Abstract
The Hippo/Yap pathway is a well-conserved signaling cascade that regulates cell proliferation and differentiation to control organ size and stem/progenitor cell behavior. Following airway injury, Yap was dynamically regulated in regenerating airway epithelial cells. To determine the role of Hippo signaling in the lung, the mammalian Hippo kinases, Mst1 and Mst2, were deleted in epithelial cells of the embryonic and mature mouse lung. Mst1/2 deletion in the fetal lung enhanced proliferation and inhibited sacculation and epithelial cell differentiation. The transcriptional inhibition of cell proliferation and activation of differentiation during normal perinatal lung maturation were inversely regulated following embryonic Mst1/2 deletion. Ablation of Mst1/2 from bronchiolar epithelial cells in the adult lung caused airway hyperplasia and altered differentiation. Inhibitory Yap phosphorylation was decreased and Yap nuclear localization and transcriptional targets were increased after Mst1/2 deletion, consistent with canonical Hippo/Yap signaling. YAP potentiated cell proliferation and inhibited differentiation of human bronchial epithelial cells in vitro. Loss of Mst1/2 and expression of YAP regulated transcriptional targets controlling cell proliferation and differentiation, including Ajuba LIM protein. Ajuba was required for the effects of YAP on cell proliferation in vitro. Hippo/Yap signaling regulates Ajuba and controls proliferation and differentiation of lung epithelial progenitor cells.
Keywords: Hippo/Yap pathway, lung, Ajuba, proliferation, differentiation
Introduction
Lung morphogenesis is a highly coordinated process categorized by distinct developmental stages, and is regulated by complex reciprocal signaling interactions between epithelial and mesenchymal progenitor cells (Maeda et al., 2007; Morrisey and Hogan, 2010; Hines and Sun, 2014). Stereotypical branching morphogenesis establishes the proximal conducting airways leading to peripheral acinar tubules/buds during the embryonic (E9–11.5 in mice) and pseudoglandular (E11.5–15.5) stages of development. Lung maturation is initiated during the canalicular (E15.5–17.5) and saccular (E17.5–PN5) stages, during which the acinar tubules/buds dilate to form saccules. While undifferentiated progenitor cells are highly proliferative during the early stages of branching morphogenesis, perinatal lung maturation is associated with dynamic changes in the expression of transcription factors and signaling molecules that function in regulatory networks to decrease proliferation and promote epithelial cell differentiation (Xu et al., 2012). The epithelium of the mature lung is comprised of multiple differentiated cell types, including basal, secretory (club, serous, and goblet), ciliated, and neuroepithelial cells in the conducting airways, and alveolar type I and type II cells in the peripheral lung that are readily distinguished by morphology and expression of cell type selective genes. Although respiratory epithelial cells of the mature lung are generally nonproliferative, multiple epithelial cell types function as facultative progenitors with remarkable regenerative activity to repair the lung after injury, including basal cells and distinct subsets of nonciliated epithelial cells in the conducting airways and alveolar type II cells (Hogan et al., 2014; Kotton and Morrisey, 2014). During repair, these epithelial progenitors undergo marked changes in cell shape, migrate, proliferate, and re-differentiate to restore the respiratory epithelium with the appropriate cell type composition and structural organization. Several pathways that regulate lung morphogenesis are also involved in regeneration of the lung epithelium following injury, including Wnt, Shh, Fgf, Tgf-beta, and Notch signaling (Shi et al., 2009; Crosby and Waters, 2010; Morrisey and Hogan, 2010). Mechanisms coordinating proliferation and differentiation of respiratory epithelial progenitor cells during lung development and repair remain unclear.
The Hippo signaling pathway plays pleiotropic roles in the regulation of cellular behavior and organ size. The Hippo pathway is comprised of kinase-adaptor protein complexes, wherein the mammalian Ste20-like serine/threonine kinases Mst1 and Mst2 (hippo in Drosophila) interact with Sav1 (salvador), and large tumor suppressor kinases Lats1 and Lats2 (warts) bind Mob1a/b (Mats). Upon stimulation, Mst1/2-Sav1 phosphorylates and activates the Lats1/2-Mob1a/b complex, which in turn phosphorylates downstream transcriptional effectors Yap (yorkie) and its paralog Taz (encoded by WWTR1) to promote their cytoplasmic localization and targeting for proteosomal degradation. In the absence of inhibitory phosphorylation by the Hippo kinase cascade, Yap/Taz translocate to the nucleus and interact with transcriptional cofactors, including the TEAD family of proteins, to regulate target genes associated with cell proliferation, apoptosis, and differentiation. Genetic models involving the loss of upstream kinase signaling or activation of Yap/Taz demonstrate that the Hippo pathway controls growth and cell fate decisions in stem/progenitor cells during embryogenesis and homeostasis (Zhao et al., 2011; Varelas, 2014). Recent studies showed that Yap is required for branching morphogenesis and epithelial differentiation in the developing lung, and interacts with p63 in basal cells to regulate cell fate and stratification of the postnatal tracheal epithelium (Mahoney et al., 2014; Zhao et al., 2014). Taz is expressed in respiratory epithelial cells of the developing mouse lung and interacts with TTF-1 to induce surfactant protein-C (Sftpc) gene expression (Park et al., 2004). Homozygous deletion of Taz in mice causes airspace enlargement, while Taz heterozygous mice are resistant to pulmonary fibrosis induced by bleomycin treatment (Mitani et al., 2009). Mst1/2 were proposed as regulators of Foxa2 protein stability to control differentiation of peripheral type I and type II pneumocytes in the embryonic lung, while signaling through the canonical transcriptional effectors Yap/Taz was unaltered (Chung et al., 2013). However, the mechanisms by which canonical Hippo/Yap/Taz signaling controls lung maturation and homeostasis remain unclear.
The present study demonstrates that Yap is dynamically regulated during regeneration of the airway epithelium following lung injury. Conditional deletion of Mst1/2 in the embryonic and adult lung and expression of YAP in primary human bronchial epithelial cells (HBECs) increased cell proliferation and inhibited differentiation of multiple epithelial cell types. Ablation of Mst1/2 reduced Yap inhibitory phosphorylation and promoted Yap nuclear localization and transcriptional activity. Ajuba LIM protein was identified as a novel target of Mst1/2–Yap signaling, and was required for the proliferative effects of Yap in vitro. Hippo/Yap signaling regulates Ajuba in respiratory epithelial cells and controls progenitor cell proliferation and differentiation in the developing and mature lung.
Results
Yap is expressed in airway epithelial cells and is dynamically regulated during bronchiolar epithelial regeneration
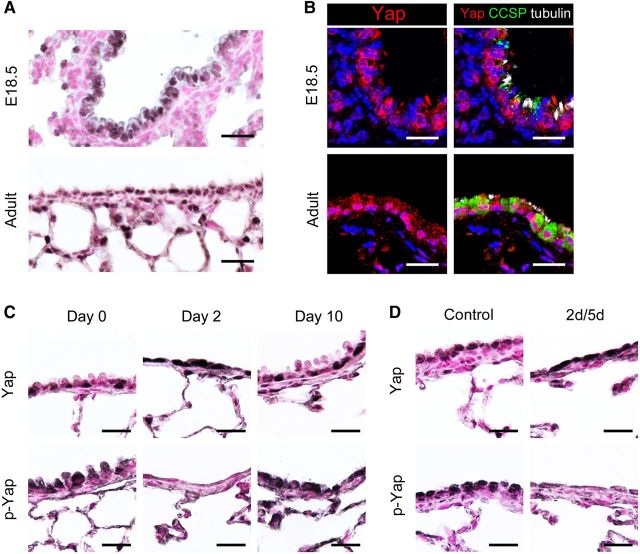
While the epithelium of the mature lung is normally quiescent, subsets of respiratory epithelial cells proliferate and migrate to regenerate the respiratory epithelium following injury. Since the Hippo/Yap pathway is an important regulator of cell proliferation, differentiation, and stem/progenitor behavior, expression of Yap, the transcriptional effector of Hippo signaling, was assessed in embryonic and mature lungs to identify cells utilizing this signaling cascade. Nuclear and cytoplasmic Yap staining was detected in airway epithelial cells of the developing and mature lungs (Figure 1A). Intense Yap staining was also present in peripheral respiratory epithelial cells in the adult lung (Figure 1A). To determine whether Yap was expressed in club or ciliated cells in the airway epithelium, immunofluorescence colabeling was performed for Yap, CCSP (club cell secretory protein), and acetylated tubulin. Diffuse nuclear and cytoplasmic Yap staining was observed in both CCSP-positive club cells and acetylated tubulin-positive ciliated cells at E18.5 and in adulthood (Figure 1B). These data demonstrate that Yap is widely expressed in respiratory epithelial cells of the embryonic and mature lung.
Figure 1.
Yap is expressed in airway epithelial cells and is dynamically regulated during bronchiolar repair. (A) Yap was detected in the nucleus and cytoplasm of bronchiolar epithelial cells in E18.5 and adult mouse lungs. Intensity of Yap staining was highest in peripheral epithelial cells in the adult lung. (B) Immunofluorescence staining for Yap (red), CCSP (green), and acetylated tubulin (white) was performed on E18.5 and adult mouse lungs. Yap was detected in the nucleus and cytoplasm of club and ciliated cells. (C and D) Yap and phospho-Yap (p-Yap) staining was assessed following naphthalene (C)- or diphtheria toxin A (D)-mediated bronchiolar cell ablation. Yap was increased and p-Yap decreased in squamous bronchiolar epithelial cells remaining on Day 2 following naphthalene exposure (C) and on Day 5 following DTA-induced cell ablation (D). Yap and p-Yap staining at 10 days following naphthalene injury were similar to controls on Day 0 (C). Scale bar, 20 µm.
To determine if the Hippo pathway is active during regeneration of the respiratory epithelium following injury, immunostaining for Yap and phospho-Yap (murine Ser112 homologous to human Ser127) was performed on adult mouse lungs in which club cells were depleted following naphthalene exposure or after their conditional ablation using diphtheria toxin A (DTA) in transgenic mice (Park et al., 2006; Perl et al., 2011). The distribution and intensity of Yap staining were increased, whereas phospho-Yap staining was decreased in the squamous bronchiolar epithelial cells remaining 2 days following naphthalene exposure (Figure 1C). Regeneration of the bronchiolar epithelium was substantially complete 10 days following naphthalene injury, at which time Yap and phospho-Yap staining were similar to that in the uninjured airway (Figure 1C). In Scgb1a1-rtTA/tetO-Cre/DTA transgenic mice, club cell ablation was mediated by acute expression of DTA initiated by administration of doxycycline for 2 days (Perl et al., 2011). After 5 days of recovery, Yap staining was increased and phospho-Yap decreased in the remaining bronchiolar epithelial cells (Figure 1D). Increased Yap and decreased phospho-Yap during lung repair is consistent with dynamic regulation of Hippo/Yap signaling in progenitor cells during regeneration of the bronchiolar epithelium.
Conditional deletion of Mst1/2 from respiratory epithelial progenitor cells impairs lung maturation
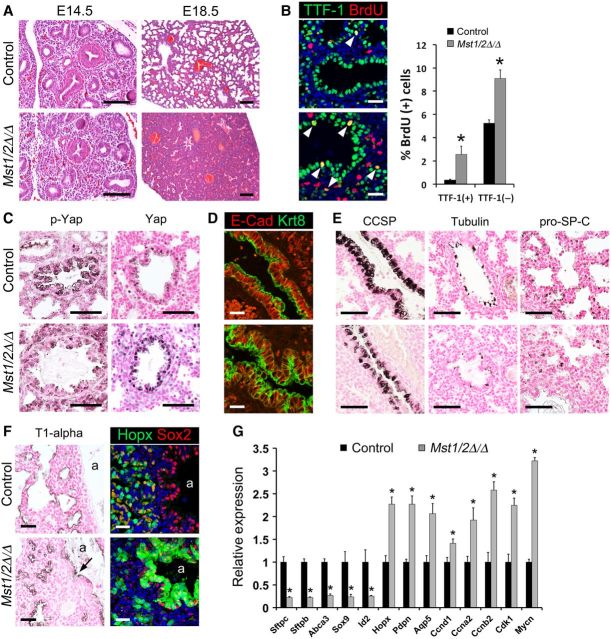
The requirement of the mammalian Hippo kinases Mst1 and Mst2 for lung morphogenesis was assessed by generating Shh-Cre/Mst1flx/flx;Mst2flx/flx mice to conditionally delete Mst1 and Mst2 from respiratory epithelial cell progenitors during lung formation. At E14.5, lung histology was similar in control and Mst1/2-deleted embryos (Figure 2A). While lobation and lung size were generally unaffected (Supplementary Figure S1B), sacculation was inhibited and lung cellularity was increased in E18.5 Mst1/2-deleted mice (Figure 2A). Lung abnormalities were only observed following deletion of both Mst1 and Mst2 and resulted in death at birth. Proliferation and apoptosis in the developing respiratory epithelium were examined by double-label immunofluorescence for TTF-1/BrdU and TTF-1/TUNEL, respectively. While undifferentiated respiratory epithelial progenitor cells are highly proliferative during the early embryonic and pseudoglandular stages of lung morphogenesis, prenatal lung maturation during the canalicular and saccular stages is associated with decreased proliferation and the induction of respiratory epithelial cell differentiation (Xu et al., 2012). BrdU incorporation was increased in both TTF-1-positive epithelial cells and TTF-1-negative mesenchymal cells of E18.5 Mst1/2-deleted lungs (Figure 2B), whereas apoptotic cells were not detected (Supplementary Figure S2). TTF-1 and Sox2 staining were not significantly changed in the developing airway epithelial cells following deletion of Mst1/2, indicating that these cells maintained bronchiolar epithelial cell identity (Supplementary Figure S1A). The bronchiolar epithelium of Mst1/2-deleted mice was hypercellular and pseudostratified in contrast to the simple columnar-cuboidal morphology typical in control mice (Supplementary Figure S1A). Immunofluorescence staining for E-cadherin and keratin 8 was utilized to assess airway epithelial cell shape and organization. While basolateral E-cadherin and apical keratin 8 localization was well-organized in epithelial cells from controls at E18.5, E-cadherin/keratin 8 revealed pseudostratification and cell shape alterations in the airway epithelium after Mst1/2 deletion (Figure 2D). These findings show that deletion of Mst1/2 from epithelial progenitors in the developing lung enhanced proliferation, causing lung hypercellularity, sacculation defects, and perinatal lethality.
Figure 2.
Conditional deletion of Mst1/2 in epithelial progenitors of the embryonic lung increases proliferation and inhibits maturation. (A–E) Control (top panels) and Shh-Cre/Mst1▵/▵;Mst2▵/▵ (Mst1/2▵/▵; bottom panels) embryo lungs were analyzed at E14.5 (A) and E18.5 (A–F). (A) Lung histology was similar in control and Mst1/2▵/▵ mice at E14.5. Deletion of Mst1/2 caused lung hypercellularity and sacculation defects at E18.5. (B) Increased BrdU labeling was observed in TTF-1-positive epithelial cells (arrowheads) and in mesenchymal cells of Mst1/2▵/▵ mice. (C) Phospho-Yap immunostaining was reduced and Yap nuclear localization was increased in epithelial cells after deletion of Mst1/2. (D) E-cadherin (E-cad, red) and keratin 8 (Krt8, green) immunofluorescence showed pseudostratification and altered cell shape in airway epithelial cells of Mst1/2▵/▵ mice. (E) Deletion of Mst1/2 caused decreased staining for CCSP, acetylated tubulin, and pro-SP-C. (F) T1-alpha immunostaining and Hopx/Sox2 immunofluorescence are shown. T1-alpha lined the saccular structures that failed to expand in Mst1/2▵/▵ embryos. T1-alpha (arrow) and Hopx were ectopically detected in the Sox2-positive conducting airway epithelium in Mst1/2-deleted embryos. a, airway lumen. (G) qPCR analysis of Epcam-positive epithelial cell mRNAs isolated from E18.5 control and Mst1/2-deleted lungs. mRNAs identifying alveolar epithelial type II cells (Sftpc, Sftpb, Abca3) and distal epithelial progenitor markers (Sox9, Id2) were decreased. Proliferation-associated mRNAs (Ccnd1, Ccna2, Ccnb2, Cdk1, Mycn) and alveolar type I cell markers (Hopx, Pdpn, Aqp5) were increased. Asterisks indicate statistical significance (P < 0.05). Scale bar, 20 µm (B, D, and F); 50 µm (C and E); 100 µm (A).
Perinatal lung maturation during the canalicular and saccular stages is associated with coordinate induction of epithelial cell differentiation and inhibition of cell proliferation prior to birth. Immunostaining of E18.5 lungs showed that CCSP, acetylated tubulin, and pro-SP-C were reduced in Mst1/2-deleted mice, indicating decreased differentiation of club, ciliated, and alveolar type II epithelial cells, respectively (Figure 2E). In the peripheral lung, staining for T1-alpha, a marker of alveolar type I cells, lined the developing saccules in controls and was detected in the saccular structures that failed to expand in Mst1/2-deleted mice (Figure 2F). Analysis of gene expression in Epcam-positive epithelial cells isolated from the lungs of E18.5 control and Mst1/2-deleted mice demonstrated that mRNAs associated with cell cycle progression (Ccnd1, Ccna2, Ccnb2, Cdk1, and Mycn) were increased and those associated with differentiated alveolar type II cells (Sftpc, Sftpb, Abca3) and secretory (Scgb1a1, Scgb3a1), goblet (Muc5ac), and ciliated (Foxj1) bronchiolar cells were decreased (Figures 2G and 4D). Surprisingly, distal epithelial progenitor markers (Sox9, Id2) were decreased and mRNAs typically associated with alveolar type I cell epithelial cells in the mature lung (Hopx, Pdpn/T1-alpha, and Aqp5) were increased following deletion of Mst1/2 (Figure 2G). Consistent with these findings, immunostaining showed aberrant expression of T1-alpha and Hopx in bronchiolar epithelial cells of Mst1/2-deleted mice (Figure 2F). Sox2 staining was normally restricted to conducting airway epithelial cells in Mst1/2-deleted lungs (Figure 2F and Supplementary Figure S1A), indicating that proximal/distal patterning of the developing lung epithelium was generally maintained despite the altered expression of distal epithelial progenitor markers. These data show that deletion of Mst1/2 in epithelial progenitor cells of the developing mouse lung inhibited sacculation and altered respiratory epithelial cell differentiation.
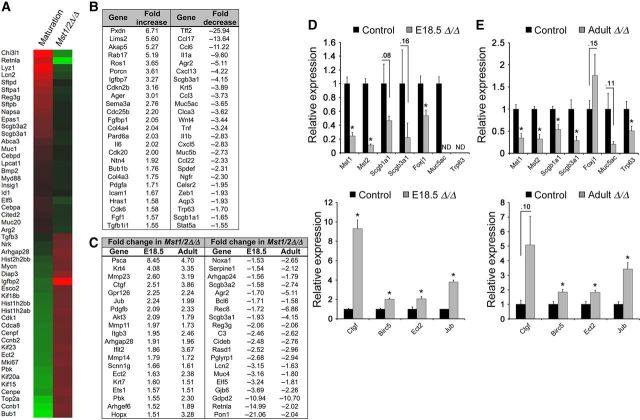
Figure 4.
Mst1/2 deletion in embryonic and mature lung epithelial cells regulates mRNAs associated with proliferation and differentiation. (A) Heat map of proliferation- and differentiation-related genes that were dynamically regulated during lung maturation (E15.5-birth, left column) compared with the mRNAs from Epcam-sorted epithelial cells from E18.5 Mst1/2▵/▵ lungs (right column). mRNAs associated with lung maturation were inversely regulated after Mst1/2 ablation. (B) RNA-seq analysis of FACS-enriched bronchiolar epithelial cells from adult lungs revealed that deletion of Mst1/2 increased mRNAs associated with proliferation and migration, and decreased bronchiolar differentiation and host defense gene expression. (C) Fold changes of selected mRNAs similarly regulated following deletion of Mst1/2 in the embryonic and adult lung are shown. (D and E) qPCR of mRNAs isolated from epithelial cells from E18.5 lungs (D) and adult conducting airways (E) are shown. Mst1, Mst2, and mRNAs encoding respiratory epithelial cell differentiation markers were significantly decreased. Yap targets (Ctgf, Birc5), Ajuba (Jub), and Ect2 mRNAs were increased. ND, not detected. Asterisks indicate statistical significance (P < 0.05).
Conditional deletion of Mst1/2 from bronchiolar epithelial cells in the mature lung causes airway hyperplasia
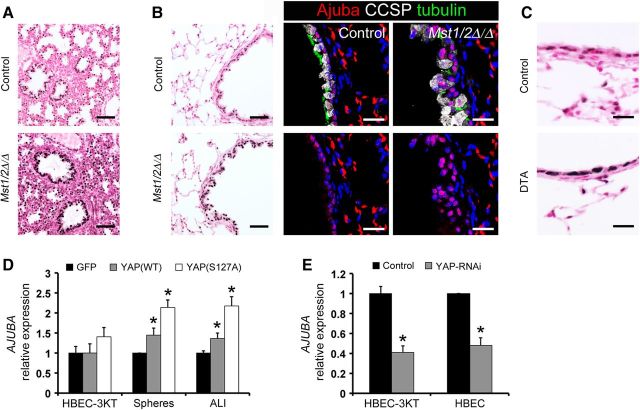
To examine whether Hippo/Yap signaling influences respiratory epithelial cell homeostasis in the mature lung, Scgb1a1-rtTA/tetO-Cre/Mst1flx/flx;Mst2flx/flx mice were generated to conditionally delete Mst1 and Mst2 from nonciliated bronchiolar epithelial cells of adult mice upon administration of doxycycline. Deletion of Mst1/2 in the adult lung caused bronchiolar epithelial hyperplasia within 1 week and gradually expanded by 6 weeks following doxycycline treatment (Figure 3A). Seven months following Mst1/2 deletion, increased subepithelial collagen deposition was evident in the hyperplastic airways (Figure 3B). Bronchiolar epithelial abnormalities were only observed following conditional deletion of both Mst1 and Mst2. While deletion of Mst1/2 in the postnatal bronchiolar epithelium induced progressive airway hyperplasia, tumor formation was not observed in the lungs of Mst1/2 ablated mice by 7 months. To determine which airway epithelial cells proliferated following deletion of Mst1/2, immunofluorescence for CCSP, acetylated tubulin, and phospho-histone H3 (pHH3) was performed on lungs 1 and 6 weeks following doxycycline administration. Increased numbers of pHH3 stained cells were detected in the hyperplastic bronchiolar epithelium 1 and 6 weeks after deletion of Mst1/2 (Figure 3C). pHH3-positive cells were only detected in CCSP-expressing club cells and not in acetylated tubulin-positive ciliated cells (Figure 3C), and bronchiolar epithelial cell counts demonstrated increased numbers of club cells while ciliated cell numbers were unchanged (Figure 3D). Apoptotic cells were not detected in the bronchiolar epithelium of control or Mst1/2-deleted mice 1 or 6 weeks following doxycycline (Supplementary Figure S2). Hyperplastic foci contained a subset of cells that did not label with differentiated club or ciliated cells markers, but maintained bronchiolar cell identity indicated by Sox2 staining (Figure 3C, D and Supplementary Figure S1C). Similar to findings in the embryonic lung, Hopx was aberrantly induced in the airway epithelium of adult Mst1/2-deleted mice (Figure 3G). Notably, Hopx-positive airway epithelial cells had reduced staining for CCSP, consistent with the concept that club cell differentiation is altered following Mst1/2 loss. Bronchiolar epithelial cell shape and polarity were assessed by immunofluorescence staining for E-cadherin, keratin 8, and the tight junction protein PAR3. Keratin 8 and PAR3 were apically localized and E-cadherin detected on basolateral surfaces in bronchiolar epithelial cells from controls (Figure 3F). Following Mst1/2 loss, E-cadherin, keratin 8, and PAR3 localization was disrupted and epithelial cell shape was altered in the hyperplastic airways (Figure 3F). Deletion of Mst1/2 from nonciliated bronchiolar epithelial cells in the adult lung induced club cell proliferation, causing airway hyperplasia associated with disrupted epithelial cell shape and polarity and the expansion of a population of undifferentiated bronchiolar cells. Thus, Hippo kinase activity is required to maintain quiescence and architecture of the postnatal bronchiolar epithelium.
Figure 3.
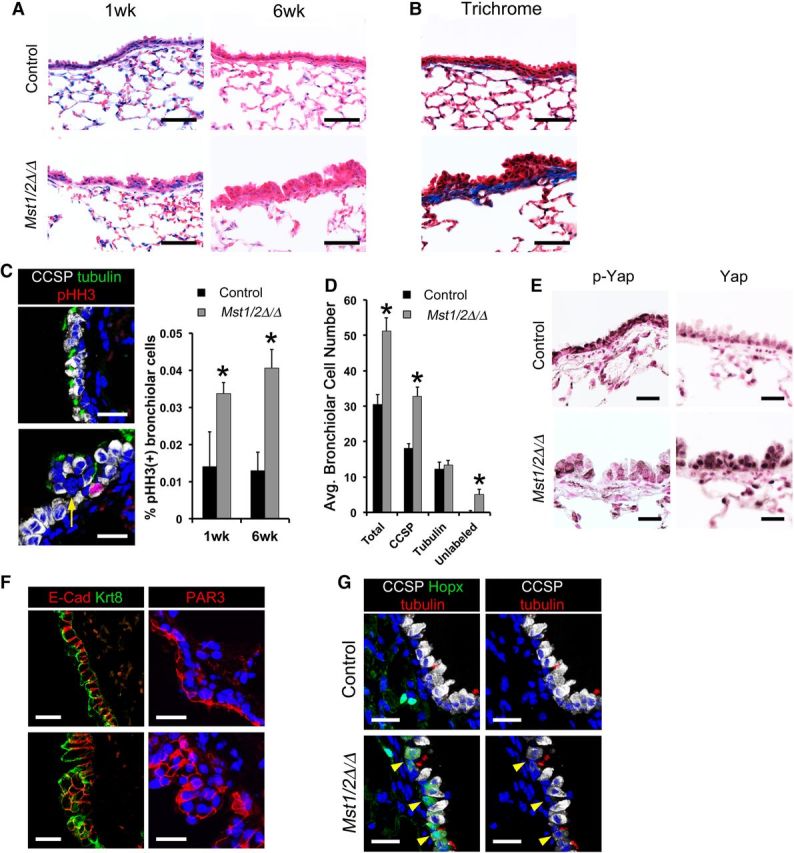
Conditional deletion of Mst1/2 in nonciliated airway epithelial cells causes bronchiolar hyperplasia. (A–G) The bronchiolar epithelium of control (top panels) and Scgb1a1-rtTA/tetO-Cre/Mst1▵/▵;Mst2▵/▵ (Mst1/2▵/▵; bottom panels) mice were examined following doxycycline treatment. (A) Deletion of Mst1/2 caused bronchiolar epithelial cell hyperplasia. (B) Trichrome staining demonstrated subepithelial fibrosis indicated by increased collagen (blue) in Mst1/2▵/▵ mice treated with doxycycline for 7 months. (C) Immunofluorescence staining and quantification of CCSP (white), acetylated tubulin (green), and phospho-histone H3 (pHH3; red) showed increased numbers of pHH3/CCSP stained cells after deletion of Mst1/2. (D) Bronchiolar cell counts demonstrated that Mst1/2-deleted airways had increased numbers of club cells after 1 week on doxycycline. Hyperplastic foci contained subsets of epithelial cells that did not label with CCSP or acetylated tubulin (yellow arrow, C). (E) Immunostaining for phospho-Yap was reduced and nuclear Yap was increased in bronchiolar epithelial cells from Mst1/2▵/▵ lungs. (F) Basolateral E-cadherin, apical keratin 8, and tight junction PAR3 localizations were disrupted and cell shape was altered in the hyperplastic airway epithelium following Mst1/2 deletion. (G) Hopx (green), CCSP (white), and acetylated tubulin (red) immunofluorescence is shown. Hopx was restricted to alveolar type 1 cells in controls and was induced in a subset of bronchiolar epithelial cells in Mst1/2 deleted mice 1 week after doxycycline treatment. Hopx-positive airway cells had reduced CCSP staining (arrowheads). Asterisks indicate statistical significance (P < 0.05). Scale bar, 20 µm (C, E, F, and G); 50 µm (A and B).
Deletion of Mst1/2 in lung epithelial cells modulates canonical Hippo/Yap signaling
In the canonical Hippo signaling pathway, Mst1/2 kinase activity induces inhibitory phosphorylation of Yap. In contrast, recent studies indicated that Foxa2 mediated the effects of Mst1/2 deletion in the embryonic lung independently of Yap/Taz (Chung et al., 2013). To determine whether Mst1/2 deletion influenced canonical versus noncanonical Hippo signaling, immunostaining for Foxa2, Yap, and phospho-Yap was performed on embryonic and adult lungs following Mst1/2 deletion. Foxa2 expression in embryonic or adult lungs was unchanged after deletion of Mst1/2 (Supplementary Figure S3). In contrast, decreased phospho-Yap and increased nuclear Yap staining was detected in respiratory epithelial cells at E18.5 and in bronchiolar epithelial cells of adult mice at 1 and 6 weeks following Mst1/2 loss (Figures 2C and 3E). Direct Yap transcriptional targets Ctgf and Birc5/survivin were increased following Mst1/2 deletion in the fetal and mature lung, further supporting Yap activation in these models (Figure 4D and E) (Dong et al., 2007; Zhao et al., 2008). These data are consistent with the concept that Mst1/2 deletion in both embryonic and adult lung epithelial cells affects canonical signaling through Yap activation.
Mst1/2 deletion in embryonic and mature lung epithelial cells regulates mRNAs associated with proliferation and differentiation
Epcam-positive epithelial cells were isolated from control and Mst1/2-deleted lungs at E18.5, and relative mRNA expression was measured by microarray. Epithelial-specific mRNAs Cdh1 (E-cadherin) and Epcam were highly enriched in the Epcam-positive cells, while lung mesenchymal markers Vimentin and Twist2 were enriched in the Epcam-negative cells (Supplementary Figure S4A). Microarray analysis identified that 741 mRNAs were significantly decreased and 551 mRNAs increased after deletion of Mst1/2 (1.5-fold). mRNAs associated with respiratory epithelial cell differentiation, metabolic and lipid biosynthetic processes were decreased, and mRNAs associated with proliferation were increased by deletion of Mst1/2 from the developing lung (Supplementary Figure S5). Lung mRNAs that change during normal perinatal lung maturation (E15.5–birth) (Xu et al., 2012) were compared with those in Mst1/2-deficient epithelial cells from E18.5 lungs. The increase in epithelial maturation markers and decrease in proliferation-related mRNAs, which occur during perinatal lung maturation, were reversed in Mst1/2-deleted mice, wherein ∼50% of the 150 top-ranked mRNAs changed during normal lung maturation were oppositely regulated (Figure 4A). These data support the concept that Hippo kinase activity and Yap inhibition are required for precise regulation of transcriptional programs during perinatal lung maturation.
Lin−/Epcam+/CD24-intermediate airway epithelial cells isolated by fluorescence cell sorting from normal adult and Mst1/2-deleted mice after 16 days of doxycycline (Supplementary Figure S4C) were used for RNA-sequence analysis. Enrichment of mRNAs for Scgb1a1 and Scgb3a1 in Lin−/Epcam+/CD24-intermediate cells validated the isolation of bronchiolar epithelial club cells by sorting (Supplementary Figure S4B). RNA-seq identified 646 significantly increased and 665 decreased mRNAs following Mst1/2 deletion in bronchiolar epithelial cells in adult mice (1.5-fold). mRNAs associated with cell migration and proliferation were increased, while those associated with host defense/immune response were decreased (Figure 4B and Supplementary Figure S5).
Comparison of the RNA profiling performed on epithelial cells isolated from fetal and adult Mst1/2-deleted mice, identified 122 similarly regulated mRNAs (64 increased and 58 decreased). In both fetal and adult lung Mst1/2-deficient epithelial cells, mRNAs activating cell proliferation and migration were induced and those associated with various aspects of epithelial cell differentiation were decreased (Figure 4C). As expected, Mst1 and Mst2 RNAs were significantly decreased (Figure 4D and E). While many proliferation-related mRNAs were induced following Mst1/2 deletion in embryonic and adult lung epithelial cells, Jub (Ajuba), Ect2, and known Yap targets Ctgf, and Birc5/survivin (Dong et al., 2007; Zhao et al., 2008) were similarly increased in both Mst1/2 deletion models (Figure 4C–E). Transcripts associated with differentiation of multiple conducting airway epithelial cell types were similarly decreased, including inhibition of mRNAs selectively identifying club cells (Scgb1a1, Scgb3a1), ciliated (Foxj1), goblet cells (Muc5ac), and basal cells (Trp63) (Figure 4C–E). Thus, activation of proliferation and inhibition of epithelial differentiation seen after deletion of Mst1/2 were consistent findings in both fetal and adult lungs.
YAP potentiates the growth of human bronchiolar epithelial cells in vitro
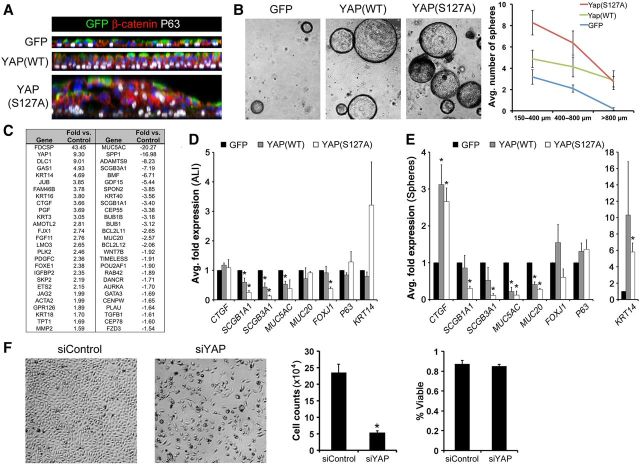
Since Yap activation was induced following Mst1/2 deletion in vivo, YAP(WT) and activated YAP(S127A) were expressed in primary HBECs cultured at air–liquid interface (ALI) or as bronchospheres to determine the direct effects of increased YAP activity. After 3 weeks at ALI, control HBEC cultures formed a well-organized pseudostratified epithelium that included P63-positive basal cells (Figure 5A). While expression of YAP(WT) increased ALI pseudostratification, YAP(S127A) caused marked hyperplasia of ALI cultures with disrupted cellular organization, aberrant localization of P63-positive cells, and altered cell shape indicated by β-catenin staining (Figure 5A). Similarly, YAP(WT) and YAP(S127A) expression increased the size and number of primary HBEC bronchospheres (Figure 5B). YAP variably increased KRT14 mRNA in ALI and sphere cultures, and decreased mRNAs associated with secretory (SCGB1A1, SCGB3A1), goblet (MUC5AC, MUC20), and ciliated (FOXJ1) airway epithelial cells (Figure 5D and E). Expression of the YAP target CTGF was increased in YAP(WT) and YAP(S127A) bronchospheres, but was not affected by YAP in HBEC cells grown at ALI (Figure 5D and E). While the basal localization of P63-positive cells was disrupted in the hyperplastic YAP(S127A) ALI cultures, neither the relative P63-positive cell fraction nor P63 mRNA were substantially affected by YAP expression in ALI or bronchosphere cultures (Figure 5D, E and Supplementary Figure S6). Inactivation of YAP in primary HBECs or HBEC-3KT cells using RNA interference caused decreased cell number after 72 h but did not alter cell viability (Figure 5F and Supplementary Figure S7), indicating that YAP is required to promote lung epithelial cell proliferation in vitro. RNA-seq analysis of YAP(S127A)-expressing bronchospheres showed that mRNAs associated with cell growth and migration were increased and those related to cellular metabolic processes, lung epithelial formation, and chromosome segregation were decreased (Figure 5C). Comparison of the microarray and RNA-seq data from YAP(S127A) bronchospheres and embryonic and adult Mst1/2-deficient epithelial cells, identified biological processes that were similarly regulated following Mst1/2 deletion in vivo and YAP activation in vitro (Supplementary Figure S5). Increased mRNAs were consistently associated with cell proliferation, migration, and adhesion, and were enriched for genes related to MAPK, Ras, and Rho signaling. Decreased mRNAs were associated with various metabolic processes related to differentiated respiratory epithelial cell functions and homeostasis. Taken together, Mst1/2 deletion in vivo and YAP expression in primary HBECs in vitro similarly activated cell proliferation and inhibited differentiation of several airway epithelial cell types.
Figure 5.
YAP promotes growth and inhibits differentiation of human bronchiolar epithelial cells in vitro. (A) Orthogonal views of whole mount immunofluorescence for β-catenin (red) and P63 (white) on lentiviral-infected (GFP, green) primary HBECs grown at ALI. YAP(WT) increased epithelial cell pseudostratification and YAP(S127A) caused hyperplasia, altered cell shape, and disrupted normal basal localization of P63 in ALI cultures. (B) YAP increased primary HBEC bronchosphere size and number. (C) Fold expression changes of selected mRNAs from RNA-seq analysis of YAP(S127A)-infected bronchospheres. (D and E) qPCR of mRNAs isolated from ALI (D) and bronchospheres cultures (E) showed that YAP variably induced KRT14 and CTGF, and inhibited mRNAs of secretory (SCGB1A1, SCGB3A1), goblet (MUC5AC, MUC20), and ciliated (FOXJ1) cells. (F) siRNA-mediated YAP inhibition blocked HBEC-3KT proliferation indicated by decreased total cell numbers while percent viability was unchanged. Asterisks indicate statistical significance (P < 0.05).
Ajuba is a target of Hippo/Yap signaling and regulates cell proliferation and differentiation
Ajuba is a member of the Zyxin/Ajuba family of LIM domain proteins that functions as an adaptor-scaffold molecule with pleiotropic effects on cell signaling, proliferation, transcription, migration and differentiation. Consistent induction of Ajuba mRNA was noted in microarray and RNA-seq analyses from both in vivo and in vitro experiments (Figures 4 and 5), indicating that it is regulated by Hippo/Yap signaling. Ajuba was detected in the nuclei of airway epithelial cells in control E18.5 and adult mouse lungs (Figure 6A and B). Epithelial Ajuba staining was increased after deletion of Mst1/2 in both developing and adult lungs (Figure 6A and B), consistent with the mRNA findings. Ajuba was increased in the nucleus of club cells in adult Mst1/2-deleted mice and was induced during airway regeneration following DTA-mediated cell ablation (Figure 6B and C). AJUBA mRNA and protein were induced by YAP(WT) and YAP(S127A) and decreased following YAP inhibition in vitro (Figures 6D, E, 7C and Supplementary Figure S7C). These data show that Ajuba is regulated by Hippo/Yap signaling in lung epithelial cells.
Figure 6.
Ajuba is regulated by Hippo/Yap signaling and is induced during bronchiolar epithelial cell repair. (A and B) Ajuba staining was increased in the nucleus of epithelial cells of Mst1/2▵/▵ lungs at E18.5 (A) and in adult mice after 1 week of doxycycline treatment (B). (B) Immunofluorescence for Ajuba, CCSP, and acetylated tubulin showed weak nuclear staining for Ajuba in ciliated and club cells in controls and increased nuclear staining in the hyperplastic lesions of Mst1/2-deleted mice. Bottom panels show Ajuba staining separated from the merged top panels. (C) Ajuba staining was increased in squamous bronchiolar epithelial cells remaining on Day 5 following DTA-mediated cell ablation. (D) YAP induced AJUBA in ALI and bronchosphere cultures. (E) AJUBA mRNA was decreased following RNAi-mediated inhibition of YAP in HBEC-3KT cells and primary HBECs. Asterisks indicate statistical significance (P < 0.05). Scale bar, 20 µm (fluorescence in B and C); 50 µm (A and immunostains in B).
Figure 7.
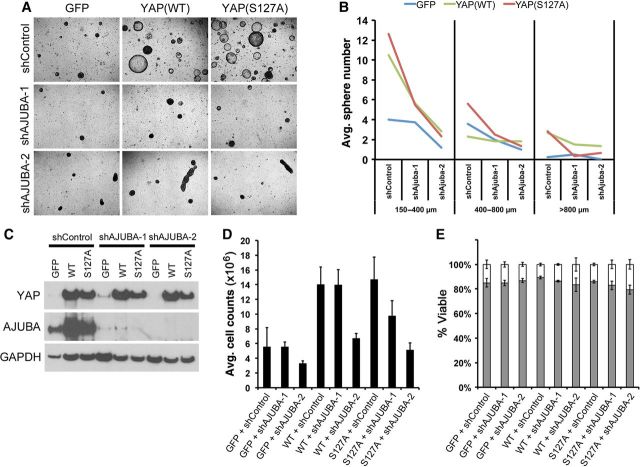
Ajuba mediates the proliferative effects of YAP in human bronchiolar epithelial cells. (A–E) Primary HBEC (A and B) and HBEC-3KT (C–E) cultures were infected with YAP-expressing or AJUBA shRNA lentiviruses. (A and B) YAP increased HBEC bronchosphere size and number, and inhibition of AJUBA blocked YAP-induced growth and altered spheroid morphology. (C) Western blot analysis of HBEC-3KT cells demonstrated that AJUBA was induced by YAP and inhibited by AJUBA shRNA. (D) YAP increased HBEC-3KT cell numbers and proliferation was inhibited by AJUBA shRNA. (E) HBEC-3KT viability was not affected by YAP or shAJUBA.
To determine if AJUBA mediated the effects of YAP on bronchiolar epithelial cell proliferation and differentiation, AJUBA was inactivated in primary HBECs and HBEC-3KT expressing YAP in vitro. AJUBA was induced by YAP(WT) and YAP(S127A) in control cultures and its expression was substantially inhibited by two separate targeted shRNAs (Figure 7C). Loss of AJUBA blocked cell proliferation induced by YAP(WT) and YAP(S127A) in both bronchospheres and HBEC3-KT cells (Figure 7A, B, D and Supplementary Figure S8). The increase in bronchosphere size and number induced by YAP was inhibited by AJUBA inactivation and loss of Ajuba disrupted spheroid morphology (Figure 7A and B). While HBEC3-KT cell number was increased by YAP and decreased by loss of AJUBA, cell viability was not affected (Figure 7D and E), consistent with regulation of cell proliferation by YAP and AJUBA. Although both AJUBA shRNA constructs abrogated YAP activity in vitro, shAJUBA-2 consistently had more potent effects on cell growth and sphere morphology. Taken together, Ajuba was induced following Mst1/2 loss or Yap activation in vivo and in vitro, and its inhibition blocked the proliferative effects of Yap in lung epithelial cells.
Discussion
The present study demonstrates that Hippo/Yap signaling regulates epithelial cell proliferation and differentiation critical for embryonic lung maturation and postnatal airway homeostasis. Deletion of Mst1/2 or activation of Yap regulated gene networks enhancing respiratory epithelial cell proliferation and migration, and inhibiting selective aspects of differentiation. Ajuba was induced following Mst1/2 deletion or Yap activation and mediated the effects of Yap on epithelial cell proliferation and tissue organization. Thus, Mst1/2 deletion caused canonical Yap activation to regulate Ajuba and influence respiratory epithelial cell proliferation and differentiation.
In the canonical Hippo signaling pathway, Mst1/2 kinase activity inhibits Yap function to restrict proliferation and influence cell fate to control organ size. The present study demonstrates that the mammalian Hippo kinases Mst1/2 are critical regulators of perinatal lung maturation. Deletion of Mst1/2 in the developing lung caused activation of Yap and broadly inhibited the transcriptional and structural changes required for lung sacculation, epithelial cell maturation, and respiration at birth. While Mst1/2 deletion caused marked lung hypercellularity at E18.5, earlier stages of lung formation were not overtly affected by Mst1/2 loss. In contrast, conditional deletion of Yap from fetal lung epithelial progenitors disrupted branching morphogenesis, causing lung hypoplasia and dilated cystic structures that were apparent by E12.5 (Mahoney et al., 2014). These findings indicate that Yap regulates epithelial progenitor cell functions during the early stages of lung morphogenesis while Mst1/2 activity is required during perinatal lung maturation to restrict proliferation and orchestrate structural and functional changes in cellular activities required for sacculation and differentiation. The expression of peripheral epithelial progenitor cell markers was altered in both Yap- and Mst1/2-deleted mice. Inactivation of Yap caused the expansion of distal epithelial progenitors and loss of proximal airway precursors (Mahoney et al., 2014), while Mst1/2 deletion caused decreased mRNAs of distal epithelial progenitor markers (Sox9, Id2) and induced ectopic expression of markers typically associated with alveolar type I cells. Hopx is expressed in airway epithelial progenitors during branching morphogenesis prior to the onset of differentiation, but later becomes increasingly restricted to alveolar type I cells in the mature lung (Yin et al., 2006; Barkauskas et al., 2013). Therefore, the aberrant expression of Hopx in airway epithelial cells in embryonic and adult Mst1/2-deleted mice may represent the induction of progenitor cell activity. Together these findings are consistent with the concept that precise temporal regulation of Mst1/2 and Yap activity during lung development coordinately balances proximal–distal epithelial progenitor cell proliferation and differentiation to control lung branching morphogenesis and maturation.
Deletion of Mst1/2 in nonciliated bronchiolar epithelial cells in the mature lung caused normally quiescent secretory/club cells to re-enter the cell cycle, causing airway epithelial hyperplasia and disrupting cell shape and polarity. These findings illustrate the remarkable plasticity and progenitor capacity of bronchiolar epithelial club cells, and are consistent with the concept that the Hippo kinases are required to suppress proliferation and maintain differentiation in the normal bronchiolar epithelium. Consistent with these findings, transgenic expression of activated Yap(S127A) in airway basal cells enhanced tracheal epithelial stratification (Zhao et al., 2014). Similarly, conditional deletion of the PI3K/AKT/mTOR pathway inhibitor Pten (Yanagi et al., 2007; Davé et al., 2008; Tiozzo et al., 2009) and transgenic expression of K14 (keratin 14; Habib Dakir et al., 2008) in bronchiolar epithelial cells caused airway hyperplasia. Although K14 mRNA was not changed in airway epithelial cells from Mst1/2-deleted mice, YAP induced K14 in primary HBEC cultures. Yap directly regulates miR-29, a known suppressor of PTEN translation, thereby integrating the Hippo/Yap and PI3K/AKT/mTOR pathways (Tumaneng et al., 2012). Transcriptional changes in miR-29 or components of the PI3K/AKT/mTOR pathway were not detected following bronchiolar epithelial deletion of Mst1/2, although posttranslation regulation of the associated networks cannot be excluded. These findings indicate a critical role for Hippo/Yap signaling in maintaining homeostasis in the postnatal airway epithelium.
Yap and phospho-Yap were dynamically regulated after epithelial Mst1/2 deletion and in regenerating epithelial cells following naphthalene and DTA-mediated club cell ablation, wherein increased Yap activity correlated with proliferation and cell shape alterations in epithelial progenitors. Present findings regarding Yap activation in vivo are consistent with in vitro findings in which YAP and YAP(S127A) induced proliferation, cell shape changes, and inhibited differentiation in primary HBECs. Deletion of Yap in developing lens progenitors caused lens hypocellularity and disrupted cell shape and polarity complexes (Song et al., 2014). In airway basal stem cells, transgenic expression of activated Yap(S127A) induced airway hyperplasia and inhibited differentiation, whereas deletion of Yap caused terminal differentiation of basal stem cells and columnar simplification of the pseudostratified epithelium (Zhao et al., 2014). These findings support a role for Hippo/Yap signaling in coordinately regulating epithelial cell proliferation, differentiation, and polarity. Hippo/Yap modulation of proliferation and differentiation in diverse fetal and adult lung stem/progenitor cell populations is consistent with studies demonstrating that Mst1/2 deletion or activation of Yap promoted the expansion of somatic stem/progenitor cells and inhibited differentiation in multiple tissues in vivo, including the intestine, skin, liver, and central nervous system (Ramos and Camargo, 2012). While Hippo/Yap signaling influences lung progenitor cell activity during development and homeostasis, further studies are necessary to determine the role of Hippo/Yap signaling during lung regeneration and in the pathogenesis of acute and chronic lung disease.
Ajuba was consistently induced following Mst1/2 deletion in vivo and after expression of YAP in vitro, demonstrating that Hippo/Yap signaling regulates Ajuba expression in respiratory epithelial cells. Ajuba has multiple adaptor protein functions in various nuclear and cytoplasmic protein complexes to regulate cell adhesion, migration, proliferation, and differentiation. Increased Ajuba staining was detected in the nucleus of respiratory epithelial cells following Mst1/2 deletion, suggesting a nuclear rather than a cytoplasmic function of Ajuba in this context. In the nucleus, Ajuba localizes to centrosomes to regulate chromosome segregation during mitosis through interactions with mitotic checkpoint kinases including Aurora-A and Lats2 (Hirota et al., 2003; Abe et al., 2006), and nuclear shuttling of Ajuba regulates proliferation and differentiation of P19 embryonal cells (Kanungo et al., 2000). Previous studies in mammalian and Drosophila cells demonstrated that Ajuba negatively regulates Hippo signaling through interactions with core pathway components Lats/Warts and WW45/salvador to inhibit phosphorylation of Yap/yorkie (Das Thakur et al., 2010). While the present findings demonstrate that increased Yap activity induced Ajuba expression, whether increased Ajuba in turn affected Lats1/2 activity remains unclear. Ajuba also forms transcriptional corepressor complexes with retinoic acid receptors, Isl1, and the SNAG domain proteins Snail and Gfi to regulate gene expression (Ayyanathan et al., 2007; Langer et al., 2008; Montoya-Durango et al., 2008; Hou et al., 2010; Witzel et al., 2012). Although Ajuba binds to TTF-1, this interaction has no effect on TTF-1 transcriptional activity (Missero et al., 2001). Thus, the transcriptional role of Ajuba in lung epithelial cells remains unknown. In the present study, inhibition of AJUBA blocked the proliferative effects of YAP in vitro, indicating that increased nuclear Ajuba may influence mitotic progression in respiratory epithelial cells. Ajuba modulated cell proliferation in vitro and immunohistochemical data showed that Yap and Ajuba were increased during lung repair in vivo. However, both Yap and Ajuba were present in normal, quiescent respiratory epithelial cells, indicating that their proliferative effects are constitutively inhibited during homeostasis. Ajuba−/− mice are viable and fertile without obvious phenotype at baseline (Pratt et al., 2005), perhaps related to compensation by related LIM proteins, Limd1 and Wtip. Thus, the role of Ajuba during normal lung morphogenesis or regeneration remains unclear.
The present study demonstrates that Hippo/Yap pathway activity is a critical regulator of cell proliferation, differentiation, and progenitor cell behavior in respiratory epithelial cells of the developing and mature lungs. While the role of Hippo signaling in the lung is in early stages of investigation, present findings show that dysregulation of core pathway kinases and activation of Yap in respiratory epithelial cells regulates transcriptional networks promoting cell proliferation, migration, and adhesion and inhibiting specific aspects of differentiation, supporting the concept that precise regulation of Hippo/Yap activity coordinates diverse cellular functions to control tissue morphogenesis and maintain homeostasis. Further elucidation of Hippo/Yap pathway upstream regulators, signaling interactions, and transcriptional targets will advance the understanding of lung progenitor cell behavior and improve targeted therapeutic strategies for lung diseases and cancer.
Materials and methods
Mice
Mst1flx and Mst2flx mice were a kind gift from Dr Randy L. Johnson (University of Texas M.D. Anderson) (Lu et al., 2010), and Shh-Cre mice were obtained from Jackson Labs (stock number 005622). Naphthalene treatment and Scgb1a1-rtTA line 2, (tetO)7CMV-Cre, and diphtheria toxin A transgenic mice have been previously described (Perl et al., 2002, 2009, 2011; Brockschnieder et al., 2006; Park et al., 2006). The presence of a copulation plug in the morning represented embryonic day (E) 0.5 for timed matings. Pregnant dams were sacrificed by CO2 inhalation and embryos were harvested at E14.5 or E18.5. Intraperitoneal injections of BrdU (50 mg/kg, Pharmingen) were administered to pregnant dams 2 h prior to harvesting embryos. Doxycycline-containing food (625 mg/kg; Harlan Teklad) was used as described for specific experiments for conditional deletion of Mst1/2 in adult mice. Isolation of epithelial cells from E18.5 and adult mouse lungs is detailed in the Supplementary methods. Adult mice were sacrificed by anesthesia using a mixture of ketamine, acepromazine, and xylazine and exsanguination by severing the inferior vena cava and descending aorta. Animals were housed in pathogen-free conditions according to the protocols approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital Research Foundation. All experiments were performed using at least four animals per group.
Immunohistochemistry and immunofluorescence
Adult mouse lungs were inflation-fixed and embryos harvested from timed matings were fixed by immersion using 4% paraformaldehyde in phosphate-buffered saline. Following overnight fixation at 4°C, tissues were processed for paraffin embedding. Histological staining, immunohistochemistry, and immunofluorescence were performed on tissue sections (6 µm) using methods and antibodies described in the Supplementary material (Supplementary Table S1). Brightfield images were obtained using a Zeiss Axio ImagerA2 microscope equipped with AxioVision Software. Fluorescent images were obtained using a Nikon A1Rsi inverted laser confocal microscope and analyzed using Imaris software (Bitplane Scientific Software). Cell proliferation and bronchiolar epithelial cell counts in control (n = 4) and Mst1/2-deleted (n = 4) embryonic and adult lungs, were determined using at least three independent images per sample and Imaris software to quantify the number of airway nuclei and immunostained cells per field.
Cell culture
Primary HBECs from normal donor lungs were obtained from Dr Scott Randell (UNC Chapel Hill, NIH Grant DK065988) cryopreserved at passage 1 and were cultured as previously described (Fulcher et al., 2005). HBEC-3KT cells, human bronchial epithelial cells immortalized by retroviral expression of Cdk4 and hTERT, were a kind gift from Dr John D. Minna (UT Southwestern) (Ramirez et al., 2004). Primary HBEC ALI or bronchosphere cultures (Rock et al., 2009) and HBEC-3KT experiments are detailed in the Supplementary methods.
RNA analyses
Total RNA was isolated from cells using a RNeasy Micro Kit (Qiagen), and reverse transcription reactions were performed using an iScript cDNA synthesis kit (BioRad) according to the manufacturer's recommendations. qPCR was performed using a StepOnePlus Real-Time PCR System and TaqMan gene expression assays listed in Supplementary Table S2 (Applied Biosystems). Relative expression was calculated using the delta-delta Ct method and statistical significance was determined by two-tailed unpaired Student's t-test (P ≤ 0.05). Microarray and RNA-seq data have been deposited in the NCBI Gene Expression Omnibus database (GEO Series accession number GSE61628). Detailed methods for microarray and RNA-seq are provided in the Supplementary methods.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
This work was supported by National Heart, Lung, and Blood Institute and Lung Repair and Regeneration Consortium (LRRC) U01-110964 to J.A.W. and A-K.P., and Lung Map U01-HL122642 to J.A.W., B.R.S., and A-K.P.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
The authors would like to thank Karen Terry (Duke University School of Medicine), Joe Kitzmiller, Ben Vidourek, Marc Lubitz, Steve Lubitz, Susan Wert, and Matt Kofron (CCHMC) for their technical assistance.
References
- Abe Y., Ohsugi M., Haraguchi K., et al. LATS2-Ajuba complex regulates gamma-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett. 2006;580:782–788. doi: 10.1016/j.febslet.2005.12.096. [DOI] [PubMed] [Google Scholar]
- Ayyanathan K., Peng H., Hou Z., et al. The Ajuba LIM domain protein is a corepressor for SNAG domain mediated repression and participates in nucleocytoplasmic Shuttling. Cancer Res. 2007;67:9097–9106. doi: 10.1158/0008-5472.CAN-07-2987. [DOI] [PubMed] [Google Scholar]
- Barkauskas C.E., Cronce M.J., Rackley C.R., et al. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockschnieder D., Pechmann Y., Sonnenberg-Riethmacher E., et al. An improved mouse line for Cre-induced cell ablation due to diphtheria toxin A, expressed from the Rosa26 locus. Genesis. 2006;44:322–327. doi: 10.1002/dvg.20218. [DOI] [PubMed] [Google Scholar]
- Chung C., Kim T., Kim M., et al. Hippo-Foxa2 signaling pathway plays a role in peripheral lung maturation and surfactant homeostasis. Proc. Natl Acad. Sci. USA. 2013;110:7732–7737. doi: 10.1073/pnas.1220603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby L.M., Waters C.M. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Thakur M., Feng Y., Jagannathan R., et al. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr. Biol. 2010;20:657–662. doi: 10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davé V., Wert S.E., Tanner T., et al. Conditional deletion of Pten causes bronchiolar hyperplasia. Am. J. Respir. Cell Mol. Biol. 2008;38:337–345. doi: 10.1165/rcmb.2007-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Feldmann G., Huang J., et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher M.L., Gabriel S., Burns K.A., et al. Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- Habib Dakir E.L., Feigenbaum L., Linnoila R.I. Constitutive expression of human keratin 14 gene in mouse lung induces premalignant lesions and squamous differentiation. Carcinogenesis. 2008;29:2377–2384. doi: 10.1093/carcin/bgn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines E.A., Sun X. Tissue crosstalk in lung development. J. Cell. Biochem. 2014;115:1469–1477. doi: 10.1002/jcb.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T., Kunitoku N., Sasayama T., et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- Hogan B.L., Barkauskas C.E., Chapman H.A., et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Peng H., White D.E., et al. LIM protein Ajuba functions as a nuclear receptor corepressor and negatively regulates retinoic acid signaling. Proc. Natl Acad. Sci. USA. 2010;107:2938–2943. doi: 10.1073/pnas.0908656107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanungo J., Pratt S.J., Marie H., et al. Ajuba, a cytosolic LIM protein, shuttles into the nucleus and affects embryonal cell proliferation and fate decisions. Mol. Biol. Cell. 2000;11:3299–3313. doi: 10.1091/mbc.11.10.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton D.N., Morrisey E.E. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat. Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer E.M., Feng Y., Zhaoyuan H., et al. Ajuba LIM proteins are snail/slug corepressors required for neural crest development in Xenopus. Dev. Cell. 2008;14:424–436. doi: 10.1016/j.devcel.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Li Y., Kim S.M., et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc. Natl Acad. Sci. USA. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y., Davé V., Whitsett J.A. Transcriptional control of lung morphogenesis. Physiol. Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- Mahoney J.E., Mori M., Szymaniak A.D., et al. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C., Pirro M.T., Simeone S., et al. The DNA glycosylase T:G mismatch-specific thymine DNA glycosylase represses thyroid transcription factor-1-activated transcription. J. Biol. Chem. 2001;276:33569–33575. doi: 10.1074/jbc.M104963200. [DOI] [PubMed] [Google Scholar]
- Mitani A., Nagase T., Fukuchi K., et al. Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am. J. Respir. Crit. Care Med. 2009;180:326–338. doi: 10.1164/rccm.200812-1827OC. [DOI] [PubMed] [Google Scholar]
- Montoya-Durango D.E., Velu C.S., Kazanjian A., et al. Ajuba functions as a histone deacetylase-dependent co-repressor for autoregulation of the growth factor-independent-1 transcription factor. J. Biol. Chem. 2008;283:32056–32065. doi: 10.1074/jbc.M802320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey E.E., Hogan B.L. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.-S., Whitsett J.A., Di Palma T., et al. TAZ interacts with TTF-1 and regulates expression of surfactant protein-C. J. Biol. Chem. 2004;279:17384–17390. doi: 10.1074/jbc.M312569200. [DOI] [PubMed] [Google Scholar]
- Park K.-S., Wells J.M., Zorn A.M., et al. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 2006;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A.-K.T., Wert S.E., Nagy A., et al. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl Acad. Sci. USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A.-K., Zhang L., Whitsett J.A. Conditional expression of genes in the respiratory epithelium in transgenic mice. Am. J. Respir. Cell Mol. Biol. 2009;40:1–3. doi: 10.1165/rcmb.2008-0011ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A.-K.T., Riethmacher D., Whitsett J.A. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am. J. Respir. Crit. Care Med. 2011;183:511–521. doi: 10.1164/rccm.201005-0744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt S.J., Epple H., Ward M., et al. The LIM protein Ajuba influences p130Cas localization and Rac1 activity during cell migration. J. Cell Biol. 2005;168:813–824. doi: 10.1083/jcb.200406083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R.D., Sheridan S., Girard L., et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- Ramos A., Camargo F.D. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22:339–346. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Onaitis M.W., Rawlins E.L., et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Chen F., Cardoso W.V. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009;6:558–563. doi: 10.1513/pats.200905-031RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.Y., Park R., Kim J.Y., et al. Dual function of Yap in the regulation of lens progenitor cells and cellular polarity. Dev. Biol. 2014;386:281–290. doi: 10.1016/j.ydbio.2013.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiozzo C., De Langhe S., Yu M., et al. Deletion of Pten expands lung epithelial progenitor pools and confers resistance to airway injury. Am. J. Respir. Crit. Care Med. 2009;180:701–712. doi: 10.1164/rccm.200901-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumaneng K., Schlegelmilch K., Russell R.C., et al. YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nat. Cell Biol. 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- Witzel H.R., Jungblut B., Choe C.P., et al. The LIM protein Ajuba restricts the second heart field progenitor pool by regulating Isl1 activity. Dev. Cell. 2012;23:58–70. doi: 10.1016/j.devcel.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Wang Y., Besnard V., et al. Transcriptional programs controlling perinatal lung maturation. PLoS One. 2012;7:e37046. doi: 10.1371/journal.pone.0037046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi S., Kishimoto H., Kawahara K., et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J. Clin. Invest. 2007;117:2929–2940. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z., Gonzales L., Kolla V., et al. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L191–L199. doi: 10.1152/ajplung.00385.2005. [DOI] [PubMed] [Google Scholar]
- Zhao B., Ye X., Yu J., et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Tumaneng K., Guan K.-L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Fallon T.R., Saladi S.V., et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev. Cell. 2014;30:151–165. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.