Abstract
This report is the first description of dosing procedures, pharmacokinetics, biochemical action, and general tolerability of the antiaging drug rapamycin in the common marmoset, a small and short-lived monkey. Eudragit-encapsulated rapamycin was given orally to trained marmosets in a short-term (3 weeks) and a long-term (14 months) study. Circulating trough rapamycin levels (mean = 5.2ng/mL; 1.93–10.73 ng/mL) achieved at roughly 1.0 mg/kg/day was comparable to those reported in studies of rodents and within the therapeutic range for humans. Long-term treated animals (6/8) indicated a reduction in mammalian target of rapamycin complex 1 signaling as noted by a decrease in the phospho rpS6 to total rpS6 ratio after 2 weeks of treatment. All long-term treated subjects had detectable concentrations of rapamycin in liver (4.7–19.9 pg/mg) and adipose tissue (2.2–32.8 pg/mg) with reduced mammalian target of rapamycin signaling in these tissues. There was no evidence of clinical anemia, fibrotic lung changes, or mouth ulcers. The observed death rate in the long-term study was as expected given the animals’ ages. The ability to rapidly and reliably dose socially housed marmosets with an oral form of rapamycin that is well tolerated and that demonstrates a suppression of the mammalian target of rapamycin pathway leads us to conclude that this species offers a viable model for rapamycin testing to establish safety and efficacy for long-term antiaging intervention.
Key Words: Rapamycin, mTOR, Marmoset, Nonhuman primate.
In 2009, Harrison and colleagues (1) reported that daily rapamycin (RAPA) dosing increased mean and maximum life span of the UM-HET3 strain of mice. This life-span extension was observed when the mice began receiving RAPA relatively late in life—600 days (about 20 months of age), roughly equivalent to 60–65 years in a human. Since that time, numerous studies have replicated the ability of RAPA to increase the life span of mice, and RAPA has also been shown to affect numerous phenotypes known to change with age in mice (2–6). Taken as a whole, these findings have led to significant interest in the potential effects of RAPA as an antiaging intervention in humans (7–9).
Currently, RAPA is already approved for use in cancer therapy and as an adjunct immunosuppressive agent for transplant patients. Reported side effects of RAPA include hyperlipidemia, hyperglycemia, anemia, renal toxicity, interstitial pneumonia, and mouth ulcers (10,11). There have, however, been no large, long-term studies of RAPA as an antiaging intervention in populations of otherwise healthy humans. Although the ultimate goal of determining whether RAPA can extend the life span of human is many decades away, studies of RAPA’s effects in a species more closely related to humans are an approachable goal that can inform as to the generalizability of the rodent findings and identification of issues likely to impede the general use of RAPA as an antiaging treatment in humans
The common marmoset (Callithrix jacchus) is a small monkey with a relatively short life span—the average and maximum life span for this species being roughly 50% of that of commonly used Old World monkeys such as macaques. Both its small size and associated shorter life span make this species a valuable nonhuman primate model for the study of aging and chronic disease (12,13). From 2011 to 2013, we conducted two independent studies: a preliminary, short-term study to determine feasibility, assess pharmacokinetics and determine the appropriate delivery and concentration for oral dosing (Trial 1), followed by a 14-month long study of daily dosing of a group of common marmosets with RAPA (Trial 2). This report describes the dosing procedure, basic pharmacokinetics, and RAPA-induced signaling changes from these studies as well as measures of general tolerability, including blood chemistry assessments of anemia, chest x-ray assessments for signs of pneumonia and monthly examinations for mouth ulcers.
Methods
Subjects
The subjects for this study were common marmoset monkeys (C. jacchus) housed at the Southwest National Primate Research Center (Table 1). Basic housing and husbandry for this colony have been described previously (14). The initial dosing determination (Trial 1) consisted of eight singly housed subjects between the ages of 5.9 and 8.1 years old. The long-term study (Trial 2) examined 13 subjects between the ages of 7.1 and 9.1 years. The subjects for Trial 2 were housed as male–female pairs, with the males having been vasectomized at least 2 months before the initiation of the study to prevent pregnancies. The 13 subjects consisted of four pairs (eight subjects) receiving RAPA and two pairs (five subjects; the male in one pair died midway through the study and was replaced with another male) receiving the empty eudragit capsules as a control.
Table 1.
Common Marmoset Subjects Tested in Trials 1 and 2
| Animal | Sex | Trial | Age at Trial Start (y) | Initial Weight (g) | Dose (mg/d) |
|---|---|---|---|---|---|
| 1 | Male | 1 | 7.6 | 435 | 0.004 |
| 2 | Female | 1 | 5.9 | 390 | 0.004 |
| 3 | Male | 1 | 6.9 | 390 | 0.04 |
| 4 | Female | 1 | 7.2 | 410 | 0.04 |
| 5 | Male | 1 | 7.6 | 369 | 0.20 |
| 6 | Female | 1 | 8.1 | 446 | 0.20 |
| 7 | Male | 1 | 6.5 | 451 | 0.40 |
| 8 | Female | 1 | 7.7 | 350 | 0.40 |
| Ctrl-1 | Female | 2 | 7.1 | 370 | 0.0 |
| Ctrl-2 | Male | 2 | 7.9 | 403 | 0.0 |
| Ctrl-3 | Female | 2 | 7.5 | 440 | 0.0 |
| Ctrl-4 | Male | 2 | 8.3 | 395 | 0.0 |
| Ctrl-5 | Male | 2 | 7.7 | 363 | 0.0 |
| Rapa-1 | Female | 2 | 7.5 | 440 | 0.40 |
| Rapa-2 | Male | 2 | 8.2 | 380 | 0.40 |
| Rapa-3 | Female | 2 | 7.2 | 340 | 0.40 |
| Rapa-4 | Male | 2 | 8.0 | 415 | 0.40 |
| Rapa-5 | Female | 2 | 7.3 | 420 | 0.40 |
| Rapa-6 | Male | 2 | 8.3 | 380 | 0.40 |
| Rapa-ob-1 | Female | 2 | 8.0 | 535* | 0.40 |
| Rapa-ob-2 | Male | 2 | 9.0 | 520* | 0.40 |
Note:
*Obese.
Dose Delivery and Training
A delivery system of microencapsulating RAPA in a eudragit coating has been described and tested in rodents (1) and is believed to prevent the mouth ulcers often reported as a negative side effect of oral dosing with nonencapsulated RAPA in humans. Therefore, to test an oral dosing regime in the marmosets, eudragit-encapsulated RAPA (hereafter referred to as e-RAPA) or empty eudragit capsules were mixed into yogurt for feeding. Yogurt is a food item with which the marmosets are familiar as they receive it regularly for dietary enrichment, and it is generally well received by the animals. Further, yogurt offered the advantage of the ability to individually dose group housed animals via a syringe delivery, and thus the intake of each daily dose could be accounted for, unlike delivery via diet.
To prepare the yogurt mix, the same brand and flavor of yogurt (Hill Country Fare low fat strawberry-banana yogurt) and the same batch of e-RAPA were used throughout both Trial 1 and Trial 2. All fruit pieces were removed from the yogurt before mixture to prevent coagulating of the e-RAPA with the fruit. E-RAPA or eudragit control were mixed into the yogurt vigorously by hand to form a homogeneous suspension. The yogurt mixture was stored for up to seven days at 4°C in a container that was shielded from light exposure—either an opaque container or a transparent container that was wrapped in aluminum foil. The stability of e-RAPA in yogurt was examined by aliquoting samples from the mixture at the time of formulation and every 24 hours after mixing for 7 days.
The marmosets were trained to lick yogurt from the offered syringe using a standard click-reward bridge training regime. Before the start of training, the marmosets were habituated to the syringes by having a syringe left in their cage overnight allowing time for the animal to freely interact with it. The animals were trained to “target” a plain syringe by receiving a reward for interacting with it. The animals were initially rewarded for approaching the syringe, then for touching it, and finally for sniffing or licking the tip. If an animal was particularly hesitant to approach a syringe, a reward (eg, piece of marshmallow) was left on the tip of the syringe, and the animal was allowed to eat it. The second step of training was to allow animals to lick yogurt from the tip of the syringe. Gradually, the amount of yogurt the animal took from the syringe was increased until the animal would consistently take a full 1 mL. A few animals were hesitant to take yogurt from the syringe following habituation and target training to the syringe. In this case, a bridge task was added by luring the animal to eat yogurt by dipping a marshmallow in it and then rewarding the animal with a clean piece of marshmallow. Once consistently consuming yogurt the tasks of yogurt consumption and the syringe were recombined. The entire training procedure took less than 2 weeks for both Trial 1 and Trial 2.
Dosing Regimen
For both trials, the marmosets were dosed every day between 07:30 and 09:30 hours in their home cages before feeding; no handling was necessary. In Trial 1 to determine an appropriate dosage for use in this species, we tested four dose concentrations with two marmosets per dose for a 3-week period (Table 1). The concentrations tested were calculated based on the typical weight of a captive common marmoset (approximately 400 g) to range from around 0.01 mg/kg/day (0.004 mg/day) to 1.0 mg/kg/day (0.40 mg/day)—see Table 1. To determine the resulting blood concentrations of RAPA and the pharmacokinetics of the oral dosing, blood samples were collected before initial dosing as well as at 30, 60, 180, 360 minutes and 24 hours after the initial dose of e-RAPA. Blood samples were also collected before the daily dosing on days 1,7,14, and 21 following the initiation of dosing with e-RAPA. For Trial 2, the subjects received a daily dose of 0.4 mg/day for 14 months. Blood samples were collected before daily dosing at 4, 5, and 6 months of the trial to determine the concentration of circulating RAPA. At the end of the 14-month trial, the animals were sacrificed and target tissues were collected immediately, flash frozen, and stored at −80°C until further analysis.
Quantifying RAPA
Quantification of RAPA in marmoset blood and tissues was performed according to the previously published procedures (1,15,16) with modifications for the use of a tandem mass spectrometer for detection.
RAPA RAPA and ascomycin (ASCO) were obtained from LC Laboratories (Woburn, MA). HPLC-grade methanol and acetonitrile were purchased from Fisher (Fair Lawn, NJ). All other reagents were purchased from Sigma Chemical Company (St Louis, MO). Milli-Q water was used for preparation of all solutions. RAPA and ASCO super stock solutions were prepared in methanol at a concentration of 1mg/mL and stored in aliquots at −80°C. A working stock solution (10 μg/mL) was prepared each day from the super stock solution and used to spike the calibrator samples.
HPLC system.
The HPLC system consisted of a Shimadzu SCL-10A Controller, LC-10AD pump with a FCV-10AL mixing chamber, SIL-10AD autosampler, and an AB Sciex API 3200 tandem mass spectrometer with turbo ion spray. The analytical column was a Grace Alltima C18 (4.6 × 150mm, 5µ) purchased from Alltech (Deerfield, IL) and was maintained at 60°C during the chromatographic runs using a Shimadzu CTO-10A column oven. Mobile phase A contained 10mM ammonium formate and 0.1% formic acid dissolved in HPLC-grade methanol:water (90:10). Mobile phase B contained 10mM ammonium formate and 0.1% formic acid dissolved in HPLC-grade methanol. The flow rate of the mobile phase was 0.5 mL/min. RAPA was eluted with a step gradient. The column was equilibrated with 100% mobile phase A. At 6.10 minutes after injection, the system was switched to 100% mobile phase B. Finally, at 15.1 minutes, the system was switched back to 100% mobile phase A in preparation for the next injection. The RAPA transition was detected at 931.6Da (precursor ion), and the daughter ion was detected at 864.5Da. ASCO was detected at 809.574Da and the daughter ion was 756.34Da.
Quantification of RAPA in whole blood and tissues.
RAPA was quantified in uncoagulated marmoset blood (ethylene diaminetetraacetic acid). Briefly, 100 µL of calibrator and unknown whole blood samples were mixed with 10 µL of 0.5 µg/mL ASCO (internal standard), and 300 µL of a solution containing 0.1% formic acid and 10mM ammonium formate dissolved in HPLC-grade methanol:water (95:5). The samples were vortexed vigorously for 2 minutes and then centrifuged at 15,000g for 5 minutes at 23°C (subsequent centrifugations were performed under the same conditions). Supernatants were transferred to 1.5-mL microfilterfuge tubes and centrifuged at 15,000g for 1 minute and then 40 µL of the final samples was injected into the liquid chromatography–tandem mass spectrometry LC/MS/MS. The ratio of the peak area of RAPA to that of the internal standard ASCO (response ratio) for each unknown sample was compared against a linear regression of calibrator response ratios at 0, 1.25, 3.13, 6.25, 12.5, 50, and 100ng/mL to quantify RAPA. The concentration of RAPA was expressed as ng/mL whole blood. For fat and liver, 100mg of calibrator and unknown tissue samples was mixed by sonication (three 5-second bursts) in the solution described earlier. After sonication, the samples were vortexed vigorously for 2 minutes and then centrifuged at 15,000g for 5 minutes at 23°C (subsequent centrifugations were performed under the same conditions). Supernatants were transferred to 1.5-mL microfilterfuge tubes and centrifuged at 15,000g for 1 minute and then 40 µL of the final extracts was injected into the LC/MS/MS. The concentration of RAPA was expressed as µg/g of tissue (parts per million).
Quantification of RAPA in yogurt.
Yogurt samples (100mg) were dissolved in 300 µL mobile phase containing 0.1% formic acid and 10mM ammonium formate dissolved in HPLC-grade methanol:water (95:5). After sonication, the samples were vortexed vigorously for 2 minutes and then centrifuged at 15,000g for 5 minutes at 23°C (subsequent centrifugations were performed under the same conditions). Supernatants were transferred to 1.5-mL microfilterfuge tubes and centrifuged at 15,000g for 1 minute and then 40 µL of the final extracts was injected into the LC/MS/MS.The ratio of the peak area of RAPA to that of the internal standard ASCO (response ratio) for each unknown sample was compared against a linear regression of calibrator response ratios of spiked yogurt samples at concentrations of 0, 0.02, 0.20, 0.60mg/g RAPA. The concentration of RAPA was expressed as mg/g of yogurt.
Quantification of mTORC1 Signaling in Response to RAPA Treatment in Whole Blood, Liver, and Adipose Tissue
Marmoset blood (~1 mL) was drawn from each animal and subjected to a Ficoll-Paque (GE Healthcare) density gradient whereby the peripheral blood mononuclear cells. fractions were subsequently isolated. The cells were lysed with a standard 1% NP40 lysis buffer containing protease and phosphatase inhibitor cocktail (Pierce). To isolate protein from adipose and liver, 50mg of tissue was homogenized in modified radioimmunoprecipitation assay buffer with phosphatase and protease inhibitors (Roche). Isolated protein samples were then separated on Nupage 4%–12% Bis-Tris gels using morpholinepropanesulfonic acid running buffer (Life Technologies), transferred to nitrocellulose membranes, and probed with phospho & total rpS6 (Cell Signaling) and Tubulin (Sigma) antibodies. Secondary IRDye 680 and 800 antibodies were then used, and the blots were analyzed with the LICOR Odyssey Infrared Imaging System. Integrated Intensity of each band was then determined and the ratios of phospho/total rpS6 and phosphor rpS6/Tubulin reported.
Health Assessments
For Trial 1, the mouths of each animal were visually inspected before the beginning of dosing and at the end of the 21-day dosing. For Trial 2, the mouths of the animals were examined monthly for lesions. Before the beginning of dosing, the animals in Trial 2 were sedated for chest x-rays to establish baseline images of the lung tissue. The animals received a second x-ray on month 10 of the trial. Blood samples collected every 6 weeks for Trial 2 were sent to the Clinical Pathology department at SNPRC for standard veterinary blood chemistry assessment.
Results
The yogurt e-RAPA mixture was made once weekly, and doses were pulled from this mixture daily. Figure 1 illustrates that the concentration of RAPA in the yogurt mix was stable over a 7-day period.
Figure 1.
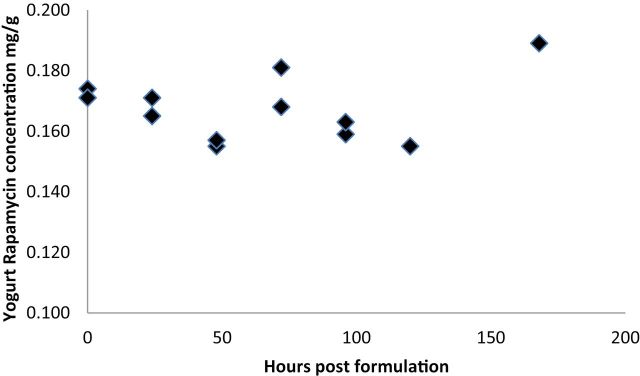
Concentrations of rapamycin in yogurt collected every 24 hours following mixing for 1 week to determine the stability of e-rapamycin in a semisolid food item.
Trial 1
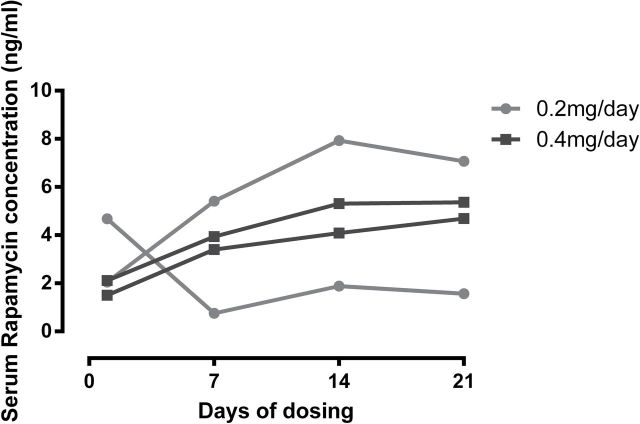
Figure 2 illustrates the serum RAPA concentration for the 24 hours following the initial dose of e-RAPA. The two subjects receiving 0.004mg/day failed to register detectable concentrations of RAPA in the blood at any time point following dosing. The two subjects receiving 0.04mg/day had returned to nondetectable concentrations at 24 hours following dosing. The subjects receiving 0.2 and 0.4mg/day displayed peak concentrations at 30 (n = 1) or 180 (n = 3) minutes postdosing and all retained detectable concentrations at 24 hours following dosing. The trough concentrations on days 1, 7, 14, and 21 for two subjects receiving 0.2mg/day and the two subjects receiving 0.4mg/day are shown in Figure 3A. Concentrations within a subject remained fairly consistent through the 3-week period, but concentrations varied appreciably among subjects. The average concentration on day 21 was 4.68 ng/mL with a range from 1.57 to 7.07 ng/mL.
Figure 2.
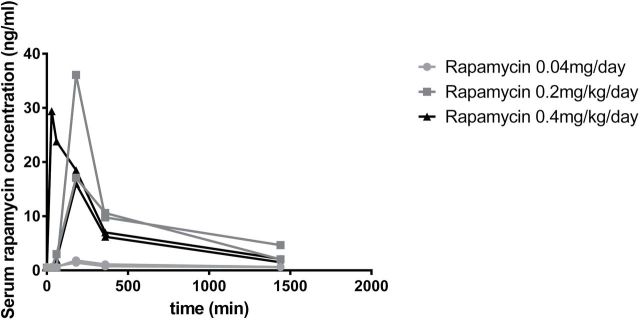
Whole blood rapamycin concentration over a 24-hour time course for animals tested in Trial 1.
Figure 3.
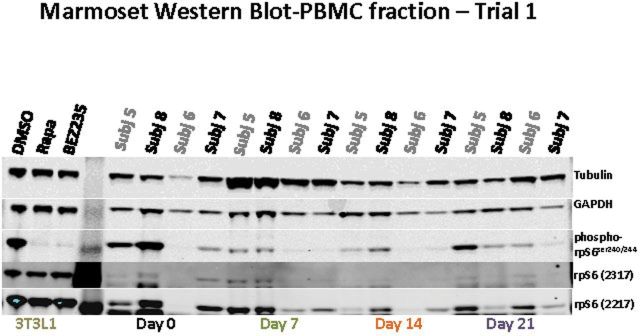
(A) Whole blood rapamycin concentrations over the 3-week dosing phase for four animals tested in Trial 1; (B) marmoset integrated intensity-PBMC fraction phospho-rpS6/total-rpS6 ratio for four animals tested in Trial 1; and (C) Western blots associated with 3-B; see Table 1 for further information on specific subjects. Subjects 5–6 were dosed at 0.20mg/day and subjects 7–8 at 0.40mg/day. A single blot was run and probed with the antibodies listed at the right. The blot was then stripped and reprobed with rpS6 (2217). The 3T3L1 cell line treated with dimethyl sulfoxide/rapamycin/BEZ235 is included as a control.
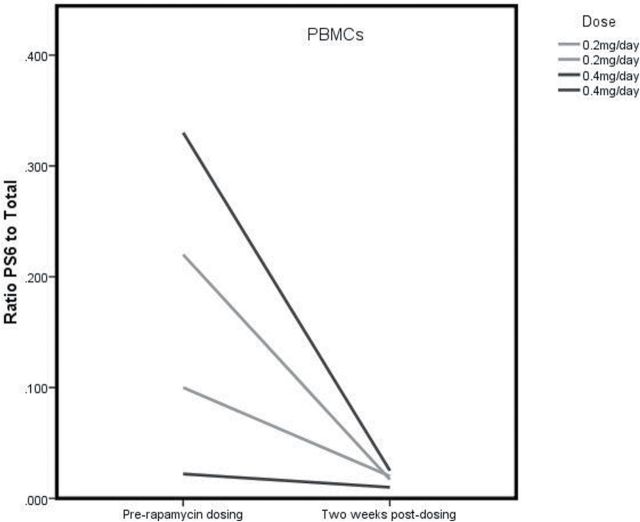
In mammalian cells, the RAPA-sensitive mammalian target of rapamycin complex 1 (mTORC1) is essential for the phosphorylation and activation of the 70kDa ribosomal protein S6 kinase (S6K) and the downstream target ribosomal protein S6 (rpS6). RAPA inhibits mTORC1 function by forming a complex with the 12-kDa FK506-binding protein (FKBP12) and then binding near the kinase domain of the mTOR resulting in a decrease in phosphorylation of S6k and consequently rpS6. Therefore, to determine if the doses of e-RAPA were biologically active, we measured the activity of mTOR in PBMCs by measuring the phosphorylated-rpS6 to total rpS6 ratio. As shown in the preliminary study (Figure 3B) for animals before and after 2 weeks, this ratio decreased. The levels of mTORC1 activity (ie, the phospho-rpS6 ratio) vary greatly in the PBMCs of untreated marmosets (Figure 3B and C). Interestingly, RAPA treatment (either at 0.2 or at 0.4mg/day) seemed to reduce mTORC1 activity to a similar basal level in all four animals.
Trial 2
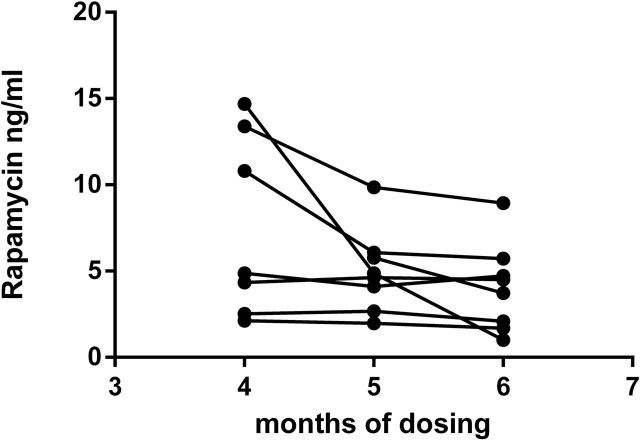
Marmosets subjected to long-term e-RAPA displayed the same variation in whole blood RAPA seen in the short-term subjects as shown in Figure 4. Monthly RAPA concentrations were fairly consistent within individuals but ranged among individuals from 1.93 to 10.73 ng/mL, with an average of 5.20 ng/mL. There was no relationship between the mean body weight of an individual and the mean RAPA concentration (r = −.521, p = .230). However, the two subjects with highest body weights (508 and 525 g) had the lowest average blood concentrations of RAPA at 1.93–2.43ng/mL. As shown in Figure 5, each treated subject was found to have detectable concentrations of RAPA in both liver and adipose tissues following the 14-month treatment. As found with the circulating concentrations of RAPA, there was a great deal of individual variability with RAPA concentrations in the liver ranging from 4.7 to 19.9 pg/mg and in adipose tissue ranging from 2.2 to 32.8 pg/mg (Figure 5).
Figure 4.
Trough whole blood rapamycin concentrations for animals tested in Trial 2.
Figure 5.
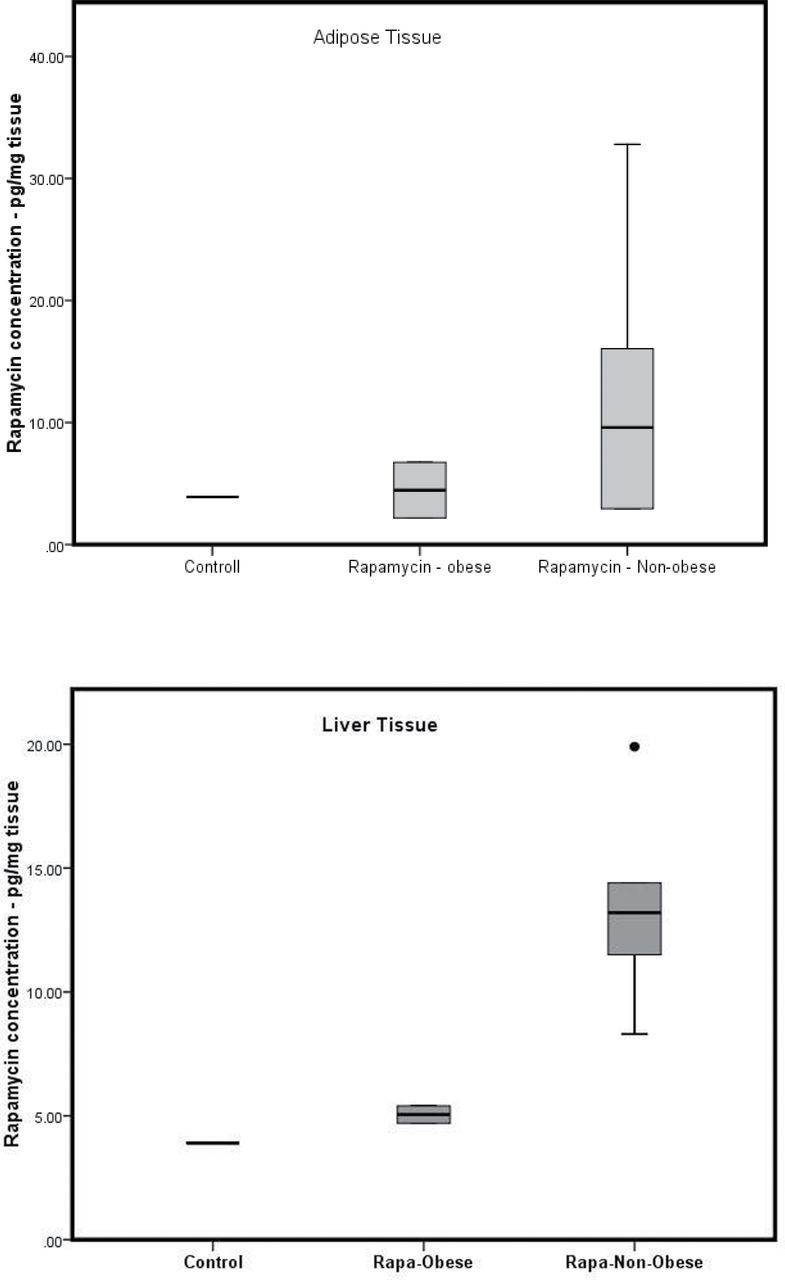
Concentrations of rapamycin in liver and adipose tissue collection following the 11 month dosing in Trial 2 in controls, obese e-rapamycin subjects, and nonobese e-rapamycin subjects.
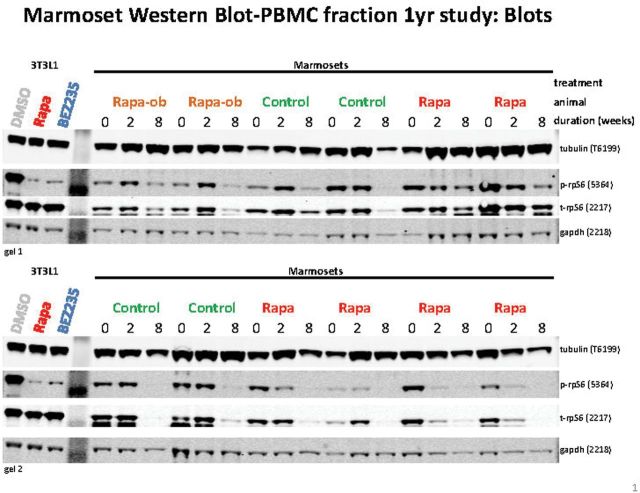
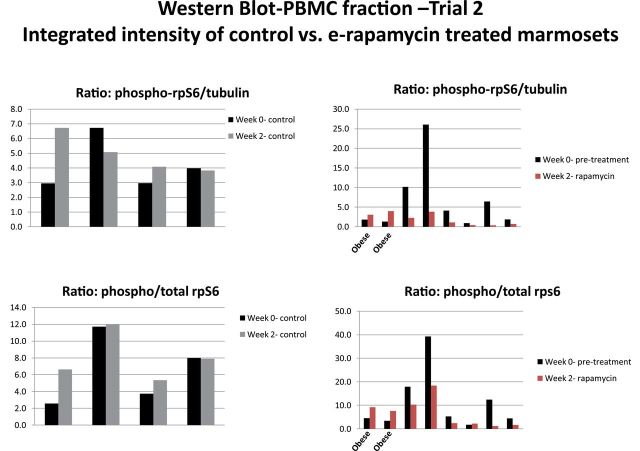
Similar to the preliminary Trial I study, blood was drawn from the marmosets prior to and after 2 weeks of administration to assess modulation of mTORC1 signaling. Assessment of the PBMC fraction from untreated control animals showed fairly consistent phospho-rpS6 to total rpS6 ratios when comparing pretreatment and posttreatment samples with some animal to animal variation (Figures 6 and 7). The integrated intensity analysis for the four untreated control animals shows no significant decrease in the ratio of intensity for phospho-rpS6 as compared with total rpS6 protein from the predosing to postdosing period. Six of the eight treated animals had a decrease in the phospho-rpS6 ratio from week 0 pretreatment to week 2 posttreatment (see Figures 6 and 7). As a second assessment, phospho-rpS6 was compared with tubulin as a loading control because the gel-loaded samples were not normalized to protein content but were all run at the same volume. Similar results were achieved for the four control animals that did not indicate significant changes in mTORC1 signaling, whereas six of the eight treated animals similarly showed a decrease in the phospho-rpS6/tubulin ratio. Two of the eight treated animals showed no reduction in the phospho/total rpS6 ratio in response to RAPA after 2 weeks of treatment. These were the two obese subjects that had the lowest circulating RAPA concentrations (Figure 4).
Figure 6.
Representative blot for phosphorylation of rpS6 in PBMC for animals treated in Trial 2.
Figure 7.
Relative change in phosphorylation of rpS6 in PBMC for control and e-rapamycin-treated animals (including the two obese subjects) before dosing and after 2 weeks of daily dosing in Trial 2.
Figure 8 illustrates the phopho-rpS6 ratios in adipose and liver tissue for controls, obese e-RAPA subjects and nonobese e-ramapycin subjects. The ratios in both the adipose and liver tissues were significantly lower in e-RAPA subjects than in control subjects (Adipose: t = 3.56, p < .001, Liver: t = 2.62, p = .026). However, within the e-RAPA subjects, the obese animals had the highest values, overlapping the range of the control subjects. In the western blot analysis, there was a large variability from animal to animal and sample to sample. This variability was addressed by quantifying the differences in the proportion of rpS6, which was phosphorylated from that which was unphosphorylated. Similarly we compared the level of phosphorylated rpS6 to tubulin. Taken together, the evidence suggested on target pathway modulation of mTORC1 by RAPA.
Figure 8.
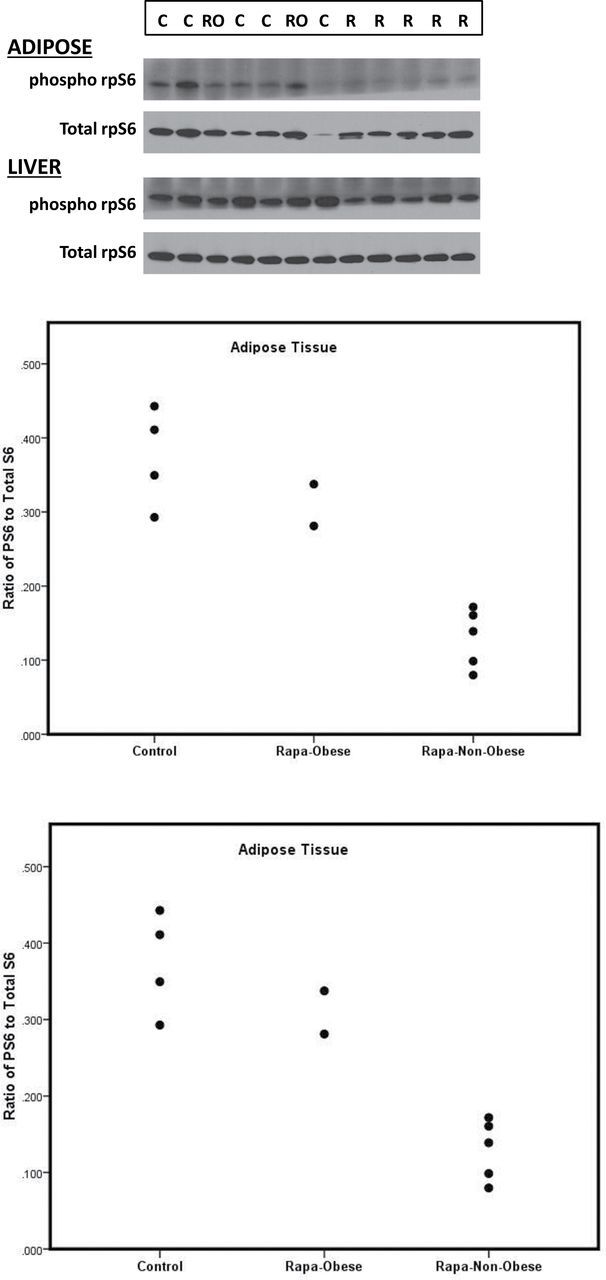
Phosphorylation of rpS6 in liver and adipose following the 14-month dosing in Trial 2 (A) Western blots; (B and C) ratios for controls, obese e-rapamycin subjects, and nonobese e-rapamycin subjects.
Daily e-RAPA treatment was well tolerated by all subjects. There was no evidence of clinical anemia. Because mouth ulcers are a common side effect of RAPA in humans, we studied the condition of the mouth in all of the marmosets in the study. No mouth ulcers were observed. In addition, we studied the condition of the lungs of the Marmosets because interstitial pneumonia, which can be serious, has been reported occasionally in humans taking RAPA. x-Ray analysis revealed no changes in lung condition after 14 months of e-RAPA treatment. One subject taking e-RAPA did display possible impaired wound healing, with minor sores on the heels and scrotum developing into a staph infection requiring treatment. The subject was treated with a topical antibiotic as well as an oral Baytril regimen and recovered. Both some control (2/5) and some e-RAPA-treated (5/8) subjects displayed occasional hematocrit values considered below normal (normal = 37–53), but no subjects in either group displayed consistently low hematocrit values. A repeated measures analysis of variance revealed no significant difference between controls and e-RAPA-treated subjects and no effects of time (ie, no difference in predosing versus post-dosing time periods) on hematocrits (F(3,24) = 0.116, n.s.) or red blood cell counts (F(3,24) = 0.928, n.s.) in e-RAPA-treated subjects.
Only one animal was reported as having white blood cell counts that were above the normal range, and it was a control animal.
The subjects in the long-term dosing study were relatively old (7.1–9.0 years at the initiation of the study). Given their age range, the expected death rate in each group during the year-long dosing was 1 out of 4 for the controls and 2 out of 9 for the e-RAPA-treated group, that is, 3 of the 12 animals in our study would be predicted to die. In fact, we observed that three animals died during the 14-month study: two control subjects (8.03 and 8.62 years of age) and one e-RAPA-treated subject (8.25 years of age). The two control deaths were attributed to acute peritonitis and septicemia and to chronic colitis. The e-RAPA subject died from cholecystitis. Thus, there was no evidence with the limited number of marmosets that we studied that 14 months of e-RAPA treatment had an adverse, toxic effect.
Discussion
We found that e-RAPA provided a reliable and efficient means to dose marmoset monkeys over both short-term and long-term studies. Subjects reliably and routinely accepted the yogurt mixtures. Using syringes to administer the yogurt, we were able to avoid a number of issues. First, no handling was required, which is easier for staff and less stressful for the animals. Second, both animals in a cage could be dosed simultaneously, ensuring that each animal received the entire 1 mL dose. It was not necessary to separate the animals from one another and wait to make sure the entire dose was ingested, as is the case with mixing RAPA into the animal’s base diet. Using the syringe method, oral dosing was quick (usually completed in under 15 minutes for all 12 animals) and it was easy to determine that each animal had received the entire dose.
Zhang and colleagues (17) reported on dosing regimens and pharmacokinetics for the daily administration of sirolimus, mixed in a gelatin and presented as a daily treat item, over a 1-month period to 10 rhesus macaques. Although the macaques readily consumed sweetened gelatin treats laced with sirolimus, their performance with presentation in a sweetened beverage licked from a syringe (a method similar to that used here) is described as poor, with several subjects refusing to take an oral dose in this form after several weeks. The marmosets, on the contrary, had a uniform positive response to the syringe presentation of the e-RAPA following training for up to 14 months. The syringe feeding method offers the advantage of allowing the investigator to more accurately quantify a subject’s drug intake, as opposed to presentation of a food item (such as the gelatin treats), which can be wholly or only partially consumed once they are taken by the animal into its cage.
The trough levels observed in both the short-term and the long-term studies that were achieved at the dose of 0.4 mg/day (roughly 1.0 mg/kg/day) were comparable with those reported in studies of C57BL/6 mice being fed a diet containing e-RAPA at a concentration of 14 ppm. Zhang and colleagues (4) report blood concentrations averaging 3–4 ng/mL and Fok and colleagues (5) report a range of 2.0–10 ng/mL. Harrison and colleagues (1) and Miller and colleagues (18), in contrast, report significantly higher circulating concentrations in the genetically heterogeneous UM-HET3 mice used in the NIA Intervention Testing Program. By comparison, the marmoset blood concentrations averaged 5.2ng/mL with a range from 1.93 to 10.73 ng/mL. The among-subject variance in trough blood concentrations, as evidence by coefficient of variation, was similar in marmosets receiving 0.40 mg/day and humans receiving 4–5mg/day (11)—marmoset c.v. = 0.667, human c.v. = 0.673–0.778.
Zhang and colleagues (17) report trough values averaging 3.13ng/mL in the rhesus macaques dosed over a 1-month period, with daily administration 0.6 mg/kg of sirolimus in a gelatin treat, suggesting a similar relationship of dose to trough values of that seen in our study. No specific health outcomes of the month long dosing of the macaques with sirolimus is reported.
The dosing regimen chosen for this study was designed to mimic a dosing regimen that might be used in humans—that is, a single dose taken once a day with the dose not weight adjusted. This approach resulted in blood concentrations that were in the desired range, consistent across months within subjects and variable among subjects. The variation observed within subjects in this study is similar to that reported for both mice and humans and places the circulating concentration at the lower end of the therapeutic range for humans (5–15ng/mL). Of particular note is the relatively low blood concentrations and limited evidence of decrease in phosphor-rpS6 in two marmosets that fell into the obese category. The subjects in this study were chosen to represent the range of weights observed in adult marmosets in a typical captive colony and, as we and others have reported previously (19,20), that includes animals that can be classified as obese. The low circulating concentrations in the obese subjects may reflect the fact that dosing, as is common in humans, was not weight adjusted. However, although the two obese subjects had the lowest circulating concentrations, there was not an overall correlation between weight and circulating concentration, suggesting the possibility that altered metabolic function associated with obesity might also be playing a role in mTOR signaling.
The higher doses of 0.2–0.4 mg/day (roughly 0.5–1.0mg/kg/day) resulted in a measureable and reproducible decrease in phospho-rpS6 indicating downregulation of mTORC1 in marmoset PBMCs as expected. In tissues, the phosphorylation of rpS6 appeared to be suppressed by the treatment of RAPA. However, the two obese subjects (with relatively low circulating RAPA concentrations) showed no decrease in phosphor-rpS6 in PBMCs at 2 weeks of dosing and had ratios that overlapped that of the controls in liver and adipose tissue taken at 14 months. These results indicate that consideration may have to be given to altered concentrations of RAPA dosing in obese subjects, including humans.
There were no signs of fibrotic changes in the lung or mouth sores in the long-term e-RAPA subjects, suggesting that oral e-RAPA is well tolerated in this species. Although occasional low hematocrit and red blood cell concentrations were seen in e-RAPA treated animals, the occurrence of occasional low values in controls as well, combined with a lack of significant difference in predosing versus postdosing values in the e-RAPA-treated subjects leads us to conclude that there is no real evidence for a RAPA effect on these variables. As opposed to rodent studies that most often take place in barrier-maintained colonies, the marmosets in this study were housed in conventional facilities. It is noteworthy that there were no indications of infectious disease in this study. Deaths occurred at a rate expected for the age of this population, and the causes of death were among those most commonly reported for captive marmosets.
The ability to dose group housed animals with an oral form of RAPA that is well tolerated and results in the apparent suppression of the mTOR pathway in a nonhuman primate leads us to conclude that the marmoset offers a viable model for RAPA testing to establish safety and efficacy for long-term antiaging intervention.
Acknowledgments
The efforts of Talia Melber and Joselyn Artavia (animal training and dosing) and Greg Friesenhahn (quantifying rapamycin in yogurt, blood, and tissues) are gratefully acknowledged. The technical assistance of Drs. Kathleen Brasky and Michael Owston at the Southwest National Primate Research Center is also gratefully acknowledged.
Funding
This research was financially supported by the Barshop Institute for Longevity & Aging Studies, the Glenn Foundation, and the San Antonio Nathan Shock Center of Excellence in the Basic Biology of Aging.
References
- 1. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. 10.1111/j.1474-9726. 2012.00832.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neff F, Flores-Dominguez D, Ryan DP, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. 10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, et al. Rapamycin extends life and health in C57Bl/6 mice. J Gerontol A Biol Sci Med Sci. 2013;69A119–130. 10.1093/Gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fok WC, Chen Y, Bokov A, et al. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One. 2014;9:e83988. 10.1371/journal.pone.0083988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sierra F. Rapamycin joins the aging fray: maybe Ponce de Leon visited Rapa Nui, not Florida. J Gerontol A Biol Sci Med Sci. 2010;65:577–579. 10.1093/gerona/glq049 [DOI] [PubMed] [Google Scholar]
- 8. Le Couteur DG, McLachlan AJ, Quinn RJ, Simpson SJ, de Cabo R. Aging biology and novel targets for drug discovery. J Gerontol A Biol Sci Med Sci. 2012;67:168–174. 10.1093/gerona/glr095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharp ZD, Strong R. The role of mTOR signaling in controlling mammalian life span: what a fungicide teaches us about longevity. J Gerontol A Biol Sci Med Sci. 2010;65:580–589. 10.1093/gerona/glp212 [DOI] [PubMed] [Google Scholar]
- 10. Stallone G, Infante B, Grandaliano G, Gesualdo L. Management of side effects of sirolimus therapy. Transplantation. 2009;87(8 suppl):S23–S26. 10.1097/TP.0b013e3181a05b7a [DOI] [PubMed] [Google Scholar]
- 11. Montalbano M, Neff GW, Yamashiki N, Meyer D, Bettiol M. A retrospective review of liver transplant patients treated with sirolimus from a single centre: an analysis of sirolimus-related complications. Transplantation. 2004;78:264–268. 10.1097/01.tp. 0000128628.31556.b1 [DOI] [PubMed] [Google Scholar]
- 12. Tardif SD, Mansfield K, Ratnam R, Ross CN, Ziegler T. The marmoset as a model of aging and age related disease. Inst Lab Anim Res J. 2011;52:54–65. 10.1093/ilar.52.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ross CN, Davis K, Dobek G, Tardif SD. Aging phenotypes of common marmosets (Callithrix jacchus). J Aging Res. 2012;2012:567143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Layne DG, Power RA. Husbandry, handling, and nutrition for marmosets. Comp Med. 2003;53:351–359. [PubMed] [Google Scholar]
- 15. Majumder S, Caccamo S, Medina DX, et al. Life-long rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and NMDA signaling. Aging Cell. 2012;11:326–335. 10.1111/j.1474-9726.2011.00791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller RA, Harrison D, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends lifespan of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2012;66:191–201. 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang S, Ye B, Zeng L, et al. Drug-containing gelatin treats as an alternative to gavage for long-term oral administration in rhesus monkeys (Macaca mulatta). J Am Assoc Lab Anim Sci. 2012;51:842–846. [PMC free article] [PubMed] [Google Scholar]
- 18. Miller RA, Harrison DE, Astle CM, et al. Rapamycin-mediated lifespan increase in mice is dose- and sex-dependent and appears metabolically distinct from dietary restriction. Aging Cell. 2013;13468–477. 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus). Obesity (Silver Spring). 2009;17:1499–1505. 10.1038/oby.2009.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wachtman LM, Kramer JA, Miller AD, Hachey AM, Curran EH, Mansfield KG. Differential contribution of dietary fat and monosaccharide to metabolic syndrome in the common marmoset (Callithrix jacchus). Obesity (Silver Spring). 2011;19:1145–1156. 10.1038/oby.2010.303 [DOI] [PMC free article] [PubMed] [Google Scholar]