Abstract
Disorders in complement regulation are a major cause of atypical haemolytic–uraemic syndrome (aHUS). Eculizumab, a monoclonal antibody targeting complement C5 and blocking the terminal complement cascade, should theoretically be useful in this disease, particularly when associated with specific complement pathway anomalies such as Factor H deficiency. Eculizumab is emerging as an effective treatment for post-transplant aHUS recurrence and may have a role in treating de novo aHUS, halting the haemolytic process. In this case report, we describe the fourth case of aHUS treated with eculizumab. In our patient, with a known complement Factor H mutation, not only has the disease process become quiescent but also this therapy has led to significantly improved renal function so that dialysis is no longer necessary.
Keywords: atypical haemolytic–uraemic syndrome, dialysis, eculizumab, plasma exchange
Introduction
Haemolytic–uraemic syndrome (HUS) is a common cause of acute renal failure and microangiopathic haemolytic anaemia (MAHA). It is associated with infections (shiga toxin-producing bacteria in diarrhoea-positive HUS and Streptococcus pneumoniae). Less commonly, it is caused by defects in complement regulation, termed atypical HUS (aHUS), and early diagnosis is vital for the initiation of plasma exchange (PEX), which can lead to improved renal outcomes [1–3]. More recently, eculizumab, a monoclonal antibody which binds complement C5 and blocks terminal complement activation, has been developed.
We describe a case of aHUS secondary to complement Factor H (CFH) mutation, a key regulator of the alternative complement cascade. The infant was PEX and dialysis dependent until treated with eculizumab.
Case report
A previously healthy 7-month-old girl presented with paleness and easy bruising, preceded by croup. Her parents were non-consanguineous. Initial investigations revealed low haemoglobin (Hb) and platelets (Plts), but normal kidney function. After 6 weeks of follow-up, she developed dark urine, oliguria, fluid overload and hypertension. Hb was 4.5 g/dL, Plts were 72 × 109/L and blood film showed MAHA with elevated urea and creatinine. There was no diarrhoea or vomiting and a clinical diagnosis of aHUS was made.
Following transfer to our Nephrology Centre, she was immediately started on haemodialysis. A plasma infusion was given on the second day of admission and PEX started on the fourth day. PEX consisted of one volume exchange with 5% albumin replacement initially and 10 mL/kg of virion-inactivated plasma (Octaplas) at the end. She received nine PEX sessions in the first 2 weeks but then developed hypertensive encephalopathy requiring ventilation, continuous veno-venous haemofiltration and sodium nitroprusside. This resolved after 5 days and PEX was recommenced thrice weekly.
Genetic testing revealed a previously undescribed sequence variant in Exon 23 of CFH (c.3568T>C; p.Tyr1190His), substituting a highly conserved amino acid. No mutations were found in CFI or CD46. Factor H autoantibodies were negative. Other complement tests were normal, including C3, C4, classical and alternative pathway activity, Factor H and Factor I levels. ADAMSTS13 activity was slightly low at 25% (normal range 55–166) but not consistent with deficiency. Other investigations for aHUS [1] were negative, including homocysteine, methylmalonic acid and autoimmune profile. Stool cultures and Escherichia coli 0157 serology were negative.
Peritoneal dialysis was commenced to manage her renal failure. Attempts at weaning PEX were unsuccessful due to haematological relapses (Figure 1). She required nine packed red blood cell transfusions in 4 months and blood priming for PEX sessions. She had three exacerbations of HUS following infectious illnesses despite regular PEX.
Fig. 1.
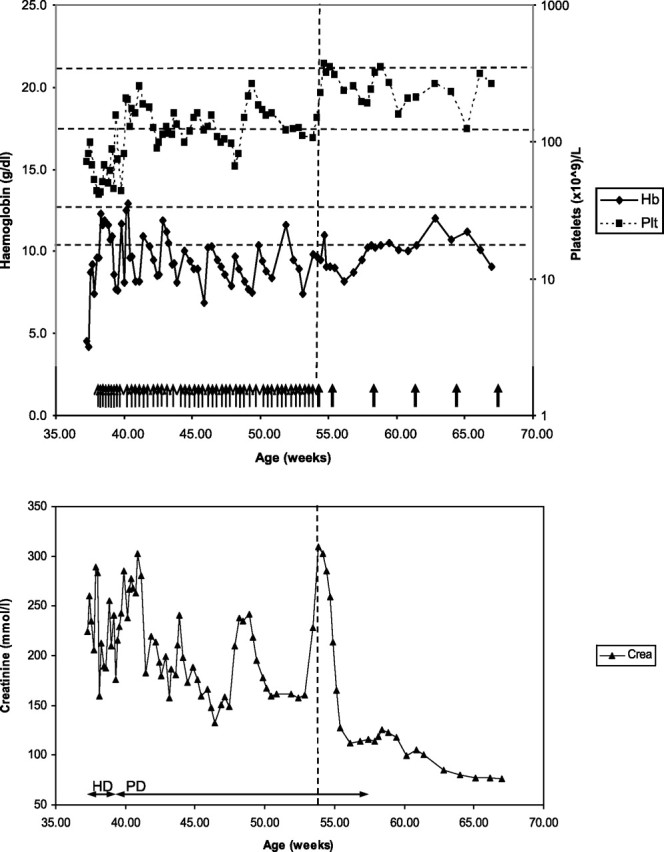
Clinical progress. Each thin arrow denotes a plasma exchange session (1 volume exchange with 5% albumin and 10 ml/kg virion inactivated plasma). Thick arrows denote eculizumab infusions. Vertical line represents the first eculizumab infusion. Horizontal dotted lines represent normal ranges for haemoglobin (bottom) and platelets (top) respectively. Time on haemodialysis (HD) and peritoneal dialysis (PD) represented by double arrowed lines.
In an attempt to modify the disease process, eculizumab was started. She received tetravalent conjugate Meningococcal ACWY vaccine 2 weeks beforehand. Eculizumab dosing was in accordance with manufacturer recommendations of 300 mg weekly for the first two doses and every 3 weeks subsequently. Following the first dose of eculizumab, there was recovery of haematological parameters enabling cessation of PEX. Renal function also improved, and dialysis was stopped 1 month after the first dose, with ongoing fall in creatinine. At last follow-up aged 18 months, she has an estimated glomerular filtration rate of 42 mL/min/1.73m2 (Schwartz formula), with excellent neurodevelopmental progress.
Discussion
Eculizumab is a humanized monoclonal antibody, which blocks the cleavage of complement C5 to C5a (a potent chemotactant) and C5b. This inhibits the progression of C5b to the terminal complement complex (TCC) C5b-9. Levels of TCC are high in aHUS, consistent with increased complement activation, and the improvement post-eculizumab supports this concept of disease pathogenesis [4].
There are four case reports describing eculizumab treatment of aHUS relapses in native kidneys [5–8]. In all cases, there was effective cessation of haemolysis and haematological recovery within 7–10 days. In three cases, there was also recovery in renal function and these patients avoided dialysis. Eculizumab was continued as maintenance therapy [5–7]. In the fourth case, there was improvement in renal function after a single dose of eculizumab. Eculizumab was used again in a subsequent relapse but there was progression to anuria and eculizumab was stopped after haemodialysis was started [8]. Two patients had identifiable mutations in CFH [7, 8]. This highlights that eculizumab is effective even if the mutation is not known.
The publication of case reports, however, is susceptible to positive bias reporting. Open-label trials in adolescents and adults have finished recruiting and a multicentre paediatric trial is ongoing though patients receiving chronic dialysis are excluded (Clinicaltrials.gov NCT01193348).
The long-term management of aHUS remains controversial. Cost considerations aside, the long-term side effects of TCC blockade in children are not known, though experiences in paroxysmal nocturnal haemoglobinuria have not highlighted any serious complications [9]. The main risk is infection from encapsulated organisms and patients require tetravalent meningococcal vaccination beforehand. However, Neisseria meningitidis serogroup B is currently not covered and prophylactic penicillin is strongly advisable [10]. An alternative strategy would be liver (with or without kidney) transplantation, which would restore production of functionally normal CFH [11]. Haller et al. successfully performed an isolated liver transplant using an established protocol with pre-transplant PEX and intratransplant plasma infusion and anticoagulation. Liver and renal function remain stable 2 years post-transplant with no aHUS relapses though the authors caution that the benefits of this treatment have to be carefully balanced against the risks of major surgery and long-term immunosuppression including calcineurin nephrotoxicity [11].
In our patient, not only did eculizumab allow immediate cessation of three times weekly PEX, it also resulted in renal recovery and allowed discontinuation of dialysis despite being dialysis dependant for 4 months before eculizumab therapy. There was a significant improvement in both the clinical condition of the child and the quality of life for the family. Although eculizumab is an expensive drug, the cost is significantly less than that of dialysis and regular PEX.
In summary, eculizumab therapy should be considered in patients with aHUS. In those with an underlying complement defect, reversal of the haematological manifestations is expected, and in addition, some dialysis-dependant patients may be able to stop dialysis.
Acknowledgments
Dr M. Taylor provided clinical insight into the appropriate use of eculizumab in this patient.
Conflict of interest statement. None declared.
(See related articles by: Fremeaux-Bacchi. Treatment of atypical uraemic syndrome in the era of eculizumab. Clin Kidney J 2012; 5: 4–6; Duran et al. Rescue therapy with eculizumab in a transplant recipient with atypical haemolytic-uraemic syndrome. Clin Kidney J 2012; 5: 28–30; and Garjau et al. Early treatment with eculizumab in atypical haemolytic uraemic syndrome. Clin Kidney J 2012; 5: 31–33.)
References
- 1.Ariceta G, Besbas N, Johnson S, et al. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol. 2009;24:687–696. doi: 10.1007/s00467-008-0964-1. [DOI] [PubMed] [Google Scholar]
- 2.Waters AM, Licht C. aHUS caused by complement dysregulation: new therapies on the horizon. Pediatr Nephrol. 2011;26:41–57. doi: 10.1007/s00467-010-1556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JJ, Goodship THJ, Tizard EJ, et al. Plasma therapy for atypical haemolytic uraemic syndrome associated with heterozygous factor H mutations. Pediatr Nephrol. 2011;26:2073–2076. doi: 10.1007/s00467-011-1944-4. [DOI] [PubMed] [Google Scholar]
- 4.Prüfer F, Scheiring J, Sautter S, et al. Terminal complement complex (C5b-9) in children with recurrent haemolytic uremic syndrome. Semin Thromb Hemost. 2006;32:121–127. doi: 10.1055/s-2006-939768. [DOI] [PubMed] [Google Scholar]
- 5.Gruppo RA, Rother RP. Eculizumab for congenital atypical haemolytic-uremic syndrome. N Engl J Med. 2009;360:544–546. doi: 10.1056/NEJMc0809959. [DOI] [PubMed] [Google Scholar]
- 6.Lapeyraque AL, Frémeaux-Bacchi V, Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatr Nephrol. 2011;26:621–624. doi: 10.1007/s00467-010-1719-3. [DOI] [PubMed] [Google Scholar]
- 7.Tschumi S, Gugger M, Bucher BS, et al. Eculizumab in atypical hemolytic uremic syndrome: long-term clinical course and histological findings. Pediatr Nephrol. 2011;26:2085–2088. doi: 10.1007/s00467-011-1989-4. [DOI] [PubMed] [Google Scholar]
- 8.Mache CJ, Acham-Roschitz B, Frémeaux-Bacchi V, et al. Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4:1312–1316. doi: 10.2215/CJN.01090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Röth A, Hock C, Konik A, et al. Chronic treatment of paroxysmal nocturnal hemoglobinuria patients with eculizumab: safety, efficacy, and unexpected laboratory phenomena. Int J Hematol. 2011;93:704–714. doi: 10.1007/s12185-011-0867-y. [DOI] [PubMed] [Google Scholar]
- 10.Bouts A, Monnens L, Davin JC, et al. Insufficient protection by Neisseria meningitidis vaccination alone during eculizumab therapy. Pediatr Nephrol. 2011 doi: 10.1007/s00467-011-1929-3. doi:10.1007/s00467-011-1929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haller W, Milford DV, Goodship TH, et al. Successful isolated liver transplantation in a child with atypical haemolytic uremic syndrome and a mutation in complement factor H. Am J Transplant. 2010;10:2142–2147. doi: 10.1111/j.1600-6143.2010.03228.x. [DOI] [PubMed] [Google Scholar]


