Background: Excessive NF-κB hyperactivation should be tightly controlled in cells.
Results: UBXN1 inhibits TNFα-triggered NF-κB signaling by sequestering cIAPs from being recruited to TNFR1.
Conclusion: UBXN1 is a novel negative regulator of TNFα-triggered NF-κB signaling.
Significance: Our study identified a novel ubiquitin-linked protein UBXN1 as a negative regulator of NF-κB signaling pathway independent of VCP/p97 and provided important insight into the new regulatory mechanism of the UBX protein family.
Keywords: Inflammation, NF-κB, Polyubiquitin Chain, Signal Transduction, Tumor Necrosis Factor (TNF), UBXN1, cIAPs
Abstract
Excessive nuclear factor κB (NF-κB) activation should be precisely controlled as it contributes to multiple immune and inflammatory diseases. However, the negative regulatory mechanisms of NF-κB activation still need to be elucidated. Various types of polyubiquitin chains have proved to be involved in the process of NF-κB activation. Many negative regulators linked to ubiquitination, such as A20 and CYLD, inhibit IκB kinase activation in the NF-κB signaling pathway. To find new NF-κB signaling regulators linked to ubiquitination, we used a small scale siRNA library against 51 ubiquitin-associated domain-containing proteins and screened out UBXN1, which contained both ubiquitin-associated and ubiquitin regulatory X (UBX) domains as a negative regulator of TNFα-triggered NF-κB activation. Overexpression of UBXN1 inhibited TNFα-triggered NF-κB activation, although knockdown of UBXN1 had the opposite effect. UBX domain-containing proteins usually act as valosin-containing protein (VCP)/p97 cofactors. However, knockdown of VCP/p97 barely affected UBXN1-mediated NF-κB inhibition. At the same time, we found that UBXN1 interacted with cellular inhibitors of apoptosis proteins (cIAPs), E3 ubiquitin ligases of RIP1 in the TNFα receptor complex. UBXN1 competitively bound to cIAP1, blocked cIAP1 recruitment to TNFR1, and sequentially inhibited RIP1 polyubiquitination in response to TNFα. Therefore, our findings demonstrate that UBXN1 is an important negative regulator of the TNFα-triggered NF-κB signaling pathway by mediating cIAP recruitment independent of VCP/p97.
Introduction
The transcription factor NF-κB3 plays significant roles in many cellular processes, such as inflammation, immune response, and cell death. The transcription factor NF-κB could be activated by many stimuli, including inflammatory factor, microbial molecules, and genotoxic stress (1–3). Following these stimulations, NF-κB inhibitor α (IκBα) is phosphorylated and then degraded. NF-κB is consequently released and translocates to the nucleus where it activates the expression of NF-κB-related genes (4, 5).
In a previous study (7), ubiquitination is a key protein post-transcriptional modification in the regulation of the canonical NF-κB signaling pathway as well as many other cellular processes. Lys-48 ubiquitination leads to the substrate degradation by the proteasomes, whereas Lys-63 ubiquitination is involved in signaling transduction. Lys-63 ubiquitination of adaptor protein receptor-interacting protein 1 (RIP1) and the NF-κB essential modulator are the key events in the cascade of canonical NF-κB signaling (6, 7). After cells are treated by TNFα, TNFR1 trimerizes and recruits TNFR-associated death domain (TRADD) protein. TRADD acts as a scaffold protein that recruits TNFR-associated factor 2 (TRAF2) and RIP1 to form a complex with TNFR1. Meanwhile, cIAP1 and cIAP2 are recruited to the TNFR1 complex through TRAF2 and act as E3 ligases of RIP1 to modify RIP1 with the Lys-63 polyubiquitin chain, which is important for TGFβ-activated kinase 1 (TAK1) and TAK1-binding proteins 2/3 (TAB2/3) heterocomplex and the IKK heterocomplex recruitment. The activated TAK1 phosphorylates and activates IKKβ, which phosphorylates IκBα and leads to its degradation and NF-κB nuclear translocation (4, 8).
However, excessive activation of the NF-κB signaling pathway extensively contributes to chronic inflammatory disease, autoimmune disease, and so on. So the NF-κB signaling pathway should be precisely regulated, and the negative regulation of NF-κB signaling is still an important issue (9). Until now, in canonical NF-κB signaling pathway, several negative regulators have been discovered. TNFα-induced protein 3 (TNFAIP3), also called A20, negatively regulates NF-κB activity by deubiquitinating Lys-63-linked polyubiquitinated RIP1. Another protein, ubiquitin C-terminal hydrolase CYLD, deubiquitinates TRAF2 to block NF-κB activation (10). CUE domain-containing protein 2 (CUEDC2) regulates IKK dephosphorylation by recruiting PP1 to inhibit NF-κB activity (11).
In the 1990s, ubiquitin regulatory X (UBX) domain had been defined as it displayed weak amino acid sequence homology to ubiquitin. In 2008, all 13 UBX domain-containing proteins were revealed to be able to bind and act as cofactors of p97, also named valosin-containing protein (VCP) (12). VCP/p97 is a central component in many ubiquitin-mediated pathways and plays pivotal roles in many cellular processes, including cell cycle, DNA damage, and hypoxia stress (13, 14). The minority of UBX domain-containing proteins are already well studied. For example, p47, also named NSFL1C, is a cofactor of VCP/p97 and regulates VCP/p97 activity in membrane fusion (15). UBXD7 helps VCP/p97 to interact with HIF1a and regulate its turnover (12). But other UBX proteins still need to be elucidated.
UBX domain-containing protein 1 (UBXN1) contains both the N-terminal UBA domain and the C-terminal UBX domain, and it was recently discovered to interfere with RIG-I-mediated antiviral immune response by targeting the mitochondrial antivirus-signaling protein (16). In this study, we used a small scale siRNA library that screened out UBXN1 as a negative regulator in TNFα-triggered NF-κB activation. Overexpression of UBXN1 inhibited TNFα-triggered NF-κB activation, and knockdown of UBXN1 potentiated TNFα-triggered NF-κB activation. UBXN1 interacting with cIAPs, but not TRAF2, prevented TNFα-mediated cIAP1 recruitment to TNFR1 and decreased RIP1 ubiquitination. To sum up, our findings reveal a new negative regulatory mechanism in the TNFα-triggered NF-κB signaling pathway and may reveal a new function of UBXN1.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Rabbit anti-UBXN1 (AB10041) was from Millipore; rabbit anti-cIAP1 (ab108361) was from Abcam; mouse anti-RIP1 (610458) was from BD Biosciences; goat anti-TNFR1 (AF225) was from R&D Systems; rabbit anti-Myc (SC-789), rabbit anti-IκBα (SC-371), mouse anti-HA (SC-7392), and mouse anti-TNFR1 (SC-8436) were from Santa Cruz Biotechnology; rabbit anti-phospho-IKKα/β (Ser-176/180) (2679) and rabbit anti-phospho-IκBα (Ser-32) were Cell Signaling Technology; mouse anti-tubulin (T5168) and mouse anti-FLAG (F1804) were from Sigma. Recombinant human TNFα (210-TA-020) was from R&D Systems.
Constructs
pNF-κB-Luc, IL-6-Luc, pRL-TK, and mammalian expression plasmids for TRADD, TRAF2, RIP1, TAK1, and IKKβ were described previously. Mammalian expression plasmids for UBXN1, UBXD7, UBXD8, FAF1, and NSFL1C were gifts from Dr. Gabriela Alexandru. Mammalian expression UBXN1 mutant plasmids were constructed from pMAL-UBXN1, and its mutants were gifts from Dr. Richard Baer by standard molecular biology techniques.
Cell Transfection and RNA Interference
Small scale siRNA library was purchased from Dharmacon SMARTpool® siRNA library, Thermo Scientific. UBXN1 siRNAs were purchased from Invitrogen, and VCP/p97 siRNA was purchased from GenePharma. The sequences targeting human UBXN1 cDNA were as follows: UBXN1-#1, 5′-GGGAGTAGGGAGGCATGCCTAGGAA-3′; UBXN1-#2, GCCGAGGAGTTAGCAGCCAGACAAA-3′. siRNAs were purchased from GenePharma. The sequence targeting human VCP/p97 cDNA was as follows: VCP/p97, 5′-GGGCACATGTGATTGTTAT-3′. Nontargeted sequence was used as control for RNAi-related experiments.
Plasmids were transfected by Lipofectamine 2000 (Invitrogen), and cells were harvested between 24 and 48 h after transfection. siRNAs from Invitrogen or Dharmacon were transfected by Lipofectamine RNAiMax (Invitrogen).
Luciferase Reporter Assays
HEK293, HeLa, and U2OS cells (1 × 105) were transfected with 0.1 μg of the luciferase reporter pNF-κB-Luc (IκBα promoter) or IL-6-Luc (IL-6 promoter) plus 0.01 μg of the Renilla reporter pRL-TK, with or without various amounts of pLPC-N-FLAG UBXN1 expression vector. After being treated for 10 h with 10 ng/ml TNFα, transfected cells were collected. Luciferase assays were performed using a dual-specific luciferase assay kit (Promega).
Quantitative Real Time PCR
HeLa and HEK293 cells were treated with TNFα. Cell pellets were collected, and RNA was extracted with TRIzol (Invitrogen). Diluted RNA was reverse-transcribed and subjected to qPCR analysis to measure mRNA expression levels of NF-κB-targeted genes.
Gene-specific primer sequences were as follows: TNFA, forward 5′-GCCGCATCGCCGTCTCCTAC-3′ and reverse 5′-CCTCAGCCCCCTCTGGGGTC; IL-6, forward 5′-TTCTCCACAAGCGCCTTCGGTC-3′ and reverse 5′-TCTGTGTGGGGCGGCTACATCT-3′; IL-8, forward 5′-TCTGGCAACCCTAGTCTGCT-3′ and reverse 5′-AAACCAAGGCACAGTGGAAC-3′; ICAM, forward 5′-TCAGTGTGACCGCAGAGGACGA-3′ and reverse 5′-TTGGGCGCCGGAAAGCTGTAGAT-3′; and IKBA, forward 5′-GCTGATGTCAATGCTCAGGA-3′ and reverse 5′-CCCCACACTTCAACAGGAGT-3′.
Coimmunoprecipitation and Immunoblot
For transient transfection and coimmunoprecipitation experiments, HEK293T cells (1 × 106) transfected with various plasmids were incubated for 24–36 h before analysis and then lysed with 1 ml of M2 lysis buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 10% glycerol, 1% Triton X-100, 0.5 mm EDTA, 0.5 mm EGTA) containing certain protease inhibitors. The cell lysate was incubated with anti-FLAG M2-agarose affinity gel (A2220, Sigma) for 4 h. Beads were washed three times with 1 ml of lysis buffer. The precipitates were analyzed by standard immunoblot procedures. For semi-endogenous immunoprecipitation experiments, lysis buffer was prepared with 50 mm HEPES-KOH, pH 7.5, 5 mm Mg(OAc)2, 70 mm KOAc, 0.2% Triton X-100, 10% glycerol, 0.2 mm EDTA. For TNFR1 immunoprecipitation experiments, lysis buffer was prepared with 20 mm Tris, pH 7.4, 250 mm NaCl, 0.5% Nonidet P-40, 3 mm EDTA, 3 mm EGTA with protease inhibitors (2 mm dithiothreitol, 50 mm NaF, 40 mm β-glycerophosphate, 5 mm tetrasodium pyrophosphate, 0.1 mm sodium vanadate, and protease inhibitor mixture (Roche Applied Science)). All other samples for immunoblotting assays were prepared in M2 lysis buffer.
RESULTS
siRNA Screen of UBA Domain-containing Proteins That Regulate TNFα-triggered NF-κB Activity
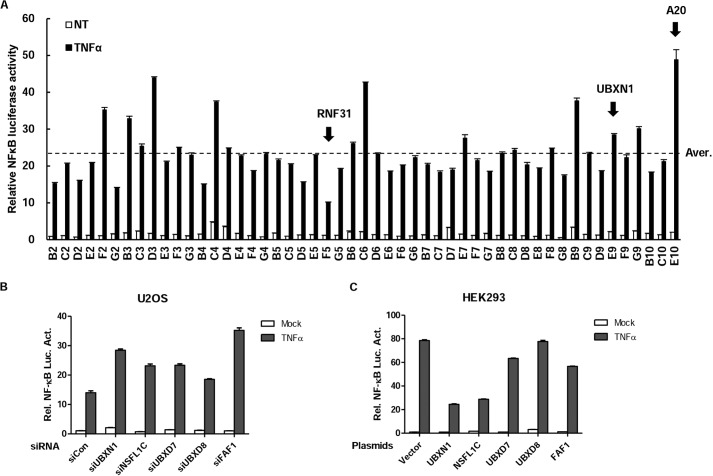
The NF-κB signaling pathway has been intensively studied for nearly 30 years. Many ubiquitin-related proteins involved in this pathway have been discovered as important regulators. To identify additional ubiquitin-related regulators in this pathway, we screened 51 Dharmacon siRNA pools for independent human genes that encode proteins containing the ubiquitin-associated domain by the NF-κB reporter assay in HeLa cells (Fig. 1 and Table 1). These efforts led to identification of UBXN1, a member of proteins containing both UBA and UBX domains. In the screen experiments, knockdown of UBXN1 markedly potentiated TNFα-triggered NF-κB activation (Fig. 1A). Among those results, RNF31 and A20 had been also screened out as positive controls (17, 18). Meanwhile, UBA and UBX domains containing proteins NSFL1C and FAF1 had been screened out, as expected. Reporter assays suggested that knockdown of UBA-UBX domain-containing proteins, UBXN1, NSFL1C, UBXD7, UBXD8, and FAF1, potentiated TNFα-triggered NF-κB activation (Fig. 1B). Overexpression of UBXN1, NSFL1C, and FAF1 consistently inhibited TNFα-triggered NF-κB activation, whereas overexpression of UBXD7 and UBXD8 barely inhibited TNFα-triggered NF-κB activation (Fig. 1C). In previous reports, NSFL1C and FAF1 had been found to be related to NF-κB signaling in 2012 and 2007 (19, 20). Those data show that UBXN1 down-regulates TNFα-triggered NF-κB activation, the same as with NSFL1C and FAF1. UBXN1 had the strongest inhibiting ability compared with NSFL1C and FAF1 in TNFα-triggered NF-κB activity.
FIGURE 1.
RNAi screen of UBA domain proteins that regulate NF-κB activity. A–C, small scale RNAi screen using a library targeting UBA domain proteins screened out UBXN1 as a potential NF-κB negative regulator. A, screening with siRNAs against 51 known/predicted UBA domain proteins was performed using the Dharmacon SMARTpool® siRNA library, in which each siRNA consisted of four individual sequences. HeLa cells were transfected with NF-κB luciferase plasmid and siRNAs. 48 h after transfection, the cells were treated with TNFα (10 ng/ml) or left untreated (NT) for 10 h before luciferase assays were performed. The gene symbols in the graph's horizontal axis represent related siRNA-targeted genes. The average raw luciferase value of the screen was inferred and indicated (Aver.). Values that are 1.2-fold higher or 1.2-fold lower than the average were marked by dashed lines. B, dual-luciferase assays of NF-κB activity in TNFα-treated HeLa cells transfected with control siRNA or siRNA against UBA-UBX family members, including UBXN1, NSFL1C, UBXD7, UBXD8, and FAF1. C, dual-luciferase assays of NF-κB activity in TNFα-treated HEK293 cells overexpressing control vector or UBA-UBX family members, including UBXN1, NSFL1C, UBXD7, UBXD8, and FAF1.
TABLE 1.
siRNA library against 51 known/predicted UBA domain proteins
| No. | Symbol | No. | Symbol | No. | Symbol | No. | Symbol |
|---|---|---|---|---|---|---|---|
| B2 | BRSK1 | C4 | MARK4 | D6 | SNF1LK | E8 | UBQLN1 |
| C2 | CBL | D4 | NACA | E6 | SNRK | F8 | UBQLN2 |
| D2 | CBLB | E4 | NACA2 | F6 | SQSTM1 | G8 | UBQLN3 |
| E2 | DHX57 | F4 | NBR1 | G6 | UBASH3A | B9 | UBQLN4 |
| F2 | FAF1 | G4 | NSFL1C | B7 | STS1 | C9 | UBXD7 |
| G2 | HIP2 | B5 | NUB1 | C7 | TDRD3 | D9 | UBXD8 |
| B3 | HUWE1 | C5 | RAD23A | D7 | TNRC6C | E9 | UBXN1 |
| C3 | ISGF3G | D5 | RAD23B | E7 | UBADC1 | F9 | USP5 |
| D3 | LATS1 | E5 | RHBDD3 | F7 | UBAC2 | G9 | USP13 |
| E3 | LATS2 | F5 | HOIP | G7 | UBAP1 | B10 | USP24 |
| F3 | MARK1 | G5 | RSC1A1 | B8 | UBAP2 | C10 | VPS13D |
| G3 | MARK2 | B6 | SIK2 | C8 | UBAP2L | E10 | A20 |
| B4 | MARK3 | C6 | SIK3 | D8 | UBL7 |
UBXN1 Negatively Regulates TNFα-triggered NF-κB Activation
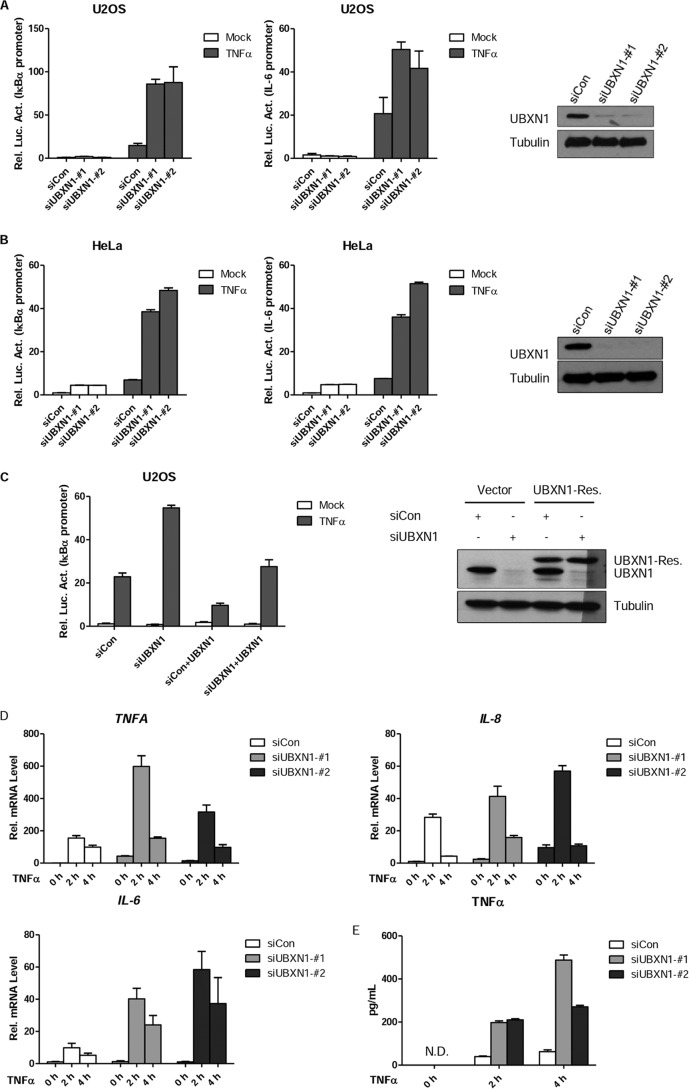
To further confirm the role of UBXN1 in TNFα-triggered NF-κB activation, we designed two different siRNAs targeting different sites of UBXN1 mRNA. As shown in Fig. 2A, transient transfection of two siRNAs could efficiently down-regulate expression of UBXN1 on a protein level in U2OS cells. In reporter assay using two different reporters, knockdown of UBXN1 markedly potentiated TNFα-triggered NF-κB activation in U2OS (Fig. 2A). Similar results were also found in HeLa cells (Fig. 2B). UBXN1 ectopic expression could also rescue UBXN1 knockdown-induced NF-κB activation upon TNFα stimuli (Fig. 2C). Real time quantitative PCR experiments demonstrated that knockdown of UBXN1 potentiated TNFα-triggered transcription of endogenous TNFA, IL-8, and IL-6 genes (Fig. 2D). Furthermore, ELISA indicated that TNFα production by knockdown of UBXN1 in U2OS cells was also up-regulated (Fig. 2E).
FIGURE 2.
Knockdown of UBXN1 potentiates TNFα-triggered NF-κB signaling. A, B, effects of UBXN1 knockdown on TNFα-triggered NF-κB activation in U2OS cells and HeLa cells. U2OS cells or HeLa cells (1 × 105) were first transfected with the pNF-κB-Luc (IκBα promoter, left), IL-6-Luc (IL-6 promoter, middle), and pRL-TK plasmids, and then control siRNA or siRNA against UBXN1-#1 and UBXN1-#2 12 h later. 48 h after siRNA transfection, cells were treated with TNFα (10 ng/ml) or left untreated for 10 h before luciferase assays were performed. Knockdown efficiencies of indicated siRNAs in U2OS cells were examined by immunoblot analysis (right). C, effects of UBXN1 knockdown on TNFα-triggered NF-κB activation could be rescued by UBXN1 expression. U2OS cells (2 × 105) were transfected with control siRNA or siRNAs against UBXN1-#1, 24 h later, UBXN1-rescue plasmid was transfected for another 24 h. Cells were treated with TNFα (10 ng/ml) or left untreated for 10 h before luciferase assays were performed. The experiments were similarly performed as in A. D, effects of UBXN1 knockdown on TNFα-induced transcription of TNFA, IL-8, and IL-6 genes. U2OS cells (2 × 105) were transfected with control siRNA or siRNAs against UBXN1-#1 and UBXN1-#2. Forty eight hours later, cells were treated with TNFα for the indicated times, and then total RNA was prepared for qPCR analysis. Expression is presented relative to GAPDH expression. E, effects of UBXN1 knockdown on TNFα-induced cytokine production of TNFα. U2OS cells (4 × 105) were transfected with control siRNA or siRNAs against UBXN1-#1 and UBXN1-#2. Forty eight hours later, cells were treated with TNFα for the indicated times, and then total supernatant was prepared for ELISA. N.D., not detected.
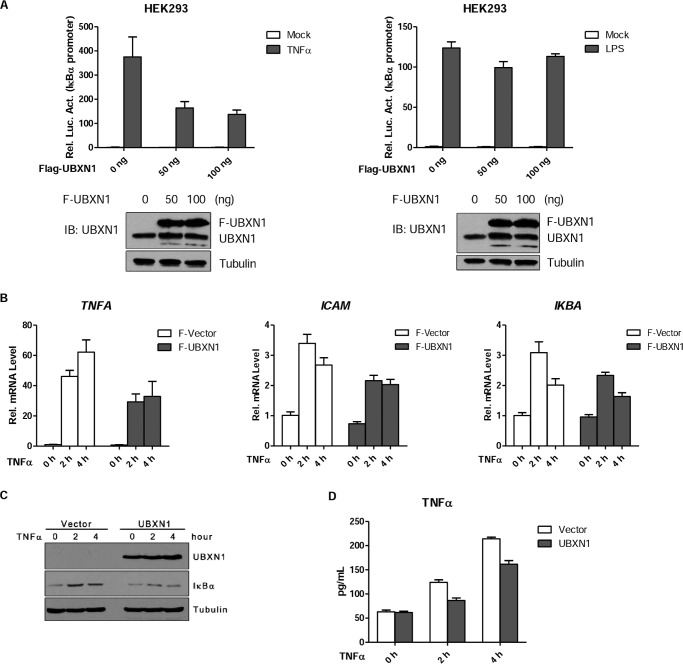
Consistently, we transiently overexpressed UBXN1 with an endogenous level in HEK293 cells, and UBXN1 had dose-dependent ability down-regulating TNFα-triggered NF-κB activation but not LPS-triggered NF-κB activation (Fig. 3A). Further qPCR assay demonstrated that overexpression of UBXN1 in HEK293 cell up-regulated TNFA, ICAM, and IκBα mRNA (Fig. 3B). ELISA indicated that overexpression of UBXN1 in HeLa cells could potentiate TNFα production in the supernatant of cultured cells (Fig. 3D). As expected, overexpression of UBXN1 also decreased IκBα protein levels in long term TNFα stimuli (Fig. 3C). These results suggest that UBXN1 is a physiological suppressor of the TNFα-triggered NF-κB signaling pathway.
FIGURE 3.
Overexpression of UBXN1 inhibits TNFα-triggered NF-κB activation. A, effects of UBXN1 overexpression on TNFα-triggered NF-κB activation in HEK293 cells were examined by dual-luciferase assays. HEK293 cells (1 × 105) were transfected with the pNF-κB-Luc, pRL-TK, and increasing amounts of FLAG-UBXN1 plasmid. 48 h after transfection, cells were treated with TNFα (10 ng/ml), LPS (1 μg/ml), or left untreated for 10 h before luciferase assays were performed. Expression of UBXN1 in HEK293 cells were examined by immunoblot analysis (bottom). B, effects of UBXN1 overexpression on TNFα-induced transcription of TNFA, ICAM, and IKBA genes. HEK293 (2 × 105) transfected with either an empty vector or FLAG-UBXN1 plasmid were treated with TNFα (10 ng/ml) for the indicated times, and then total RNA was prepared for qPCR analysis. The mRNA expression is presented relative to GAPDH expression. C, protein expression of UBXN1 and IκBα at indicated times after TNFα (10 ng/ml) treatment was examined by immunoblot analysis. D, effects of UBXN1 overexpression on TNFα-induced cytokine production of TNFα. HeLa cells (4 × 105) were transfected with either an empty vector or FLAG-UBXN1 plasmid. 36 h later, cells were treated with TNFα for the indicated times, and then total supernatant was prepared for ELISA.
UBXN1 Suppresses NF-κB Signaling at TNFR Complex Level
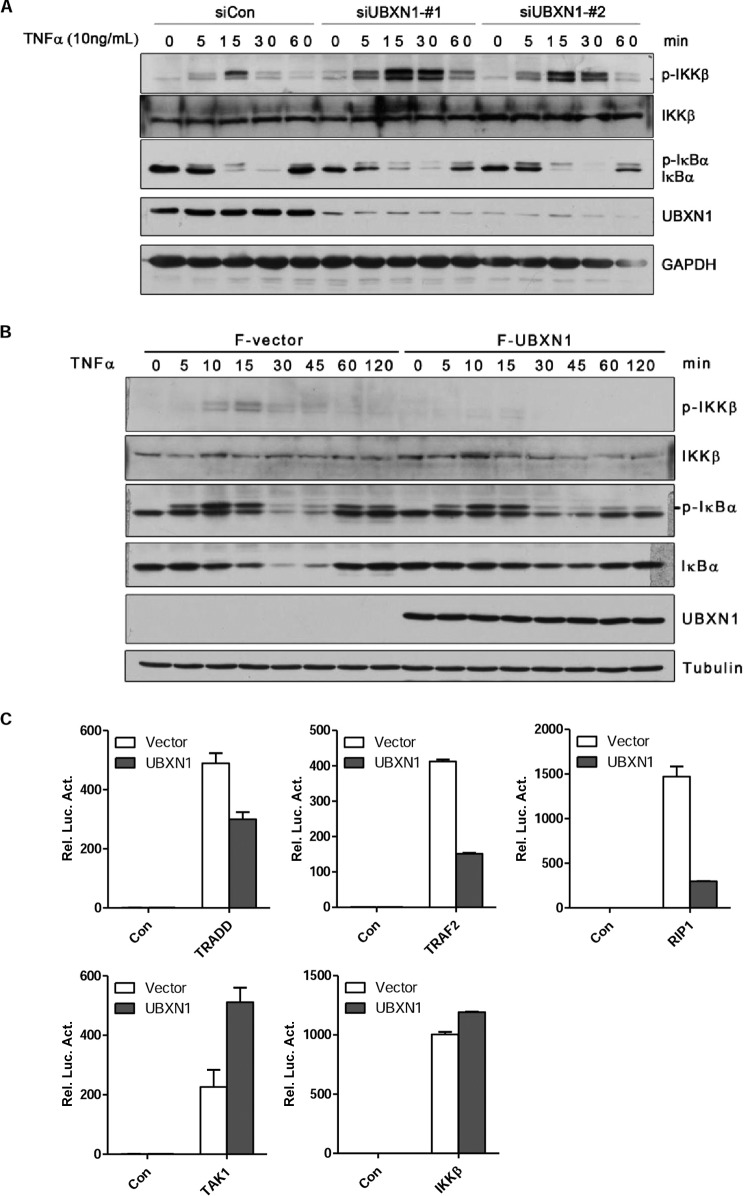
We next investigated whether UBXN1 affects IKK activation in NF-κB signaling. In response to TNFα treatment in U2OS cells, phosphorylation of IKKβ was much more robust when UBXN1 was down-regulated by respective siRNAs than when cells were transfected with control siRNA (Fig. 4A). Specifically, silencing UBXN1 expression resulted in prolonged IKKβ activation and accelerated IκBα degradation (Fig. 4A). Consistently, in response to TNFα treatment, we barely detected phosphorylation of IKKβ in HEK293 cells transfected with the UBXN1 plasmid. In addition, overexpression of UBXN1 blocked TNFα-induced IκBα degradation (Fig. 4B). These results indicated that UBXN1 has an inhibitory effect on IKK activation and may act as a regulator upstream of IKK level.
FIGURE 4.
UBXN1 inhibits TNFα-triggered NF-κB signaling at TNF receptor level. A and B, UBXN1 inhibits the process of TNFα-triggered NF-κB activation. A, U2OS cells (2 × 105) were transfected with control siRNA, UBXN1-#1, and UBXN1-#2. After 72 h, the cells were treated with TNFα (10 ng/ml) for indicated times. The whole cell lysate was analyzed by immunoblotting with the indicated antibodies. B, HEK293T cells (2 × 105) were transfected with either an empty vector or FLAG-UBXN1 plasmid. After 36 h, cells were treated with TNFα (10 ng/ml) for the indicated times. The whole cell lysate was analyzed by immunoblotting with the indicated antibodies. C, UBXN1 inhibits TRADD-, TRAF2-, and RIP1-mediated but not TAK1- or IKKβ-mediated NF-κB activation. HEK293T cells (1 × 105) were transfected with reporter plasmid, either an empty vector, or FLAG-UBXN1 plasmid together with TRADD, TRAF2, RIP1, TAK1, IKKβ, respectively. Luciferase assay was assessed 24 h after transfection.
To further confirm at which level UBXN1 negatively regulates NF-κB signaling, we examined the effect of UBXN1 on NF-κB activation mediated by important adaptor proteins and kinases TRADD, TRAF2, RIP1, TAK1, and IKKβ. Overexpression of UBXN1 markedly blocked the NF-κB activation mediated by TRADD, TRAF2, and RIP1 in the reporter assay (Fig. 4C). In contrast, UBXN1 did not affect TAK1 and IKKβ-mediated NF-κB activation in the reporter assay (Fig. 4C). As TRADD, TRAF2, and RIP1 were recruited to TNFR1 to form the TNFR complex, these results collectively indicated that UBXN1 may act as a negative regulator upstream of the TAK1 and IKK complex.
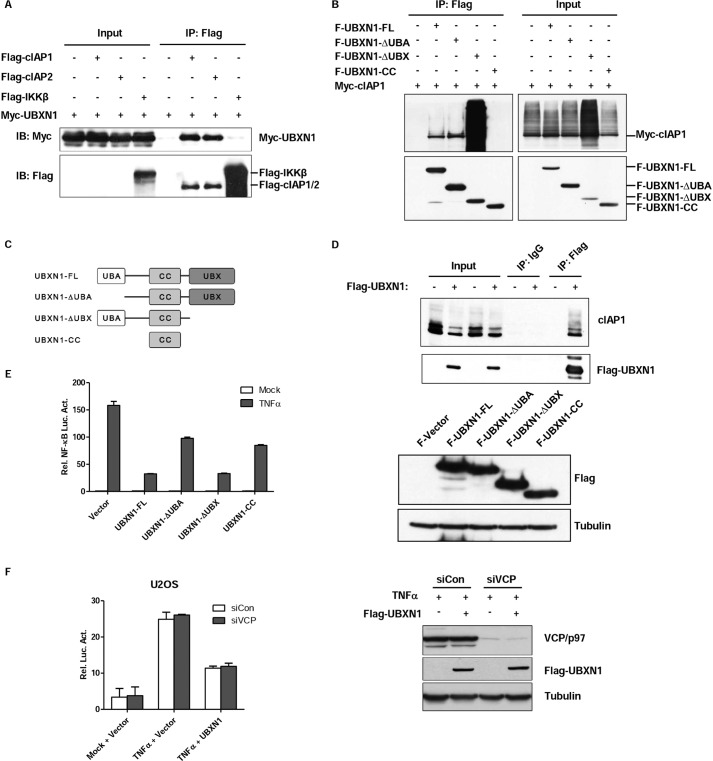
UBA Domain of UBXN1 Is Crucial for UBXN1-mediated NF-κB Inhibition
The results above indicate that UBXN1 functions upstream of TAK1 and IKK level in the TNFα-triggered NF-κB signaling pathway. Considering that the TNFR complex was upstream of the TAK1 complex and UBXN1 was reported that it may interact with cIAP1 by mass spectrometry (12), we therefore hypothesized that UBXN1 functioned at the TNFR complex level and interacted with cIAP1. Next, we confirmed the interaction between cIAP1 and UBXN1 by coimmunoprecipitation of tagged proteins expressed in HEK293T (Fig. 5A). Both TNFR complex subunits, cIAP1 and cIAP2, were able to bind to UBXN1, whereas IKKβ did not show any detectable association with UBXN1. Next, semi-endogenous immunoprecipitation suggested that UBXN1 interacted with cIAP1 supporting the notion that UBXN1 functioned at cIAPs of the TNFR complex (Fig. 5D). UBXN1 has an N-terminal UBA domain and a C-terminal UBX domain with a coiled-coil domain between them (Fig. 5C). UBXN1 domain mapping analysis indicated that either UBA or UBX domain is essential for the interaction between UBXN1 and cIAP1. Interestingly, UBX domain depletion seemingly potentiated the interaction of UBXN1 and cIAP2 (Fig. 5B). Then, to test that these UBXN1 truncations mediated NF-κB signaling inhibition, reporter assays suggested that the UBA domain-depleted UBXN1 had marked decreased ability to inhibit TNFα-triggered NF-κB activation (Fig. 5E). Because the UBX domain-depleted UBXN1 could still inhibit TNFα-triggered NF-κB activation, we supposed that UBXN1 acted as an NF-κB negative regulator independent of VCP/p97. Reporter assay indicated that knockdown of VCP/p97 did not have any effect on UBXN1-mediated NF-κB inhibition (Fig. 5F). These data collectively indicated that UBXN1 interacted with cIAPs by either the UBA domain or UBX domain, and the UBA domain of UBXN1 is crucial for UBXN1-mediated NF-κB inhibition.
FIGURE 5.
UBA domain of UBXN1 is crucial for UBXN1-mediated NF-κB inhibition. A–D, UBXN1 interacts with cIAPs. A, immunoassay of lysates of HEK293T cells cotransfected with vectors expressing FLAG-tagged (Flag-) cIAP1, cIAP2, or IKKβ and Myc-tagged (Myc-) UBXN1, immunoprecipitated (IP) with anti-FLAG, and analyzed by immunoblotting (IB) with anti-Myc. B, UBXN1 interacts with cIAP1 through either the UBA domain or UBX domain. Coimmunoprecipitation of cIAP1 and UBXN1 from HEK293T cells expressing Myc-cIAP1 and FLAG-tagged UBXN1 mutants using anti-FLAG-agarose beads was followed by immunoblot using a FLAG or Myc antibody. C, schematic representation of UBXN1 deletion mutants. D, immunoblot analysis of the interaction between endogenous cIAP1 proteins and exogenous UBXN1 in lysates of HEK293T cells transfected with either an empty vector or FLAG-UBXN1 plasmid after immunoprecipitation with mouse IgG or anti-FLAG (right) or with the same amount of cell lysate used for immunoprecipitation (Input; left). E, UBA domain of UBXN1 is essential for inhibiting TNFα-triggered NF-κB activation. Dual-luciferase assays of NF-κB activity in TNFα-treated HEK293T cells overexpressing FLAG-tagged UBXN1 truncates as described in C. The expression levels of FLAG-UBXN1 truncated mutants were examined by immunoblots. F, knockdown of VCP/p97 does not have effect on UBXN1-mediated NF-κB inhibition. The experiment was similarly performed as in Fig. 2A.
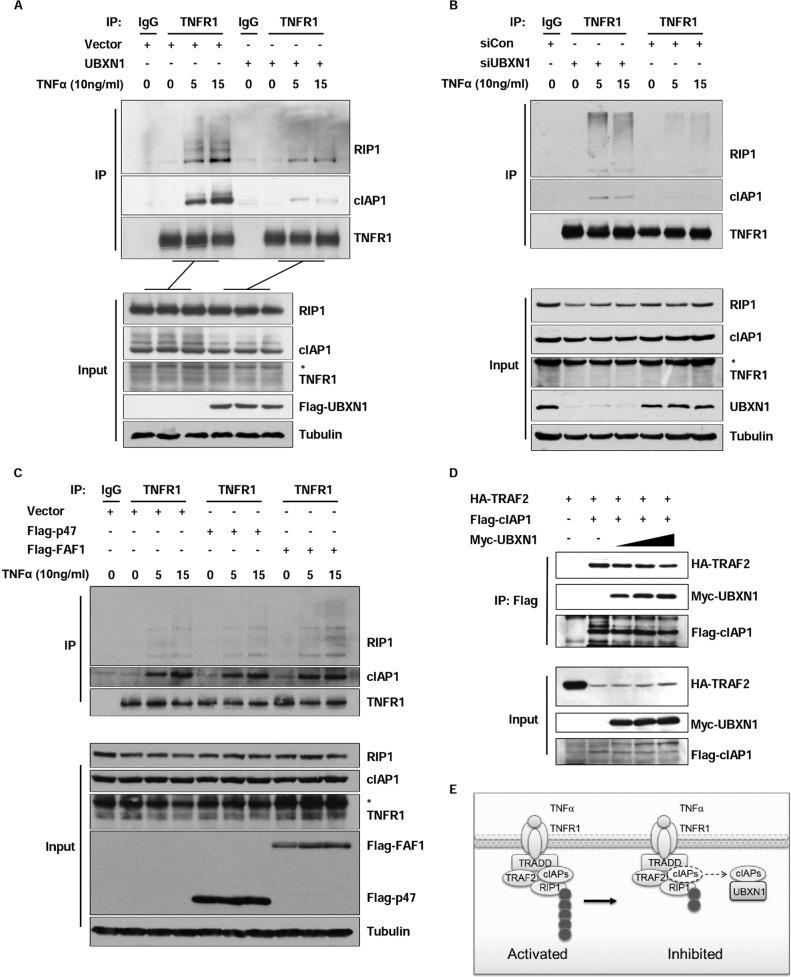
UBXN1 Sequesters cIAPs from TRAF2 and Decreases RIP1 Ubiquitination
In response to TNFα stimuli, RIP1, TRAF2, and cIAP1/2 were recruited to TNFR1 and act as important upstream adaptors for this signaling pathway. cIAP1/2 interacted with TRAF2 and functioned as the main E3 ligases of RIP1 for its Lys-63 ubiquitination, which was significant for downstream TAK1 and IKK complex recruitment (6). So, we examined whether TNFR complex formation would be affected by the UBXN1 protein level. In co-immunoprecipitation experiments by TNFR1 antibody, cIAP1 recruited to TNFR1 was substantially decreased in UBXN1-overexpressing HEK293 cells compared with those cells transfected with empty vector following TNFα stimulation. Moreover, RIP1 ubiquitination was also markedly decreased during TNFα stimulation (Fig. 6A). Consistently, transient knockdown of UBXN1 in U2OS cells promoted cIAP1 recruitment to TNFR1 by endogenous immunoprecipitation. RIP1 ubiquitination was also markedly increased by UBXN1 knockdown in U2OS cells (Fig. 6B). To test UBXN1 specificity on cIAP recruitment, we overexpressed two other UBA domain-containing UBX proteins, NSFL1C/p47 and FAF1, in the TNFR1 recruitment system. Expression of NSFL1C/p47 and FAF1 did not impair cIAP1 recruitment to TNFR1 in response to TNFα (Fig. 6C). Because UBXN1 could interact with cIAPs, we hypothesized that UBXN1 might sequester the cIAP1 and prevent its recruitment to TRAF2. Then we found that when UBXN1 was overexpressed in a dose-dependent manner, cIAP2-interacting TRAF2 was decreased (Fig. 6D). Thus, these results suggested that UBXN1 may functioned as another docking site of cIAP1 and sequester cIAP1 from recruiting to TNFR1 to ubiquitinate RIP1 following TNFα stimulation (Fig. 6E).
FIGURE 6.
UBXN1 sequesters cIAPs from TRAF2 and decreases RIP1 ubiquitination. A, overexpression of UBXN1 inhibits cIAP1 recruitment to TNFR1 and RIP1 ubiquitination upon TNFα stimulation. HEK293T cells transfected with vectors or FLAG-tagged (Flag-) UBXN1 plasmids were left untreated or treated with TNFα (10 ng/ml) for the indicated times. Immunoprecipitation (IP) and immunoblot analysis were performed with the indicated antibodies. B, UBXN1 deficiency potentiates cIAP1 recruitment to TNFR1 and RIP1 ubiquitination upon TNFα stimulation. U2OS cells transfected with control siRNA or siRNA against UBXN1 were left untreated or treated with TNFα (10 ng/ml) for the indicated times. Immunoprecipitation and immunoblot analysis were performed with the indicated antibodies. C, overexpression of NSFL1C/p47 and FAF1 barely inhibit cIAP1 recruitment to TNFR1 and RIP1 ubiquitination upon TNFα stimulation. Similar experiments were performed as in A. D, immunoassay of HEK293T cells transfected with HA-tagged TRAF2, FLAG-tagged cIAP1, and increasing amounts (wedges) of Myc-tagged UBXN1; cIAP1 in lysates was immunoprecipitated with anti-FLAG, followed by immunoblot analysis of immunoprecipitates (top) and input lysate (bottom). E, model illustrating UBXN1-mediated TNFα-triggered NF-κB signaling inhibition. Upon TNF-α stimulation, TRAF2 and RIP1 were recuited to TNFR1. cIAPs associate with TRAF2 and catalyze the conjugation of Lys-63-linked polyubiquitin chains to RIP1. UBXN1 binds to cIAPs and sequesters cIAPs from TRAF2 and decreases RIP1 ubiquitination.
DISCUSSION
The transcription factor NF-κB is a critical regulator of diverse physiological and pathological processes, including development, immune response, inflammation, and cancer. Many inflammation-related diseases and cancer are correlated with excessive NF-κB activation. To keep inflammatory homeostasis, NF-κB activation must be precisely regulated to keep it at a normal level. Therefore, elucidating the negative regulatory mechanisms in the NF-κB-signaling pathway is a key task. We screened 51 siRNA pools for independent proteins containing the UBA domain in TNFα-triggered NF-κB activation, as TNFα is a potent proinflammatory cytokine that can robustly activate the NF-κB signaling pathway. We identified UBXN1, one of 13 UBX domain proteins, as a possible negative regulator.
As we know, 13 UBX domain proteins had been identified that may interact with VCP/p97 as cofactors to maintain VCP/p97 function. Among the 13 UBX proteins, UBXN1, NSFL1C, UBXD7, UBXD8, and FAF1 contained both the N-terminal UBA domain and the C-terminal UBX domain, although others contained only the C-terminal UBX domain. We found that knockdown of these five proteins, UBXN1, NSFL1C, UBXD7, UBXD8, and FAF1, more or less potentiated TNFα-triggered NF-κB activation in the reporter assay. Meanwhile, only overexpression of UBXN1, NSFL1C, and FAF1 but not UBXD7 and UBXD8 could markedly inhibit TNFα-triggered NF-κB activation. Unexpectedly, UBXN1 had a stronger negative regulatory ability as NSFL1C and FAF1 had been reported as the NF-κB negative regulator in 2012 and 2007.
We next confirmed UBXN1 as a negative regulator in the TNFα-triggered NF-κB signaling pathway. Overexpression of UBXN1 down-regulated TNFα-triggered NF-κB activation in reporter assay, qPCR, and ELISAs, whereas knockdown of UBXN1 had consistent opposite effects on TNFα-triggered NF-κB activation. Furthermore, we found that knockdown of UBXN1 markedly potentiated IKKβ phosphorylation under TNFα stimulation. As TAK1 phosphorylated IKKβ in response to TNFα, we conferred that UBXN1 acted as a negative regulator upstream of the TAK1 complex.
Most regulators of the NF-κB signaling pathway regulate the key protein post-translational modification to down-regulate NF-κB activation. Well studied deubiquitinases, A20 and CYLD, have been reported to inhibit NF-κB signaling as deubiquitinase targeted the NF-κB essential modulator and TRAF2. CUEDC2 has been reported to act as an adaptor to target IKK for dephosphorylation by recruiting PP1 to inhibit NF-κB activation. In this study, we found that UBXN1 could interact with cIAPs. which are the main E3 ligases of RIP1. cIAPs together with TRAF2 ubiquitinate RIP1 for its Lys-63 ubiquitination, and the Lys-63 ubiquitination chain of RIP1 acts as the docking sites for IKK complex and TAK1 complex. Our results indicated that UBXN1 limits the interaction between TRAF2 and cIAPs, so that cIAPs cannot efficiently bind to TRAF2 and ubiquitinate RIP1, as our data showed. In a previous study, the UBX domain is essential for interacting with VCP/p97 (21), and the UBA domain has the ability to associate with ubiquitin chain (22, 23). Domain mapping assay showed that either UBA domain or UBX domain could help UBXN1 interact with cIAP1. However, UBA domain depletion of UBXN1 is not capable of inhibiting NF-κB activation, although UBX domain depletion of UBXN1 is still capable. Knockdown of VCP/p97 in the reporter assay indicated that VCP/p97 is simply not involved in UBXN1-mediated NF-κB inhibition. Another study (24) revealed that UBXN1 may block the function of BRCA1 by directly binding the ubiquitin chain. In our data, UBXN1 interacts with cIAPs through the UBA domain and prevents the interaction between TRAF2 and cIAPs as the negative regulator of NF-κB signaling pathway. Generally, UBXN1 acts as a steric hindrance of cIAPs in the NF-κB signaling pathway.
In conclusion, we have shown a new negative regulatory mechanism of the NF-κB signaling pathway. UBXN1 has been identified as cIAPs' interacting protein to block recruitment of cIAPs to TNFR1 from TRAF2 and sequentially inhibit RIP1 ubiquitination.
Acknowledgments
We thank Dr. Gabriela Alexandru and Dr. Richard Baer for generously providing materials.
This work was supported by National Basic Research Program of China Grants 2013CB910302, 2012CB910701, and 2012CB910801, National Natural Science Foundation of China Grants 81325014, 81130037, 31370761, 81221004, and 81025010, National Science and Technology Major Projects for “Major New Drugs Innovation and Development” Grant 2012ZX09301003-001-001, International S&T Corporation Program of China Grant 2013DFA31710, and National Science and Technology Support Program Grant 2012BAK25B00.
- NF-κB
- nuclear factor κB
- IKK
- IκB kinase
- qPCR
- quantitative PCR
- UBA
- ubiquitin-associated
- cIAP
- cellular inhibitors of apoptosis protein
- TNFR
- TNF receptor
- TRADD
- TNFR-associated death domain
- UBX
- ubiquitin regulatory X
- VCP
- valosin-containing protein.
REFERENCES
- 1. Karin M. (2006) Nuclear factor-κB in cancer development and progression. Nature 441, 431–436 [DOI] [PubMed] [Google Scholar]
- 2. Hayden M. S., Ghosh S. (2012) NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26, 203–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiDonato J. A., Mercurio F., Karin M. (2012) NF-κB and the link between inflammation and cancer. Immunol. Rev. 246, 379–400 [DOI] [PubMed] [Google Scholar]
- 4. Chen G., Goeddel D. V. (2002) TNF-R1 signaling: a beautiful pathway. Science 296, 1634–1635 [DOI] [PubMed] [Google Scholar]
- 5. Wajant H., Pfizenmaier K., Scheurich P. (2003) Tumor necrosis factor signaling. Cell Death Differ. 10, 45–65 [DOI] [PubMed] [Google Scholar]
- 6. Chen Z. J. (2012) Ubiquitination in signaling to and activation of IKK. Immunol. Rev. 246, 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chernorudskiy A. L., Gainullin M. R. (2013) Ubiquitin system: direct effects join the signaling. Sci. Signal. 6, pe22. [DOI] [PubMed] [Google Scholar]
- 8. Ghosh S., Hayden M. S. (2012) Celebrating 25 years of NF-κB research. Immunol. Rev. 246, 5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ben-Neriah Y., Karin M. (2011) Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 12, 715–723 [DOI] [PubMed] [Google Scholar]
- 10. Harhaj E. W., Dixit V. M. (2012) Regulation of NF-κB by deubiquitinases. Immunol. Rev. 246, 107–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li H. Y., Liu H., Wang C. H., Zhang J. Y., Man J. H., Gao Y. F., Zhang P. J., Li W. H., Zhao J., Pan X., Zhou T., Gong W. L., Li A. L., Zhang X. M. (2008) Deactivation of the kinase IKK by CUEDC2 through recruitment of the phosphatase PP1. Nat. Immunol. 9, 533–541 [DOI] [PubMed] [Google Scholar]
- 12. Alexandru G., Graumann J., Smith G. T., Kolawa N. J., Fang R., Deshaies R. J. (2008) UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1α turnover. Cell 134, 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer H., Bug M., Bremer S. (2012) Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat. Cell Biol. 14, 117–123 [DOI] [PubMed] [Google Scholar]
- 14. Meyer H., Weihl C. C. (2014) The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J. Cell Sci. 127, 3877–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kondo H., Rabouille C., Newman R., Levine T. P., Pappin D., Freemont P., Warren G. (1997) p47 is a cofactor for p97-mediated membrane fusion. Nature 388, 75–78 [DOI] [PubMed] [Google Scholar]
- 16. Wang P., Yang L., Cheng G., Yang G., Xu Z., You F., Sun Q., Lin R., Fikrig E., Sutton R. E. (2013) UBXN1 interferes with Rig-I-like receptor-mediated antiviral immune response by targeting MAVS. Cell Rep. 3, 1057–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tokunaga F., Sakata S., Saeki Y., Satomi Y., Kirisako T., Kamei K., Nakagawa T., Kato M., Murata S., Yamaoka S., Yamamoto M., Akira S., Takao T., Tanaka K., Iwai K. (2009) Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nat. Cell Biol. 11, 123–132 [DOI] [PubMed] [Google Scholar]
- 18. Wertz I. E., O'Rourke K. M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R., Boone D. L., Ma A., Koonin E. V., Dixit V. M. (2004) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 [DOI] [PubMed] [Google Scholar]
- 19. Shibata Y., Oyama M., Kozuka-Hata H., Han X., Tanaka Y., Gohda J., Inoue J. (2012) p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO. Nat. Commun. 3, 1061. [DOI] [PubMed] [Google Scholar]
- 20. Park M. Y., Moon J. H., Lee K. S., Choi H. I., Chung J., Hong H. J., Kim E. (2007) FAF1 suppresses IκB kinase (IKK) activation by disrupting the IKK complex assembly. J. Biol. Chem. 282, 27572–27577 [DOI] [PubMed] [Google Scholar]
- 21. Schuberth C., Buchberger A. (2008) UBX domain proteins: major regulators of the AAA ATPase Cdc48/p97. Cell. Mol. Life Sci. 65, 2360–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hurley J. H., Lee S., Prag G. (2006) Ubiquitin-binding domains. Biochem. J. 399, 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hofmann K., Bucher P. (1996) The UBA domain: a sequence motif present in multiple enzyme classes of the ubiquitination pathway. Trends Biochem. Sci. 21, 172–173 [PubMed] [Google Scholar]
- 24. Wu-Baer F., Ludwig T., Baer R. (2010) The UBXN1 protein associates with autoubiquitinated forms of the BRCA1 tumor suppressor and inhibits its enzymatic function. Mol. Cell Biol. 30, 2787–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]