Abstract
Atypical haemolytic uraemic syndrome (aHUS) is a rare and life-threatening disease caused by complement system dysregulation leading to uncontrolled complement activation and thrombotic microangiopathy. We report the case of an adult patient with plasmaphaeresis-resistant aHUS and hypertension treated with the complement inhibitor eculizumab. Eculizumab was shown to completely inhibit haemolysis, normalize thrombocyte levels and increase diuresis. Full recovery of renal function was not possible due to irreversible renal damage prior to eculizumab initiation. These findings highlight the importance of early treatment with eculizumab in patients with poor response to standard therapy, in order to avoid irreversible renal damage.
Keywords: atypical haemolytic uraemic syndrome, complement system, eculizumab, plasmaphaeresis-resistant
Background
Haemolytic uraemic syndrome (HUS) is a thrombotic microangiopathy characterized by Coombs-negative haemolytic anaemia, thrombocytopenia and microvascular thrombosis, with many patients also experiencing acute renal failure [1]. Approximately 90% of cases have typical HUS, which is secondary to infection by a Shiga-like toxin-producing Escherichia coli [1]. Atypical HUS (aHUS) is a relatively rare non-Shiga toxin-associated form of HUS and accounts for the remaining 10% of cases [1]. aHUS is associated with dysregulation of the complement system, which causes chronic uncontrolled complement activation and leads to a pro-coagulant, platelet-activation state endothelial swelling and, ultimately, thrombotic microangiopathy [1, 2]. In approximately 50% of aHUS cases, mutations in genes encoding complement regulatory proteins [e.g. membrane cofactor protein (MCP)] have been identified [1–5]. aHUS has a poor prognosis, with ∼50% of patients progressing to end-stage renal disease or dying within the first year of diagnosis, with a high risk of recurrence after kidney transplantation [1].
Patients with aHUS do not always respond to plasma exchange [6]. As aHUS is linked to complement system dysregulation, inhibition of this system has been suggested as a rational therapeutic approach [7]. Eculizumab (Soliris; Alexion Pharmaceuticals) is a humanized monoclonal antibody that binds to the complement protein C5, preventing cleavage of C5 to C5a and C5b, thereby inhibiting the generation of the terminal complement complex C5b-9a [7].
Eculizumab is approved for the treatment of paroxysmal nocturnal haemoglobinuria [8] and case reports indicate that eculizumab may also be beneficial in aHUS [2, 7, 9, 10].
Case report
A 44-year-old man was admitted to hospital with prolonged diarrhoea (lasting 1 week), fever and anuria. On admission, blood pressure was 220/150 mmHg with no signs of malignant hypertension upon retinal fundoscopy. Blood analysis showed haemolytic anaemia: haemoglobin 6.9 g/dL, schistocytes in the blood smear, lactate dehydrogenase elevated to 1837 U/L and platelet count 111 000/dL. The direct Coombs test was negative. The patient displayed acute renal failure and creatinine levels of 20.7 mg/dL.
The patient was transfused and treated with haemodialysis and plasmaphaeresis from Day 1. There was no Shiga-like toxin in the stool sample. C3, C4 and CH50 levels and ADAMTS-13 activity were normal (57%). Anti-nuclear, anti-phospholipid, anti-topoisomerase III and anti-ADAMTS-13 antibodies were negative. Three weeks after being admitted, the patient still required haemodialysis despite receiving 4 U of fresh frozen plasma and initiating plasmaphaeresis. Following 21 sessions of plasmaphaeresis, haemolysis, high blood pressure and renal failure persisted.
A molecular genetic study was performed. Classical and alternative complement pathway analysis revealed normal plasmatic factor H (26 mg/mL, normal range 12–56), 70% plasmatic concentration of factor I (normal range 71–115), without antibodies against factor H. The expression of MCP on leucocytes was 51% compared to control (normal range 91–109) and heterozygous mutation of intron 2 (c.286+1G>C) of the MCP gene.
Eculizumab was initiated 90 days after hospital admission at a dose of 900 mg weekly for 5 weeks, then 1200 mg every 2 weeks until Week 27. The patient received an anti-meningococcal vaccine 15 days prior to starting eculizumab.
During eculizumab treatment, haemolysis was inhibited completely, thrombocytes and platelets returned to normal levels and diuresis increased slowly to 1.5 L/day. The patient no longer required haemodialysis, although he continued renal replacement therapy. Blood pressure control improved, but anti-hypertensive medication was continued.
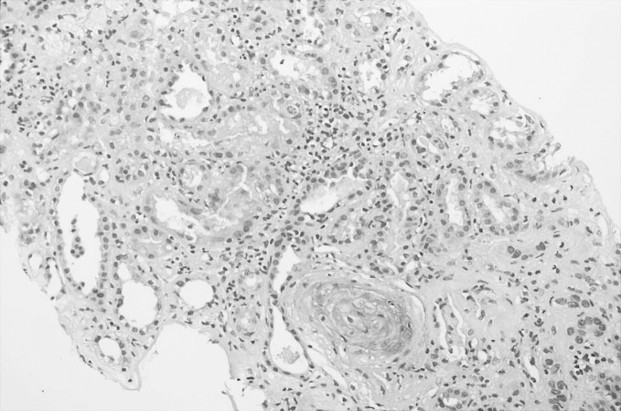
Renal biopsy (Figure 1) conducted the second week after admission revealed occlusive thrombosis in several small- and medium-sized arteries with secondary ischaemic glomerular changes. There was extensive acute tubular necrosis in the renal tubules with focal areas of tubular atrophy. The interstitium showed moderate fibrosis and mild chronic inflammatory infiltrate. Direct immunofluorescence was negative. A second renal biopsy (Figure 2) performed 2.5 months after admission, and 3 weeks before starting eculizumab, showed changes similar to those observed at the first biopsy: thrombotic microangiopathic lesions persisted and there was deterioration of interstitial fibrosis and tubular atrophy.
Fig. 1.
Renal arteriolar wall thickening, mild interstitial fibrosis and tubular atrophy areas.
Fig. 2.

Ischaemic glomeruli and artery wall thickening with thrombotic microangiopathy changes.
Discussion
aHUS is a rare and devastating disease linked to complement dysregulation and chronic uncontrolled complement activation [1]. MCP mutations occur in ∼10–15% of aHUS patients and may be familial or sporadic with incomplete disease penetrance in families [1]. aHUS usually manifests in childhood, but sporadic cases have been reported that have variable onset and are often related to infections, drugs or other clinical situations that trigger complement activation. In the case reported here, the clinical disease onset seems to have a been triggered by an infection. Patients with aHUS, particularly those with MCP mutations, often have a poor response to plasmaphaeresis and/or fresh frozen plasma infusions [1, 6] and require alternative or additional therapy.
As an inhibitor of terminal complement activation, eculizumab may offer an effective treatment option.
Our patient presented with typical HUS features, but a lack of response to plasmaphaeresis and no evidence of Shiga toxin suggested aHUS, although no family history of the disease was apparent. Based on positive results from other cases [2, 7, 9, 10], we began eculizumab therapy 90 days post-admission. Eculizumab was associated with inhibition of haemolysis and normalization of thrombocyte and platelet levels, allowing cessation of haemodialysis.
Unfortunately, renal function recovery was not possible with eculizumab in our patient. Renal biopsy revealed that irreversible damage to the kidney had already been established by the time eculizumab was initiated. Our case supports previous findings that initiation of eculizumab late in disease progression is unable to reverse pre-existing renal damage [2]. Early use of eculizumab in patients with aHUS may prevent the thrombotic microangiopathy resulting from uncontrolled complement activation and could therefore help to avoid irreversible renal damage. Indeed, eculizumab treatment has resulted in recovery of renal function in other aHUS cases [7, 10].
Our patient is currently on a waiting list for renal transplantation. aHUS caused by a mutation in the MCP gene has a good prognosis post-transplant, as the normal kidney corrects the defect and complement activation is maintained within the normal range [1].
In conclusion, this case describes how eculizumab normalized the haematological parameters in a patient with aHUS. Probably due to the late initiation of treatment, eculizumab was unable to have an impact on the irreversible renal damage that had already occurred. Consequently, we propose that early treatment initiation with eculizumab is warranted in patients with aHUS in order to avoid irreversible renal damage.
Acknowledgments
Editing assistance funded by Alexion Pharmaceuticals Inc. was provided by Helen Beaumont and Tamsin Williamson of Bioscript Stirling Ltd.
Conflict of interest statement. None declared.
(See related articles by: Fremeaux-Bacchi. Treatment of atypical uraemic syndrome in the era of eculizumab. Clin Kidney J 2012; 5: 4–6; Duran et al. Rescue therapy with eculizumab in a transplant recipient with atypical haemolytic-uraemic syndrome. Clin Kidney J 2012; 5: 28–30; and Kim et al. Eculizumab in atypical haemolytic uraemic syndrome allows cessation of plasma exchange and dialysis. Clin Kidney J 2012; 5: 34–36.)
References
- 1.Noris M, Remuzzi G. Atypical hemolytic uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 2.Mache C, Acham-Roschitz B, Fremeaux-Bacchi V, et al. Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Nephrol. 2009;4:1312–1316. doi: 10.2215/CJN.01090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirt-Minkowski P, Dickenmann M, Schifferli JA. Atypical hemolytic uremic syndrome: update on the complement system and what is new. Nephron Clin Pract. 2010;114:c219–c235. doi: 10.1159/000276545. [DOI] [PubMed] [Google Scholar]
- 5.Fremeaux-Bacchi V, Miller EC, Liszewski MK, et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caprioli J, Noris M, Brioshi S, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentations response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nürnberger J, Witzke O, Saez AO, et al. Eculizumab for atypical hemolytic uremic syndrome. N Engl J Med. 2009;360:542–544. doi: 10.1056/NEJMc0808527. [DOI] [PubMed] [Google Scholar]
- 8.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 9.Gruppo RA, Rother RP. Eculizumab for congenital atypical hemolytic uremic syndrome. N Engl J Med. 2009;360:544–546. doi: 10.1056/NEJMc0809959. [DOI] [PubMed] [Google Scholar]
- 10.Lapeyraque AL, Frémeaux-Bacchi V, Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatr Nephrol. 2011;26:621–624. doi: 10.1007/s00467-010-1719-3. [DOI] [PubMed] [Google Scholar]