Abstract
Haemolytic–uraemic syndrome is a clinical syndrome characterized by thrombocytopaenia, non-autoimmune haemolytic anaemia and renal impairment. Pathological alterations in kidney samples show thrombotic microangiopathy. The underlying pathogenesis is endothelial cell injury with thrombotic occlusion of the arterioles and capillaries. A variety of causes have been identified, associated with infection of Escherichia coli O157:H7, environmental factors as immunosuppressive drugs and genetic deficiencies in complement regulatory factors. The latter is called atypical haemolytic–uraemic syndrome (aHUS). Here, we present a patient with severe aHUS with complement factor H deficiency triggered by cocaine use and recurrence after kidney transplantation. The patient restarted haemodialysis for severe renal insufficiency and anti-C5 antibody eculizumab was used as salvage treatment with progressive recovery of graft function and suppression of dialysis.
Keywords: atypical haemolytic-uraemic syndrome, kidney transplantation, monoclonal antibody
Case report
A 28-year-old man presented to the emergency department with renal failure, lactate dehydrogenase (LDH) elevation, low complement levels; C3 0.584 (0.820–1.870 g/L) and C4 0.136 (0.110–0.450 g/L) and low haptoglobin level. Renal biopsy showed thrombotic microangiopathy. Genetic analysis revealed a mutation on complement H factor gene (c.3514G>T; Glu1172Stop ) with low concentration levels of the protein 14.78 mg/dL (normal 12–56 mg/dL). He was diagnosed with atypical haemolytic–uraemic syndrome (aHUS), and plasma exchange therapy was administered every-other-day for 1 month with no response. The patient started chronic daily haemodialysis in November 2005. Cocaine consumption was considered the only risk factor that could trigger the disease, which was also associated with severe dilated cardiomyopathy.
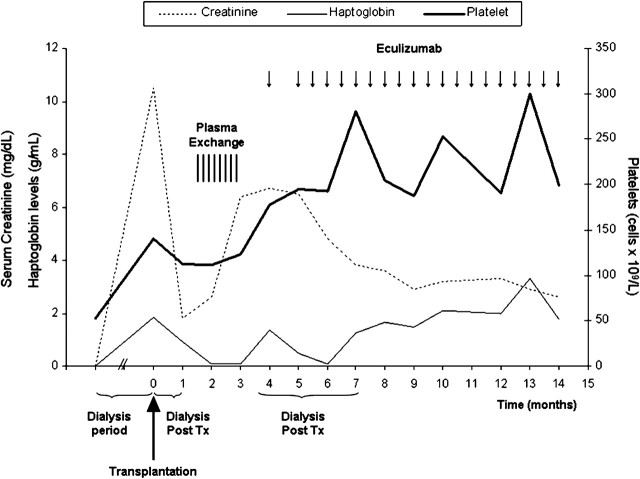
Four years later (August 2009), the patient received a non-heart beating donor kidney graft. Immunosuppressive therapy consisted of thymoglobulin, prednisone, mycophenolic acid and sirolimus. He developed delayed graft function and was on haemodialysis for 20 days. Renal biopsy confirmed the presence of acute tubular necrosis and absence of both acute rejection and thrombotic microangiopathy. Diuresis and renal function progressively recovered, and dialysis was stopped 25 days after transplantation. A serum creatinine concentration of 1.8 mg/dL was reached before hospital discharge at 40 days after transplantation.
One month later, a substantial increase in serum creatinine concentration (2.8 mg/dL) was detected and the patient was admitted after sporadic cocaine consumption. Blood test parameters and renal biopsy confirmed the recurrence of aHUS with severe renal involvement. Intense plasma exchange (eight every-other-day sessions) was started, achieving mild control of haemolytic activity and renal function stabilized with a serum creatinine concentration of 3 mg/dL.
One month later, the patient was re-admitted to hospital because of uraemic syndrome, with a serum creatinine concentration of 6.4 mg/dL, LDH 1100 UI/dL, haemoglobin 7.6 g/dL and platelet count 105 000/mm3. He admitted sporadic cocaine consumption. A new renal biopsy confirmed the severity of the thrombotic microangiopathy with glomerular capillary thrombosis and Grade I chronic allograft nephropathy. The patient started chronic dialysis (5 × weeks), and treatment with eculizumab (900 mg weekly for 4 weeks followed by 1200 mg every other week thereafter until now) was initiated. Anti-meningococcal vaccination was administered before the first dose of eculizumab. Renal function was followed up with an isotopic renogram. Two months after starting dialysis and eculizumab treatment, the patient reported a progressive increase in diuresis. Isotope studies confirmed a marked and progressive improvement in renal parameters. Dialysis was gradually reduced, and 3.5 months after restarting dialysis, the patient ceased replacement therapy with a serum creatinine concentration of 5 mg/dL [creatinine clearance (CrCl) 12 mL/min]. Renal function progressively improved, with a current serum creatinine concentration of 2.6 mg/dL (CrCl 35 mL/min), the patient being in good health (Figure 1). Eculizumab treatment (1200 mg per intravenous/2 weeks) has been maintained with good tolerance. No haematological parameters of aHUS have been detected in the last 10 months. Strict control of blood/urine cocaine has been negative.
Fig. 1.
Ten months evolution of renal function after eculizumab initiation.
Discussion
Haemolytic–uraemic syndrome is characterized by non-immune haemolytic anaemia, thrombocytopaenia and renal impairment with thrombotic microangiopathy [1]. aHUS is generally caused by mutations in genes of the alternative pathway of the complement system [2]. These mutations have been described in genes encoding complement factor H (CFH), I (CFI), B (CFB), C3 and membrane cofactor protein [2]. Recently, the presence of autoantibodies against complement factor H has been described and genes encoding thrombomodulin have been associated with aHUS [1].
Identification of the genetic mutation is an important prognostic factor in kidney transplantation. More than 50% of transplant recipients with some mutations have disease recurrence and 90% develop renal insufficiency. Patients with CFH or CFI have the least favourable prognosis after kidney transplantation [1, 2].
Recently, Tanhehco et al. [3] reported that cocaine could increase complement protein expression in isolated rabbit hearts. Cocaine can trigger inflammation and complement activation, upregulating the expression of adhesion molecules and promoting leucocyte migration. The final result is platelet activation with severe endothelial toxicity [3].
Eculizumab is a humanized monoclonal antibody that is specific for human C5 complement protein and prevents the activation of terminal complement complex C5b-9. This drug was approved for the treatment of paroxysmal nocturnal haemoglobinuria. Recent and sporadic cases have suggested the efficacy of eculizumab in the treatment of aHUS and post-transplantation aHUS recurrence [4]. Eculizumab could be a good therapeutic alternative in patients with resistant aHUS and may be effective in the prevention of aHUS recurrence after renal transplantation in patients with genetic mutations [5].
The present case illustrates a patient with severe aHUS with CFH deficiency triggered by cocaine use and recurrence after kidney transplantation. The patient re-started haemodialysis for severe renal insufficiency and anti-C5 antibody eculizumab was used as salvage treatment. Although the patient has been on haemodialysis for >3 months, absolute control of haemolytic parameters with the use of eculizumab allowed progressive recovery of graft function with suppression of dialysis. Ten months later, the patient is stable with acceptable kidney function and no sign of haemolytic activity. Based on this experience, eculizumab treatment should be considered in patients with severe aHUS despite the presence of renal insufficiency or dialysis dependency. Renal biopsy may be required to define the viability of the renal parenchyma and reversibility of aHUS and renal insufficiency. Prospective studies are mandatory to define the role of eculizumab in the treatment of aHUS but preliminary experiences are highly encouraging in terms of the drug’s efficacy and tolerability.
Acknowledgments
Conflict of interest statement. None declared.
(See related articles by: Fremeaux-Bacchi. Treatment of atypical uraemic syndrome in the era of eculizumab. Clin Kidney J 2012; 5: 4–6; Garjau et al. Early treatment with eculizumab in atypical haemolytic uraemic syndrome. Clin Kidney J 2012; 5: 31–33; and Kim et al. Eculizumab in atypical haemolytic uraemic syndrome allows cessation of plasma exchange and dialysis. Clin Kidney J 2012; 5: 34–36.)
References
- 1.Noris M, Remuzzi G. Atypical hemolytic–uremic syndrome. N Engl J Med. 2009;361:1676. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 2.Bienaime F, Dragon-Durey MA, Regnier CH, et al. Mutations in components of complement influence the outcome of Factor I-associated atypical hemolytic uremic syndrome CFI mutations in aHUS. Kidney Int. 2010;77:339. doi: 10.1038/ki.2009.472. [DOI] [PubMed] [Google Scholar]
- 3.Tanhehco EJ, Yasojima K, McGeer PL, et al. Acute cocaine exposure up-regulates complement expression in rabbit heart. J Pharmacol Exp Ther. 2000;292:201. [PubMed] [Google Scholar]
- 4.Larrea CF, Cofan F, Oppenheimer F, et al. Efficacy of eculizumab in the treatment of recurrent atypical hemolytic-uremic syndrome after renal transplantation. Transplantation. 2010;89:903. doi: 10.1097/TP.0b013e3181ccd80d. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerhackl L, Hofer J, Cortina G, et al. Prophylactic eculizumab after renal transplantation in atypical hemolytic–uremic syndrome. N Engl J Med. 2010;362:1746. doi: 10.1056/NEJMc1001060. [DOI] [PubMed] [Google Scholar]