Fbw7-D).
Immunoprecipitation and Immunoblotting
Total cell lysates were immunoprecipitated with 2 μg of antibodies and protein G Plus-Sepharose 4FF (GE Healthcare) at 4 °C for 2 h. Immunocomplexes were washed four times with lysis buffer. Immunoprecipitated samples and original cell lysates (input) were separated by SDS-PAGE and transferred from the gel onto a PVDF membrane (Millipore), followed by immunoblotting (IB). Proteins were visualized using an enhanced chemiluminescence system (Bio-Rad).
Immunoprecipitation under Denaturing Conditions
Cells lysates which were prepared with lysis buffer as described above were added to equal volume of 2× denaturing IP buffer (100 mm Tris-HCl, pH 7.5, 2% SDS, 10 mm dithiothreitol) and incubated at 100 °C for 8 min and then centrifuged for 13,000 rpm for 10 min. The supernatants were diluted with five volumes of lysis buffer and immunoprecipitated with 2 μg of antibodies and protein G Plus-Sepharose 4FF (GE healthcare) at 4 °C for 2 h. Immunocomplexes were washed four times with lysis buffer. Immunoprecipitated samples were separated by SDS-PAGE and transferred from the gel onto a PVDF membrane (Millipore), followed by IB.
DiPIUS-NL
DiPIUS-NL analysis was performed as described (42). Briefly, Neuro2A cells expressing mouse Fbw7α or its ΔF mutant were incubated for 6 h in the presence of the proteasome inhibitor MG132 (10 μm; Peptide Institute) and were then lysed in 8 ml of a solution containing 20 mm HEPES-NaOH (pH 7.5), 150 mm NaCl, 1% digitonin, 10 mm NaF, 10 mm Na4P2O7, 0.4 mm Na3VO4, 0.4 mm EDTA, leupeptin (20 μg/ml), aprotinin (10 μg/ml), and 1 mm PMSF. The lysates were centrifuged at 2200 × g for 20 min at 4 °C. The supernatants (20 mg of protein in 8 ml of solution) were incubated for 1 h at 4 °C with 120 μl of beads conjugated with M2 antibodies to FLAG. The beads were washed three times with 4 ml of a solution containing 10 mm HEPES-NaOH (pH 7.5), 150 mm NaCl, and 0.1% Triton X-100, and bead-bound proteins were then eluted with the FLAG peptide (500 μg/ml; Sigma), precipitated with ice-cold 20% trichloroacetic acid, and washed with acetone. The concentrated immunoaffinity-purified proteins were dissolved in SDS sample buffer, fractionated by SDS-PAGE, and stained with silver. Individual lanes of the stained gel were sliced into 12 pieces, and proteins within these pieces were subjected to in-gel digestion with trypsin as described previously (43). The resulting peptides were analyzed by an ion trap mass spectrometer (LTQ-XL, Thermo Finnigan). Peak lists were generated with lcq_dta.exe (Thermo Finnigan) and were compared with the use of the MASCOT algorithm (version 2.2.1) with the “Target-decoy” Mouse IPI database (version 3.4.4, released in June 2008; with 55,078 target sequences, searched against a total of 110,156 sequences (target and reverse/decoy)), maintained by the European Bioinformatics Institute. Assigned high scoring peptide sequences (MASCOT score >35) were processed with in-house software. If the MASCOT score was <45 (peptides for which the MS2 score was above the 95th percentile of significance), assigned sequences were manually confirmed by comparison with the corresponding collision-induced dissociation spectra on the basis of the following criteria: (i) a Δ score of >15 or (ii) at least six successive matches for y- or b-ions or at least three blocks of three successive matches for y- or b-ions. Identified peptides from independent experiments were integrated and regrouped by IPI accession number. Proteins identified in only one experiment or with a single-peptide assignment were removed from spectral counting data. Estimated false discovery rates were zero at the protein level.
In Vivo Degradation Assay
pcDNA3.1-GATA2-myc-His or pcDNA3.1-GATA2-T176A-myc-His was transfected with or without pcDNA3-FLAG-Fbw7 into HeLa cells. To analyze the effect of Fbw7 on the stability of endogenous GATA2, siRNA for Fbw7 or control siRNA was transfected into K562 cells using RNAiMax. After 48 h, cells were treated with 20 μg/ml cycloheximide for the indicated times. Cell lysates were subjected to IB with the indicated antibodies. The intensity of the bands was measured using image analysis software ImageJ, and the signal intensity of GATA2 in samples was normalized using levels of β-actin as a loading control.
In Vitro Phosphorylation Assay
GST-fused WT or T176A mutant of GATA2 was produced in Escherichia coli BL21 and purified using glutathione-Sepharose beads (GE Healthcare). In vitro phosphorylation was described previously (23, 44). Each recombinant GATA2 was incubated with the indicated kinase sources, including recombinant cyclin D3-CDK4 (Abcam), cyclin E1-CDK2 (Abcam), cyclin A2-CDK2 (Abcam), cyclin B1-CDK1 (Millipore), or synchronized HeLa cell lysates at 30 °C for 30 min in reaction buffer containing 1 mm ATP. The reaction was terminated by boiling with SDS-sample buffer for 8 min. For in vitro phosphorylation following binding assays, phosphorylated mixtures were incubated for an additional 1 h at 4 °C with lysates from HEK293 cells exogenously expressing Fbw7. GST-fused proteins were then precipitated using glutathione-Sepharose beads. The mixtures were treated with precision protease (GE Healthcare) for 30 min to cleave GATA2 from the GST tag. All reaction mixtures were analyzed by IB with the indicated antibodies.
Quantitative Real-time RT-PCR (RT-qPCR) Analysis
Total RNA was isolated from cells using RNAiso (Takara) and subjected to reverse transcription with random hexanucleotide primers and SuperScript Reverse Transcriptase II (Invitrogen). The resulting complementary DNA was subjected to RT-qPCR using the Rotor-Gene 3000 system (Corbett Research) and the SYBR premix Ex Taq kit (Takara). The sequences of PCR primers were as follows: 5′-CCCACCTACCCCTCCTATGT-3′ (sense) and 5′-TGCCCATTCATCTTGTGGTA-3′ (antisense) for GATA2 and 5′-GTAACCCGTTGAACCCCATT-3′ (sense) and 5′-CCATCCAATCGGTAGTAGCG-3′ (antisense) for 18 S rRNA. The amount of transcripts was normalized against that of 18 S rRNA as an internal standard.
Conditional Knock-out Mice
Generation of Mx1-Cre/Fbw7Flx/Flx mice was described previously (45). Fbw7Flx/Flx mice were used as controls. Expression of Cre recombinase in transplant recipients was induced by intraperitoneal injection of polyinosinic-polycytidylic acid (pIpC) (Sigma) to 8 and 9 weeks of age at a dose of 20 mg/kg of body weight on 7 alternate days. A Lin− fraction of bone marrow (BM) cells was isolated 1 week after the last injection of pIpC. All mice were treated according to the protocols approved by the Hamamatsu University School of Medicine Animal Care Committees at the Center Animal Care Facility. The genotype of gene-targeted mice and deletion of exon 5 of the floxed Fbw7 allele were verified by PCR with DNA subjects prepared by CellEase Tissue II (Biocosm) and the primers 5′-GTGTTCTTCACTTGGGAAGTGC-3′ (forward) and 5′-TGAACAGACGCAGACGCATTCT-3′ (reverse) for the Fbw7 allele and 5′-AGGTTCGTTCACTCATGGA-3′ (forward) and 5′-TCGACCAGTTTAGTTACCC-3′ (reverse) for the Cre recombinase transgene.
FACS Analysis
Isolation of mononuclear cells from BM was performed by Ficoll-Paque PLUS (GE Healthcare). Subsequently, lineage cells and common lymphoid progenitor populations were magnetically depleted. After staining with anti-c-Kit antibody, Lin− cells were fixed and permeabilized with the Foxp3 staining kit (eBioscience) prior to intracellular staining with the labeled anti-GATA2 antibody. The labeled Lin− cells were scored and sorted by FACS Aria instruments (BD Biosciences).
Statistical Analysis
Quantitative data were presented as means ± S.D. and were analyzed by Student's t test.
RESULTS
Fbw7 Binds to and Ubiquitylates GATA2 in a CPD-dependent Manner
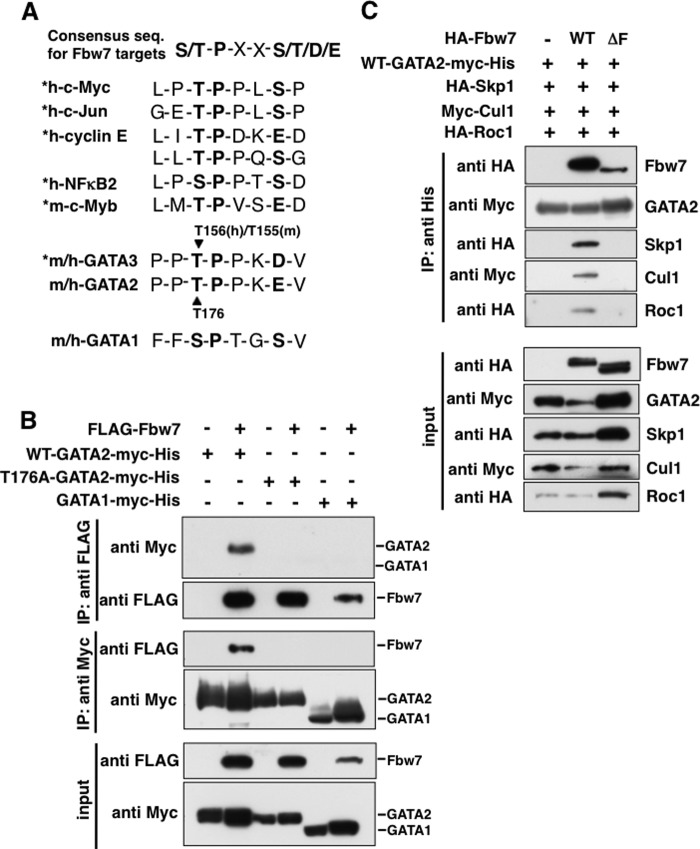
In many cases, Fbw7 recognizes phosphorylation of Ser/Thr residues in the CPD, which is the consensus sequence for recognition by Fbw7 in the selective substrates (19, 32). The CPD is speculated to be Ser(P)/Thr(P)-Pro-X-X-Ser(P)/Thr(P)/Glu/Asp. It has been reported that Fbw7 targets many substrates, such as c-Myc (8, 9), c-Jun (10, 11), cyclin E (7), NFκB2 (22), c-Myb (17–19), and Notch (12, 13), in a CPD-dependent manner (2, 32). For c-Myc, c-Jun, cyclin E, and NFκB2, their secondary Ser residues participate as the priming phosphorylation sites for GSK3-mediated phosphorylation of the first Ser/Thr residues in the CPD (18, 23). Recently, we found that GATA3, a GATA family transcriptional factor, has a CPD motif and is an Fbw7 target (23). Furthermore, putative CPD motifs were identified in both mouse and human GATA1 and GATA2 (Fig. 1A). Therefore, it was speculated that they were also the substrates for SCF-Fbw7. We investigated whether Fbw7 binds to GATA1 and GATA2. HEK293 cells were transfected with GATA1 or GATA2 in the presence or absence of Fbw7 and were treated with MG132. Fbw7 or GATAs were reciprocally immunoprecipitated following immunoblotting (IP/IB) to evaluate the binding of E3 and the substrates. As shown in Fig. 1B, Fbw7 bound to wild type GATA2 (WT-GATA2) but not to GATA1. Among GATA family members, GATA2 has the highest homology with GATA3 by aa sequence. Especially, a 14-aa sequence around the CPD region (from Thr156 to Asp160) of GATA3 that is recognized by Fbw7 in a phosphorylation-dependent manner, is almost completely conserved in GATA2. Therefore, we focused on a region (from Thr176 to Glu180) of GATA2, corresponding to CPD of GATA3. To validate the importance of Thr176, which might be phosphorylated, we prepared a GATA2 mutant with aa substitution (T176A), in which the Thr176 residue was replaced by Ala. As shown in Fig. 1B, the T176A-GATA2 did not bind to Fbw7. This result suggests that Fbw7 binds to GATA2 in a Thr176-dependent manner. Moreover, we found that GATA2 interacted with Skp1, Cul1, and Roc1/Rbx1 in the presence of wild type Fbw7 but not its mutant form lacking the entire F-box domain (ΔF) (Fig. 1C). This indicated that GATA2 binds to the SCFFbw7 complex.
FIGURE 1.
Fbw7 binds to GATA2 in a Thr176-dependent manner. A, the CPD, the consensus sequence for recognition by Fbw7, is indicated in the top panel. Sequences surrounding the CPD-like motif of GATA1 and GATA2 were aligned to CPD motifs in the reported Fbw7 substrates (*). Conserved amino acid residues within CPD are shown in boldface type. B, Fbw7 binds to WT-GATA2 but not T176A-GATA2 and GATA1. HEK293 cells were transfected with the indicated plasmids, and then were incubated with MG132 for 6 h. Total cell lysates were subjected to IP with the indicated antibody, followed by IB with the indicated antibodies. The original cell lysates (input) were subjected to IB with indicated antibody to confirm protein expression. C, GATA2 binds to the SCFFbw7 complex. HEK293 cells were transfected with the indicated plasmids and then were incubated with MG132 for 6 h. Total cell lysates were subjected to IP with anti-His antibodies, followed by IB with the indicated antibodies.
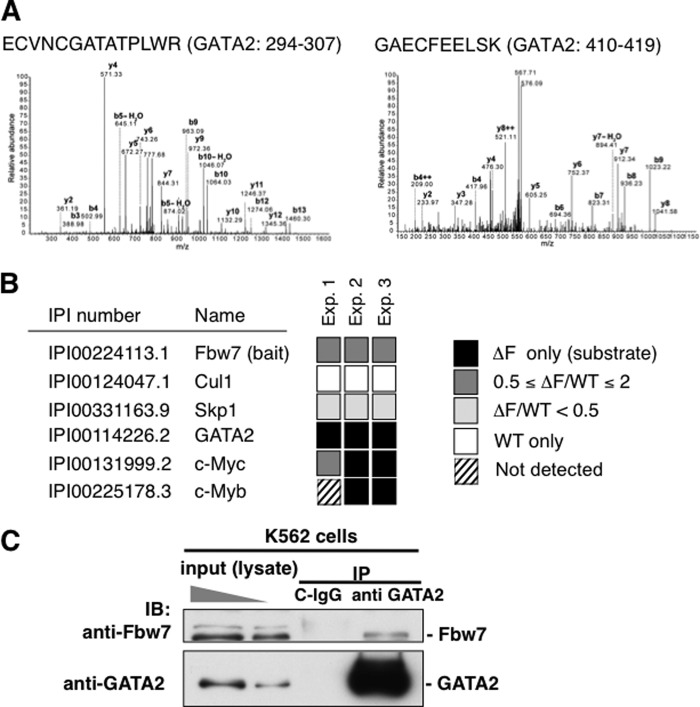
To determine whether endogenous GATA2 bound to Fbw7 in cells, we used two different approaches: differential proteomics analysis and immunoprecipitation analysis following immunoblotting (IP/IB) assay. To isolate the substrates for a given F-box protein, we used a differential proteomics approach termed DiPIUS-NL (differential proteomics-based identification of ubiquitylation substrates, nonlabeling) (42). A substrate is expected to be ubiquitylated and degraded upon its recognition by a ubiquitin ligase, resulting in a decrease in its cellular concentration. In contrast, an F-box-deleted ligase is expected to retain the ability to associate with a substrate but to have lost the ability to mediate ubiquitin conjugation, resulting in accumulation of the substrate in the cell. Both WT Fbw7 and the ΔF mutant of Fbw7 were tagged at their NH2 termini with the FLAG epitope and expressed separately in Neuro2A cells. Cell lysates were subjected to immunoprecipitation with antibodies to FLAG, and the immunoprecipitated proteins were analyzed by LC-MS/MS. We identified peptides coding m-GATA2 protein as a binding protein to the ΔF mutant of Fbw7 (Fig. 2A). The amounts of proteins that bound to the WT or mutant F-box proteins in three independent experiments were compared by semiquantitative spectral counting (42). The known Fbw7 substrates, such as c-Myc and c-Myb, were identified (42). Moreover, this assay reproducibly identified GATA2 in the three independent experiments (Fig. 2B). These results strongly suggest that Fbw7 targets GATA2 in cells.
FIGURE 2.
Endogenous GATA2 binds to Fbw7. A and B, differential proteomic analysis termed DiPIUS-NL indicated that GATA2 is a potential target of Fbw7. Neuro2A cells expressing mouse 3×FLAG-Fbw7 (WT) or its ΔF mutant (ΔF) were treated with MG132. The lysates were immunoprecipitated with anti-FLAG antibody and then eluted with FLAG peptide. The immunopurified proteins were separated by SDS-PAGE, and the silver-stained gel was sliced and subjected to in-gel digestion with trypsin. The resulting peptides were analyzed by LC-MS/MS. The indicated peptides bound to Fbw7-ΔF mutant were identified as mouse GATA2-derived peptide using MASCOT analysis (A). The abundance of proteins that bound to the WT or mutant F-box proteins in three independent experiments were compared by semiquantitative spectral counting, respectively (B). GATA2 was reproducibly identified as a strong candidate of Fbw7 substrate. C, endogenous GATA2 binds to endogenous Fbw7 in K562 cells. K562 cells treated with MG132 were lysed with proteasome inhibitors, protease inhibitors, and phosphatase inhibitors. Cell lysates were immunoprecipitated with anti-GATA2 rabbit monoclonal antibody (ab109241) or control rabbit IgG following immunoblotting with anti-Fbw7 rabbit polyclonal antibody (301-720A-1) or anti-GATA2 rat monoclonal antibody (RC1.1.1). Endogenous Fbw7 was co-precipitated with endogenous GATA2.
An IP/IB assay to evaluate the interaction between endogenous GATA2 and Fbw7 was performed. K562 cells treated with MG132 were lysed in the presence of proteasome inhibitors, protease inhibitors, and phosphatase inhibitors. Cell lysates were immunoprecipitated with anti-GATA2 antibody or control IgG, and immunoblotting analysis with the indicated antibodies was performed. This result clarified the binding of endogenous Fbw7 to endogenous GATA2 (Fig. 2C).
Fbw7 Ubiquitylates GATA2 in a CPD-dependent Manner
We investigated whether Fbw7 ubiquitylated GATA2 using IP/IB. WT-GATA2 but not T176A-GATA2 was ubiquitylated by Fbw7 (Fig. 3A). Furthermore, we performed a ubiquitylation assay under denaturing conditions to avoid the detection of ubiquitylated GATA2-associated proteins by Fbw7 (see “Experimental Procedures”). Under these conditions, Fbw7 clearly enhanced the ubiquitylation of GATA2, whereas GATA2 was only slightly ubiquitylated in cells without the ectopic expression of Fbw7 (Fig. 3B). However, ubiquitylation levels of GATA1 were not improved by Fbw7 (Fig. 3C). These results strongly suggest that Fbw7 promotes the ubiquitylation of GATA2 in a CPD-dependent manner.
FIGURE 3.
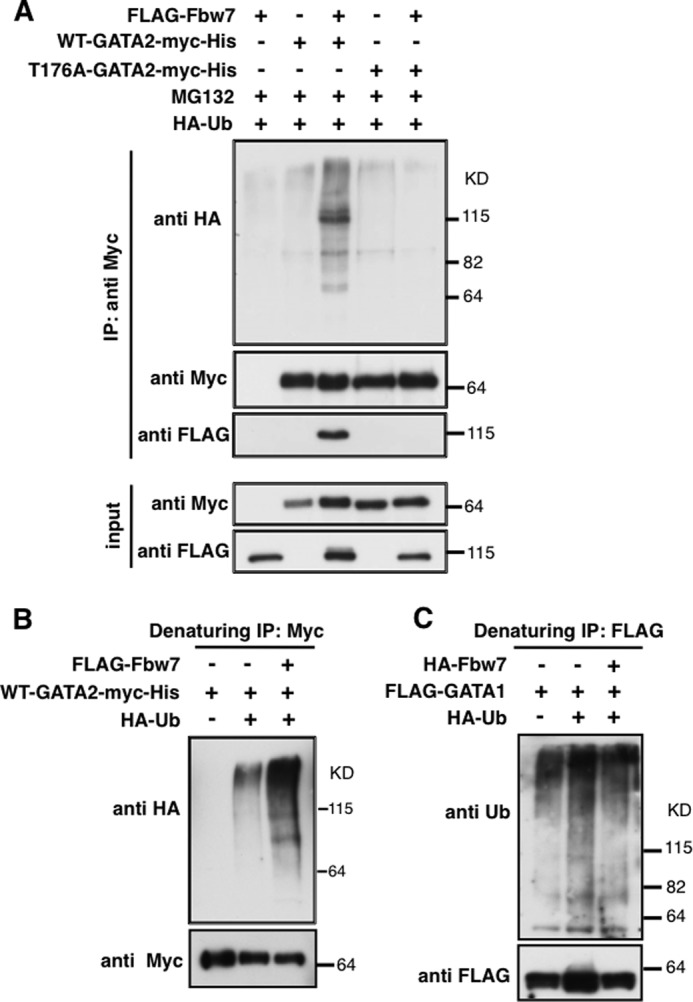
Fbw7 promotes ubiquitylation of GATA2 but not GATA1. HEK293 cells were transfected with indicated expression plasmids and were then incubated with MG132 for 6 h. Total cell lysates were subjected to IP with the indicated antibodies under native (A) or denaturing conditions (B and C), followed by immunoblotting with the indicated antibodies (see “Experimental Procedures”).
Fbw7 Promotes Proteasome-mediated Degradation of GATA2
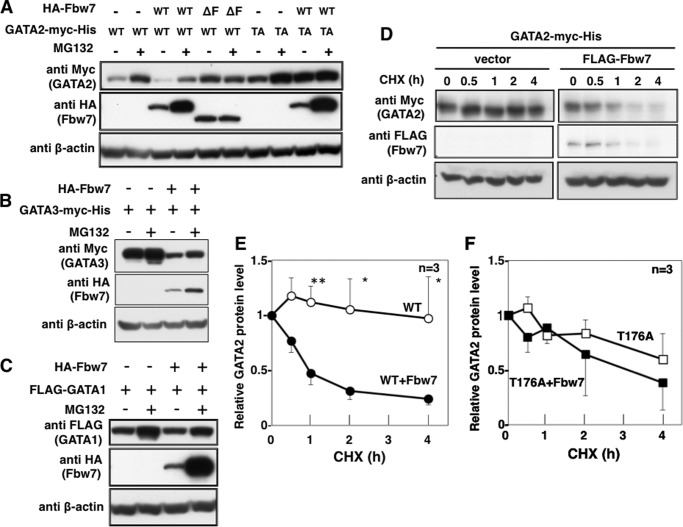
We next investigated whether Fbw7 destabilized GATA2. WT- or T176A-GATA2-myc-His (WT or TA, respectively, in Fig. 4) was transfected with or without HA-Fbw7 into HeLa cells with or without MG132 treatment. Forced expression of WT-Fbw7 but not ΔF-Fbw7 suppressed WT-GATA2 expression (Fig. 4A, lane 1 versus lanes 3 and 5), and that was recovered by MG132 treatment (Fig. 4A, lane 3 versus lane 4). Expression of T176A-GATA2 was not decreased by the co-expression of Fbw7 (Fig. 4A, lane 7 versus lane 9). We also confirmed that expression of GATA3 but not that of GATA1 was suppressed by Fbw7 (Fig. 4, B and C). Moreover, we evaluated the effect of Fbw7 on GATA2 stability using cycloheximide (CHX) to inhibit protein synthesis. Forced expression of Fbw7 destabilized WT-GATA2 in HeLa cells (Fig. 4, D and E). In contrast, the T176A-GATA2 was not affected by co-expression of Fbw7 (Fig. 4F). There was a tendency for a higher turnover of the T176A mutant than WT-GATA2. Although we evaluated whether the tendency was statistically significant by Student's t test, it was not significant. Therefore, degradation speeds of wild type GATA2 and GATA2-T176A were not significantly different. These results suggested that Fbw7 promotes proteasome-mediated degradation of GATA2 in a CPD-dependent manner.
FIGURE 4.
Fbw7-mediated degradation of GATA2 is Thr176-dependent. A, effects of Fbw7 on GATA2 stability. HeLa cells were transfected with WT-GATA2-myc-His or T176A-GATA2-myc-His in the absence or presence of Fbw7 or ΔF-box mutant (ΔF). After 41 h of transfection, cells were treated with or without 10 μm MG132 for 7 h and then harvested. Total cell lysates were subjected to immunoblotting with the indicated antibodies. B and C, effects of Fbw7 on GATA3 and GATA1 stability. HeLa cells were transfected with GATA3-myc-His or FLAG-GATA1 in the absence or presence of HA-Fbw7 and then were treated with or without 10 μm MG132 for 7 h. Levels of GATA3 (B) and GATA1 (C) were analyzed by immunoblotting with the indicated antibodies. D, effects of ectopic expression of Fbw7 on degradation of WT-GATA2 or T176A-GATA2. HeLa cells were transfected with WT-GATA2-myc-His in the absence or presence of FLAG-Fbw7, treated with 20 μg/ml CHX for the indicated periods, and harvested. Total cell lysates were subjected to immunoblotting. The representative data of WT-GATA2 are indicated (D). E and F, levels of WT-GATA2 and T176A-GATA2 in the absence or presence of FLAG-Fbw7 after the various chase times were quantitated by image analysis and normalized against β-actin. The percentages of remaining GATA2 protein were calculated as the mean ± S.D. (error bars) from three independent experiments. The p value was determined by Student's t test. *, p < 0.05; **, p < 0.01.
Cyclin B-CDK1 Phosphorylates Thr176 of GATA2
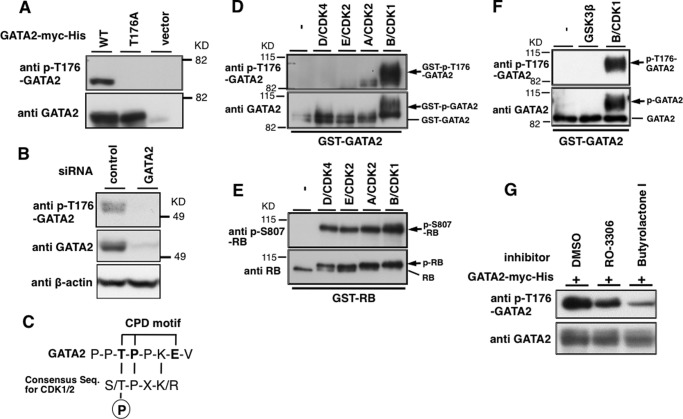
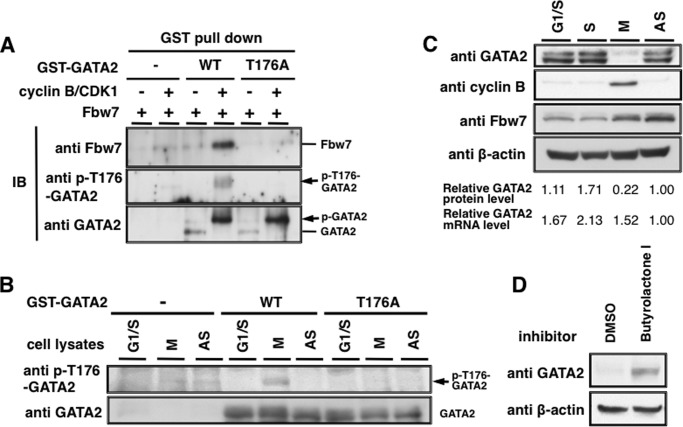
Fbw7 often recognizes phosphorylated CPD in substrates it binds to. We speculated that, as for other Fbw7 substrates, the regulation of GATA2 by Fbw7 would be mediated by phosphorylation of Thr176 in its CPD. To evaluate whether Thr176 of GATA2 was phosphorylated in intact cells, we generated a specific antibody against phosphorylated Thr176 of GATA2 (anti-p-T176-GATA2) as described under “Experimental Procedures.” Anti-p-T176-GATA2 recognized phospho-Thr176-containing antigen peptides but not unphosphorylated Thr176-containing peptides by ELISA (data not shown). WT-GATA2 expressed in HEK293 cells treated with phosphatase and proteasome inhibitors was detected by anti-p-T176-GATA2 antibody, but no signal was detected when using the T176A-GATA2 (Fig. 5A). Thus, the Thr176 of exogenous GATA2 is phosphorylated in vivo. Furthermore, we detected endogenous GATA2 and its phosphorylation on Thr176 in HeLa cells transfected with control siRNA but not with GATA2 siRNA (Fig. 5B), suggesting that endogenous GATA2 was phosphorylated at Thr176 in HeLa cells. The CPD motif in GATA2, Thr176-Pro-Pro-Lys-Glu, corresponds to a consensus motif for CDKs that contains Ser/Thr-Pro-X-Lys/Arg (Fig. 5C). To determine the kinase responsible for phosphorylation of GATA2 Thr176, we performed an in vitro phosphorylation assay using purified GST-GATA2 protein and recombinant cyclin-CDK complexes. Phosphorylation of Thr176 by kinases, including cyclin D3-CDK4 and cyclin E1-CDK2, was not detected, whereas all used CDK complex phosphorylated RB protein at almost the same efficiency (Fig. 5, D and E). Cyclin B1-CDK1 efficiently and cyclin A2-CDK2 slightly phosphorylated GATA2 at Thr176 (Fig. 5D). We also examined whether recombinant GSK3 phosphorylates GST-GATA2 in vitro (Fig. 5F). The slow migrating form of GST-GATA2 detected with anti-GATA2 was observed in the presence of cyclin B-CDK1 but not of GSK3β (Fig. 5F, bottom). Furthermore, GST-GATA2 was phosphorylated at Thr176 by cyclin B-CDK1 but not GSK3β (Fig. 5F, top), although glutamic acid at position +4 in T176PPKE might function as a mimic for priming phosphorylation required for GSK3β. We determined that GSK3β did not phosphorylate Thr176 in GATA2 in vitro and thus might not be the kinase responsible for CPD of GATA2. We further investigated whether phosphorylation of Thr176 of GATA2 was inhibited by CDK inhibitors in intact cells. Phosphorylation of Thr176 in HEK293 cells was inhibited by RO-3306, a selective CDK1 inhibitor, as well as by butyrolactone I (39, 40), which inhibits CDK1, -2, -3, and -5 (Fig. 5G). Thus, cyclin B-CDK1 might be a major kinase of Thr176 of GATA2.
FIGURE 5.
Cyclin B-CDK1 participates in phosphorylation of Thr176 of GATA2. A, evaluation of antibodies against phospho-Thr176-GATA2 (p-T176-GATA2). WT-GATA2-myc-His, or T176A-GATA2-myc-His were transiently expressed in HEK293 cells. Cell lysates prepared with lysis buffer containing phosphatase inhibitors were subjected to immunoblotting with anti-GATA2 or anti-p-T176-GATA2. B, Thr176 of endogenous GATA2 protein is phosphorylated in HeLa cells. Endogenous GATA2 protein was depleted by GATA2 siRNA, and lysates were subjected to immunoblotting with the indicated antibodies. C, putative consensus motif for phosphorylation by CDK1 and CDK2 in the CPD motif containing Thr176 in GATA2. D–F, Thr176 in GATA2 is efficiently phosphorylated by cyclin B1-CDK1 in vitro. Recombinant GST-WT-GATA2 (D and F) or GST-RB (E) was incubated with the indicated recombinant kinases in reaction buffer with 1 mm ATP for 30 min and then subjected to immunoblotting with the indicated antibodies. G, inhibition of Thr176 phosphorylation of GATA2 by CDK inhibitors. HEK293 cells were transfected with WT-GATA2-myc-His and then treated with CDK inhibitor (1 μm RO-3306 or 1 μm butyrolactone I) for 5 h. Cell lysates were subjected to immunoblotting with anti-p-T176-GATA2 or anti-GATA2.
Furthermore, we investigated whether phosphorylation of Thr176 in GATA2 was required for recognition by Fbw7 using a GST pull-down assay. Purified GST-WT-GATA2 or GST-T176A-GATA2 was phosphorylated by cyclin B1-CDK1 and incubated with cell lysates expressing FLAG-Fbw7. Then the mixtures were pulled down with glutathione beads following IB analysis with anti-FLAG to detect the binding of Fbw7 to GATA2. In addition to Thr176, because there are some putative phosphorylation sites for CDK1 in GATA2, it is speculated that GATA2 underwent multiple phosphorylations by cyclin B-CDK1 in vitro. GATA2 in the presence of cyclin B-CDK1 migrated more slowly than the unphosphorylated form of GATA2, even when Thr176 was substituted to alanine (T176A) (Fig. 6A, bottom). Finally, we found that Fbw7 bound to GST-WT-GATA2, which was detected by anti-p-T176-GATA2 antibody after treatment with cyclin B1-CDK1 (Fig. 6A, middle), but not to GST-GATA2 without cyclin B1-CDK1 or not to GST-T176A-GATA2 with or without cyclin B1-CDK1 (Fig. 6A, top). These results suggested that phosphorylation of Thr176 in GATA2 by cyclin B-CDK1 participates in the recognition by Fbw7.
FIGURE 6.
Phosphorylation of Thr176 of GATA2 participates in binding with Fbw7 and GATA2 is decreased in M phase. A, Thr176-phosphorylated GATA2 binds Fbw7 in vitro. Purified GST-fused WT- or T176A-GATA2 or GST protein using glutathione beads was incubated with or without recombinant cyclin B1-CDK1 in reaction buffer containing 1 mm ATP for 30 min. The reaction mixtures were incubated with lysates from HEK293 cells expressing FLAG-Fbw7 and then precipitated using glutathione-Sepharose beads and subjected to IB with the indicated antibodies. B, Thr176 of WT-GATA2 protein was phosphorylated by cell lysates prepared from synchronized HeLa cells at the M phase in vitro. Purified GST-fused WT- or T176A- GATA2 or GST proteins with glutathione beads were incubated with lysates prepared from HeLa cells arrested in G1/S or M phase or asynchronized (AS) HeLa cells in reaction buffer containing 1 mm ATP for 30 min. The mixtures were treated with precision protease for 30 min to cleave out GATA2 from the GST tag and then subjected to immunoblotting with indicated antibodies. C, endogenous GATA2 is decreased in the M phase. Lysates prepared from HeLa cells arrested at G1/S, S, or M phase or asynchronized HeLa cells were subjected to immunoblotting with indicated antibodies. mRNA levels in synchronized cells were measured by RT-qPCR. Total RNA was isolated from cells and subjected to reverse transcription with random hexanucleotide primers. The resulting complementary DNA was subjected to RT-qPCR. The amount of transcripts was normalized against that of 18 S rRNA as an internal standard. Relative protein and mRNA levels of GATA2 are indicated below. D, effect of CDK inhibitor treatment on GATA2 levels in M phase cells. HeLa cells synchronized at M phase were treated with 10 μm butyrolactone I for 6 h, and then GATA2 levels were analyzed by immunoblotting.
GATA2 Is Phosphorylated and Destabilized in M Phase during the Cell Cycle
Cyclin B-CDK1 is activated in the M phase and phosphorylates its substrates. To determine when Thr176 is phosphorylated during the cell cycle, we prepared total cell lysates from synchronized HeLa cells at G1/S or M phase or asynchronized HeLa cells and incubated with recombinant WT-GATA2 or T176A-GATA2. Thr176 of WT-GATA2 was phosphorylated only when incubated with M phase cell lysates, whereas that of T176A-GATA2 did not respond to any cell lysates tested (Fig. 6B). Moreover, endogenous GATA2 protein level was low in the M phase, during which cyclin B is expressed (Fig. 6C). GATA2 mRNA levels in the M phase were moderately increased compared with that in the asynchronized cells but were similar to that in G1/S phase cells, whereas GATA2 protein levels in the M phase were dramatically reduced (Fig. 6C). This suggested that the enhanced post-transcriptional degradation of GATA2 was involved in decreased GATA2 levels in the M phase. In addition, the low GATA2 protein levels in the M phase were increased by butyrolactone I treatment (Fig. 6D). In M phase cells, it was expected that butyrolactone I would selectively inhibit cyclin B-CDK1, although it inhibits both cyclin B-CDK1 and cyclin A/E-CDK2 in asynchronized cells. This finding suggests that inhibition of cyclin B-CDK1 activity by butyrolactone I increased the stability of GATA2 in the M phase. Together, this suggests that GATA2 protein is degraded in the M phase in a cyclin-B-CDK1-dependent manner.
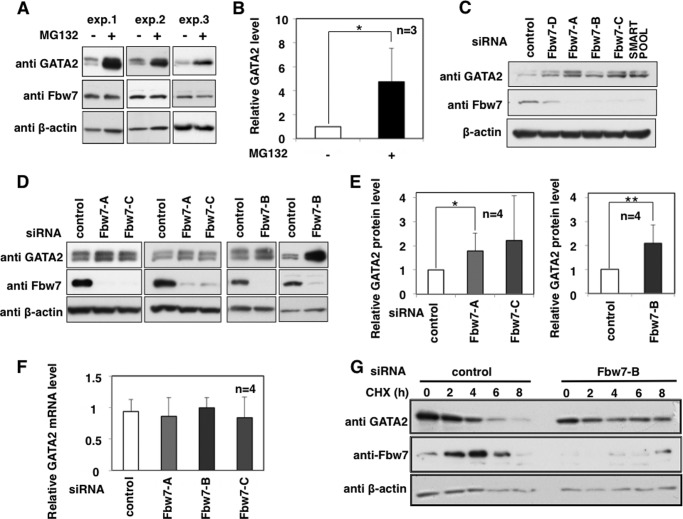
Depletion of Fbw7 Stabilizes Endogenous GATA2
Next, we addressed whether Fbw7 participated in stability control of endogenous GATA2 in intact cells. K562 cells endogenously expressed both GATA2 and Fbw7. GATA2 significantly accumulated in K562 cells treated with a proteasome inhibitor, MG132, suggesting that endogenous GATA2 degradation is proteasome-dependent (Fig. 7, A and B). Next, the contribution of Fbw7 to GATA2 stability was evaluated. Endogenous GATA2 was accumulated by depletion of Fbw7 using various siRNAs for Fbw7 in K562 cells (Fig. 7C). In addition, we repeated the knockdown experiments using siRNA-Fbw7-A, -B, and -C (Fig. 7, D and E). All siRNAs had a tendency to accumulate GATA2 protein, especially siRNA-Fbw7-A and Fbw7-B, which significantly accumulated GATA2 protein in K562 cells. To exclude the possibility that Fbw7 depletion may increase the transcription of GATA2, we evaluated the effects of Fbw7 depletion on GATA2 mRNA expression. As shown in Fig. 7F, GATA2 mRNA expression was not significantly influenced by treatment with siRNAs for Fbw7. We attempted to confirm the CHX chase experiment result. Although GATA2 was degraded in a time-dependent manner by treating CHX, depletion of Fbw7 decelerated its degradation rate in K562 cells (Fig. 7G). These results strongly suggest that endogenous Fbw7 functions as an E3 ligase for the degradation of GATA2 in intact cells.
FIGURE 7.
Depletion of endogenous Fbw7 stabilizes endogenous GATA2 protein in K562 cells. A and B, GATA2 is degraded in a proteasome-dependent manner. K562 cells were treated with or without 20 μm MG132 for 6 h. The cells were harvested, and the lysates were subjected to immunoblotting with the indicated antibodies (A). GATA2 levels normalized to β-actin levels were calculated from three independent experiments (B). C–F, effects of Fbw7 depletion on GATA2 stability. The lysates were subjected to immunoblotting with the indicated antibodies. K562 cells were transfected with the indicated siRNA or control siRNA for 48 h and harvested. The lysates were subjected to immunoblotting with the indicated antibodies. Representative data are shown (D). Relative GATA2 levels in the absence or presence of siRNA for Fbw7 were normalized to β-actin levels and calculated from four independent experiments (E). Relative mRNA levels of GATA2 were measured by RT-qPCR (F). Total RNA was isolated from cells and subjected to reverse transcription with random hexanucleotide primers. The resulting complementary DNA was subjected to RT-qPCR. The amount of transcripts was normalized against that of 18 S rRNA as an internal standard. G, K562 cells were transfected with siRNA for Fbw7 or control siRNA for 48 h and then treated with 20 μg/ml CHX for the indicated times. Cells were harvested, and lysates were subjected to immunoblotting with the indicated antibodies. p values were determined using Student's t test. *, p < 0.05; **, p < 0.01 (B and E). The relative GATA2 levels were calculated as the mean ± S.D. (error bars).
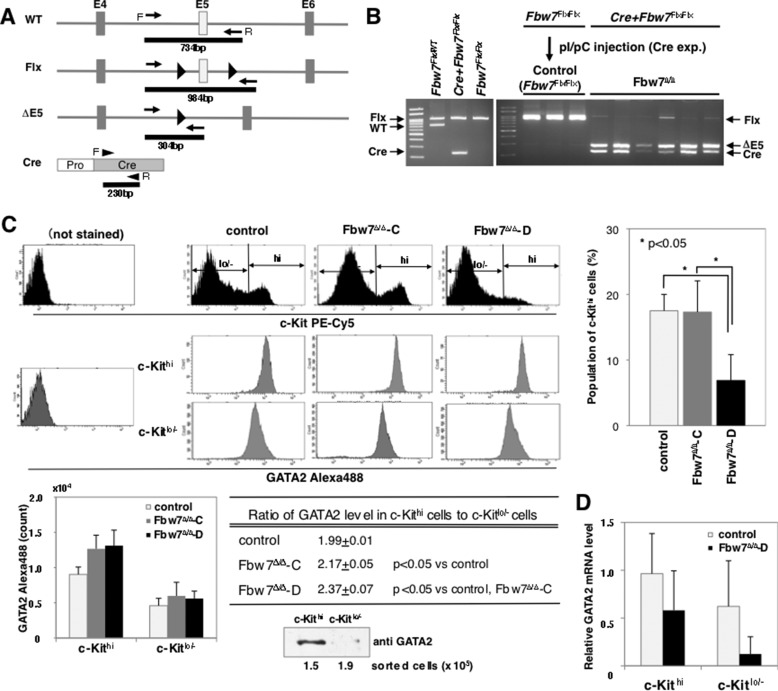
Effects of Fbw7 Depletion in Hematopoietic Stem Cells
Next, we addressed whether Fbw7 participated in stability control of GATA2 in mice. GATA2 is highly expressed in HSCs and progenitors, but its expression declines after erythroid commitment of progenitors (28). To address the in vivo contribution of Fbw7 to GATA2 stability during the early development of hematopoietic cells, we used conditional knock-out Mx1-Cre/Fbw7F/F mice, in which Fbw7 expression is lost by genetic deletion using Cre recombinase under control of the Mx1 gene promoter (Fig. 8A). Fbw7F/F mice were used for controls. Both groups were injected with pIpC on 7 alternate days to yield the Fbw7Δ/Δ genotype. One week after the final injection of pIpC, we confirmed that almost all floxed alleles of Fbw7 in Mx1-Cre/Fbw7F/F mice were exchanged to Fbw7Δ/Δ (Fig. 8B). Lineage-negative (Lin−) populations derived from BM monocyte were subjected to FACS analysis. A histogram of c-Kit analysis indicated that two peaks (c-Kithi and c-Kitlo/−) in control mice, besides the peak pattern derived from Fbw7Δ/Δ mice, were classifiable into two types (Fbw7Δ/Δ-C and Fbw7Δ/Δ-D). The expression pattern of c-Kit in Lin− cells from three of six Fbw7Δ/Δ mice (Fbw7Δ/Δ-C) corresponded with that from control mice (Fig. 8C, top). Meanwhile, a significant decrease in the c-Kithi subpopulation in the Lin− population was observed in the remaining three Fbw7Δ/Δ mice (Fbw7Δ/Δ-D) compared with that in other mice (Fig. 8C, top). The expression of GATA2 in the Lin− population was examined by FACS and IB analysis of sorted cells. The c-Kithi fraction had a higher expression of GATA2 than the c-Kitlo/− fraction in all examined subjects (Fig. 8C, middle, bottom left, and table). The tendency was similar between three subgroups of mice (Fig. 8C, middle and bottom left). Nevertheless, GATA2 levels in both fractions were higher in Fbw7Δ/Δ mice compared with those of control mice (Fig. 8C, bottom left). Interestingly, the ratio of GATA2 levels in c-Kithi cells to c-Kitlo/− cells was significantly increased in the fraction from Fbw7Δ/Δ mice compared with that of control mice; moreover, the ratio in the fraction from Fbw7Δ/Δ-D mice was significantly higher than in that from Fbw7Δ/Δ-C mice (Fig. 8C, table). There are two explanations for the increased GATA2 ratio. First, GATA2 levels might be repressed in c-Kitlo/− cells from Fbw7Δ/Δ mice, although there was a tendency toward increased GATA2 levels in both c-Kit fractions of Fbw7Δ/Δ mice. Second, a large quantity of GATA2 might exist during the c-Kithi stage derived from Fbw7Δ/Δ mice. In addition, c-Kithi subpopulation was significantly repressed in Fbw7Δ/Δ-D mice (Fig. 8C, top right). Conditional depletion of Fbw7 in BM cells often resulted in the enhanced accumulation of GATA2. It might depend on the undifferentiated degree in the progenitors. Therefore, excess GATA2 beyond the threshold in a primitive progenitor might cause the consumption of c-Kithi cells. We observed a higher accumulation of GATA2 in c-Kithi cells from Fbw7Δ/Δ mice compared with control mice, although its difference was not prominent. The depletion of Fbw7 did not increase GATA2 mRNA in either c-Kithi or c-Kitlo/− fractions (Fig. 8D). Taken together, we speculate that the depletion of Fbw7 caused increased GATA2 levels at the early stages of hematopoietic cell development, and subsequently, when amounts of GATA2 exceed the threshold, the cells may not be sustained in an undifferentiated state.
FIGURE 8.
GATA2 increases in Lin−/c-Kithi BM cells by Fbw7 depletion. A, schematic representations of the wild type mouse Fbw7 allele (WT), floxed Fbw7 allele (Flx), and floxed Fbw7 allele after removal of exon 5 by Cre recombinase (ΔE5). Triangles within Flx and ΔE5 alleles indicate LoxP genes. The positions of forward (F) and reverse (R) primer annealing corresponding to WT, Flx, and ΔE5 alleles are indicated by arrows, and those corresponding to the Cre recombinase gene are indicated by arrowheads, with each PCR product size. B, PCR analysis of genomic DNA from mouse tails (left) and mononuclear cells isolated from BM 1 week after the last injection of pIpC (right) were performed for genotyping of transgenic mice and verifying deletion of exon 5 (Fbw7Δ/Δ) in Cre+FbwFlx/Flx mice after pIpC treatment, respectively. C, the top graphs show representative profiles of c-Kit staining in Lin− BM cells from pIpC-injected mice. Cells were classified by expression levels of c-Kit, into c-Kithi and c-Kitlo/− subsets, and subsequently each subpopulation was analyzed for expression of GATA2 protein by FACS (middle) or subjected to immunoblot analysis (bottom right). Fbw7Δ/Δ mice were categorized into two classes based upon c-Kit profiles. Those with same levels as control mice and those with significantly decreased c-Kithi populations were named Fbw7Δ/Δ-C and Fbw7Δ/Δ-D, respectively (top right). Mean counts of GATA2 Alexa488 (bottom left) and ratio of GATA2 levels in c-Kithi cells to c-Kitlo/− cells (table) were compared among the three subgroups. Data are means ± S.D. (error bars) from three mice of each subgroup. D, GATA2 mRNA does not increase in Lin− BM cells by Fbw7 depletion. c-Kithi and c-Kitlo/− subsets from Lin− BM cells of control and Fbw7Δ/Δ mice were sorted and were analyzed for expression of GATA2 mRNA by RT-qPCR. Data are means ± S.D. from four mice of each genotype.
DISCUSSION
This study identified cyclin B-CDK1 as the CPD kinase responsible for phosphorylation of Thr176 in GATA2 in the M phase. Moreover, Fbw7 participated in stability control of GATA2 via the ubiquitin-proteasome system in both cultured cell lines and mouse HSCs. Therefore, we propose that Fbw7 is a bona fide E3 ligase for GATA2.
Many reported substrates of Fbw7 do not contain a lysine residue in their CPD, and some reports indicate that the existence of a lysine residue in the CPD does not enhance recognition by Fbw7. However, conflicting findings have been reported. The sequence of h-cyclin E contains two CPD motifs. One of them codes T62PDKE, which contains a lysine residue and is an active degron recognized by Fbw7 (46–48). Furthermore, we detected a functional CPD site in human GATA3, which belongs to the substrates of Fbw7 (23). It codes T156PPKD, which is similar to the site in h-cyclin E. In this report, we detected CPD in human GATA2, which codes T176PPKE. This is similar to that of human GATA3. Therefore, these findings provide compelling evidence that CPD functions as an Fbw7 recognition motif even if a lysine residue is present. Furthermore, the capacity of CPD that we detected in human GATA2 is supported by these data. It has been reported that GSK3β phosphorylates CPD motifs in the cognate substrates of Fbw7 (25, 32). GSK3β phosphorylates serine or threonine at the consensus sequence for GSK3β (Ser/Thr-X-X-X-Ser(P)/Thr(P)) after priming phosphorylation of the later Ser/Thr residue by other kinases. In addition, GSK3β functions on Ser/Thr-X-X-X-Glu/Asp without priming phosphorylation as for mouse c-Myb (18). Because the CPD motif overlaps with the GSK3β recognition consensus sequence, CPD motifs in Fbw7 substrates are often phosphorylated by GSK3β. In contrast, our studies indicated that CPDs in GATA2 and GATA3 (23) were phosphorylated by cyclin B-CDK1 and cyclin A-CDK2, respectively. The aa residue before the priming phosphorylation site in GATA2 and GATA3 is lysine, consistent with a consensus sequence (Ser/Thr-Pro-X-Lys/Arg) for phosphorylation by CDK (44). In contrast to GSK3β responding to signal transduction (32), CDKs are activated by the expression of specific cyclins in a cell cycle-dependent manner (49), and cyclin B-CDK1 functions in M phase. This is the first report that collaboration between cyclin B-CDK1 and SCFFbw7 contributes to protein degradation in M phase.
Koga et al. (38) previously reported the cell cycle-dependent regulation of GATA2 expression. In their study, GATA2 levels were high in S phase but low in G1/S and M phases. GATA2 protein contains some consensus sequences (Ser/Thr-Pro-X-Lys/Arg) for phosphorylation by CDKs. In vitro GATA2 phosphorylation assays suggested that cyclin D1-CDK4, cyclin A-CDK2, and cyclin B1-Cdc2 (CDK1) were candidates for the responsible kinases, although the individual phosphorylation sites were not identified. They also described that the aa 1–70, 153–256, and 412–480 regions were important for degradation of GATA2 and that Ser227 participated in destabilizing GATA2 in M phase. They speculated that CDKs mediated phosphorylation of Ser227, but the specific CDKs were not identified. Furthermore, it was not clarified why degradation of GATA2 was required in the M phase. The current study also did not answer this question; thus, further studies are required. Because sequences around Ser227 do not contain a CPD motif, it is not expected that Fbw7 recognizes phosphorylated Ser227 in GATA2 for ubiquitin-dependent degradation. Our study is the first to identify an E3 ligase for GATA2 and to clarify the recognition mechanism of GATA2 by Fbw7. Cellular proteins regulated by the ubiquitin-proteasome system are often targeted by E3 ligases. Moreover, depletion of Fbw7 increased the stability of GATA2, although it was still degraded. Accordingly, Fbw7 and other E3 ligases might target other sites, including Ser227, for the ubiquitin-dependent degradation of GATA2. Each E3 ligase might be specific for various cell types.
Fbw7 participates in the degradation of many cell cycle accelerators in hematopoietic cells. Fbw7 may coordinate their cellular levels via the ubiquitin-proteasome system to systemically control proliferation and differentiation of HSCs. Matsuoka et al. (50) studied the influence of depletion of Fbw7 in the development of BM HSCs using conditional knock-out Mx1-Cre/Fbw7F/F mice. In their report, leukocytes, hemoglobin, and platelets from peripheral blood cells, Lin− fraction of BM, and Lin−Sca-1+c-Kit+ (LSK) CD34− HSCs, respectively, were decreased in number in Fbw7-deleted mice compared with control mice. This suggests that disturbance of HSCs causes a diminution of differentiated cells. Moreover, increased c-Myc and Notch1 proteins involved in cell cycle progression were present in LSK cells from Fbw7-deleted mice. The authors explained that cell cycle promotion of HSCs was one cause of premature depletion of normal BM HSCs when Fbw7 was depleted. In addition to c-Myc and Notch1, GATA2 also functions as a cell cycle accelerator in HSCs. We observed its accumulation in the Lin− fraction of Fbw7Δ/Δ mice and speculated that c-Kithi cells might exhaust themselves by excessive GATA2 expression. However, it was also reported that enforced high expression of GATA2 inhibited proliferation, cell cycle entry from a quiescent stage, and functions of stem and progenitor cells (51–53). Therefore, accumulation of GATA2 in Fbw7Δ/Δ mice might inhibit cell cycle entry and cell proliferation of HSCs, and thereby c-Kithi HSC might be preferentially decreased. In addition, the depletion of Fbw7 promotes c-Myc accumulation and eliminates leukemia-initiating cells via apoptosis (45, 54). The Fbw7-mediated control of GATA2 and other Fbw7 substrates, including c-Myc, might participate in self-renewal and maintenance of hematopoietic/leukemic stem cells. Accordingly, GATA2 as well as c-Myc and Notch1 in HSCs from Fbw7Δ/Δ mice might perturb cell homeostasis because of their excessive accumulation.
Acknowledgments
We thank M. Matsumoto, M. Yoshida, and M. Hakamata for technical support and members of our laboratory for useful discussions.
This work was supported in part by Ministry of Education, Culture, Sports, Science, and Technology of Japan Grants-in-aid 24570151 (to K. K.) and 25112508 (to M. K.).
- SCF
- Skp1, Cullin, F-box-containing complex
- BM
- bone marrow; hematopoietic stem cell
- CPD
- Cdc4 phosphodegron
- aa
- amino acid(s)
- IB
- immunoblot
- pIpC
- polyinosinic-polycytidylic acid
- IP
- immunoprecipitation
- CHX
- cycloheximide
- RT-qPCR
- real-time quantitative RT-PCR.
REFERENCES
- 1. Hershko A., Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 [DOI] [PubMed] [Google Scholar]
- 2. Kitagawa K., Kotake Y., Kitagawa M. (2009) Ubiquitin-mediated control of oncogene and tumor suppressor gene products. Cancer Sci. 100, 1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lipkowitz S., Weissman A. M. (2011) RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat. Rev. Cancer 11, 629–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheffner M., Kumar S. (2014) Mammalian HECT ubiquitin-protein ligases: biological and pathophysiological aspects. Biochim. Biophys. Acta 1843, 61–74 [DOI] [PubMed] [Google Scholar]
- 5. Hatakeyama S., Nakayama K.-I. I. (2003) U-box proteins as a new family of ubiquitin ligases. Biochem. Biophys. Res. Commun. 302, 635–645 [DOI] [PubMed] [Google Scholar]
- 6. Nakayama K. I., Nakayama K. (2006) Ubiquitin ligases: cell-cycle control and cancer. Nat. Rev. Cancer 6, 369–381 [DOI] [PubMed] [Google Scholar]
- 7. Koepp D. M., Schaefer L. K., Ye X., Keyomarsi K., Chu C., Harper J. W., Elledge S. J. (2001) Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294, 173–177 [DOI] [PubMed] [Google Scholar]
- 8. Welcker M., Orian A., Jin J., Grim J. A., Harper J. W., Eisenman R. N., Clurman B. E. (2004) The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. U.S.A. 101, 9085–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yada M., Hatakeyama S., Kamura T., Nishiyama M., Tsunematsu R., Imaki H., Ishida N., Okumura F., Nakayama K., Nakayama K. I. (2004) Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23, 2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nateri A. S., Riera-Sans L., Da Costa C., Behrens A. (2004) The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 303, 1374–1378 [DOI] [PubMed] [Google Scholar]
- 11. Wei W., Jin J., Schlisio S., Harper J. W., Kaelin W. G., Jr. (2005) The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 8, 25–33 [DOI] [PubMed] [Google Scholar]
- 12. Tetzlaff M. T., Yu W., Li M., Zhang P., Finegold M., Mahon K., Harper J. W., Schwartz R. J., Elledge S. J. (2004) Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc. Natl. Acad. Sci. U.S.A. 101, 3338–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsunematsu R., Nakayama K., Oike Y., Nishiyama M., Ishida N., Hatakeyama S., Bessho Y., Kageyama R., Suda T., Nakayama K. I. (2004) Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J. Biol. Chem. 279, 9417–9423 [DOI] [PubMed] [Google Scholar]
- 14. Sundqvist A., Bengoechea-Alonso M. T., Ye X., Lukiyanchuk V., Jin J., Harper J. W., Ericsson J. (2005) Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCFFbw7. Cell Metab. 1, 379–391 [DOI] [PubMed] [Google Scholar]
- 15. Punga T., Bengoechea-Alonso M. T., Ericsson J. (2006) Phosphorylation and ubiquitination of the transcription factor sterol regulatory element-binding protein-1 in response to DNA binding. J. Biol. Chem. 281, 25278–25286 [DOI] [PubMed] [Google Scholar]
- 16. Fu L., Kim Y. A., Wang X., Wu X., Yue P., Lonial S., Khuri F. R., Sun S. Y. (2009) Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 69, 8967–8976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanei-Ishii C., Nomura T., Takagi T., Watanabe N., Nakayama K. I., Ishii S. (2008) Fbxw7 acts as an E3 ubiquitin ligase that targets c-Myb for nemo-like kinase (NLK)-induced degradation. J. Biol. Chem. 283, 30540–30548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kitagawa K., Hiramatsu Y., Uchida C., Isobe T., Hattori T., Oda T., Shibata K., Nakamura S., Kikuchi A., Kitagawa M. (2009) Fbw7 promotes ubiquitin-dependent degradation of c-Myb: involvement of GSK3-mediated phosphorylation of Thr-572 in mouse c-Myb. Oncogene 28, 2393–2405 [DOI] [PubMed] [Google Scholar]
- 19. Kitagawa K., Kotake Y., Hiramatsu Y., Liu N., Suzuki S., Nakamura S., Kikuchi A., Kitagawa M. (2010) GSK3 regulates the expressions of human and mouse c-Myb via different mechanisms. Cell Div. 5, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inuzuka H., Shaik S., Onoyama I., Gao D., Tseng A., Maser R. S., Zhai B., Wan L., Gutierrez A., Lau A. W., Xiao Y., Christie A. L., Aster J., Settleman J., Gygi S. P., Kung A. L., Look T., Nakayama K. I., DePinho R. A., Wei W. (2011) SCFFBW7 regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471, 104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wertz I. E., Kusam S., Lam C., Okamoto T., Sandoval W., Anderson D. J., Helgason E., Ernst J. A., Eby M., Liu J., Belmont L. D., Kaminker J. S., O'Rourke K. M., Pujara K., Kohli P. B., Johnson A. R., Chiu M. L., Lill J. R., Jackson P. K., Fairbrother W. J., Seshagiri S., Ludlam M. J., Leong K. G., Dueber E. C., Maecker H., Huang D. C., Dixit V. M. (2011) Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471, 110–114 [DOI] [PubMed] [Google Scholar]
- 22. Fukushima H., Matsumoto A., Inuzuka H., Zhai B., Lau A. W., Wan L., Gao D., Shaik S., Yuan M., Gygi S. P., Jimi E., Asara J. M., Nakayama K., Nakayama K. I., Wei W. (2012) SCFFbw7 modulates the NFκB signaling pathway by targeting NFκB2 for ubiquitination and destruction. Cell Rep. 1, 434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitagawa K., Shibata K., Matsumoto A., Matsumoto M., Ohhata T., Nakayama K. I., Niida H., Kitagawa M. (2014) Fbw7 targets GATA3 through CDK2-dependent proteolysis and contributes to regulation of T-cell development. Mol. Cell. Biol. 34, 2732–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welcker M., Clurman B. E. (2008) FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer 8, 83–93 [DOI] [PubMed] [Google Scholar]
- 25. Kitagawa K., Kitagawa M. (2012) The SCF ubiquitin ligases involved in hematopoietic lineage. Curr. Drug Targets 13, 1641–1648 [DOI] [PubMed] [Google Scholar]
- 26. Wang Z., Inuzuka H., Fukushima H., Wan L., Gao D., Shaik S., Sarkar F. H., Wei W. (2012) Emerging roles of the FBW7 tumour suppressor in stem cell differentiation. EMBO Rep. 13, 36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Z., Inuzuka H., Zhong J., Wan L., Fukushima H., Sarkar F. H., Wei W. (2012) Tumor suppressor functions of FBW7 in cancer development and progression. FEBS Lett. 586, 1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodrigues N. P., Tipping A. J., Wang Z., Enver T. (2012) GATA-2 mediated regulation of normal hematopoietic stem/progenitor cell function, myelodysplasia and myeloid leukemia. Int. J. Biochem. Cell Biol. 44, 457–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vicente C., Conchillo A., García-Sánchez M. A., Odero M. D. (2012) The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit. Rev. Oncol. Hematol. 82, 1–17 [DOI] [PubMed] [Google Scholar]
- 30. Nawijn M. C., Ferreira R., Dingjan G. M., Kahre O., Drabek D., Karis A., Grosveld F., Hendriks R. W. (2001) Enforced expression of GATA-3 during T cell development inhibits maturation of CD8 single-positive cells and induces thymic lymphoma in transgenic mice. J. Immunol. 167, 715–723 [DOI] [PubMed] [Google Scholar]
- 31. Onoyama I., Tsunematsu R., Matsumoto A., Kimura T., de Alborán I. M., Nakayama K., Nakayama K. I. (2007) Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J. Exp. Med. 204, 2875–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skaar J. R., Pagan J. K., Pagano M. (2013) Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell Biol. 14, 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ting C. N., Olson M. C., Barton K. P., Leiden J. M. (1996) Transcription factor GATA-3 is required for development of the T-cell lineage. Nature 384, 474–478 [DOI] [PubMed] [Google Scholar]
- 34. Shimizu R., Yamamoto M. (2012) Contribution of GATA1 dysfunction to multi-step leukemogenesis. Cancer Sci. 103, 2039–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishida H., Imai K., Honma K., Tamura S.., Imamura T., Ito M., Nonoyama S. (2012) GATA-2 anomaly and clinical phenotype of a sporadic case of lymphedema, dendritic cell, monocyte, B-and NK-cell (DCML) deficiency, and myelodysplasia. Eur. J. Pediatr. 171, 1273–1276 [DOI] [PubMed] [Google Scholar]
- 36. Spinner M. A., Sanchez L. A., Hsu A. P., Shaw P. A., Zerbe C. S., Calvo K. R., Arthur D. C., Gu W., Gould C. M., Brewer C. C., Cowen E. W., Freeman A. F., Olivier K. N., Uzel G., Zelazny A. M., Daub J. R., Spalding C.D., Claypool R. J., Giri N. K., Alter B. P., Mace E. M., Orange J. S., Cuellar-Rodriguez J., Hickstein D. D., Holland S. M. (2014) GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 123, 809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Minegishi N., Suzuki N., Kawatani Y., Shimizu R., Yamamoto M. (2005) Rapid turnover of GATA-2 via ubiquitin-proteasome protein degradation pathway. Genes Cells 10, 693–704 [DOI] [PubMed] [Google Scholar]
- 38. Koga S., Yamaguchi N., Abe T., Minegishi M., Tsuchiya S., Yamamoto M., Minegishi N. (2007) Cell-cycle-dependent oscillation of GATA2 expression in hematopoietic cells. Blood 109, 4200–4208 [DOI] [PubMed] [Google Scholar]
- 39. Kitagawa M., Okabe T., Ogino H., Matsumoto H., Suzuki-Takahashi I., Kokubo T., Higashi H., Saitoh S., Taya Y., Yasuda H. (1993) Butyrolactone I, a selective inhibitor of cdk2 and cdc2 kinase. Oncogene 8, 2425–2432 [PubMed] [Google Scholar]
- 40. Kitagawa M., Higashi H., Takahashi I. S., Okabe T., Ogino H., Taya Y., Hishimura S., Okuyama A. (1994) A cyclin-dependent kinase inhibitor, butyrolactone I, inhibits phosphorylation of RB protein and cell cycle progression. Oncogene 9, 2549–2557 [PubMed] [Google Scholar]
- 41. Nakayama K., Nagahama H., Minamishima Y. A., Matsumoto M., Nakamichi I., Kitagawa K., Shirane M., Tsunematsu R., Tsukiyama T., Ishida N., Kitagawa M., Nakayama K., Hatakeyama S. (2000) Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yumimoto K., Matsumoto M., Oyamada K., Moroishi T., Nakayama K. I. (2012) Comprehensive identification of substrates for F-box proteins by differential proteomics analysis. J. Proteome Res. 11, 3175–3185 [DOI] [PubMed] [Google Scholar]
- 43. Matsumoto M., Hatakeyama S., Oyamada K., Oda Y., Nishimura T., Nakayama K. I. (2005) Large-scale analysis of the human ubiquitin-related proteome. Proteomics 5, 4145–4151 [DOI] [PubMed] [Google Scholar]
- 44. Kitagawa M., Higashi H., Jung H.-K., Suzuki-Takahashi I., Ikeda M., Tamai K., Kato J., Segawa K., Yoshida E., Nishimura S., Taya Y. (1996) The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 15, 7060–7069 [PMC free article] [PubMed] [Google Scholar]
- 45. Takeishi S., Matsumoto A., Onoyama I., Naka K., Hirao A., Nakayama K. I. (2013) Ablation of Fbxw7 eliminates leukemia-initiating cells by preventing quiescence. Cancer Cell 23, 347–361 [DOI] [PubMed] [Google Scholar]
- 46. Welcker M., Singer J., Loeb K. R., Grim J., Bloecher A., Gurien-West M., Clurman B. E., Roberts J. M. (2003) Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol. Cell 12, 381–392 [DOI] [PubMed] [Google Scholar]
- 47. Ye X., Nalepa G., Welcker M., Kessler B. M., Spooner E., Qin J., Elledge S. J., Clurman B. E., Harper J. W. (2004) Mechanisms of Signal Transduction: Recognition of Phosphodegron Motifs in Human Cyclin E by the SCFFbw7 Ubiquitin Ligase. J. Biol. Chem. 279, 50110–50119 [DOI] [PubMed] [Google Scholar]
- 48. Hao B., Oehlmann S., Sowa M. E., Harper J. W., Pavletich N. P. (2007) Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol. Cell 26, 131–143 [DOI] [PubMed] [Google Scholar]
- 49. Pines J. (1993) Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem. Sci. 18, 195–197 [DOI] [PubMed] [Google Scholar]
- 50. Matsuoka S., Oike Y., Onoyama I., Iwama A., Arai F., Takubo K., Mashimo Y., Oguro H., Nitta E., Ito K., Miyamoto K., Yoshiwara H., Hosokawa K., Nakamura Y., Gomei Y., Iwasaki H., Hayashi Y., Matsuzaki Y., Nakayama K., Ikeda Y., Hata A., Chiba S., Nakayama K. I., Suda T. (2008) Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 22, 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Persons D. A., Allay J. A., Allay E. R., Ashmun R. A., Orlic D., Jane S. M., Cunningham J. M., Nienhuis A. W. (1999) Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood 93, 488–499 [PubMed] [Google Scholar]
- 52. Minegishi N., Suzuki N., Yokomizo T., Pan X., Fujimoto T., Takahashi S., Hara T., Miyajima A., Nishikawa S., Yamamoto M. (2003) Expression and domain-specific function of GATA-2 during differentiation of the hematopoietic precursor cells in midgestation mouse embryos. Blood 102, 896–905 [DOI] [PubMed] [Google Scholar]
- 53. Tipping A. J., Pina C., Castor A., Hong D., Rodrigues N. P., Lazzari L., May G. E., Jacobsen S. E., Enver T. (2009) High GATA-2 expression inhibits human hematopoietic stem and progenitor cell function by effects on cell cycle. Blood 113, 2661–2672 [DOI] [PubMed] [Google Scholar]
- 54. Reavie L., Buckley S. M., Loizou E., Takeishi S., Aranda-Orgilles B., Ndiaye-Lobry D., Abdel-Wahab O., Ibrahim S., Nakayama K. I., Aifantis I. (2013) Regulation of c-Myc ubiquitination controls chronic myelogenous leukemia initiation and progression. Cancer Cell 23, 362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]