Chronic granulomatous disease is a genetic immunodeficiency characterized by a limited spectrum of recurrent bacterial and fungal infections. Genetically determined superoxide production is linked to overall survival as well as severity of infections.
Keywords: bacterial infection, fungal infection, CGD, superoxide production, survival
Abstract
Background. Chronic granulomatous disease (CGD) is due to defective nicotinamide adenine dinucleotide phosphate oxidase activity and characterized by recurrent infections with a limited spectrum of bacteria and fungi as well as inflammatory complications. To understand the impact of common severe infections in CGD, we examined the records of 268 patients followed at a single center over 4 decades.
Methods. All patients had confirmed diagnoses of CGD, and genotype was determined where possible. Medical records were excerpted into a standard format. Microbiologic analyses were restricted to Staphylococcus, Burkholderia, Serratia, Nocardia, and Aspergillus.
Results. Aspergillus incidence was estimated at 2.6 cases per 100 patient-years; Burkholderia, 1.06 per 100 patient-years; Nocardia, 0.81 per 100 patient-years; Serratia, 0.98 per 100 patient-years, and severe Staphylococcus infection, 1.44 per 100 patient-years. Lung infection occurred in 87% of patients, whereas liver abscess occurred in 32%. Aspergillus incidence was 55% in the lower superoxide-producing quartiles (quartiles 1 and 2) but only 41% in the higher quartiles (rate ratio, <0.0001). Aspergillus and Serratia were somewhat more common in lower superoxide producing gp91phox deficiency. The median age at death has increased from 15.53 years before 1990 to 28.12 years in the last decade. Fungal infection carried a higher risk of mortality than bacterial infection and was the most common cause of death (55%).Gastrointestinal complications were not associated with either infection or mortality.
Conclusions. Fungal infections remain a major determinant of survival in CGD. X-linked patients generally had more severe disease, and this was generally in those with lower residual superoxide production. Survival in CGD has increased over the years, but infections are still major causes of morbidity and mortality.
(See the Editorial Commentary by Gennery on pages 1184–5.)
Chronic granulomatous disease (CGD) is a genetic immunodeficiency characterized by recurrent infections and inflammatory complications. It is caused by defective function of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, the enzyme responsible for the phagocyte respiratory burst and superoxide production. Defects in the NADPH complex can be inherited through mutations in any of 5 phagocyte oxidase (phox) genes: X-linked gp91phox (cytochrome b-245 beta polypeptide), autosomal recessive p22phox (cytochrome b-245 alpha polypeptide), autosomal recessive p47phox (neutrophil cytosolic factor 1), autosomal recessive p67phox (neutrophil cytosolic factor 2), and autosomal recessive p40phox (neutrophil cytosolic factor 4) [1–3]. A minimal US rate is approximately 1 per 250 000 live births; rates in other countries are similar but differ in the prevalence of recessive mutations, depending on the rates of consanguinity [4, 5].
Since the first cases described in 1954, prophylactic antibiotics, antifungals, and interferon (IFN)–γ, along with aggressive surgical and medical management, have improved outcomes, but infections still cause significant morbidity and mortality [6–9]. The genetic type of CGD and superoxide production are clearly linked to overall survival, but the reasons are unclear [10]. Currently, bone marrow transplant is the only cure.
While some infections are harder to treat than others, it is unclear whether all infections are equally deleterious or equally distributed by CGD genotype. Furthermore, it remains unclear whether different infections affect survival differently. To understand the relative roles of genetics and superoxide generation in susceptibility to specific infections and to determine the relative roles of specific infections on morbidity and mortality, we examined the infections of a large cohort of CGD subjects followed at a single center over 4 decades.
METHODS
Subjects
Patients with CGD have been followed at the Clinical Center of the National Institutes of Health (NIH; Bethesda, Maryland) on 1 or more approved protocols involving the natural history or treatment of CGD.
Data Collection
Information was retrospectively retrieved through systematic review of CGD patient hospital records from 1969 to 2012. Data collected included demographics (age, sex, and race/ethnicity), genotype (where available), superoxide production (where available), and causative microorganisms. CGD was confirmed by either nitroblue tetrazolium reduction or dihydrorhodamine oxidation, depending on the year of diagnosis. The specific gene lesioned was determined by using immunoblotting or gene sequencing. Genes to be sequenced were guided by immunoblotting results; for p47phox deficiency, only immunoblotting was done. Gene determination was performed on 85% of the total group. Microbiology and electronic medical records (Biomedical Translational Research Information System, NIH) were reviewed to identify patients with documented bacterial or fungal infections. Infection was defined as isolation or detection of a pathogen associated with CGD that was relevant to the symptoms and the site. Severe infection was defined as 1 for which intravenous therapy was given, required hospitalization, or caused death. Although there are numerous organisms that can cause infection in CGD, we focused on the prevalent pathogens in CGD—Staphylococcus aureus, Burkholderia (Pseudomonas) cepacia complex, Serratia marsescens, Nocardia species, and Aspergillus species—to be able to compare across genotypes and over decades. Organisms clinically suspected of being colonizers or detected in routine surveillance cultures or blood cultures thought to be due to contamination were not considered as infections. All fungal cases met the standard definition for proven invasive fungal infection [11]. When possible, the diagnosis of lung infection was based on medical notes or direct tissue sampling (open or percutaneous lung biopsy); bronchoalveolar lavage was reported only as confirmation data in concordance with clinical history. Liver, skin, soft tissue, bone, and lymph node infections were collected based on medical reports and microbiologic isolation at biopsy. Staphylococcus infection was only analyzed when it was reported from an abscess by invasive culture or biopsy. For this study, the older designation Pseudomonas cepacia was recoded to Burkholderia cepacia complex. Patients who had had ≥2 biopsies from the same episode (defined as the same hospitalization or process within 3 months) were included as only 1 event.
Incidence rates were calculated as the number of events divided by the sum of patient-years within the cohort. The prevalence of pathogen-specific infections was calculated for each CGD genotype and by superoxide production quartiles [10]. All events were included from the first dates available. Overall patient-years were from date of birth to last completed follow-up visit, date of death, or date of bone marrow transplant.
Statistical Analysis
Student's t test was used to compare continuous variables, and χ2 test or Fisher exact test were used to test for significant differences among proportions of genotypes or between categories of interest, respectively. All P values were 2-tailed, and P < .05 was considered statistically significant.
CGD genotype, superoxide production, and gastrointestinal manifestations were considered to be potential risk factors for infection. Rate ratios (RRs) with 95% confidence intervals (CIs) for incidence were also calculated for recurrences of specific infections among genotypes.
Mortality is shown as a crude rate, a cause-specific rate, and a fatality rate. Fatality and cause-specific rates were defined by autopsy or confirmed cultures from medical notes. Fatality rate was calculated as [(proportions of deaths due to specific infection/total number of patients with the same infection) × 100]. Cause-specific rate was calculated as [number of deaths due to a cause/population].
Differences in survival were analyzed using Kaplan–Meier curves followed by log-rank Mantel–Cox test. All tests were performed with Prism 6 software for Mac OSX, and OpenEpi for person-time rates.
RESULTS
Cohort Characteristics
We summarized severe infections in 268 patients with confirmed diagnoses of CGD between 1969 and 2012, accounting for 2571 patient-years of direct observation, but reflecting 6188 patient-years of overall survival from birth. This retrospective cohort consisted of 184 X-linked (gp91phox) patients, 70 patients with p47phox deficiency, 8 patients with p22phox deficiency, 6 patients with p67phox deficiency, and 1 patient with p40phox deficiency.
The mean follow-up per patient was 10.01 ± 8.2 years, ranging from a single visit to 32 years of follow-up. Twenty-seven percent of the patients have been incorporated into the cohort since 2007. The median age of the active cohort at the time of this report was 25 years, ranging from 3 to 55 years. The overall population was diagnosed at a mean age of 5.4 years. The mean age of diagnosis for X-linked CGD was 3.2 years, whereas the mean age for autosomal recessive CGD (mostly p47phox deficient) was 11 years (P ≤ .0001), similar to previous reports [12, 13].
Most CGD patients were treated with long-term antibiotic and antifungal prophylaxis. Ninety-seven percent of patients reported using antibiotic prophylaxis. Most used trimethoprim-sulfamethoxazole (6 mg/kg/day of trimethoprim divided twice daily) [14], but some used cephalosporin or ciprofloxacin prophylaxis due to adverse events or intolerance. Continuous antifungal prophylaxis was used in 70% of patients, mostly with itraconazole 100 mg or 200 mg daily according to weight [15]. In the last several years, some patients have used posaconazole or voriconazole as secondary prophylaxis. Among all CGD patients, 40% received steroids at some point as treatment for inflammation or as an adjuvant to specific treatment. In the surviving active cohort of 170 patients, 40% were on IFN-γ prophylaxis 50 µg/m2 subcutaneously 3 times weekly. Forty-one patients received bone marrow transplant, of whom 31 remain alive. In general, the decision to pursue bone marrow transplant was based on recurrent or severe infections or severe refractory colitis [16].
Severe Infections
At the time of this report, only 3% of patients had not had a severe infection at some point during life: 1 patient with p40phox deficiency and 4 X-linked patients, with predominantly gastrointestinal manifestations. The small group in which no severe infections occurred had predominantly gastrointestinal complications that started early in life, leading to transplant. Only 5% had infections limited to skin, without vital organs involved, all of whom had autosomal recessive forms of CGD. A small group (17%) has suffered only 1 severe infection (Figure 1).
Figure 1.
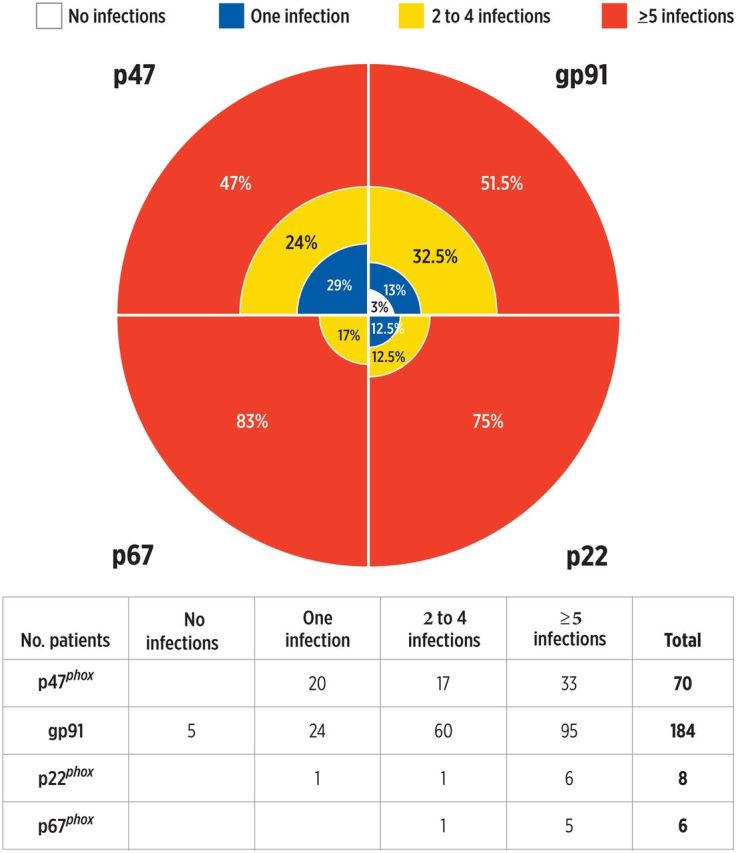
Overall proportion of severe infections. The percentage of patients is shown for each genotype.
Localization
The lung was the most common site of infection. At least 1 episode of pulmonary infection occurred in 87% of patients; 48% had >1 infection. Lung biopsy in general had a 50% (161 of 323) rate of positive organism isolation. Bronchoalveolar lavage (BAL) alone had a 30% (44 of 143) rate of positive isolation. When both were performed for the same event, BAL had only a 44% concordance rate with biopsy isolation over 63 episodes recorded.
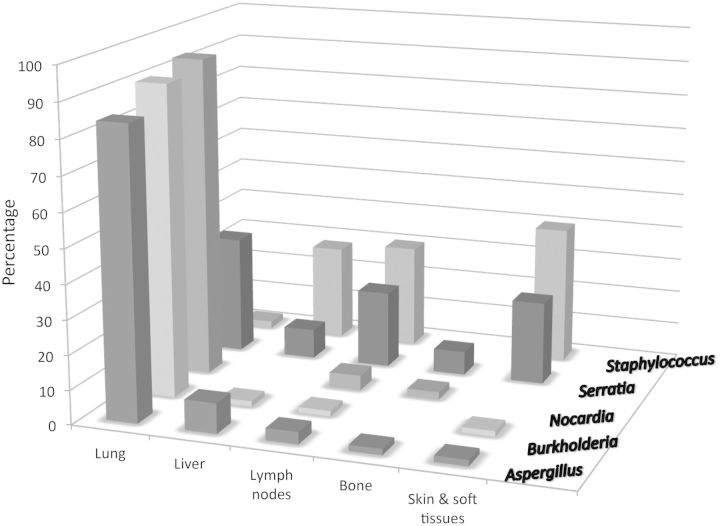
Liver abscess occurred in 32% of the cohort. Over the last 10 years, 21 patients (of an active cohort of 170) had confirmed liver abscess by biopsy, 47% of whom had recurrent liver infection. Forty-eight percent of the biopsies yielded a positive culture, and S. aureus was the predominant organism isolated (86%); the other organisms isolated were coagulase-negative staphylococci (3 cases) and a gram-negative rod (1 case). Liver infections were recorded predominantly in the X-linked genotype (71% of the cases), corresponding to their proportion of the overall cohort (Figure 2).
Figure 2.
Frequency (%) of organisms isolated according to site of infection.
At least 25% of the cohort had an episode of lymphadenitis for which an invasive culture was performed. The rate of positive culture was 62%, and S. aureus was the predominant organism isolated (48% of the positive cultures). Therefore, 31% of episodes of lymphadenitis grew S. aureus.
Pathogens
Overall, there were 429 episodes of infection, for a total infection rate of 7.26 per 100 patient-years, 4 times less than the previously reported rate during the IFN-γ prophylaxis protocol [17]. Aspergillus incidence was 2.6 cases per 100 patient-years, Burkholderia 1.06 per 100 patient-years, Nocardia 0.81 per 100 patient-years; Serratia 0.98 per 100 patient-years, and severe Staphylococcus infection 1.44 per 100 patient-years (Table 1).
Table 1.
Incidence Rate of Specific Severe Infections
| Group: cases/patient year (events) | Aspergillus Infection | Burkholderia Infection | Serratia Infection | Nocardia Infection | Staphylococcus Infection |
|---|---|---|---|---|---|
| Total cohort: cases/py (events) n = 268 % affected |
0.026 (161) n = 125 46% |
0.0106 (66) n = 46 17% |
0.0098 (61) n = 42 15% |
0.0081 (51) n = 49 18% |
0.0181 (112) n = 90 33% |
| Age at first event (overall) | 13 y (range, 0.1–62 y) |
16 y (range, 0.6–41 y) |
14 y (range, 0.1–40 y) |
17 y (range, 1.5–53 y) |
16 y (range, 3–37 y) |
| gp91phox: cases/py (events) n = 184 % affected |
0.0325 (118)* n = 90 48% |
0.0088 (32) n = 27 14% |
0.013 (47)* n = 36 19% |
0.0093 (34) n = 30 16% |
0.0190 (69) n = 62 30% |
| Autosomal recessive: cases/py (events) n = 84 % affected |
0.0171 (43) n = 35 41% |
0.0135 (34) n = 19 22% |
0.0055 (14) n = 12 14% |
0.0067 (17) n = 16 19% |
0.0171 (43) n = 27 32% |
| p47phox: cases/py (events) n = 70 % affected |
0.0160 (35) n = 28 40% |
0.0141 (31) n = 16 23% |
0.0046 (10) n = 9 13% |
0.0064 (14) n = 13 18% |
0.0178 (39) n = 25 35% |
| p22phox: cases/py (events) n = 8 % affected |
0.0246 (5) n = 5 62% |
0.0147 (3) n = 3 37% |
0.0147 (3) n = 2 25% |
0.0147 (3) n = 3 37% |
0.0098 (2) n = 1 12% |
| p67phox: cases/py (events) n = 6 % affected |
0.0224 (3) n = 2 33% |
… | 0.0074 (1) n = 1 16% |
… | 0.0149 (2) n = 1 16% |
| Quartiles 1–2: cases/py (events) (lower superoxide) n = 105 % affected |
0.041 (91)* n = 58 55% |
0.0096 (21) n = 18 17% |
0.0156 (34)* n = 25 23% |
0.0101 (22) n = 20 19% |
0.0257 (56)* n = 26 25% |
| Quartiles 3–4: cases/py (events) (higher superoxide) n = 117 % affected |
0.0197 (62) n = 49 41% |
0.0124 (39) n = 21 18% |
0.0070 (22) n = 19 16% |
0.0060 (19) n = 19 16% |
0.014 (44) n = 42 36% |
Data are presented as cases per patient-years (No. of events). Quartiles were calculated based on superoxide production, used as a continuous variable [10]. The comparisons are in relation to quartiles 3–4 (higher superoxide producing).
Statistically significant (P ≤ .05).
In 44% (125 patients), Aspergillus species were isolated at least once, and 20% had >1 Aspergillus infection, making this the most common pathogen in our cohort. Aspergillus fumigatus was the most prevalent, with lower incidences for other Aspergillus species (A. nidulans, A. terreus, A. tanneri). The median age at first fungal infection was lower in the gp91phox-deficient patients (12 years [range, 0.1–38 years]) than in the p47phox-deficient patients (17 years [range, 4–62 years]) (P = .014). The incidence of Aspergillus infection was 55% in the lower superoxide production quartiles and 41% in the higher superoxide production quartiles, independent of genotype (P ≤ .0001).
Ninety percent of the 60 cases of Burkholderia infection were identified by isolation from lung biopsy. Two patients presented with lymph node infections, and 4 patients presented with bacteremia. Burkholderia had a 26% rate of recurrence of infection [18]. Among 16 patients with p47phox genotype who had Burkholderia infections, 8 had >1; only 3 patients with X-linked CGD had >1 episode due to this organism (P = .0064).
Nocardia infections were predominantly pulmonary, but only 10% of patients had recurrences.
Serratia marsescens had a recurrence rate of 18%, the sites of which were diverse. The predominant sites of infection were lymph nodes and skin abscesses in 44% of cases; 36% were isolated from lung biopsy, and 8% were osteomyelitis. Nocardia, Burkholderia, and Aspergillus were present in lymph node culture in 10% of the cases reported here, mostly in the setting of disseminated infection.
Severe infection caused by S. aureus was confirmed in 33% (90 patients) of the whole population (268 patients), being cultured from lymph nodes or liver abscesses. No pneumonia exclusively due to S. aureus was identified on our review, but S. aureus grew from lung biopsies in 4 patients, concomitant with fungal infection. The patients who had Staphylococcus species infections had a recurrence rate of 25%. Staphylococcus epidermidis was isolated as the probable cause of infection in 38 patients, most of whom (80%) had histories of S. aureus infection as well.
Risk Factor Association
As reported previously, but still to our surprise, we found no evidence that colitis or inflammatory bowel disease was associated with higher risks of severe infection with the organisms described (RR, 1.3 [95% CI, .9–1.99]). There was a slight but significant difference in the rates of infection between gp91phox- and p47phox-deficient patients (RR, 1.4 [95% CI, 1.1–1.74]). Aspergillus and Serratia were somewhat more common in gp91phox-deficient patients in the lower superoxide production quartiles (1 and 2) than in those in the upper quartiles (3 and 4). Burkholderia infection was more recurrent in the p47phox cohort (P = .06). No cofactors or clinical factors could be associated with the recurrence. Nocardia infection did not segregate by genotype. Age at first Aspergillus infection was earlier in the gp91phox- than the p47phox-deficient groups (P = .006), but age at first infection for the other organisms did not significantly differ.
Mortality
Forty-seven (17%) of the patients had died by the time of data extraction. Nine patients were excluded from analysis due to having received bone marrow transplant. Among the remaining 38 patients, 29 (76%) were X-linked and 9 had autosomal recessive CGD (8 p47phox, 1 p67phox). Infection was the cause of death in 81% of the cases. The remaining 7 deaths were unrelated to infection.
Of 21 autopsies, the lung was the predominant site of infection in 76%, as confirmed by culture or pathology. Of 26 patients with available data, 70% of the fatalities occurred in the lower superoxide production quartiles [10]. However, superoxide production data were not available for all fatal cases, and most of those lacking data were p47phox patients. However, estimating that all the p47phox fatalities would be in the third or fourth quartiles, mortality is still higher at the lower levels of superoxide production.
Fungal infection accounted for 21 deaths (21/38 [55%]). Overall Aspergillus-specific mortality was 9%. Death due to fungal infection was confirmed in 37% of the p47phox and 62% of the gp91phox cases. Aspergillus fumigatus was isolated from 10 patients, A. tanneri was isolated from 2 patients, and A. nidulans was identified from 1 fatal fungal infection. The fatality rate for Aspergillus infections within the X-linked group was 11%, but only 5.7% within the p47phox group (P = .14).
Other fatal fungi isolated were Neosartorya species (1 patient) and Phaeoacremonium parasiticum (2 patients). In 3 cases, Candida was isolated as a possible cause of death. In a few cases, coinfections might have been related to cause of death: 1 patient with human immunodeficiency virus had multiple organisms recovered in autopsy culture (A. fumigatus, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Candida glabrata). Another patient who had been treated with infliximab had fatal pan-lobar pneumonia that grew Acinetobacter baumannii, P. aeruginosa, and mucoid Burkholderia multivorans [19]. Fifteen percent of deaths were related to bacterial infections, yielding a specific mortality of 4%. Fatal Burkholderia pneumonia occurred in 5 patients, 4 with B. cepacia, for a Burkholderia fatality rate of 6% [20]. Only 1 death was due to Nocardia infection, in an X-linked patient. Overall, fungal infections had a higher risk of mortality than bacterial infections (P = .03), and mortality rates were higher among those who had histories of fungal infections than in those who did not (RR, 1.8 [95% CI, 1.06–3.06]). Staphylococcus infections were higher overall in those patients who died independent of genotype (46% vs 30%; P = .12).
The age at death has increased substantially over time in CGD. The median age at death in this cohort before 1991 was 15.53 years; by 2001 it was 20.17 years; and by 2012 it was 28.12 years. (P = .003; Figure 3). Although the crude mortality rates by genotype were not statistically different (18% for X-linked, 15% for autosomal recessive; P = .59), the median age at death was quite different between the groups. Autosomal recessive p47phox-deficient patients had a median age at death of 32 years, whereas X-linked patients died at a median of 23 years (P = .03).
Figure 3.
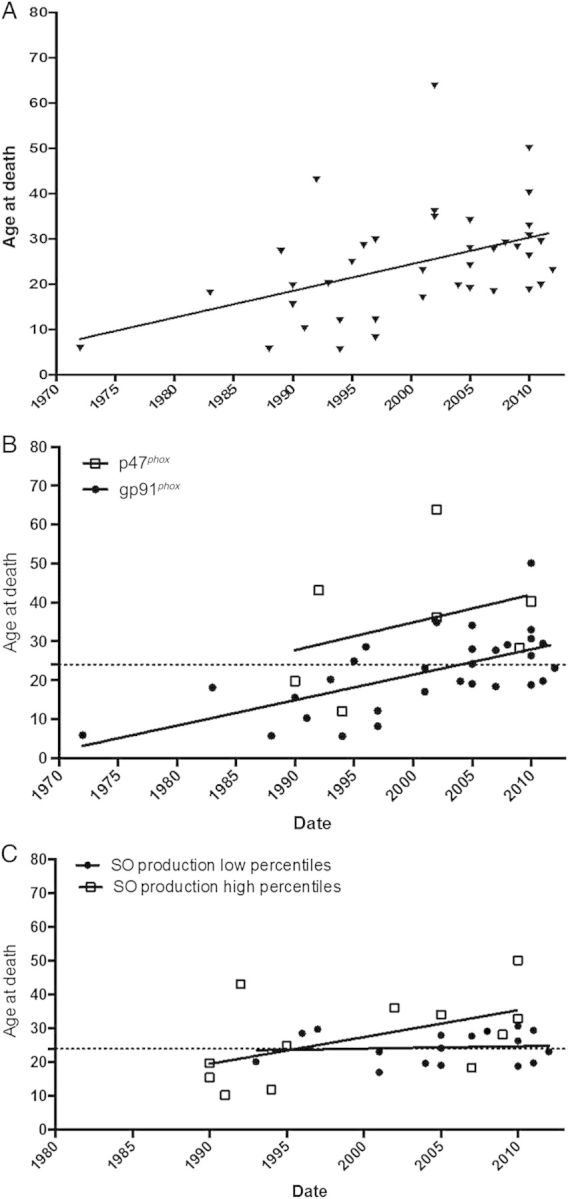
CGD Deaths over the period 1970–2012. A, Ages at death for the entire cohort over time. B, Ages at death separated by p47phox (open squares) and gp91phox (closed circles). C, Ages at death separated by higher (open squares) and lower (closed circles) superoxide production. The Y axis is age at death, the X axis is years. The dotted line in figures B and C is average age at death (23 years) over the entire cohort over the entire period. The solid lines represent the fitted correlations. Abbreviations: CGD, chronic granulomatous disease; SO, superoxide.
DISCUSSION
We examined severe infections in a large cohort of CGD patients followed over a long period of time in an effort to identify genetic or functional features that would inform how and why infections contribute to morbidity and mortality in CGD. In previous reports Aspergillus was the most commonly encountered pathogen, and this remains the case [19, 21–23]. Burkholderia, Nocardia, and Serratia infections occurred at lower rates than Aspergillus infections, but the total infection frequency was still significant. Among those organisms, severity was most marked for Burkholderia species, whereas only 1 case of fatal Nocardia infection and no fatal Serratia infections were recorded.
Invasive or severe infections continued to occur throughout life [24]. Both inheritance pattern and superoxide production confirmed previous observations that X-linked patients generally have more severe disease, and this generally in those with lower superoxide production.
Whereas infection continued to be the cause of death in most cases, the ages at which patients died have increased significantly. There have been differences in the timeline of development of infections as well as differences in treatment over the last decade, reflected in a considerable increase in age at death [12, 13, 25]. Despite the different mortality rates between the groups, both p47phox- and gp91phox-deficient patients suffered mostly from the same pathogens. However, fungal infections, as determined by both incidence and mortality, were worse in the gp91phox cohort. Although antifungal therapies and more generalized prophylaxis are likely to have contributed to the improvement in survival over the last decade [26–29], the probability of getting an invasive infection remains high. Therefore, determining the causal pathogen is essential to effective management. The age at death has continued to increase over time, with survival curves higher than previous reports [4, 13, 25]. Gastrointestinal involvement remains independent of the history of infections and unlinked to mortality, suggesting that it is a manifestation of an aspect of CGD that is distinct from bacterial susceptibility and separate from the issues driving liver disease, another critical cause of mortality in CGD [30].
There are several limitations to this report. First, because microbiologically documented infections were required for inclusion, only prevalent organisms were analyzed. Therefore, the number of actual infections is necessarily higher. We limited our analysis to confirmed cases to be certain about infections per se and their impact. Incidence estimates were limited to the prevalent organisms in CGD patients, and diagnoses were obtained from biopsies in the majority of the events. Second, retrospectively searching over a long period of time encompasses a broad and changing spectrum of prophylaxis, antimicrobials, and therapies [31, 32].
Severe infections continue to affect patients with CGD, with fungal infections still a major determinant of survival [33]. Bacterial infections showed a modest impact on overall mortality, but their recurrence may contribute to chronic lung, liver, or kidney disease [34]. The reasons for the remarkable rate of Burkholderia recurrence in p47phox deficiency remain elusive, but suggest another distinct aspect of p47phox deficiency, in addition to the higher rates of diabetes and heart disease [35]. Mortality was higher in gp91phox-deficient patients, but mostly in the lower quartile of superoxide production, suggesting that the critical determinant of mortality remains superoxide production, even after adjusting for rates of infection. Gaining a better understanding of the rates and consequences of infections in CGD is a step toward determining whether there are windows of increased risk identifying possible targets for intensified prophylaxis and treatment.
Notes
Financial support. This project was supported by Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) and the National Cancer Institute, NIH (contract number HHSN261200800001E) and the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, NIH.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Matute JD, Arias AA, Wright NA, et al. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood 2009; 114:3309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roos D, Kuhns DB, Maddalena A, et al. Hematologically important mutations: X-linked chronic granulomatous disease (third update). Blood Cells Mol Dis 2010; 45:246–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roos D, Kuhns DB, Maddalena A, et al. Hematologically important mutations: the autosomal recessive forms of chronic granulomatous disease (second update). Blood Cells Mol Dis 2010; 44:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolach B, Gavrieli R, de Boer M, et al. Chronic granulomatous disease in Israel: clinical, functional and molecular studies of 38 patients. Clin Immunol 2008; 129:103–14. [DOI] [PubMed] [Google Scholar]
- 5.Fattahi F, Badalzadeh M, Sedighipour L, et al. Inheritance pattern and clinical aspects of 93 Iranian patients with chronic granulomatous disease. J Clin Immunol 2011; 31:792–801. [DOI] [PubMed] [Google Scholar]
- 6.Janeway CA, Craig J, Davidson M, Doroney W, Gitlin D, Sullivan JC. Hypergammaglobulinemia associated with severe recurrent and chronic nonspecific infections. AMA Am J Dis Child 1954; 388. [Google Scholar]
- 7.Quie PG. Chronic granulomatous disease of childhood. Adv Pediatr 1969; 16:287–300. [PubMed] [Google Scholar]
- 8.Bassiri-Jahromi S, Doostkam A. Fungal infection and increased mortality in patients with chronic granulomatous disease. J Mycol Med 2012; 22:52–7. [DOI] [PubMed] [Google Scholar]
- 9.Holland SM. Chronic granulomatous disease. Hematol Oncol Clin North Am 2013; 27:89–99, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhns DB, Alvord WG, Heller T, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med 2010; 363:2600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkelstein JA, Marino MC, Johnston RB, Jr, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000; 79:155–69. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg JM, van Koppen E, Ahlin A, et al. Chronic granulomatous disease: the European experience. PLoS One 2009; 4:e5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman AF, Holland SM. Antimicrobial prophylaxis for primary immunodeficiencies. Curr Opin Allergy Clin Immunol 2009; 9:525–30. [DOI] [PubMed] [Google Scholar]
- 15.Gallin JI, Alling DW, Malech HL, et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med 2003; 348:2416–22. [DOI] [PubMed] [Google Scholar]
- 16.Kang EM, Marciano BE, DeRavin S, Zarember KA, Holland SM, Malech HL. Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol 2011; 127:1319–26; quiz 1327–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marciano BE, Wesley R, De Carlo ES, et al. Long-term interferon-gamma therapy for patients with chronic granulomatous disease. Clin Infect Dis 2004; 39:692–9. [DOI] [PubMed] [Google Scholar]
- 18.Guide SV, Stock F, Gill VJ, et al. Reinfection, rather than persistent infection, in patients with chronic granulomatous disease. J Infect Dis 2003; 187:845–53. [DOI] [PubMed] [Google Scholar]
- 19.Beaute J, Obenga G, Le Mignot L, et al. Epidemiology and outcome of invasive fungal diseases in patients with chronic granulomatous disease: a multicenter study in France. Pediatr Infect Dis J 2011; 30:57–62. [DOI] [PubMed] [Google Scholar]
- 20.Dorman SE, Gill VJ, Gallin JI, Holland SM. Burkholderia pseudomallei infection in a Puerto Rican patient with chronic granulomatous disease: case report and review of occurrences in the Americas. Clin Infect Dis 1998; 26:889–94. [DOI] [PubMed] [Google Scholar]
- 21.Segal BH, Romani LR. Invasive aspergillosis in chronic granulomatous disease. Med Mycol 2009; 47(suppl 1):S282–90. [DOI] [PubMed] [Google Scholar]
- 22.Antachopoulos C. Invasive fungal infections in congenital immunodeficiencies. Clin Microbiol Infect 2010; 16:1335–42. [DOI] [PubMed] [Google Scholar]
- 23.Blumental S, Mouy R, Mahlaoui N, et al. Invasive mold infections in chronic granulomatous disease: a 25-year retrospective survey. Clin Infect Dis 2011; 53:e159–69. [DOI] [PubMed] [Google Scholar]
- 24.Mouy R, Fischer A, Vilmer E, Seger R, Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediatr 1989; 114(4 pt 1):555–60. [DOI] [PubMed] [Google Scholar]
- 25.Jones LB, McGrogan P, Flood TJ, et al. Special article: chronic granulomatous disease in the United Kingdom and Ireland: a comprehensive national patient-based registry. Clin Exp Immunol 2008; 152:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kepenekli E, Soysal A, Kuzdan C, Ermerak NO, Yuksel M, Bak RM. Refractory invasive aspergillosis controlled with posaconazole and pulmonary surgery in a patient with chronic granulomatous disease: case report. Ital J Pediatr 2014; 40:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen-Wolff A, Koch A, Friedrich W, Hahn G, Gahr M, Roesler J. Successful elimination of an invasive Aspergillus nidulans lung infection by voriconazole after failure of a combination of caspofungin and liposomal amphotericin B in a boy with chronic granulomatous disease. Pediatr Infect Dis J 2004; 23:584–6. [DOI] [PubMed] [Google Scholar]
- 28.Welzen ME, Bruggemann RJ, Van Den Berg JM, et al. A twice daily posaconazole dosing algorithm for children with chronic granulomatous disease. Pediatr Infect Dis J 2011; 30:794–7. [DOI] [PubMed] [Google Scholar]
- 29.Segal BH, Barnhart LA, Anderson VL, Walsh TJ, Malech HL, Holland SM. Posaconazole as salvage therapy in patients with chronic granulomatous disease and invasive filamentous fungal infection. Clin Infect Dis 2005; 40:1684–8. [DOI] [PubMed] [Google Scholar]
- 30.Feld JJ, Hussain N, Wright EC, et al. Hepatic involvement and portal hypertension predict mortality in chronic granulomatous disease. Gastroenterology 2008; 134:1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Disease TICG, Group. CS. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. The International Chronic Granulomatous Disease Cooperative Study Group. N Engl J Med 1991; 324:509–16. [DOI] [PubMed] [Google Scholar]
- 32.Margolis DM, Melnick DA, Alling DW, Gallin JI. Trimethoprim-sulfamethoxazole prophylaxis in the management of chronic granulomatous disease. J Infect Dis 1990; 162:723–6. [DOI] [PubMed] [Google Scholar]
- 33.Falcone EL, Holland SM. Invasive fungal infection in chronic granulomatous disease: insights into pathogenesis and management. Curr Opin Infect Dis 2012; 25:658–69. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg DE, Goldberg JB, Stock F, Murray PR, Holland SM, Lipuma JJ. Recurrent Burkholderia infection in patients with chronic granulomatous disease: 11-year experience at a large referral center. Clin Infect Dis 2009; 48:1577–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leiding JW, Marciano BE, Zerbe CS, Deravin SS, Malech HL, Holland SM. Diabetes, renal and cardiovascular disease in p47 phox-/- chronic granulomatous disease. J Clin Immunol 2013; 33:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]