Abstract
Henoch–Schönlein purpura (HSP) is a systemic small-vessel leucocytoclastic vasculitis with deposition of immune complexes containing Immunoglobulin A (IgA). IgA Nephropathy (IgAN) is a glomerulonephritis caused by mesangial deposition of IgA. The onset of HSP, but not IgAN, has been linked to influenza vaccination.
We report the first case of HSP with glomerular involvement, in a renal transplant recipient following influenza vaccination. The patient had prior end-stage renal failure (ESRF) secondary to IgAN, without clinical evidence of IgAN recurrence after transplantation. This is of clinical relevance as influenza vaccination is regarded safe, effective, and recommended after renal transplantation.
Nephrologists should be aware of the potential for influenza vaccination to have adverse effects in renal transplant recipients, especially if the primary renal disease is HSP or IgAN.
Keywords: Henoch–Schönlein purpura (HSP), Immunoglobulin A nephropathy (IgAN), influenza vaccination, renal transplant
Background
Henoch-Schönlein Purpura (HSP) is a systemic small vessel, leucocytoclastic vasculitis with deposition of immune complexes containing Immunoglobulin A (IgA) [1]. It is rare in adults, with incidence 1.3 per 100 000 [1]. HSP is often considered the systemic form of IgA Nephropathy (IgAN), a common glomerulonephritis characterized by diffuse mesangial deposition of abnormally glycosylated IgA1 [2].
Aetiology of HSP remains uncertain, with various environmental, infectious, neoplastic and pharmacological triggers implicated in the pathogenesis. The onset of HSP following influenza vaccination has been described [3–5], but not in renal transplant recipients. Influenza vaccination is considered to be safe, effective, and recommended in renal transplant patients [6, 7].
We report the first case of HSP and biopsy proven-graft IgAN in a renal transplant recipient, whose primary disease was IgAN, following influenza vaccination.
Case report
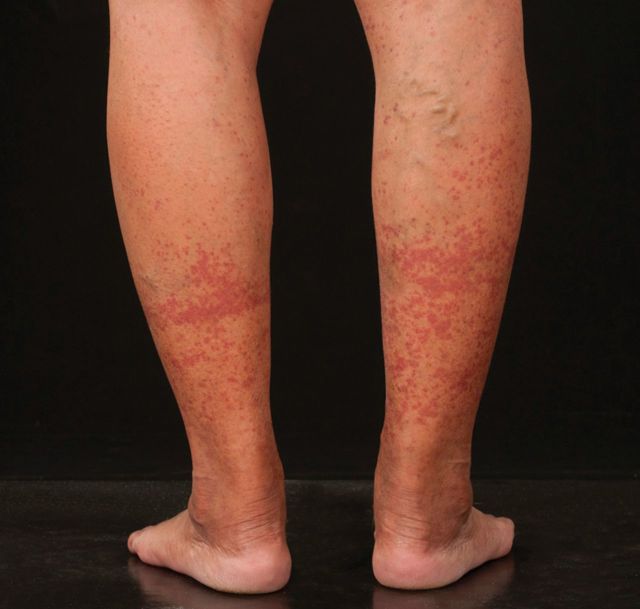
A 49-year-old female of South East Asian ethnicity presented with a progressive, palpable, purpuric rash involving the limbs, 10 days after annual seasonal influenza (‘Fluvac’) vaccination (Figure 1). There was no arthralgia, fever, abdominal pain, haematuria, recent infection, or overseas travel. She was afebrile, normotensive (132/77 mmHg) with no other examination abnormalities.
Fig. 1.
Lower limb purpuric rash.
Medical history included hypertension, gastro-oesophageal reflux disease and biopsy-proven IgAN at age 33 that progressed to end-stage renal failure (ESRF) over 6 years, without features of HSP. At age 41, she underwent uncomplicated 3/6 human leucocyte antigen mismatch, living-related donor, kidney transplant. Medications included ciclosporin A 75 mg twice daily, prednisone 10 mg daily, azathioprine 125 mg daily, diltiazem 360 mg daily, and ranitidine 150 mg daily. Baseline creatinine after transplant was 90–105 μmol/L, without microscopic haematuria or proteinuria. She had previously recieved ‘Fluvac’ after transplantation without incident.
On initial presentation, full blood count and coagulation profile were normal. Serum creatinine was 100 μmol/L, and the estimated glomerular filtration rate (eGFR) was 51 mL/min/1.73 m2 [Modification of Diet in Renal Disease (MDRD) formula]. Urinalysis revealed <10 × 106/L white and <10 × 106/L red blood cells (RBCs), no casts and total urine protein:creatinine ratio (TPCR) 17.8 g/mol. Anti-neutrophil cytoplasmic antibodies, anti-glomerular basement membrane antibody, anti-nuclear antibody, rheumatoid factor, complement, serum cryoglobulins and streptococcal serology were unremarkable. Skin biopsy revealed leucocytoclastic vasculitis with positive immunofluorescence for IgA. A diagnosis of HSP was made. Purpura resolved at 4-months follow-up.
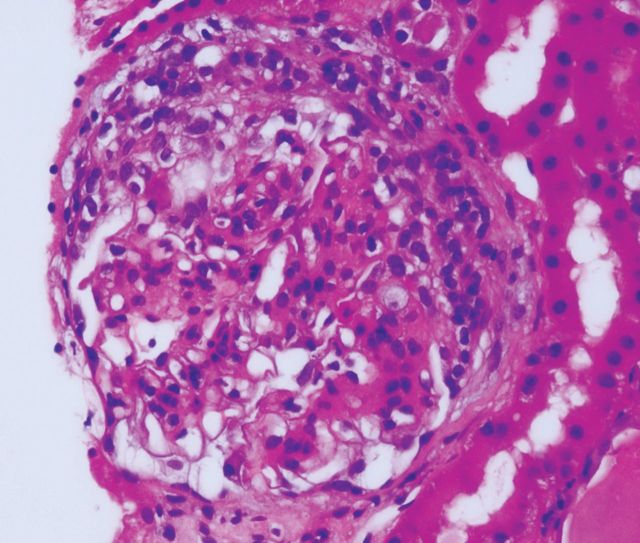
Seven weeks after the onset of purpura, there was microscopic haematuria (50–100 × 106/L RBCs) which persisted for 6 months, along with the development of cellular casts, dysmorphic RBCs, nephrotic range proteinuria (urine TPCR 658.1 g/mol), oedema and worsening hypertension (148/96 mmHg). Renal function remained stable [creatinine 102 μmol/L, eGFR (MDRD) 50 mL/min/1.73 m2]. Allograft biopsy showed active IgAN, with focal cellular or fibrocellular crescents in 4 of 25 glomeruli (Figure 2), and moderate (2+) mesangial staining for IgA. There was minor interstitial fibrosis and no features of rejection.
Fig. 2.
Renal transplant biopsy—active IgA nephropathy with focal crescent formation.
Cilazapril 1 mg daily and frusemide 40 mg daily were introduced for hypertension, proteinuria and oedema. Influenza vaccination was considered to be the trigger of HSP with subsequent development of IgAN. Microscopic haematuria and proteinuria persisted for 12 months without deterioration in renal function. ‘Fluvac’ successfully prevented influenza infection, but she was advised to avoid future influenza vaccination.
Discussion
This is the first reported case of HSP and IgAN in a renal transplant recipient following influenza vaccination. The onset of HSP has been linked to influenza vaccination in non-renal transplant patients [3–5]. An immune reaction between influenza vaccine components and native IgA antibodies is thought to result in vascular endothelial injury and HSP [3, 5]. A recent literature review outlined 11 cases of HSP following influenza vaccination [3]. Like our patient with IgAN, five had immune-mediated disease. One developed ESRF, and another chronic glomerulonephritis. The authors recommended that patients with influenza vaccine-induced HSP should not be revaccinated.
Patients with IgAN may have excessive IgA1 responses following influenza vaccination, and IgA1 is implicated in the pathogenesis of IgAN [2, 8]. However, there have been no reports of influenza vaccination triggering de novo or recurrent IgAN, and our patient had previously received ‘Fluvac’ after transplantation without evidence of IgAN recurrence.
One could deduce that the renal biopsy changes in this case could be explained by HSP nephritis or de novo or recurrent IgAN post-transplant. Renal biopsy positivity for fibrin may have helped to differentiate between IgAN (without fibrin) and HSP nephritis (with fibrin) [9], but our pathologists did not perform this staining technique. Nonetheless, given the sequence of vaccination, purpura and urinary abnormalities, we believe mesangial IgA deposition occurred as a consequence of influenza vaccine-induced HSP nephritis rather than de novo or recurrent IgAN. The temporal relationship (10 days) between vaccination and purpura was consistent with the reported latency of 11.7 days (range 1–22 days) [3]. Diagnostic criteria of HSP were met (purpura, IgA staining of dermal capillary walls and renal involvement) [10].
Approximately one-third of patients with ESRF secondary to IgAN experience histological or clinical recurrence following transplantation [11], with the 10-year cumulative incidence of graft loss from recurrent IgAN being 4.3% [12]. In regards to HSP, histological recurrence is common (61%) when performing routine kidney biopsy post-transplant, but this seldom leads to clinical consequences [13]. Kanaan et al. reported that the actuarial risk of clinical recurrence of HSP (histological recurrence plus microscopic haematuria and proteinuria ± extra-renal signs of HSP) in a first graft was 2.5% and 11.5%, and the risk of first graft loss due to recurrent HSP was 2.5% and 7.5% at 5 and 10 years, respectively [14]. The median follow-up in this study was 105 months.
At the 12 month follow-up, our patient had evidence of active glomerulonephritis with enduring microscopic haematuria and proteinuria. These urinary abnormalities, along with crescents, represent risks of ESRF in HSP nephritis and recurrent IgAN [15–19] and could portend allograft failure earlier than otherwise expected in this case. In one study, 10 of 10 patients with recurrent or de novo crescentic IgAN post-transplant developed progressive renal dysfunction, oedema, proteinuria and haematuria, and despite alterations in immunosuppression, 9 of 10 patients advanced to ESRF after 6–36 months [17]. Similarly, steroid treatment was unsuccessful in abating renal failure over 6 months in a patient with recurrent crescentic IgAN 10 months post-transplant [16]. However, resolution of urinary abnormalities with normal renal function may occur in up to 20% of adult patients with HSP nephritis [15], and some with recurrent IgAN or HSP will have a benign renal course, despite the presence of crescents [18, 19].
Although crescents and minimal interstitial fibrosis were seen on the renal biopsy, a trial of high-dose corticosteroids ± cyclophosphamide was not considered when balancing the risk of these treatments with that of progressive allograft failure. Our patient had remained well, without deterioration in renal function after 1 year. Moreover, there is no randomized-controlled evidence that any immunosuppressive agent favourably alters renal outcomes in HSP nephritis [19, 20] and recurrent IgAN [10]. An angiotensin converting enzyme (ACE) inhibitor was commenced as this is effective in reducing proteinuria and blood pressure in recurrent IgAN [21].
In conclusion, nephrologists should be cognizant of the potential for allograft dysfunction following influenza vaccination in renal transplant recipients, especially if the primary cause of ESRF was immune-mediated, such as IgAN or HSP. Due to the benefits of influenza vaccination and the apparent rarity of this complication, we do not support limiting its use in kidney transplant recipients. In patients with influenza vaccine-induced HSP, repeat vaccination should be avoided. Aside from ACE inhibition for proteinuria and blood pressure control, evidence-based immunosuppressive treatment options that improve renal outcomes in crescentic HSP nephritis and recurrent IgAN remain elusive.
Conflict of interest statement
None declared.
References
- 1.Hung SP, Yang YH, Lin YT, et al. Clinical manifestations and outcomes of Henoch-Schönlein purpura: comparison between adults and children. Pediatr Neonatal. 2009;50:162–168. doi: 10.1016/S1875-9572(09)60056-5. [DOI] [PubMed] [Google Scholar]
- 2.van den Wall Bake AW, Beyer WE, Evers-Schouten JH, et al. Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J Clin Invest. 1989;84:1070–1075. doi: 10.1172/JCI114269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe T. Henoch-Schönlein purpura following influenza vaccinations during the pandemic of influenza A (H1N1) Pediatr Nephrol. 2011;26:795–798. doi: 10.1007/s00467-010-1722-8. [DOI] [PubMed] [Google Scholar]
- 4.Pimentel MI, Vasconcellos Ede C, Cerbino-Neto J. Henoch–Schönlein purpura following influenza A H1N1 vaccination. Rev Soc Bras Med Trop. 2011;44:531. doi: 10.1590/s0037-86822011000400029. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T, Onda H. Henoch-Schönlein purpura with antiphospholipid antibodies following an influenza vaccination. Pediatr Nephrol. 2001;16:458–459. doi: 10.1007/s004670100569. [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Zeier MG, Craig JC, et al. KDIGO Clinical Practice Guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S41–S43. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 7.Scharpé J, Evenepoel P, Maes B, et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant. 2008;8:332–337. doi: 10.1111/j.1600-6143.2007.02066.x. [DOI] [PubMed] [Google Scholar]
- 8.Kashem A, Endoh M, Yano N, et al. In vivo antibody response to mucosal (NASAL) and subcutaneous stimulation of influenza virus in patients with IgA nephropathy. Tokai J Exp Clin Med. 1992;17:23–28. [PubMed] [Google Scholar]
- 9.Davin J, Berge IJ, Weening JJ. What is the difference between IgA nephropathy and Henoch–Schönlein purpura nephritis? Kidney Int. 2001;59:823–834. doi: 10.1046/j.1523-1755.2001.059003823.x. [DOI] [PubMed] [Google Scholar]
- 10.Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936–941. doi: 10.1136/ard.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol. 2010;5:2363–2372. doi: 10.2215/CJN.06720810. [DOI] [PubMed] [Google Scholar]
- 12.Clayton P, McDonald S, Chadban S. Steroids and recurrent IgA nephropathy after kidney transplantation. Am J Transplant. 2011;11:1645–1649. doi: 10.1111/j.1600-6143.2011.03667.x. [DOI] [PubMed] [Google Scholar]
- 13.Thervet E, Aouizerate J, Noel LH, et al. Histological recurrence of Henoch–Schonlein purpura nephropathy after renal transplantation on routine allograft biopsy. Transplantation. 2011;92:907–912. doi: 10.1097/TP.0b013e31822e0bcf. [DOI] [PubMed] [Google Scholar]
- 14.Kanaan N, Mourad G, Thervet E, et al. Recurrence and graft loss after kidney transplantation for Henoch–Schönlein purpura nephritis: a multicenter analysis. Clin J Am Soc Nephrol. 2011;6:1768–1772. doi: 10.2215/CJN.00520111. [DOI] [PubMed] [Google Scholar]
- 15.Pillebout E, Thervet E, Hill G, et al. Henoch–Schönlein purpura in adults: outcome and prognostic factors. Am Soc Nephrol. 2002;13:1271–1278. doi: 10.1097/01.asn.0000013883.99976.22. [DOI] [PubMed] [Google Scholar]
- 16.Benabdallah L, Rerolle JP, Peraldi MN, et al. An unusual recurrence of crescentic nephritis after renal transplantation for IgA Nephropathy. Am J Kidney Dis. 2002;40:E20. doi: 10.1053/ajkd.2002.36931. [DOI] [PubMed] [Google Scholar]
- 17.Tang Z, Ji SM, Chen DR, et al. Recurrent or de novo IgA nephropathy with crescent formation after renal transplantation. Ren Fail. 2008;30:611–616. doi: 10.1080/08860220802134516. [DOI] [PubMed] [Google Scholar]
- 18.Kowalewska J, Yuan S, Sustento-Reodica N, et al. IgA nephropathy with crescents in kidney transplant recipients. Am J Kidney Dis. 2005;45:167–175. doi: 10.1053/j.ajkd.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Shrestha S, Sumingan N, Tan J, et al. Henoch–Schönlein purpura with nephritis in adults: adverse prognostic indicators in a UK population. QJM. 2006;99:2053–2065. doi: 10.1093/qjmed/hcl034. [DOI] [PubMed] [Google Scholar]
- 20.López Meiller MJ, Cavallasca JA, Maliandi Mdel R, et al. Henoch–Schönlein Purpura in adults. Clinics (Sao Paulo) 2008;63:273–276. doi: 10.1590/s1807-59322008000200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oka K, Imai E, Moriyama T, et al. A clinicopathological study of IgA nephropathy in renal transplant recipients: Beneficial effect of angiotensin-converting enzyme inhibitor. Nephrol Dial Transplant. 2000;15:689–695. doi: 10.1093/ndt/15.5.689. [DOI] [PubMed] [Google Scholar]